Consuming Blackberry as a Traditional Nutraceutical Resource from an Area with High Anthropogenic Impact
Abstract
1. Introduction
2. Materials and Methods
2.1. The History of the Pollution
2.2. Sampling Design
2.3. Processing the Material
2.4. Data Processing
3. Results and Discussions
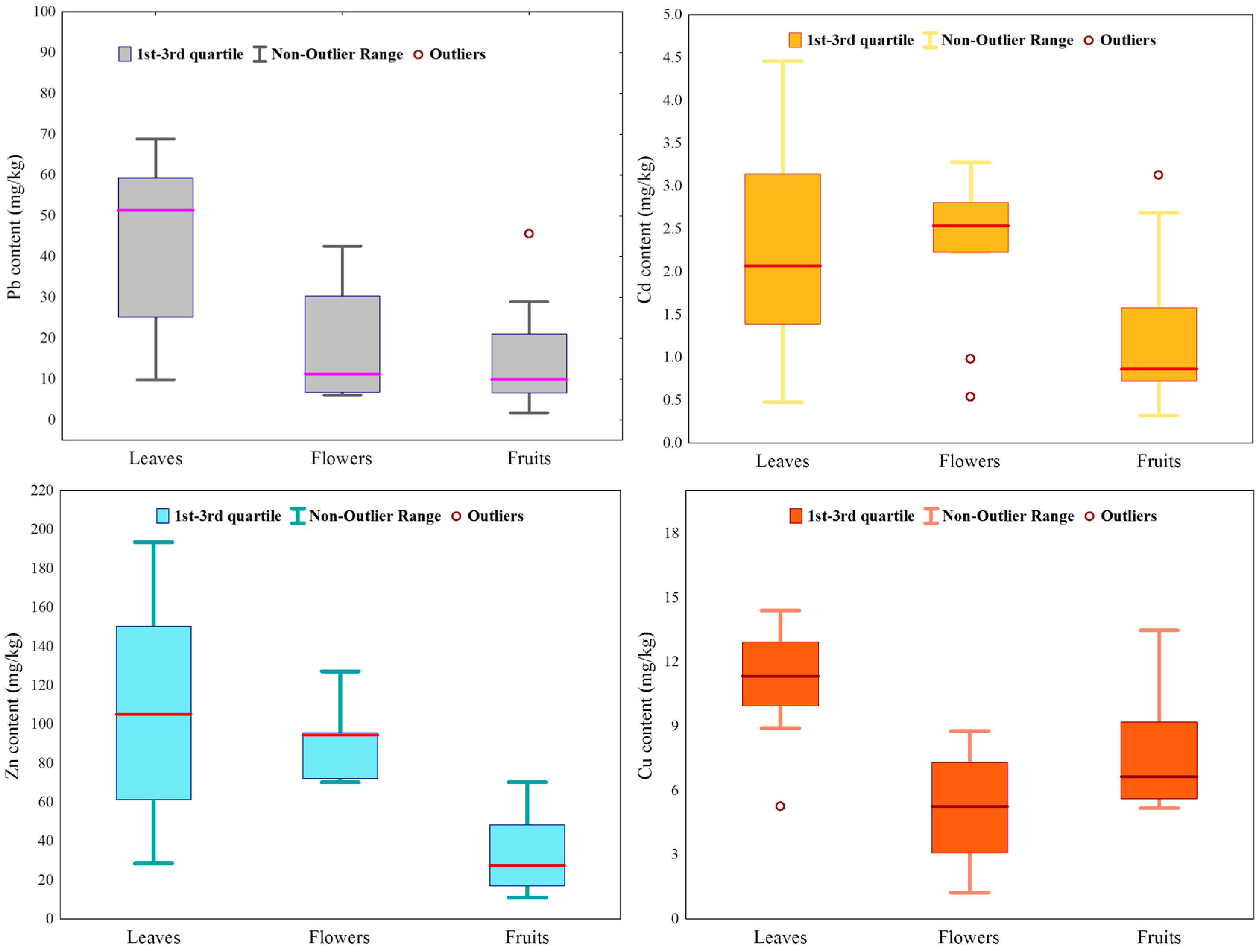
3.1. The Level of Heavy Metal Contamination in Blackberry
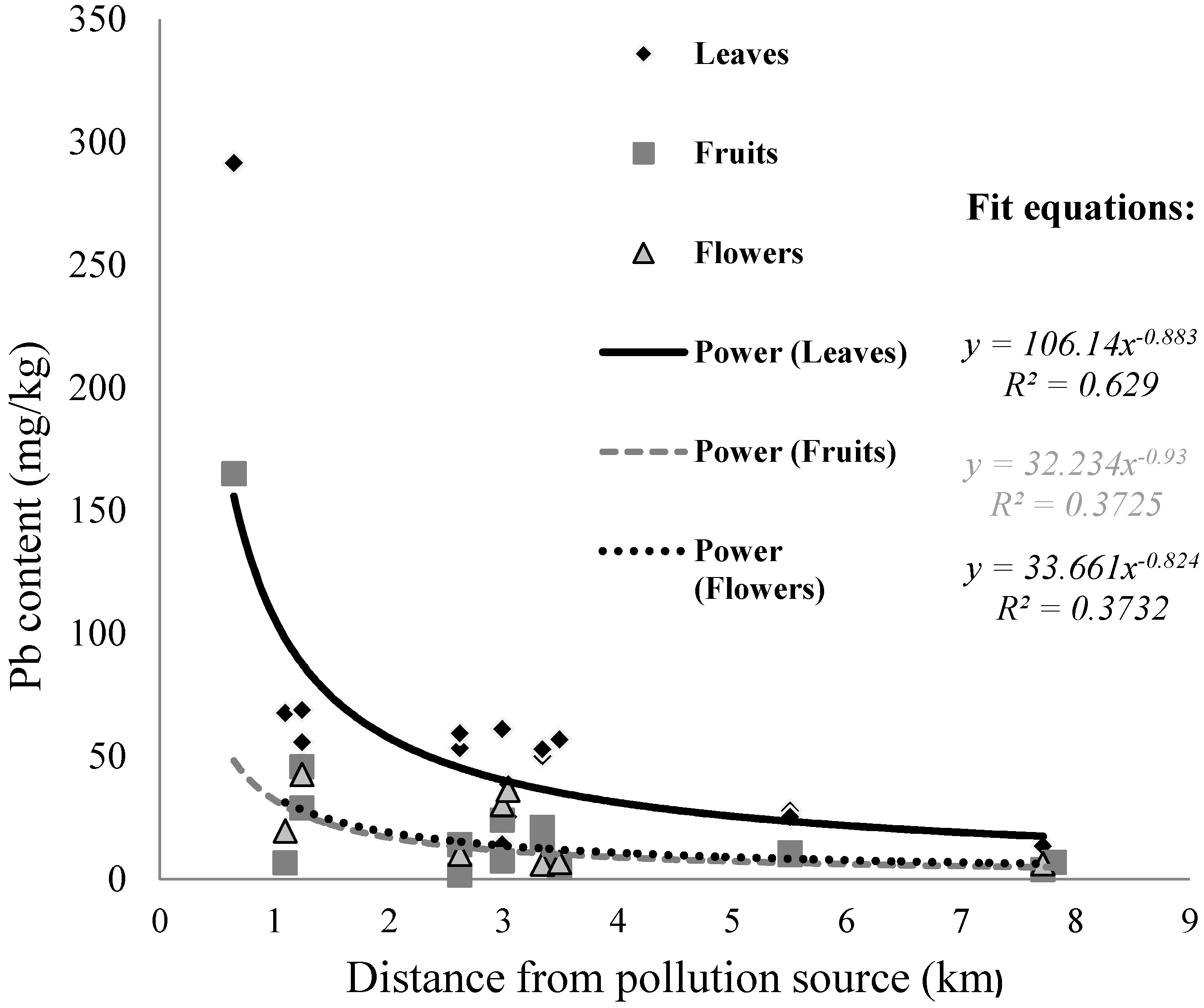
3.2. The Spatial Variability of the Heavy Metal Content in Blackberry
3.3. Safety in Herbal Medicine
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Ekor, M. The Growing Use of Herbal Medicines: Issues Relating to Adverse Reactions and Challenges in Monitoring Safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Butură, V. Encyclopaedia of Romanian Ethnobotany; Științifică și Enciclopedică Publishing House: Bucharest, Romania, 1979; pp. 159–202. [Google Scholar]
- Lim, T.K. Edible Medicinal and Non-Medicinal Plants—Fruits; Springer: Berlin, Germany, 2012; pp. 559–569. [Google Scholar]
- Alonso, R.; Cavidad, I.; Calleja, J.M. A preliminary study of hypoglycemic activity of Rubus fruticosus. Planta Med. 1980, 40, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Leonti, M.; Casu, L.; Sanna, F.; Bonsignore, L. A comparison of medicinal plant use in Sardinia and Sicily-De Materia Medica revisited? J. Ethnopharmacol. 2009, 121, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Guarrera, P.M. Food medicine and minor nourishment in the folk traditions of Central Italy (Marche, Abruzzo and Latium). Fitoterapia 2003, 74, 515–544. [Google Scholar] [CrossRef]
- Lust, J. The Herb Book; Dover Publications Inc.: New York, NY, USA, 2014; pp. 298–300. [Google Scholar]
- Chevallier, A. Encyclopedia of Herbal Medicine, 3rd ed.; Dorling Kindersley Publishing: New York, NY, USA, 2016; pp. 264–265. [Google Scholar]
- Beldeanu, E.C. Forest Species of Sanogenic Interest; Transilvania University Press: Braşov, Romania, 2004; pp. 145–147. [Google Scholar]
- Sher, H. Ethnoecological evaluation of some medicinal and aromatic plants of Kot Malakand Agency, Pakistan. Sci. Res. Essays 2011, 6, 2164–2173. [Google Scholar]
- Daliu, P.; Santini, A.; Novellino, E. From pharmaceuticals to nutraceuticals: Bridging disease prevention and management. Expert Rev. Clin. Pharmacol. 2018, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E.; Armini, V.; Ritieni, A. State of the art of Ready-to-Use Therapeutic Food: A tool for nutraceuticals addition to foodstuff. Food Chem. 2013, 140, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E. To Nutraceuticals and Back: Rethinking a Concept. Foods 2017, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Novellino, E. Nutraceuticals: Shedding light on the grey area between pharmaceuticals and food. Expert Rev. Clin. Pharmacol. 2018, 11, 545–547. [Google Scholar] [CrossRef] [PubMed]
