Atmospheric Nitrogen Dioxide Improves Photosynthesis in Mulberry Leaves via Effective Utilization of Excess Absorbed Light Energy
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. NO2 Fumigation Treatment
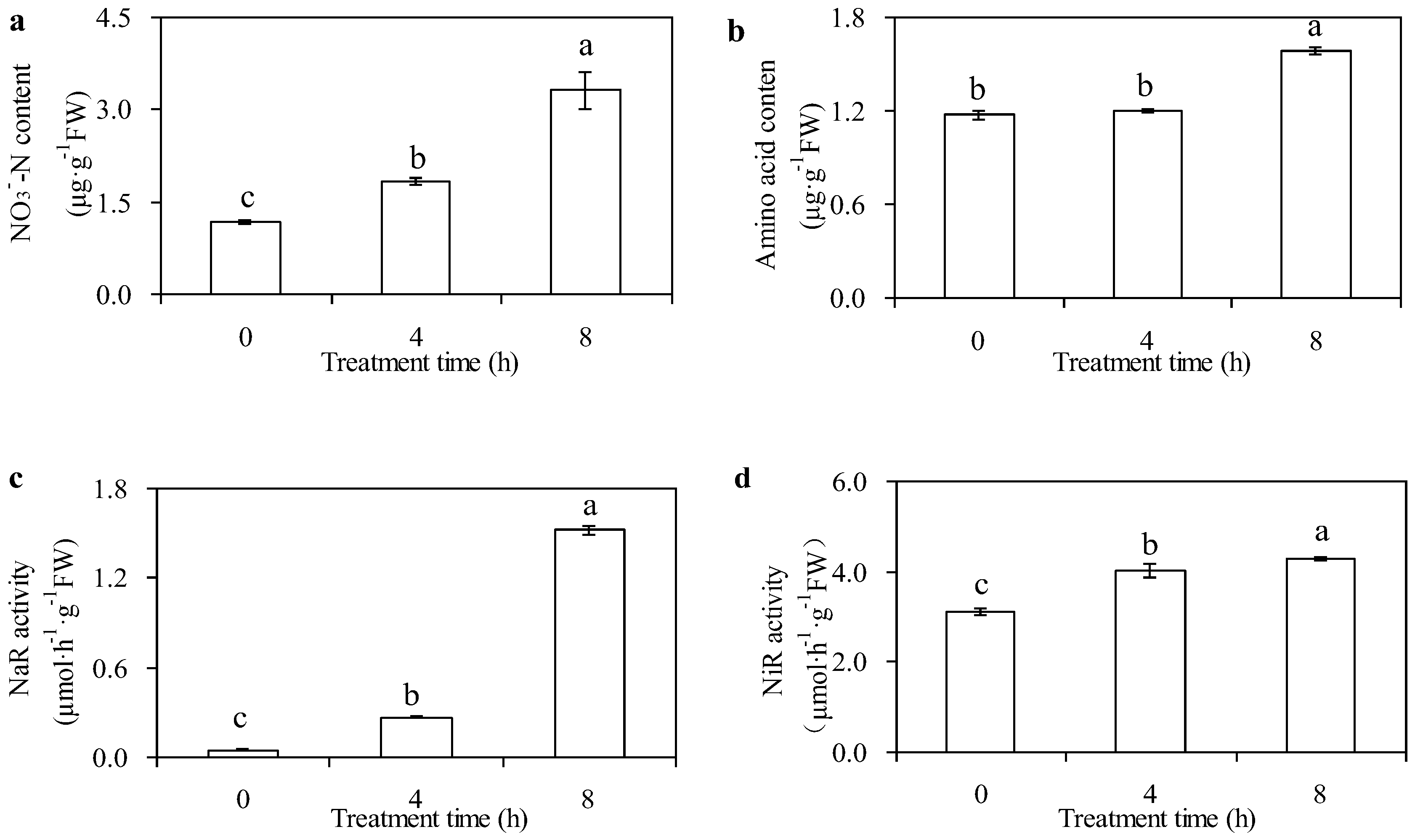
2.3. Measurement of NO3−-N Content
2.4. Measurement of Amino Acid Content
2.5. Measurement of Nitrate Reductase Activity
2.6. Measurement of Nitrite Reductase Activity
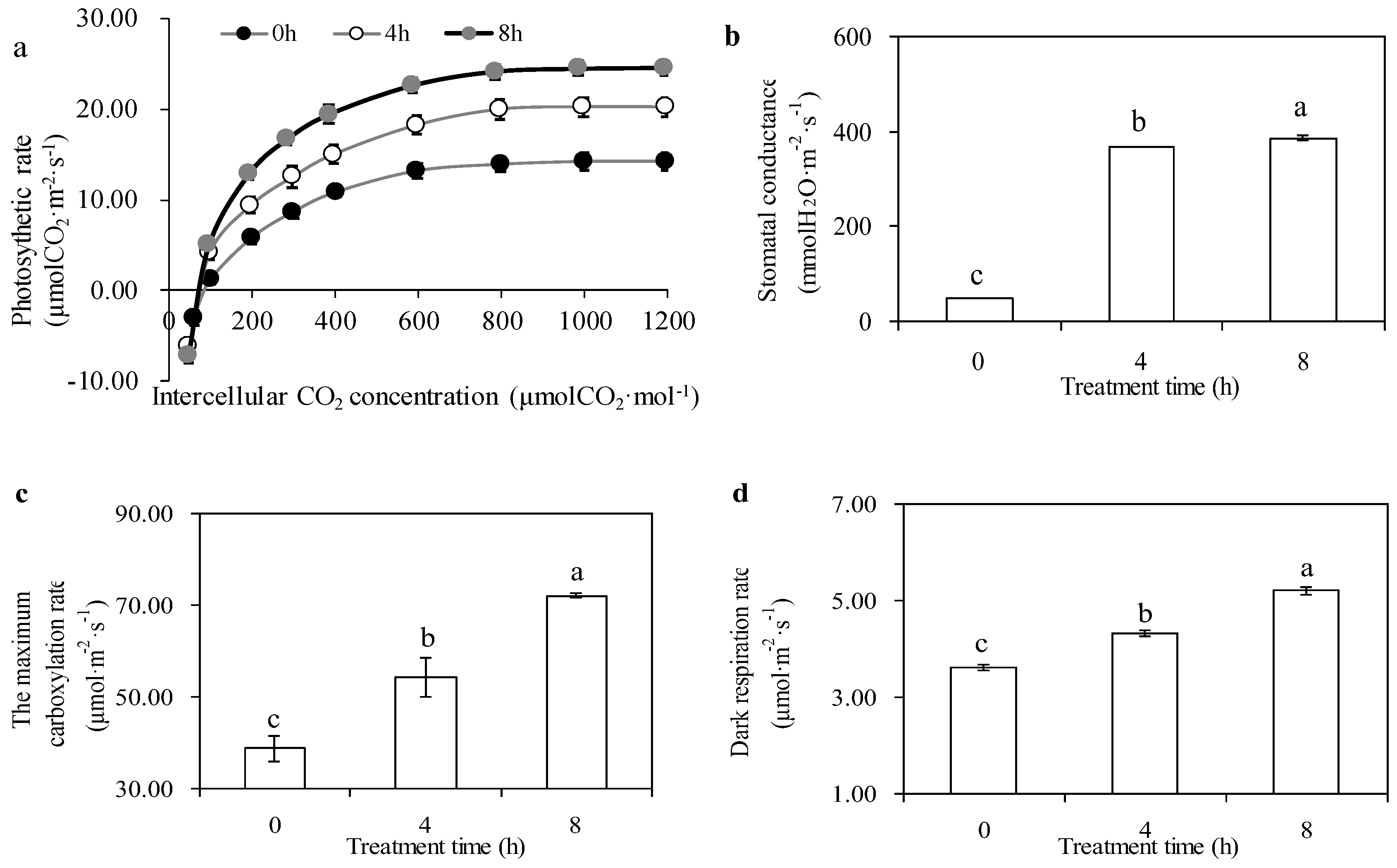
