The Resistance of Scots Pine (Pinus sylvestris L.) Half-sib Families to Heterobasidion annosum
Abstract
1. Introduction
2. Materials and Methods
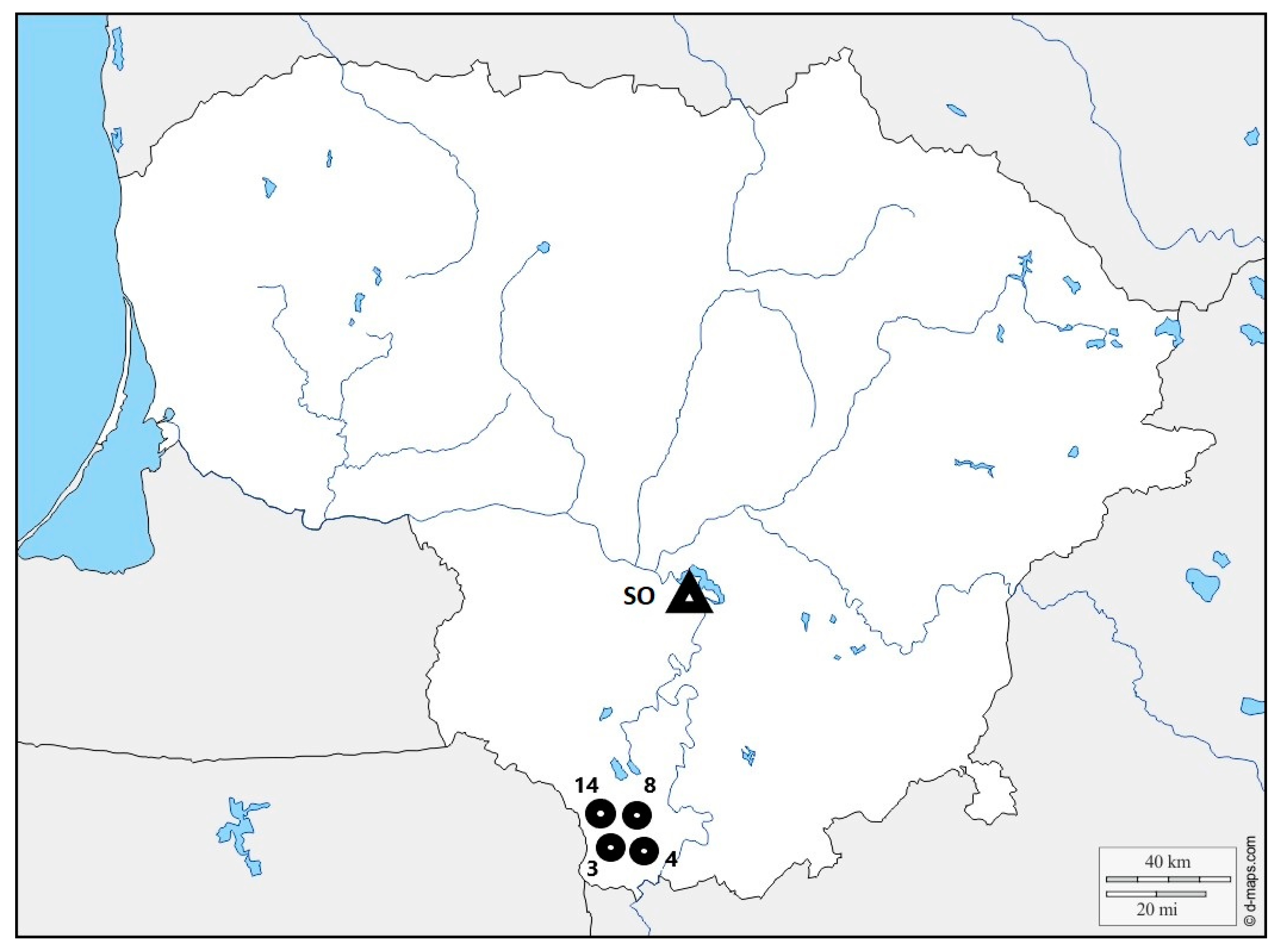
2.1. Isolates of Heterobasidion annosum
2.2. Inoculation and Inspection Methods
2.3. Quantification of Phenolic Compounds
2.4. Statistical Analysis
3. Results
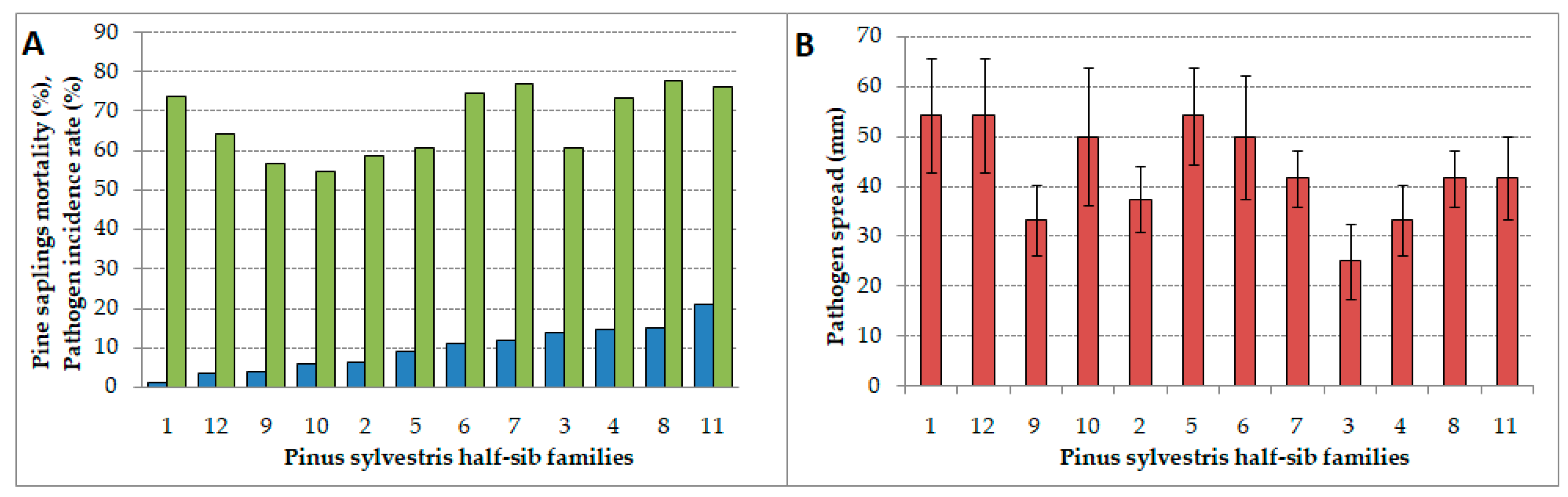
3.1. Incidence Rate, Pathogen Spread, and Pine Seedlings Mortality
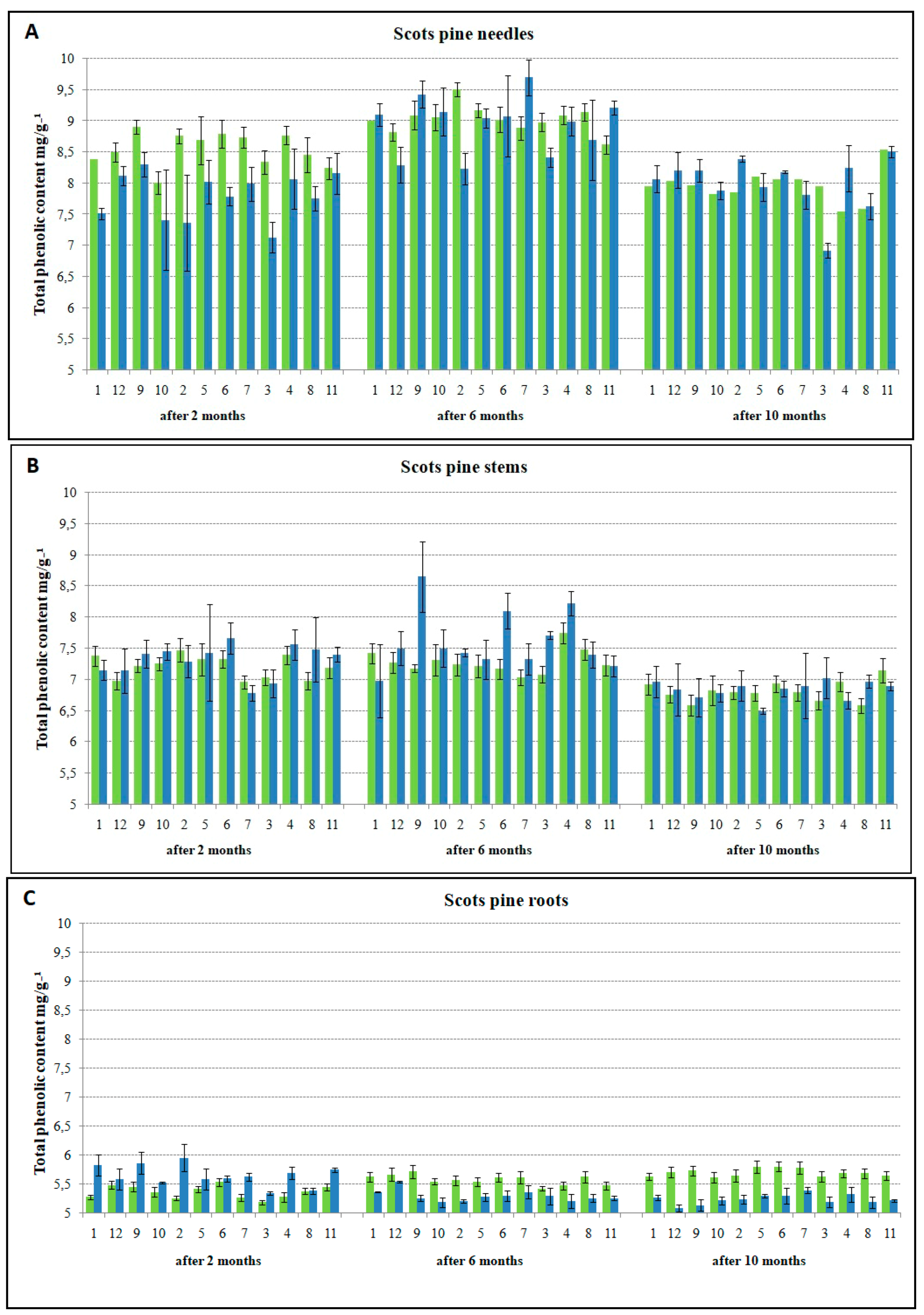
3.2. The Relationship between TPC Accumulation and Susceptibility to H. annosum
4. Discussion
