Wood Density Variations of Legume Trees in French Guiana along the Shade Tolerance Continuum: Heartwood Effects on Radial Patterns and Gradients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Study Species
2.3. Selected Trees
2.4. Tree Logging, Collection, and Preparation of Wood Discs
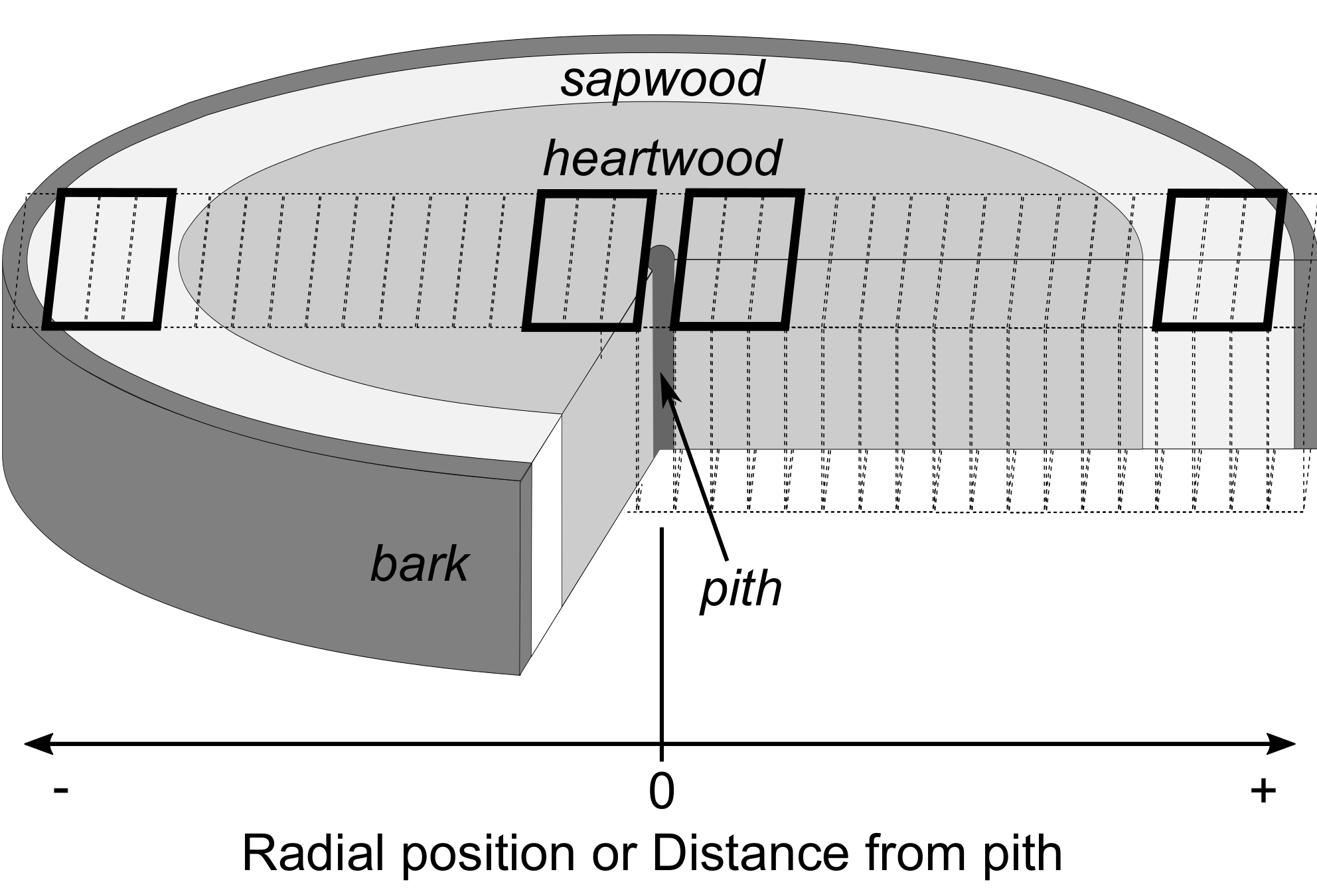