2.7. Measurement of Gas Exchange Parameters
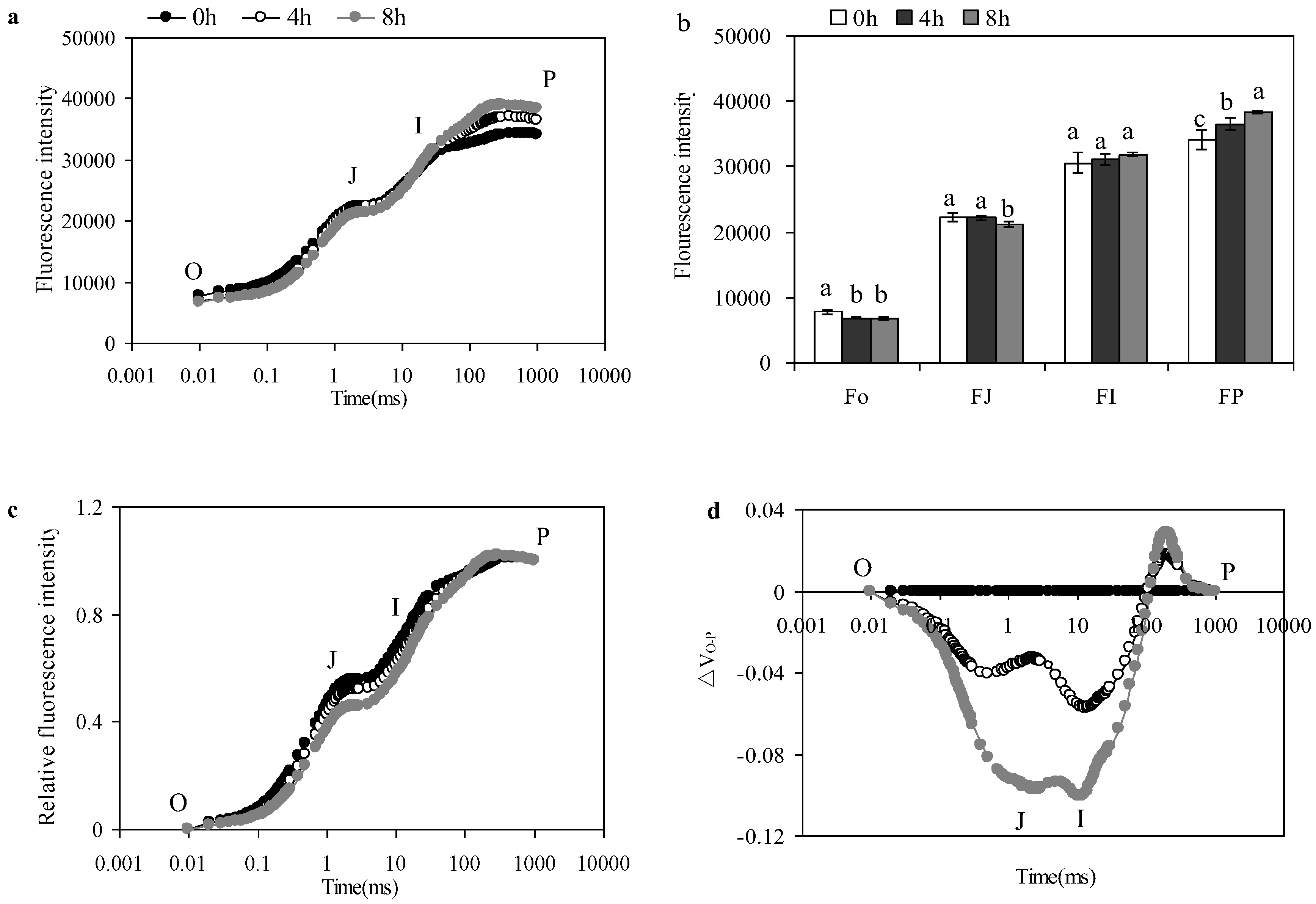
2.8. Measurement of Chlorophyll A Fluorescence Transient and Light Absorbance at 820 nm
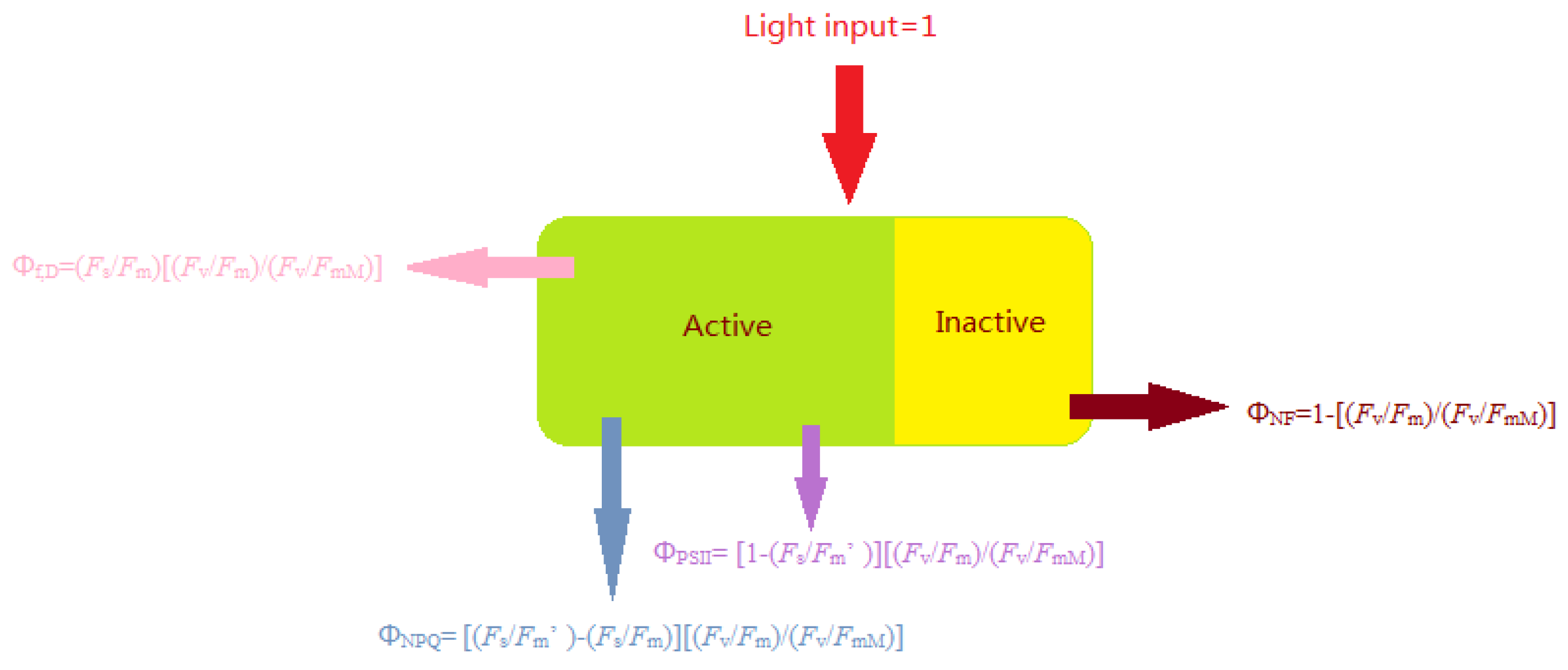
2.9. Measurement of Photochemical Quenching, Electron Transfer Rate, and Absorbed Energy of the PSII Reaction Center
ΦNPQ = [(Fs/Fm′) − (Fs/Fm)] [(Fv/Fm)/(Fv/FmM)]
Φf,D = (Fs/Fm) [(Fv/Fm)/(Fv/FmM)]
ΦNF = 1 − [(Fv/Fm)/(Fv/FmM).