- De Felice, S.L. The nutraceutical revolution: Its impact on food industry R&D. Trends Food Sci. Technol. 1995, 6, 59–61. [Google Scholar]
- Santini, A.; Tenore, G.C.; Novellino, E. Nutraceuticals: A paradigm of proactive medicine. Eur. J. Pharm. Sci. 2017, 96, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Santini, A.; Cammarata, S.M.; Capone, G.; Ianaro, A.; Tenore, G.C.; Pani, L.; Novellino, E. Nutraceuticals: Opening the debate for a regulatory framework. Br. J. Clin. Pharmacol. 2018, 84, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Tzvetkov, N.T.; El-Tawil, O.S.; Bungǎu, S.G.; Abdel-Daim, M.M.; Atanasov, A.G. Antioxidants: Scientific Literature Landscape Analysis. Oxid. Med. Cell. Longev. 2019, 2019, 8278454. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; D’Addezio, L.; Camilli, E.; Piccinelli, R.; Turrini, A.; Marletta, L.; Marconi, S.; Lucarini, M.; Lisciani, S.; Gabrielli, P.; et al. From Plant Compounds to Botanicals and Back: A Current Snapshot. Molecules 2018, 23, 1844. [Google Scholar] [CrossRef] [PubMed]
- Andrew, R.; Izzo, A.A. Principles of pharmacological research of nutraceuticals. Br. J. Pharmacol. 2017, 174, 1177–1194. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.S.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Patel, J.D.; Mumper, R.J. Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. J. Med. Food 2007, 10, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Hager, T.J.; Howard, L.R.; Liyanage, R.; Lay, J.O.; Prior, R.L. Ellagitannin composition of blackberry as determined by HPLC-ESI-MS and MALDI-TOF-MS. J. Agric. Food Chem. 2008, 56, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, I.M.; Meyer, A.S.; Frankel, E.N. Antioxidant activity of berry phenolics on human low-density lipoprotein and liposome oxidation. J. Agric. Food Chem. 1998, 46, 4107–4112. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Feng, R.; Wang, S.Y.; Bowman, L.; Lu, Y.; Qian, Y.; Castranova, V.; Jiang, B.H.; Shi, X. Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J. Biol. Chem. 2006, 281, 17359–17368. [Google Scholar] [CrossRef] [PubMed]
- Pantelidis, G.E.; Vasilakakis, M.; Manganaris, G.A.; Diamantidis, G.R. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and cornelian cherries. Food Chem. 2007, 102, 777–783. [Google Scholar] [CrossRef]
- Elisia, I.; Kitts, D.D. Anthocyanins inhibit peroxyl radical-induced apoptosis in Caco-2 cells. Mol. Cell. Biochem. 2008, 312, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Forbes, C.S.; Zhang, Y.; Nair, M.G. Anthocyanin content, antioxidant, anti-inflammatory and anticancer properties of blackberry and raspberry fruits. J. Food Compos. Anal. 2010, 23, 554–560. [Google Scholar] [CrossRef]