4.1. Incidence Rate, Pathogen Spread, and Mortality Differences among Half-sib Families
4.2. Infection-Induced Phenolic Response
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mason, W.L.; Alia, R. Current and future status of Scots pine (Pinus sylvestris L.) forests in Europe. For. Syst. 2000, 1, 317–336. [Google Scholar]
- ME/SFS (Ministry of Environment, State Forest Service). Lithuanian Statistical Yearbook of Forestry 2018; Butkus, A., Dumčienė, V., Eigirdas, M., Kuliešis, A., Vižlenskas, D., Eds.; Lututė: Kaunas, Lithuania, 2018.
- Johannesson, H.; Stenlid, J. Nuclear reassortment between vegetative mycelia in natural populations of the basidiomycete Heterobasidion annosum. Fungal Genet. Biol. 2004, 41, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Woodward, S.; Stenlid, L.; Karjalainen, R.; Hüttermann, A. Heterobasidion annosum: Biology, Ecology, Impact and Control; CAB International: Wallingford, CT, USA, 1998; pp. 387–403. [Google Scholar]
- ME/SFS (Ministry of Environment, State Forest Service). Lithuanian Statistical Yearbook of Forestry 2016; Butkus, A., Dumčienė, V., Eigirdas, M., Kuliešis, A., Vižlenskas, D., Eds.; Lututė: Lithuania, Kaunas, 2016.