2.10. Statistical Analysis
3. Results
3.1. Effects of N Metabolism Indicators
3.2. Effects of Gas Exchange Parameters
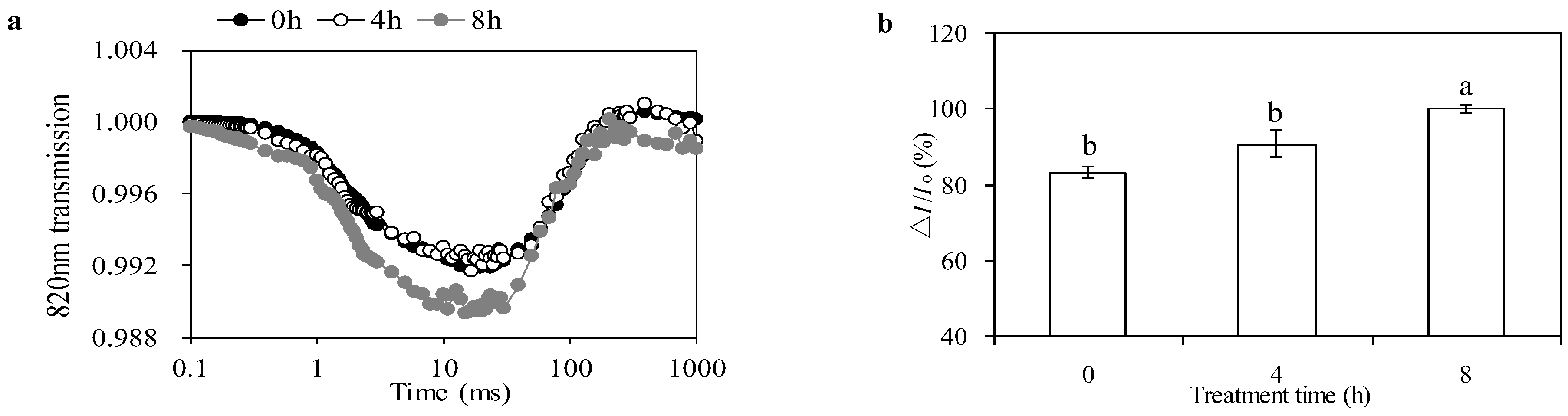
3.3. Effects of Distribution of Light Absorbed by PSII
3.4. Effects of Chlorophyll A Fluorescence Transient
3.5. Effects of PSII activity
3.6. Effects of PSI Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xia, Y.; Zhao, Y.; Nielsen, C.P. Benefits of China’s efforts in gaseous pollutant control indicated by the bottom-up emissions and satellite observations 2000–2014. Atmos. Environ. 2016, 136, 43–53. [Google Scholar] [CrossRef]
- Nouchi, I. Responses of whole plants to air pollutants. In Air Pollution and Plant Biotechnology; Springer: Tokyo, Japan, 2002; pp. 3–39. [Google Scholar]
- Wang, Y.; Li, L.J.; Liu, Y. Characteristics of atmospheric NO2 in the Beijing-Tianjin-Hebei region and the Yangtze River delta analyzed by satellite and ground observations. Chin. J. Environ. Sci. 2012, 33, 3685–3692. [Google Scholar]
- Wang, Y.; Zhang, X.L.; Hu, Y.B.; Teng, Z.Y.; Zhang, S.B.; Chi, Q.; Sun, G.Y. Phenotypic response of tobacco leaves to simulated acid rain and its impact on photosynthesis. Int. J. Agric. Biol. 2019, 21, 391–398. [Google Scholar]
- Fang, H.; Mo, J.M. Reactive nitrogen increasing: A threat to our environment. Ecol. Environ.Sci. 2006, 15, 164–168. [Google Scholar]
- Cheng, Y.F.; Zheng, G.J.; Wei, C.; Mu, Q.; Zheng, B.; Wang, Z.B.; Gao, M.; Zhang, Q.; He, K.B.; Carmichael, G.; et al. Reactive nitrogen chemistry in aerosol water as a source of sulfate during haze events in China. Sci. Adv. 2016, 2, e1601530. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.A.; Dai, Q.Q. Investigation report of an acute poisoning of nitrogen dioxide. Occup. Health 2004, 20, 31. [Google Scholar]
- United States Environmental Protection Agency Home Page. Available online: http://www3.epa.gov/ttn/naaqs/criteria.html (accessed on 8 December 2018).