- Jing, P.; Giusti, M. Contribution of berry anthocyanins to their chemopreventive properties. In Berries and Cancer Prevention; Stoner, G.D., Seeram, N.P., Eds.; Springer: New York, NY, USA, 2011; pp. 1–38. [Google Scholar]
- Kolevski, G.; Ivic-Kolevska, S. Antioxidants in fruits and human medical research: An overview. J. Hyg. Eng. Des. 2012, 1, 271–274. [Google Scholar]
- Tavares, L.; Figueira, I.; McDougall, G.J.; Vieira, H.L.A.; Stewart, D.; Alves, P.M.; Ferreira, R.B.; Santos, C.N. Neuroprotective effects of digested polyphenols from wild blackberry species. Eur. J. Nutr. 2013, 52, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Masood, S.; Sutana, S.; Ben Hadda, T.; Bader, A.; Zafar, M. Antioxidant and nutraceutical value of wild medicinal Rubus berries. Pak. J. Pharm. Sci. 2015, 28, 241–247. [Google Scholar] [PubMed]
- Predná, L.; Habánová, M. Antioxidant potential in selected species of small berry fruits. Acta Fytotech. Zootech. 2015, 18, 116–118. [Google Scholar] [CrossRef]
- Bhattarai, N.; Karki, M. Medicinal and aromatic plants: Ethnobotany and conservation status. In Encyclopedia of Forest Sciences; Burley, J., Evans, J., Youngquist, J.A., Eds.; Elsevier Ltd.: Kidlington, UK, 2004; pp. 523–532. [Google Scholar]
- Strik, B.C. Berry crops: Worldwide area and production systems. In Berry Fruit: Value-Added Products for Health Promotion, 1st ed.; Zhao, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 3–51. [Google Scholar]
- Farmaki, E.G.; Thomaidis, N.S. Current status of the metal pollution of the environment of Greece—A review. Glob. NEST J. 2008, 10, 366–375. [Google Scholar]
- Mohammed, A.S.; Kapri, A.; Goel, R. Heavy metal pollution: Source, impact and remedies. In Biomanagement of Metal-Contaminated Soils, Environmental Pollution; Khan, M.S., Zaidi, A., Goel, R., Musarrat, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 20, pp. 1–29. [Google Scholar]
- Dietrich, K.N.; Ris, M.D.; Succop, P.A.; Berger, O.G.; Bornschein, R.L. Early exposure to lead and juvenile delinquency. Neurotoxicol. Teratol. 2001, 23, 511–518. [Google Scholar] [CrossRef]
- Needleman, H.L.; McFarland, C.; Ness, R.B.; Fienberg, S.E.; Tobin, M.J. Bone lead levels in adjudicated delinquents - a case control study. Neurotoxicol. Teratol. 2002, 24, 711–717. [Google Scholar] [CrossRef]
- Kim, S.; Moon, C.; Eun, S.; Ryu, P.; Jo, S. Identification of ASK1, MKK4, JNK, c-Jun, and caspase-3 as a signaling cascade involved in cadmium-induced neuronal cell apoptosis. Biochem. Biophys. Res. Commun. 2005, 328, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Monroe, R.K.; Halvorsen, S.W. Cadmium blocks receptor-mediated Jak/STAT signaling in neurons by oxidative stress. Free Radic. Biol. Med. 2006, 41, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ji, X.J.; Wang, H.D.; Pan, H.; Chen, M.; Lu, T.J. Zinc neurotoxicity to hippocampal neurons in vitro induces ubiquitin conjugation that requires p38 activation. Brain Res. 2012, 1438, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Caito, S.; Aschner, M. Developmental neurotoxicity of lead. Adv. Neurobiol. 2017, 18, 3–12. [Google Scholar] [PubMed]