- Delatour, C.; von Weissenberg, K.; Dimitri, L. Host resistance. In Heterobasidion annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 143–166. [Google Scholar]
- Fiodorov, N.I. Eastern Europe and Baltic countries. In Heterobasidion Annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 387–403. [Google Scholar]
- Lygis, V.; Vasiliauskas, R.; Stenlid, J.; Vasiliauskas, A. Silvicultural and pathological evaluation of Scots pine afforestations mixed with deciduous trees to reduce the infections by Heterobasidion annosum s. For. Ecol. Manag. 2004, 201, 275–285. [Google Scholar] [CrossRef]
- Stenlid, J.; Rönnberg, J.; Vollbrecht, G. Rutten forskning hjäl pers skogen. Skog Forsk. 2000, 1, 31–35. (In Swedish) [Google Scholar]
- Thor, M. Heterobasidion Root Rot in Norway Spruce: Modelling Incidence, Control Efficacy and Economic Consequences in Swedish Forestry. Ph.D. Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2005. [Google Scholar]
- Swedjemark, G.; Karlsson, B. Genotypic variation in susceptibility following artificial Heterobasidion annosum inoculation of Picea abies clones in a 17-year-old field test. Scand. J. For. Res. 2004, 19, 103–111. [Google Scholar] [CrossRef]
- Redfern, D.B.; Stenlid, J. Spore dispersal and infection. In Heterobasidion annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Huttermann, A., Eds.; CAB International: Wallingford, UK, 1998; pp. 105–124. [Google Scholar]
- Hadfield, F.S.; Goheen, D.J.; Filip, G.M.; Schmitt, C.L.; Harvey, R.D. Root Diseases in Washington and Oregon Conifers; R6-FPM-250-86; USDA Forest Service, State and Private Forestry, Forest Pest Management: Portland, OR, USA, 1986.
- Lygis, V.; Vasiliauskas, R.; Stenlid, J. Planting Betula pendula on pine sites infested by Heterobasidion annosum: Disease transfer, silvicultural evaluation, and community of wood-inhabiting fungi. Can. J. For. Res. 2004, 34, 120–130. [Google Scholar] [CrossRef]
- Vasiliauskas, R.; Lygis, V.; Thor, M.; Stenlid, J. Impact of biological (Rotstop) and chemical(urea) treatments on fungal community structure in freshly Picea abies stumps. Biol. Control 2004, 31, 405–413. [Google Scholar] [CrossRef]
- Brandtberg, P.O.; Johansson, M.; Seeger, P. Effects of season and urea treatment on infection of stumps of Picea abies by Heterobasidion annosum in stands on former arable land. Scand. J. For. Res. 1996, 11, 261–268. [Google Scholar] [CrossRef]
- Von Weissenberg, K. Variation in relative resistance to spread of Fomes annosus in four clones of Picea abies. Eur. J. For. Pathol. 1975, 5, 112–117. [Google Scholar] [CrossRef]
- Swedjemark, G.; Stenlid, J. Variation in spread of Heterobasidion annosum in clones of Picea abies grown at different vegetation phases under greenhouse conditions. Scand. J. For. Res. 1996, 11, 137–144. [Google Scholar] [CrossRef]
- Swedjemark, G.; Stenlid, J. Between-tree and between isolate variation for growth of S-group Heterobasidion annosum in sapwood of Picea abies cuttings. Can. J. For. Res. 1997, 27, 711–715. [Google Scholar] [CrossRef]
- Skrøppa, T.; Solheim, H.; Steffenrem, A. Genetic variation, inheritance patterns and parent–offspring relationships after artificial inoculations with Heterobasidion parviporum and Ceratocystis polonica in Norway spruce seed orchards and progeny tests. Silva Fennica 2015, 49, 1191. [Google Scholar] [CrossRef]
- Swedjemark, G.; Johannesson, H.; Stenlid, J. Intraspecific variation in Heterobasidion annosum for growth in sapwood of Picea abies and Pinus sylvestris. For. Pathol. 2008, 29, 249–258. [Google Scholar] [CrossRef]
- Swedjemark, G.; Karlsson, B. Mycelial growth and exclusion of Heterobasidion parviporum inoculated in branches of 15-year-old Picea abies clones. Scand. J. For. Res. 2006, 36, 209–214. [Google Scholar] [CrossRef]