- Adam, S.E.; Shigeto, J.; Sakamoto, A.; Takahashi, M. Atmospheric nitrogen dioxide at ambient levels stimulates growth and development of horticultural plants. Botany 2008, 86, 213–217. [Google Scholar] [CrossRef]
- Gheorghe, I.F.; Ion, B. The effects of air pollutants on vegetation and the role of vegetation in reducing atmospheric pollution. In The Impact of Air Pollution on Health, Economy, Environment and Agricultural Sources; InTech: Timisoara, Romania, 2011; Volume 12, pp. 241–280. [Google Scholar]
- Ashenden, T.W. The effects of long-term exposures to SO2 and NO2 pollution on the growth of Dactylis glomerata L. and Poa pratensis L. Environ. Pollut. 1979, 18, 249–258. [Google Scholar] [CrossRef]
- Ashenden, T.W.; Bell, S.A.; Rafarel, C.R. Effects of nitrogen dioxide pollution on the growth of three fern species. Environ. Pollut. 1990, 66, 301–308. [Google Scholar] [CrossRef]
- Takahashi, M.; Nakagawa, M.; Sakamoto, A.; Ohsumi, C.; Matsubara, T.; Morikawa, H. Atmospheric nitrogen dioxide gas is a plant vitalization signal to increase plant size and the contents of cell constituents. New Phytol. 2005, 168, 149–154. [Google Scholar] [CrossRef]
- Takahashi, M.; Morikawa, H. Kinematic evidence that atmospheric nitrogen dioxide increases the rates of cell proliferation and enlargement to stimulate leaf expansion in Arabidopsis. Plant Signal. Behav. 2015, 10, e1022011. [Google Scholar] [CrossRef][Green Version]
- Takahashi, M.; Furuhashi, T.; Ishikawa, N.; Horiguchi, G.; Sakamoto, A.; Tsukaya, H.; Hiromichi, M. Nitrogen dioxide regulates organ growth by controlling cell proliferation and enlargement in Arabidopsis. New Phytol. 2014, 201, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhou, B.; Ma, C.; Xu, X.; Xu, J.; Jiang, Y.B.; Liu, C.; Li, G.Z.; Herbert, S.J.; Hao, L. Salicylic acid-altering arabidopsis mutants response to NO2 exposure. Bull. Environ. Contam. Toxicol. 2010, 84, 106–111. [Google Scholar] [CrossRef]
- Morikawa, H.; Takahashi, M.; Kawamura, Y. Metabolism and genetics of atmospheric nitrogen dioxide control using pollutant-philic plants. Phytoremediat. Transform. Control Contam. 2004, 763–786. [Google Scholar]
- Takahashi, M.; Sakamoto, A.; Ezura, H.; Morikawa, H. Prolonged exposure to atmospheric nitrogen dioxide increases fruit yield of tomato plants. Plant Biotechnol. 2012, 28, 485–487. [Google Scholar] [CrossRef]
- Wang, Y.; Teng, Z.Y.; Zhang, X.L.; Che, Y.H.; Sun, G.Y. Research progress on the effects of atmospheric nitrogen dioxide on plant growth and metabolism. Chin. J. Appl. Ecol. 2019, 30, 316–324. [Google Scholar]
- Evans, J.R. Improving photosynthesis. Plant Physiol. 2013, 162, 1780–1793. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, A.; Takeba, G. Photorespiration protects C3 plants from photooxidation. Nature 1996, 384, 557–560. [Google Scholar] [CrossRef]
- Kaiser, W.M.; Spill, D. Rapid modulation of spinach leaf nitrate reductase activity by photosynthesis II. In vitro modulation by ATP and AMP. Plant Physiol. 1991, 96, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Yamori, W.; Shikanai, T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu. Rev. Plant Biol. 2016, 67, 81–106. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.P.; Peng, H.X.; Zhu, Y.Y.; Zhang, X.L.; Sun, G.Y. Effect of SO2 wet deposition on morphology and light energy utilization in mulberry leaves. Pratacult. Sci. 2017, 34, 2080–2089. [Google Scholar]