- von Hoffen, L.P.; Säumel, I. Orchards for edible cities: Cadmium and lead content in nuts, berries, pome and stone fruits harvested within the inner city neighbourhoods in Berlin, Germany. Ecotoxicol. Environ. Saf. 2014, 101, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Wisłocka, M.; Krawczyk, J.; Klink, A.; Morrison, L. Bioaccumulation of heavy metals by selected plant species from uranium mining dumps in the Sudety Mts., Poland. Pol. J. Environ. Stud. 2006, 15, 811–818. [Google Scholar]
- Teofilova, T.; Kodzabashev, N.; Gherasimov, S.; Markova, E. Comparative characterization of the heavy metal contents in samples from two regions in Bulgaria with different anthropogenic load. Nat. Montenegrina 2010, 9, 897–912. [Google Scholar]
- Shikhova, N.S. Some regularities in the accumulation of lead in urban plants (by example of Vladivostok). Contemp. Probl. Ecol. 2012, 5, 215–222. [Google Scholar] [CrossRef]
- Yedoyan, R.; Yedoyan, T.V. The study of heavy metals (Ni, Zn, Cu, Pb) in the vegetative organs, harvest and growing soil of potatoes, wheat, and wild blackberry. Food Environ. Saf. J. Fac. Food Eng. 2012, 11, 38–42. [Google Scholar]
- Vollmanova, A.; Zupka, S.; Bajcan, D.; Medvecky, M.; Daniel, J. Dangerous heavy metals in soil and small forest fruit as a result of old environmental loads. In Proceedings of the 14th International Conference on Environmental Science and Technology, Rhodes, Greece, 3–5 September 2015; pp. 698–703. [Google Scholar]
- Micu, L.M.; Petanec, D.I.; Iosub-Ciur, M.D.; Andrian, S.; Popovici, R.A.; Porumb, A. The heavy metals content in leave of the forest fruits (Hippophae rhamnoides and Rubus fruticosus) from the tailings dumps mining. Rev. Chim. 2016, 67, 64–68. [Google Scholar]
- Kekedy-Nagy, L.; Ionescu, A. Characterization and classification of tea herbs based on their metal content. Acta Univ. Sapientiae Agric. Environ. 2009, 1, 11–19. [Google Scholar]
- Amidžić, D.; Klarić, I.; Velić, D.; Vedrina Dragojević, I. Evaluation of mineral and heavy metal contents in Croatian blackberry wines. Czech J. Food Sci. 2011, 29, 260–267. [Google Scholar] [CrossRef]
- Smejkal, G. The Forest and the Industrial Pollution; Ceres: Bucharest, Romania, 1982; p. 195. [Google Scholar]
- Micu, M.O. The Influence of Pollution in the Area Copşa Mică and Its Ecological Implications. Ph.D. Thesis, Transilvania University of Brașov, Braşov, Romania, 2001. [Google Scholar]
- National Meteorological Agency. Catalog. Available online: http://www.meteoromania.ro/catalog/?tip=1&cod_geo=614436&cod_clasa=CLIMATOLOGICA&cod_subclasa=1|18&pas=5&tipulLor=LUNARE&pagina=2 (accessed on 22 February 2019).