- Oliva, J.; Gonthier, P.; Stenlid, J. Gene flow and inter-sterility between allopatric and sympatric populations of Heterobasidion abietinum and H. parviporum in Europe. For. Pathol. 2010, 41, 243–252. [Google Scholar] [CrossRef]
- Zaluma, A.; Gailis, A.; Burnevica, N.; Korhonen, K.; Gaitnieks, T. Susceptibility of Picea abies and Pinussylvestris seedlings of various origins to Heterobasidion annosum and H. parviporum. Proc. Latv. Acad. Sci. Sect. B 2016, 70, 29–33. [Google Scholar] [CrossRef]
- Hammerschmidt, R. Phenols and plant-pathogen interactions: The saga continues. Physiol. Mol. Plant Pathol. 2005, 66, 77–78. [Google Scholar] [CrossRef]
- Witzell, J.; Martín, J.A. Phenolic metabolites in the resistance of northern forest trees to pathogens–past experience sand future prospects. Can. J. For. Res. 2008, 38, 2711–2727. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plan Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Pan, H.; Lundgren, L.N. Phenolic extractives from root bark of Picea abies. Phytochemistry 1995, 39, 1423–1428. [Google Scholar] [CrossRef]
- Slimestad, R. Flavonoids in buds and young needles of Picea, Pinus and Abies. Biochem. Syst. Ecol. 2003, 31, 1247–1255. [Google Scholar] [CrossRef]
- Sallas, L.; Kainulainen, P.; Utriainen, J.; Holopainen, T.; Holopainen, J.K. The influence of elevated O3 and CO2 concentrations on secondary metabolites of Scots pine (Pinus sylvestris L.) seedlings. Glob. Chang. Biol. 2001, 7, 303–311. [Google Scholar] [CrossRef]
- Schultz, T.P.; Nicholas, D.D. Naturally durable heartwood: Evidence for a proposed dual defensive function of the extractives. Phytochemistry 2000, 54, 47–52. [Google Scholar] [CrossRef]
- Venäläinen, M.; Harju, A.M.; Kainulainen, P.; Viitanen, H.; Nikulainen, H. Variation in decay resistance and its relationship with other wood characteristics in old Scots pines. Ann. For. Sci. 2003, 60, 409–417. [Google Scholar] [CrossRef]
- Lieutier, F.; Sauvard, D.; Brignolas, F.; Picron, V.; Yart, A.; Bastien, C.; Jay-Allemand, C. Changes in phenolics metabolites of Scots pine induced by Ophiostomabrunneo-ciliatum, a bark beetle associated fungus. For. Pathol. 1996, 26, 145–216. [Google Scholar] [CrossRef]
- Bois, E.; Lieutier, F. Phenolic response of Scots pine clones to inoculation with Leptographium wingfieldii, a fungus associated with Tomicus piniperda. Plant Physiol. Biochem. 1997, 35, 819–825. [Google Scholar]
- Bonello, P.; Heller, W.; Sanderman, H. Ozone effects on root-disease susceptibility and defense responses in mycorrhizal and non-mycorrhizal seedlings of Scots pine (Pinus sylvestris L.). New Phytol. 1993, 124, 653–663. [Google Scholar] [CrossRef]
- Bonello, P.; Blodgett, J.T. Pinus nigra—Sphaeropsis sapinea as a model pathosystem to investigate local and systemic effects of fungal infection of pines. Physiol. Mol. Plant Pathol. 2003, 63, 249–261. [Google Scholar] [CrossRef]
- Wallis, C.; Eyles, A.; Chorbadjian, R.; McSpadden Gardener, B.; Hansen, R.; Cipollini, D.; Herms, D.A.; Bonello, P. Systemic induction of phloem secondary metabolism and its relationship to resistance to a canker pathogen in Austrian pine. New Phytol. 2008, 177, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Edenius, L.; Grzegorz, M.; Witzell, J.; Berghd, J. Effects of repeated fertilization of young Norway spruce on foliar phenolics and arthropods: Implications for insectivorous birds’ food resources. For. Ecol. Manag. 2012, 277, 38–45. [Google Scholar] [CrossRef]
- Andrew, R.L.; Wallis, I.R.; Harwood, C.E.; Henson, M.; Foley, W.J. Heritable variation in the foliar secondary metabolite sideroxylonal in Eucalyptus confers cross-resistance to herbivores. Oecologia 2007, 153, 891–901. [Google Scholar] [CrossRef]