- Wang, Y.; Xu, B.T.; Teng, Z.Y.; Zhang, S.B.; Zhang, X.L.; Sun, G.Y. Effect of simulated acid rain on the growth and photosystemII in the leaves of mulberry seedlings. Pratacult. Sci. 2018, 35, 2220–2229. [Google Scholar]
- Gu, C.H.; Wang, Y.X.; Bai, S.B.; Wu, J.Q.; Yang, Y. Tolerance and accumulation of four ornamental species seedlings to soil cadmium contamination. Acta Ecol. Sin. 2015, 35, 2536–2544. [Google Scholar]
- Xu, N.; Zhang, X.S.; Zhang, X.L.; Zhang, H.H.; Zhu, W.X.; Li, X.; Yue, B.B.; Sun, G.Y. Effects of different N application levels on yield and physiological characteristics of leaf in mulberry. Nonwood For. Res. 2011, 29, 45–49. [Google Scholar]
- Xu, N.; Zhang, H.H.; Zhu, W.X.; Li, X.; Yue, B.B.; Jin, W.W.; Wang, L.Z.; Sun, G.Y. Effects of nitrogen forms on growth and photosynthetic characteristics. Pratacult. Sci. 2012, 29, 1574–1580. [Google Scholar]
- Xu, N.; Ni, H.W.; Zhong, H.X.; Sha, W.; Wu, Y.N.; Xing, J.H.; Sun, G.Y. Growth and photosynthetic characteristics of forage mulberry in response to different nitrogen application levels. Jinagsu J. Agric. Sci. 2015, 4, 865–870. [Google Scholar]
- Xu, N.; Zhang, H.X.; Sha, W.; Wu, Y.N.; Zhang, H.H.; Sun, G.Y. Different N forms on energy allocation in photosystem of mullberry seedlings leaves. Chin. Agric. Bull. 2015, 31, 18–25. [Google Scholar]
- Hu, Y.; Bellaloui, N.; Sun, G.; Tigabu, M.; Wang, J. Exogenous sodium sulfide improves morphological and physiological responses of a hybrid populus, species to nitrogen dioxide. J. Plant Physiol. 2014, 171, 868–875. [Google Scholar] [CrossRef]
- Gao, J.F. Guidance for Plant Physiology Experiment; Beijing Higher Education Press: Beijing, China, 2006; pp. 68–70. [Google Scholar]
- Li, H.S. Principle and Technology of Plant Physiology and Biochemical Experiments; Beijing Higher Education Press: Beijing, China, 2000; pp. 192–194. [Google Scholar]
- Ethier, G.J.; Livingston, N.J. On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ. 2004, 27, 137–153. [Google Scholar] [CrossRef]
- Schansker, G.; Srivastava, A.; Strasser, R.J. Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct. Plant Biol. 2003, 30, 785–796. [Google Scholar] [CrossRef]
- Strasser, B.J.; Strasser, R.J. Measuring fast fluorescence transients to address environmental questions: The JIP-Test. Photosynth. Light Biosph. 1995, 5, 977–980. [Google Scholar]
- Hendrickson, L.; Furbank, R.T.; Chow, W.S. A simple alternative approach to assessing the fate of absorbed light energy using chlorophyll fluorescence. Photosynth. Res. 2004, 82, 73–81. [Google Scholar] [CrossRef]
- Zhou, Y.; Lam, H.M.; Zhang, J. Inhibition of photosynthesis and energy dissipation induced by water and high light stresses in rice. J. Exp. Bot. 2007, 58, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Zeevaart, A.J. Induction of nitrate reductase by NO2. Acta Bot. Neerl. 1974, 23, 345–346. [Google Scholar] [CrossRef]
- Rowland, A.J.; Drew, M.C.; Wellburn, A.R. Foliar entry and incorporation and incorporation of atmospheric nitrogen dioxide into barley plants of different nitrogen status. New Phytol. 1987, 107, 357–371. [Google Scholar] [CrossRef]