- Stefan, K.; Raitio, H.; Bartels, U.; Fürst, A.; Rautio, P. Sampling and analysis of needles and leaves—Manual part IV. In Manual on Methods and Criteria for Harmonized Sampling, Assessment, Monitoring and Analysis of the Effects of Air Pollution on Forests; UNECE-ICP Forests Programme Co-ordinating Centre: Hamburg, Germany, 2005; pp. 1–25. [Google Scholar]
- Luyssaert, S.; Raitio, H.; Vervaeke, P.; Mertens, J.; Lust, N. Sampling procedure for the foliar analysis of decidous trees. J. Environ. Monit. 2002, 4, 858–864. [Google Scholar] [CrossRef] [PubMed]
- Djingova, F.; Kuleff, I. Instrumental techniques for trace analysis. In Trace Elements: Their Distribution and Effects in the Environment; Markert, B., Friese, K., Eds.; Elsevier Science Ltd.: London, UK, 2000; pp. 137–185. [Google Scholar]
- Hansen, M.D.; Nøst, T.H.; Heimstad, E.S.; Evenset, A.; Dudarev, A.A.; Rautio, A.; Myllynen, P.; Dushkina, E.V.; Jagodic, M.; Christensen, G.N.; et al. The impact of a Nickel-Copper smelter on concentrations of toxic elements in local wild food from the Norwegian, Finnish, and Russia border regions. Int. J. Environ. Res. Public Health 2017, 14, 694. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. Kelp, Monograph 1426, 6th ed.; Council of Europe: Strasbourg, France, 2007; Volume 2. [Google Scholar]
- Sun, H.; Li, L. Investigation of Distribution for Trace Lead and Cadmium in Chinese Herbal Medicines and Their Decoctions by Graphite Furnace Atomic Absorption Spectrometry. Am. J. Anal. Chem. 2011, 2, 217–222. [Google Scholar] [CrossRef]
- Thomsen, V.; Schatzlein, D.; Mercuro, D. Limits of Detection in Spectroscopy. Spectroscopy 2003, 18, 112–114. [Google Scholar]
- Chan, C.C. Analytical method validation: Principles and practices. In Pharmaceutical Manufacturing Handbook: Regulations and Quality; Gad, S.C., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 727–742. [Google Scholar]
- Frank, V.; Tölgyessy, J. The chemistry of soil. In Chemistry and Biology of Water, Air and Soil: Environmental Aspects; Tölgyessy, J., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 621–698. [Google Scholar]
- World Health Organization. WHO Guidelines for Assessing Quality of Herbal Medicines with Reference to Contaminants and Residues; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Kabelitz, L. Heavy metals in herbal drugs. Pharm. Ind. 1998, 60, 444–451. [Google Scholar]
- European Commission. Regulation (EC) No 396/2005 of the European Parliament and of the Council of 23 February 2005 on Maximum Residue Levels in or on Food and Feed of Plant and Animal Origin and Amending Council Directive 91/414/EEC; European Commission: Brussels, Belgium, 2005; pp. 1–16. [Google Scholar]
- Gasser, U.; Klier, B.; Kühn, A.V.; Steinhoff, B. Current findings on the heavy metal content in herbal drugs. Pharm. Sci. Notes 2009, 1, 37–50. [Google Scholar]
- Mohite, R.D.; Basavaiah, N.; Singare, P.U.; Reddy, A.V.R.; Singhal, R.K.; Blaha, U. Assessment of Heavy Metals Accumulation in Washed and Unwashed Leafy Vegetables Sector-26 Vashi, Navi Mumbai, Maharashtra. J. Chem. Biol. Phy. Sci. Sec. D 2016, 6, 1130–1139. [Google Scholar]
- Ataabadi, M.; Hoodaji, M.; Najafi, P. Assessment of washing procedure for determination some of airborne metal concentrations. Afr. J. Biotechnol. 2012, 11, 4391–4395. [Google Scholar] [CrossRef]