- Külheim, C.; Yeoh, S.H.; Wallis, I.R.; Laffan, S.; Moran, G.F.; Foley, W.J. The molecular basis of quantitative variation in foliar secondary metabolites in Eucalyptus globulus. New Phytol. 2011, 191, 1041–1053. [Google Scholar] [CrossRef] [PubMed]
- Ganthaler, A.; Stoggl, W.; Mayr, S.; Kranner, I.; Schuler, S.; Wischnitzki, E. Association genetics of phenolic needle compounds in Norway spruce with variable susceptibility to needle bladder rust. Plant Mol. Biol. 2017, 94, 229–251. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, S.C.; Wheeler, N.C.; Ersoz, E.; Dana Nelson, C.; Neale, D.B. Association genetics in Pinus taeda L. I. Wood property traits. Genetics 2007, 175, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Dillon, S.K.; Nolan, M.; Li, W.; Bell, C.; Wu, H.X.; Southerton, S.G. Allelic variation in cell wall candidate genes affecting solid wood properties in natural populations and land races of Pinus radiata. Genetics 2010, 185, 1477–1487. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.; Doerksen, T.; Boyle, B.; Clément, S.; Deslauriers, M.; Beauseigle, S.; Blais, S.; Poulin, P.L.; Lenz, P.; Caron, S.; et al. Association genetics of wood physical traits in the conifer white spruce and relationships with gene expression. Genetics 2011, 188, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Westbrook, J.W.; Resende, M.F.R.; Muñoz, P.D.R.; Walker, A.R.; Wegrzyn, J.L.; Nelson, C.D.; Neale, D.B.; Kirst, M.; Huber, D.A.; Gezan, S.A.; Peter, G.F.; et al. Association genetics of oleoresin flow in loblolly pine: Discovering genes and predicting phenotype for improved resistance to bark beetles and bioenergy potential. New Phytol. 2013, 199, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Lepoittevin, C.; Harvengt, L.; Plomion, C.; Garnier-Géré, P. Association mapping for growth, straightness and wood chemistry traits in the Pinus pinaster Aquitaine breeding population. Tree Genet. Genom 2012, 8, 113–126. [Google Scholar] [CrossRef]
- Budde, K.B.; Heuertz, M.; Hernández-Serrano, A.; Pausas, J.G.; Vendramin, G.G.; Verdú, M.; González-Martínez, S.C. In situ genetic association for serotiny, a fire-related trait, in Mediterranean maritime pine (Pinus pinaster). New Phytol. 2014, 201, 230–241. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, S.C.; Huber, D.; Ersoz, E.; Davis, J.M.; Neale, D.B. Association genetics in Pinus taeda L. II. Carbon isotope discrimination. Heredity 2008, 101, 19–26. [Google Scholar] [CrossRef]
- Cumbie, W.P.; Eckert, A.; Wegrzyn, J.; Whetten, R.; Neale, D.; Goldfarb, B. Association genetics of carbon isotope discrimination, height and foliar nitrogen in a natural population of Pinus taeda L. Heredity 2011, 107, 105–114. [Google Scholar] [CrossRef]
- Eckert, A.J.; Bower, A.D.; Wegrzyn, J.L.; Pande, B.; Jermstad, K.D.; Krutovsky, K.V.; St Clair, J.B.; Neale, D.B. Association genetics of coastal Douglas fir (Pseudotsuga menziesii var. menziesii, Pinaceae). I. Cold-hardiness related traits. Genetics 2009, 182, 1289–1304. [Google Scholar] [CrossRef] [PubMed]
- Holliday, J.A.; Ritland, K.; Aitken, S.N. Widespread, ecologically relevant genetic markers developed from association mapping of climate related traits in Sitka spruce (Picea sitchensis). New Phytol. 2010, 188, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.J.; Wegrzyn, J.L.; Cumbie, W.P.; Goldfarb, B.; Huber, D.A.; Tolstikov, V.; Fiehn, O.; Neale, D.B. Association genetics of the loblolly pine (Pinus taeda, Pinaceae) metabolome. New Phytol. 2012, 193, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Quesada, T.; Gopal, V.; Cumbie, W.P.; Eckert, A.J.; Wegrzyn, J.L.; Neale, D.B.; Goldfarb, B.; Huber, D.A.; Casella, G.; Davis, J.M. Association mapping of quantitative disease resistance in a natural population of loblolly pine (Pinus taeda L.). Genetics 2010, 186, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Swedjemark, G.; Stenlid, J. Population dynamics of the root rot fungus Heterobasidion annosum following thinning of Picea abies. Oikos 1993, 66, 247–254. [Google Scholar] [CrossRef]