- Wingsle, G.N.; Sholm, T.; Lundmark, T.; Ericsson, A. Induction of nitrate reductase in needles of Scots pine seedlings by NOX and NO3−. Physiol. Plant. 2010, 70, 399–403. [Google Scholar] [CrossRef]
- Norby, R.J.; Weerasuriya, Y.; Hanson, P.J. Induction of nitrate reductase activity in red spruce needles by NO2. Can. J. For. Res. 1989, 19, 889–896. [Google Scholar] [CrossRef]
- Hisamatsu, S.; Nihira, J.; Takeuchi, Y.; Satoh, S.; Kondo, N. NO2 suppression of light-induced nitrate reductase in Squash Cotyledons. Plant Cell Physiol. 1988, 29, 395–401. [Google Scholar]
- Wang, Y.H.; He, X.Y.; Zhou, G.S. Characteristics and quantitative simulation of stomatal conductance of Aneurolepidium chinense. Chin. J. Appl. Ecol. 2001, 12, 517–521. [Google Scholar]
- Jiao, N.Y.; Ning, T.Y.; Yang, M.K.; Fu, G.Z.; Ying, F.; Xu, G.W. Effect of maize and peanut intercropping on photosynthetic characters and yield forming of intercropped maize. Acta Ecol. Sin. 2013, 33, 4324–4330. [Google Scholar] [CrossRef]
- Bilger, W.; Schreiber, U.; Bock, M. Determination of the quantum efficiency of photosystem II and of non-photochemical quenching of chlorophyll fluorescence in the field. Oecologia 1995, 102, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Lavaud, J.; Kroth, P.G. In diatoms, the transthylakoid proton gradient regulates the photoprotective non-photochemical fluorescence quenching beyond its control on the xanthophyll cycle. Plant Cell Physiol. 2006, 47, 1010–1016. [Google Scholar] [CrossRef]
- Demmig-adams, B.; Adams, W.W.; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA, redox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, L.; Förster, B.; Pogson, B.J.; Chow, W.S. A simple chlorophyll fluorescence parameter that correlates with the rate coefficient of photoinactivation of photosystem II. Photosynth. Res. 2005, 84, 43–49. [Google Scholar] [CrossRef]


| 0 h | 4 h | 8 h | |
|---|---|---|---|
| Fv/Fo | 3.20 ± 0.50b | 4.27 ± 0.12a | 4.46 ± 0.32a |
| Fv/Fm | 0.82 ± 0.00c | 0.83 ± 0.00b | 0.84 ± 0.01a |
| qP | 0.20 ± 0.05b | 0.26 ± 0.01a | 0.27 ± 0.00a |
| ETR | 43.99± 3.32b | 58.79 ± 0.92a | 60.56 ± 0.17a |
| RC/CSm | 20501.38 ± 377.35c | 26050.96 ± 1194.94b | 27341.97 ± 206.688a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Jin, W.; Che, Y.; Huang, D.; Wang, J.; Zhao, M.; Sun, G. Atmospheric Nitrogen Dioxide Improves Photosynthesis in Mulberry Leaves via Effective Utilization of Excess Absorbed Light Energy. Forests 2019, 10, 312. https://doi.org/10.3390/f10040312
Wang Y, Jin W, Che Y, Huang D, Wang J, Zhao M, Sun G. Atmospheric Nitrogen Dioxide Improves Photosynthesis in Mulberry Leaves via Effective Utilization of Excess Absorbed Light Energy. Forests. 2019; 10(4):312. https://doi.org/10.3390/f10040312
Chicago/Turabian StyleWang, Yue, Weiwei Jin, Yanhui Che, Dan Huang, Jiechen Wang, Meichun Zhao, and Guangyu Sun. 2019. "Atmospheric Nitrogen Dioxide Improves Photosynthesis in Mulberry Leaves via Effective Utilization of Excess Absorbed Light Energy" Forests 10, no. 4: 312. https://doi.org/10.3390/f10040312
APA StyleWang, Y., Jin, W., Che, Y., Huang, D., Wang, J., Zhao, M., & Sun, G. (2019). Atmospheric Nitrogen Dioxide Improves Photosynthesis in Mulberry Leaves via Effective Utilization of Excess Absorbed Light Energy. Forests, 10(4), 312. https://doi.org/10.3390/f10040312