- Aksoy, A.; Åžahin, U. Elaeagnus angustifolia L. as a biomonitor of heavy metal pollution. Turk. J. Bot. 1999, 23, 83–87. [Google Scholar]
- Yusuf, K.A.; Oluwole, S.O. Heavy Metal (Cu, Zn, Pb) Contamination of Vegetables in Urban City: A Case Study in Lagos. Res. J. Environ. Sci. 2009, 3, 292–298. [Google Scholar] [CrossRef]
- Felix-Henningsen, P.; Urushadze, T.; Steffens, D.; Kalandadze, B.; Narimanidze, E. Uptake of heavy metals by food crops from highly-polluted Chernozem-like soils in an irrigation district south of Tbilisi, eastern Georgia. Agron. Res. 2010, 8, 781–795. [Google Scholar]
- Aghaei, A.; Khademi, H.; Eslamian, S. Comparison of Three Tree Leaves as Biomonitors of Heavy Metals Contamination in Dust, A Case Study of Isfahan. Helix Int. J. 2017, 7, 1873–1887. [Google Scholar]
- Hoffman, D.J.; Rattner, B.A.; Burton, G.A.; Cairns, J. Handbook of Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2003; p. 1315. [Google Scholar]
- Alexa, B.; Cotârlea, I.; Bărbătei, R. The Pollution of Forests in Mediaş Forest District and the Ecological Restoration Done; Constant Publishing House: Sibiu, Romania, 2004; p. 145. [Google Scholar]
- Mantovi, P.; Bonazzi, G.; Maestri, E.; Marmiroli, N. Accumulation of copperand zinc from liquid manure in agricultural soils and crop plants. Plant Soil 2003, 50, 249–257. [Google Scholar] [CrossRef]
- Sadhu, A.; Upadhyay, P.; Singh, P.K.; Agrawal, A.; Ilango, K.; Karmakar, D.; Singh, G.P.I.; Dubey, G.P. Quantitative analysis of heavy metals in medicinal plants collected from environmentally diverse locations in India for use in a novel phytopharmaceutical product. Environ. Monit. Assess. 2015, 187, 542. [Google Scholar] [CrossRef] [PubMed]
- Nookabkaew, S.; Rangkadilok, N.; Satayavivad, J. Determination of trace elements in herbal tea products and their infusions consumed in Thailand. Agric. Food Chem. 2006, 54, 6939–6944. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans—Inorganic and Organic Lead Compounds; World Health Organization: Lyon, France, 2006; Volume 87. [Google Scholar]
- Goyer, R. Lead toxicity: Current concerns. Environ. Health Perspect. 1993, 100, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Silbergeld, E.K.; Sauk, J.; Somerman, M.; Todd, A.; McNeill, F.; Fowler, B.; Fontaine, A.; van Buren, J. Lead in bone: Storage site, exposure source, and target organ. Neurotoxicology 1993, 14, 225–236. [Google Scholar] [PubMed]
- Sakai, T. Biomarkers of lead exposure. Ind. Health 2000, 38, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Kalia, K.; Flora, S.J. Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J. Occup. Health 2005, 47, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Navas-Acien, A.; Guallar, E.; Silbergeld, E.K.; Rothenberg, S.J. Lead exposure and cardiovascular disease - a systematic review. Environ. Health Perspect. 2007, 115, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.J.S.; Pachauri, V.; Saxena, G. Arsenic, cadmium and lead. In Reproductive and Developmental Toxicology; Gupta, R.C., Ed.; Academic: New York, NY, USA, 2011; Volume 33, pp. 415–438. [Google Scholar]