- Stenlid, J. Population structure of Heterobasidion annosum as determined by somatic incompatibility, sexual incompatibility, and isoenzyme patterns. Can. J. Bot. 1985, 63, 2268–2273. [Google Scholar] [CrossRef]
- Johannsson, M.; Stenlid, J. Infection of roots of Norway spruce (Picea abies) by Heterobasidion annosum. Eur. J. For. Pathol. 1985, 15, 32–45. [Google Scholar] [CrossRef]
- Swedjemark, G.; Stenlid, J. Susceptibility of conifer and broadleaf seedlings to Swedish S- and P-strains of Heterobasidion annosum under greenhouse conditions. Plant Pathol. 1995, 44, 73–79. [Google Scholar] [CrossRef]
- Stenlid, J.; Swedjemark, G. Differential growth of S- and P-isolates of Heterobasidion annosum in Picea abies and Pinus sylvestris. Trans. Br. Mycol. Soc. 1988, 90, 209–213. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Kenward, M.G.; Roger, J.H. Small Sample Inference for Fixed Effects from Restricted Maximum Likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef] [PubMed]
- Werner, A.; Lakomy, P. Host specialization of IS-group isolates of Heterobasidion annosum to Scots pine, Norway spruce and common fir in field inoculation experiments. Dendrobiology 2002, 47, 59–68. [Google Scholar]
- Werner, A.; Lakomy, P. Intraspecific variation in Heterobasidion annosum for mortality rate on Pinus sylvestris and Picea abies seedlings grown in pure culture. Mycologia 2002, 94, 856–861. [Google Scholar] [CrossRef]
- Stenlid, J. Controlling and predicting the spread of Heterobasidion annosum from infected stumps and trees of Piceaabies. Scand. J. For. Res. 1987, 2, 187–198. [Google Scholar] [CrossRef]
- Swedjemark, G.; Stenlid, J.; Karlsson, B. Variation in growth of Heterobasidion annosum among clones of Picea abies incubated for different periods of time. For. Pathol. 2001, 31, 163–175. [Google Scholar] [CrossRef]
- Lakomy, P.; Kwasna, H.; Dalke-Swiderska, M. The virulence of Heterobasidion parviporum population from Norway spruce stand in Suvalki forest district. Acta Sci. Polon. Silvarum Colendarum Ratio Ind. Lig. 2011, 10, 27–36. [Google Scholar]
- Ganthaler, A.; Mayr, S. Temporal variation in airborne spore concentration of Chrysomyxa rhododendri: Correlation with weather conditions and consequences for Norway spruce infection. For. Pathol. 2015, 45, 443–449. [Google Scholar] [CrossRef]
- Ganthaler, A.; Stöggl, W.; Kranner, I.; Mayr, S. Foliar Phenolic Compounds in Norway Spruce with Varying Susceptibility to Chrysomyxa rhododendri: Analyses of Seasonal and Infection-Induced Accumulation Patterns. Front. Plant Sci. 2017, 8, 1173. [Google Scholar] [CrossRef] [PubMed]
- Hakulinen, J.; Sorjonen, S.; Julkunen-Tiitto, R. Leaf phenolics of three willow clones differing in resistance to Melampsora rust infection. Physiol. Plant 1999, 105, 662–669. [Google Scholar] [CrossRef]
- Hjältén, J.; Niemi, L.; Wennström, A.; Ericson, L.; Roininen, H.; Julkunen-Tiitto, R. Variable responses of natural enemies to Salix triandra phenotypes with different secondary chemistry. Oikos 2007, 116, 751–758. [Google Scholar] [CrossRef]
- Slimestad, R. Amount of flavonols and stilbenes during needle development of Picea abies; variation between provenances. Biochem. Syst. Ecol. 1998, 26, 225–238. [Google Scholar] [CrossRef]
- Evensen, P.C.; Solheim, H.; Hoiland, K.; Stenersen, J. Induced resistance of Norway spruce, variation of phenolic compounds and their effects on fungal pathogen. For. Pathol. 2000, 30, 97–108. [Google Scholar] [CrossRef]