- Schwartz, G.G.; Ilyasova, D.; Ivanova, A. Urinary cadmium, impaired fasting glucose, and diabetes in the NHANES III. Diabetes Care 2003, 26, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Eum, K.D.; Lee, M.S.; Paek, D. Cadmium in blood and hypertension. Sci. Total Environ. 2008, 407, 147–153. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans—Beryllium, Cadmium, Mercury, and Exposures in the Glass Manufacturing Industry; World Health Organization: Lyon, France, 1993; Volume 58. [Google Scholar]
- World Health Organization. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans—Arsenic, Metals, Fibres and Dusts; World Health Organization: Lyon, France, 2012; Volume 100. [Google Scholar]
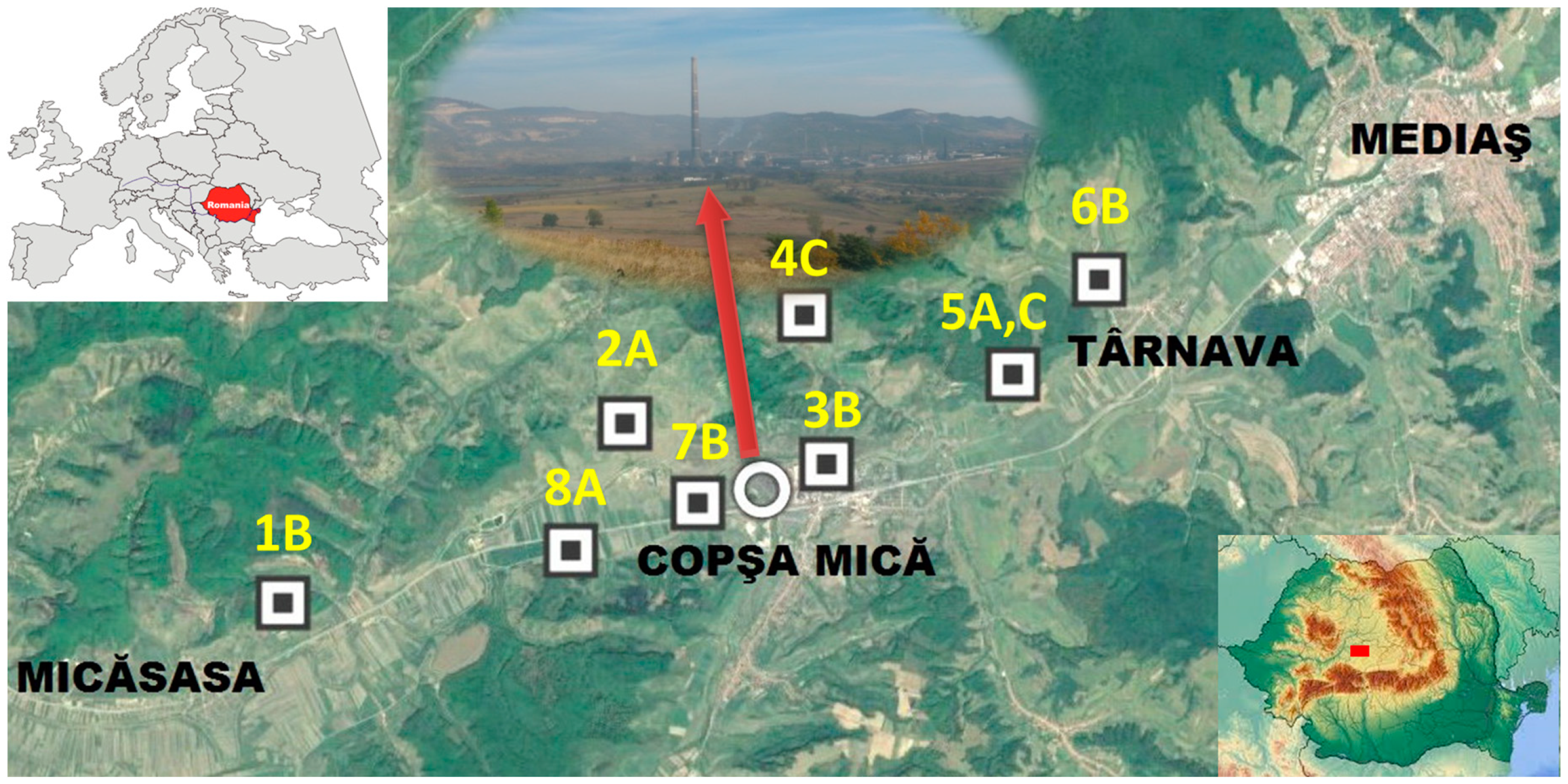
| Resource | Utilization | Information Source | |||
|---|---|---|---|---|---|
| Part of Plant | Species | Range | Product | Uses/Disease | |
| Whole plant | Rubus fruticosus | Romanian folk medicine | tea, decoction | leukorrhea | [2] |
| Rubus sp. | Native American folk medicine | extract from fruit, root, and leaves | hair and fabric dye | [3] | |
| Aerial parts | Rubus fruticosus | Europe | various | hypoglycemia | [4] |
| Stem | Rubus sp. | Native American practices | rope | transport | [3] |
| Young shoots | Rubus fruticosus, R. ulmifolius Schott | Sardinian and Sicilian traditional medicine | decoction | menstrual pain | [5] |
| Rubus fruticosus | Romanian folk medicine | decoction | bronchitis, diarrhea, dysentery | [2] | |
| Leaves | Rubus fruticosus, R. ulmifolius | Sardinian and Sicilian traditional medicine | fresh leaves for chewing | strengthening spongy gums | [5] |
| Rubus fruticosus | Central Italy folk medicine | maceration | cicatrizant for skin, fungal infections, skin abscesses | [6] | |
| Rubus villosus Aiton. | around the world | leaves for chewing | bleeding gums | [7] | |
| Rubus fruticosus | European folk medicine | mouthwash, decoction | strengthening spongy gums, mouth ulcers, sore throats, diarrhea, hemorrhoids | [8] | |
| Fruits | Rubus sp. | around the world | jam, syrup, jelly, marmalade, cake stuffing, wine, liqueur, ice cream, in yoghurt, drink and chewing gum dye | [3,9] | |
| Rubus fruticosus | Romanian folk medicine | decoction in lard | tuberculosis | [2] | |
| wine | leukorrhea | ||||
| Rubus fruticosus | Ancient Greeks | fresh fruit | gout | [3] | |
| Fruits and leaves | Rubus fruticosus | Pakistani traditional medicine | various | skin diseases, itching, scabies, eczema | [10] |
| Roots | Rubus villosus | around the world | dried root tea used for edema, leaves and roots used for diarrhea, enteritis, chronic appendicitis, leukorrhea, expectorant properties | [7] | |
| Type of Site | Site Description |
|---|---|
| A | Site located in the main valley (where the source of pollution is found) with frontal exposure to the source of pollution (slope facing the flue-gas stack). |
| B | Site located in the main valley (where the source of pollution is found) with tangential exposure to the source of pollution (slope not facing the flue-gas stack). |
| C | Site located in a secondary valley with frontal exposure to the local circulation of air mass. |
| D | Site located in a secondary valley, partially protected from the source of pollution. |
| Temperature (°C) | 145 | 180 | 100 |
|---|---|---|---|
| Power (%) | 75 | 90 | 40 |