- Lieutier, F.; Brignolas, F.; Sauvard, D.; Yart, A.; Galet, C.; Brunet, M.; van de Sype, H. Intra- and inter-provenance variability in phloem phenols of Picea abies and relationship to a bark beetle-associated fungus. Tree Physiol. 2003, 23, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Fossdal, C.G.; Nagy, N.E.; Hietala, A.M.; Kvaalen, H.; Slimestad, R.; Woodward, S.; Solheim, H. Indications of heightened constitutive or primed host response affecting the lignin pathway transcripts and phenolics in mature Norway spruce clones. Tree Physiol. 2012, 32, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Bonello, P.; Capretti, P.; Luchi, N.; Martini, V.; Michelozzi, M. Systemic effects of Heterobasidion annosums. s. infection on severity of Diplodia pinea tip blight and terpenoid metabolism in Italian stone pine (Pinus pinea). Tree Physiol. 2008, 28, 1653–1660. [Google Scholar] [CrossRef] [PubMed]
- Lieutier, F.; Garcia, J.; Romary, P.; Yart, A.; Jactel, H.; Sauvard, D. Inter-tree variability in the induced defense reaction of Scots pine to single inoculations by Ophiostomabrunneo-ciliatum, a bark beetle-associated fungus. For. Ecol. Manag. 1993, 59, 257–270. [Google Scholar] [CrossRef]
- Schmidt, A.; Zeneli, G.; Hietala, A.M.; Fossdal, C.G.; Krokene, P.; Christiansen, E.; Gershenzon, J. Induced chemical defenses in conifers: Biochemical and molecular approaches to studying their function. In Chemical Ecology and Phytochemistry of Forest Ecosystems; Romeo, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–28. [Google Scholar]
- Phillips, M.A.; Croteau, R.B. Resin-based defenses in conifers. Trends Plant Sci. 1999, 4, 184–190. [Google Scholar] [CrossRef]


| Pairs of Treatments for Comparison | Wilcoxon Z | DSCF Value | Pr > DSCF |
|---|---|---|---|
| Variable: incidence rate of H. annosum infection after six months | |||
| Control vs. strain No. 3 | −6.92 | 9.79 | <0001 |
| Control vs. strain No. 4 | −6.33 | 8.96 | <0001 |
| Control vs. strain No. 8 | −4.86 | 6.88 | <0001 |
| Control vs. strain No. 14 | −7.54 | 10.65 | <0001 |
| Strain No. 3 vs. strain No. 4 | 0.83 | 1.17 | 0.9225 |
| Strain No. 3 vs. strain No. 8 | 2.70 | 3.82 | 0.0534 |
| Strain No. 3 vs. strain No. 14 | −0.98 | 1.38 | 0.8662 |
| Strain No. 4 vs. strain No. 8 | 1.92 | 2.72 | 0.3063 |
| Strain No. 4 vs. strain No. 14 | −1.77 | 2.51 | 0.3887 |
| Strain No. 8 vs. strain No. 14 | −3.56 | 5.03 | 0.0034 |
| Variable: H. annosum mycelium spread after six months | |||
| Control vs. strain No. 3 | −6.72 | 9.51 | <0001 |
| Control vs. strain No. 4 | −6.24 | 8.82 | <0001 |
| Control vs. strain No. 8 | −4.86 | 6.88 | <0001 |
| Control vs. strain No. 14 | −7.43 | 10.50 | <0001 |
| Strain No. 3 vs. strain No. 4 | 1.31 | 1.85 | 0.6880 |
| Strain No. 3 vs. strain No. 8 | 3.49 | 4.94 | 0.0044 |
| Strain No. 3 vs. strain No. 14 | 0.68 | 0.96 | 0.9615 |
| Strain No. 4 vs. strain No. 8 | 2.41 | 3.40 | 0.1134 |
| Strain No. 4 vs. strain No. 14 | −0.93 | 1.32 | 0.8848 |
| Strain No. 8 vs. strain No. 14 | −3.77 | 5.34 | 0.0015 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marčiulynas, A.; Sirgedaitė-Šėžienė, V.; Žemaitis, P.; Baliuckas, V. The Resistance of Scots Pine (Pinus sylvestris L.) Half-sib Families to Heterobasidion annosum. Forests 2019, 10, 287. https://doi.org/10.3390/f10030287
Marčiulynas A, Sirgedaitė-Šėžienė V, Žemaitis P, Baliuckas V. The Resistance of Scots Pine (Pinus sylvestris L.) Half-sib Families to Heterobasidion annosum. Forests. 2019; 10(3):287. https://doi.org/10.3390/f10030287
Chicago/Turabian StyleMarčiulynas, Adas, Vaida Sirgedaitė-Šėžienė, Povilas Žemaitis, and Virgilijus Baliuckas. 2019. "The Resistance of Scots Pine (Pinus sylvestris L.) Half-sib Families to Heterobasidion annosum" Forests 10, no. 3: 287. https://doi.org/10.3390/f10030287
APA StyleMarčiulynas, A., Sirgedaitė-Šėžienė, V., Žemaitis, P., & Baliuckas, V. (2019). The Resistance of Scots Pine (Pinus sylvestris L.) Half-sib Families to Heterobasidion annosum. Forests, 10(3), 287. https://doi.org/10.3390/f10030287