| Time (min) | 5 | 10 | 10 |
| Standard Conditions | Element | |||
|---|---|---|---|---|
| Cd | Cu | Pb | Zn | |
| Wavelength, λ (nm) | 228.8 | 324.8 | 283.3 | 213.9 |
| Slit width (nm) | 1.2 | 1.2 | 1.2 | 0.5 |
| Hollow-cathode lamp current (mA) | 3 | 3 | 3 | 4 |
| Background correction | Deuterium | Deuterium | Deuterium | Deuterium |
| Flame | C2H2/air | C2H2/air | C2H2/air | C2H2/air |
| Fuel flow (N L/h) | 50 | 50 | 65 | 50 |
| Parameter | Element | |||
|---|---|---|---|---|
| Cd | Zn | Pb | Cu | |
| Linear working range (mg/L) | 0–1 | 0–1 | 0–1 | 0–3 |
| Limit of detection (mg/L) | 0.012 | 0.013 | 0.083 | 0.036 |
| Limit of quantification (mg/L) | 0.039 | 0.042 | 0.276 | 0.119 |
| Reference | Pb (mg/kg) | Cd (mg/kg) | Zn (mg/kg) | Cu (mg/kg) |
|---|---|---|---|---|
| [68] | 10.0 | 0.5 | - | - |
| [67] | 10.0 | 0.3 | - | - |
| [62] | 5 | 4 | - | - |
| [69] | - | - | - | 5 (berries and small fruits) |
| [62] | 5 | 0,5 | - | - |
| Metal | The Significance of the Differences between Individual Values * | Range | Arithmetic Mean | Median | Coefficient of Variation (%) | Relative Frequency (%) of Values Which Exceed the World Health Organization Threshold | The Significance of the Differences between Blackberry Organs ** (Kruskal–Wallis Test) | ||
|---|---|---|---|---|---|---|---|---|---|
| t | p | H | p | ||||||
| Pb (mg/kg dry weight) | 4.64 | <0.001 | 1.67–291.39 | 34.72 | 20.27 | 141.59 | 70.5 | 14.27 | <0.001 |
| Cd (mg/kg d.w.) | 11.49 | <0.001 | 0.32–4.46 | 1.86 | 1.61 | 57.08 | 100.0 | 8.18 | 0.02 |
| Zn (mg/kg d.w.) | 10.01 | <0.001 | 10.91–193.54 | 76.03 | 70.29 | 65.49 | - | 23.05 | <0.001 |
| Cu (mg/kg d.w.) | 7.84 | <0.001 | 1.23–34.08 | 8.51 | 7.18 | 69.89 | 83.3 | 9.34 | 0.01 |
| Independent Variable | Dependent Variable | |||
|---|---|---|---|---|
| Pb | Cd | Zn | Cu | |
| p from Kruskal–Wallis Test (0.05 is the Threshold Value for Statistical Significance) * | ||||
| Distance from source of pollution | 0.005 | 0.13 | 0.36 | 0.80 |
| Altitude | 0.01 | 0.31 | 0.27 | 0.07 |
| Aspect | 0.08 | 0.92 | 0.59 | 0.70 |
| Exposure to air circulation | 0.09 | 0.01 | 0.15 | 0.48 |
| Year of sampling | 0.26 | 0.41 | 0.89 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlad, I.A.; Goji, G.; Dinulică, F.; Bartha, S.; Vasilescu, M.M.; Mihăiescu, T. Consuming Blackberry as a Traditional Nutraceutical Resource from an Area with High Anthropogenic Impact. Forests 2019, 10, 246. https://doi.org/10.3390/f10030246
Vlad IA, Goji G, Dinulică F, Bartha S, Vasilescu MM, Mihăiescu T. Consuming Blackberry as a Traditional Nutraceutical Resource from an Area with High Anthropogenic Impact. Forests. 2019; 10(3):246. https://doi.org/10.3390/f10030246
Chicago/Turabian StyleVlad, Ioana Andra, Győző Goji, Florin Dinulică, Szilard Bartha, Maria Magdalena Vasilescu, and Tania Mihăiescu. 2019. "Consuming Blackberry as a Traditional Nutraceutical Resource from an Area with High Anthropogenic Impact" Forests 10, no. 3: 246. https://doi.org/10.3390/f10030246
APA StyleVlad, I. A., Goji, G., Dinulică, F., Bartha, S., Vasilescu, M. M., & Mihăiescu, T. (2019). Consuming Blackberry as a Traditional Nutraceutical Resource from an Area with High Anthropogenic Impact. Forests, 10(3), 246. https://doi.org/10.3390/f10030246