2.5. Assignment of Radial Positions and Measurement of Wood Density
2.6. Comparisons of the Inner, Outer, and Overall Wood Density at Species Level
2.7. Decoupling the Effect of Xylogenesis from that of Heartwood Formation on WD
2.8. Estimation of Wood Density Corrected by Extractive Content
2.9. Selected Young Trees of Three Species
2.10. Successional Status and Demographic Variables
2.11. Modeling the Radial Pattern
2.12. Model Selection Procedure
2.13. Determination of the Species Corrected WD Radial Pattern
2.14. Calculation of Observed and Corrected Radial Gradients
2.15. Correlations among WD Variables and between WD Variables and Demographic Variables
2.16. Linking Successional Status and Corrected WD Radial Pattern to WD and Demographic Variables
3. Results
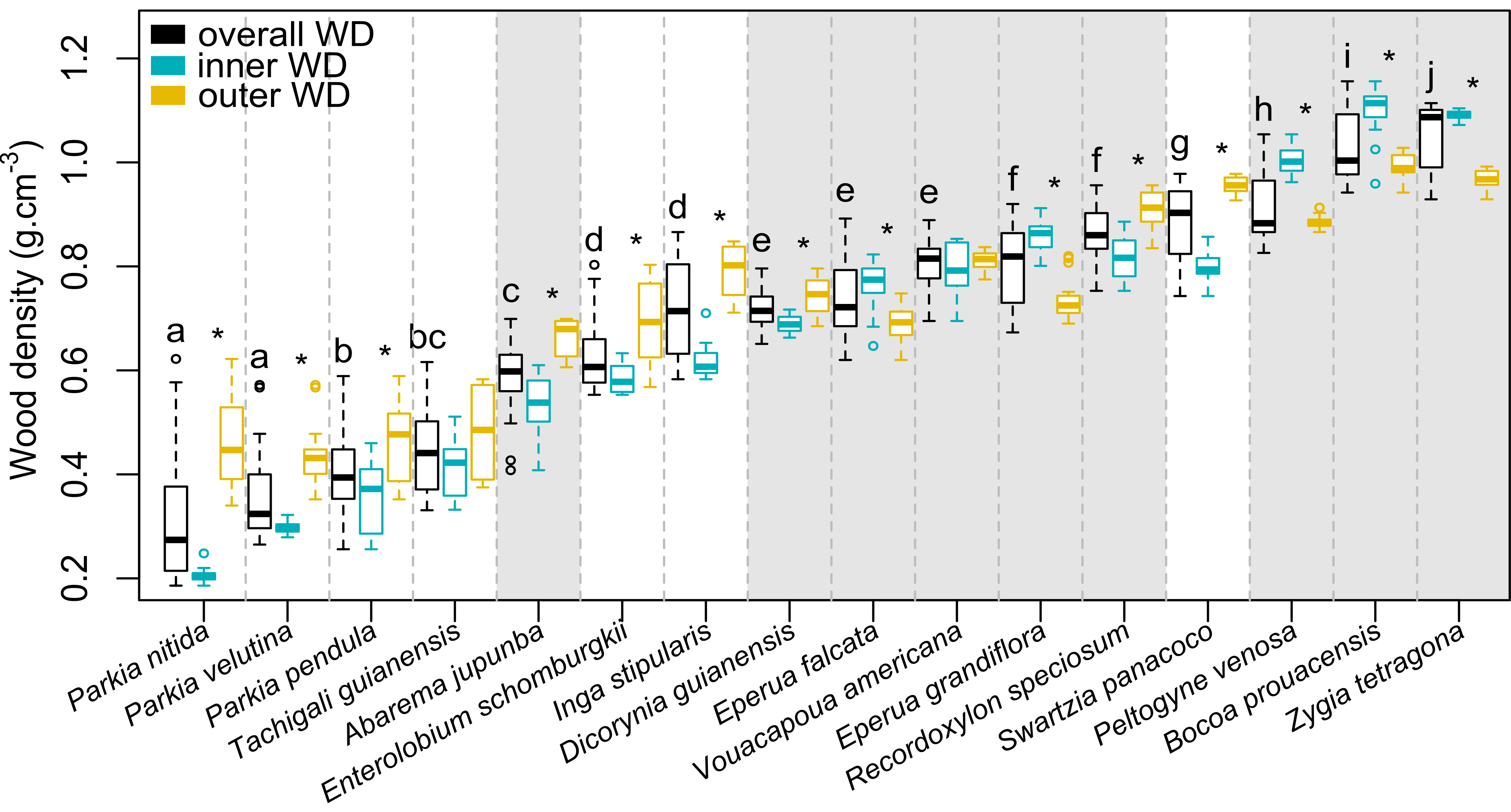
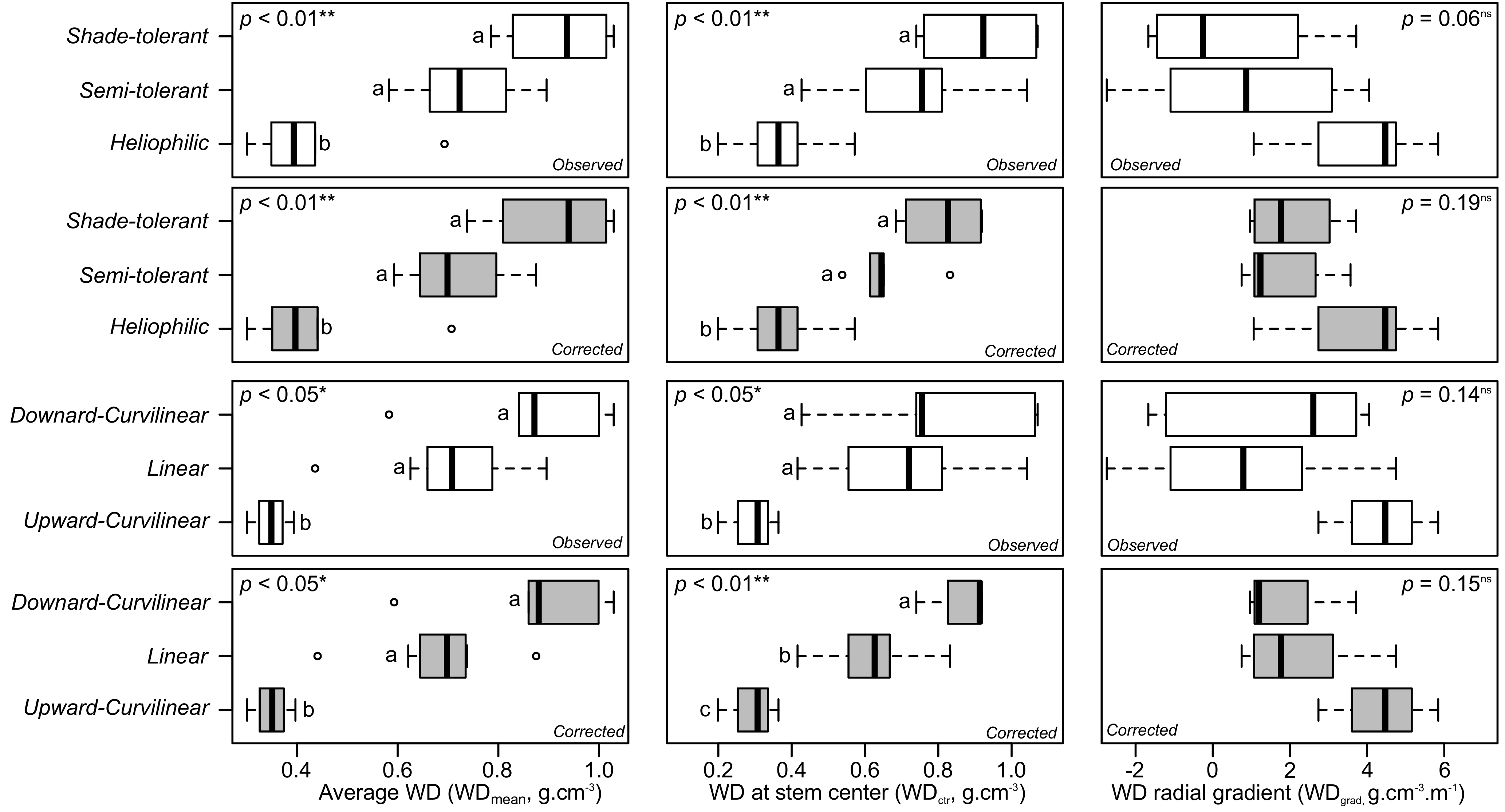
3.1. Observed Overall, Inner and Outer Wood Density at the Species Level
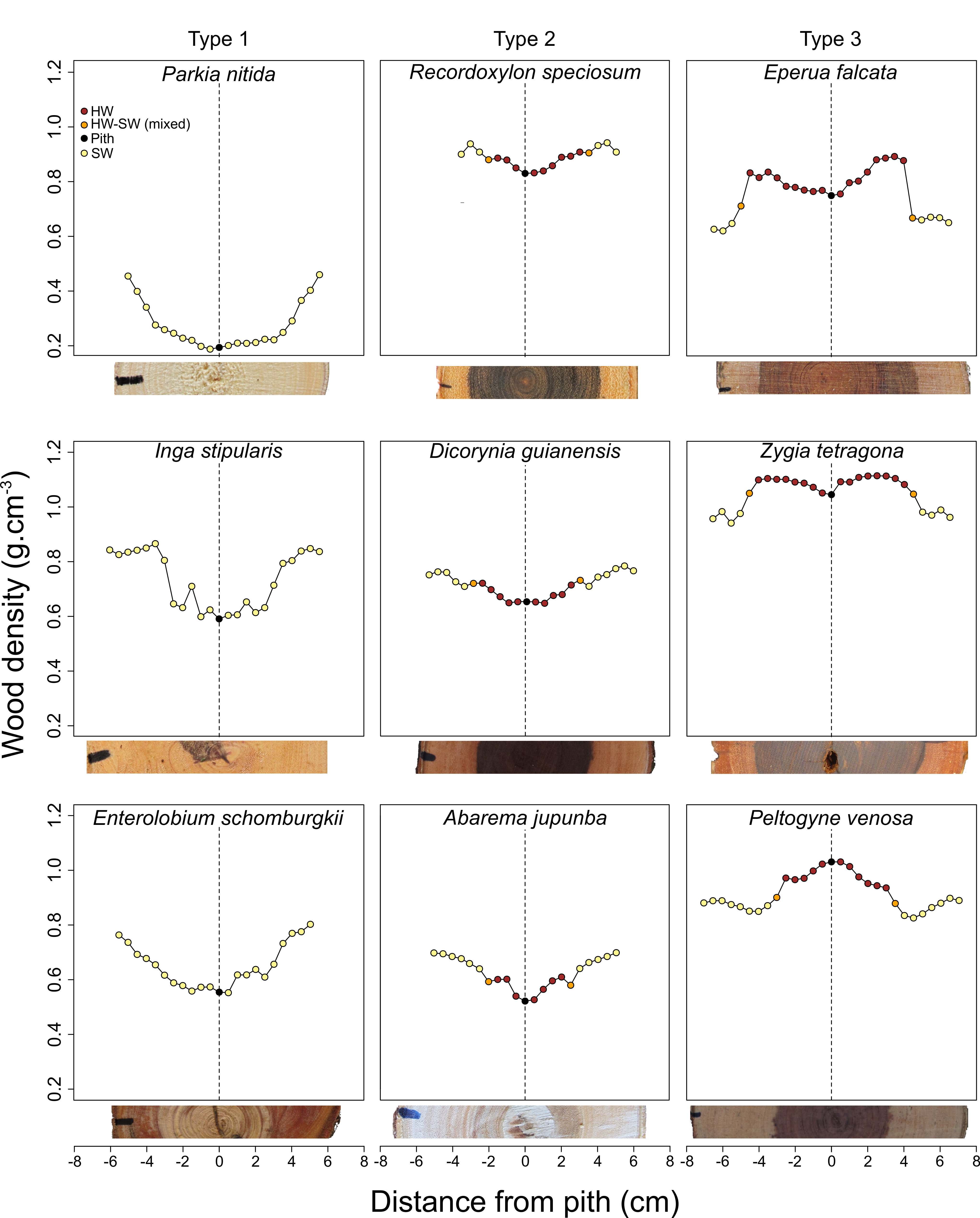
3.2. Qualitative Assessment of WD Radial Patterns in Light of the Presence of Heartwood
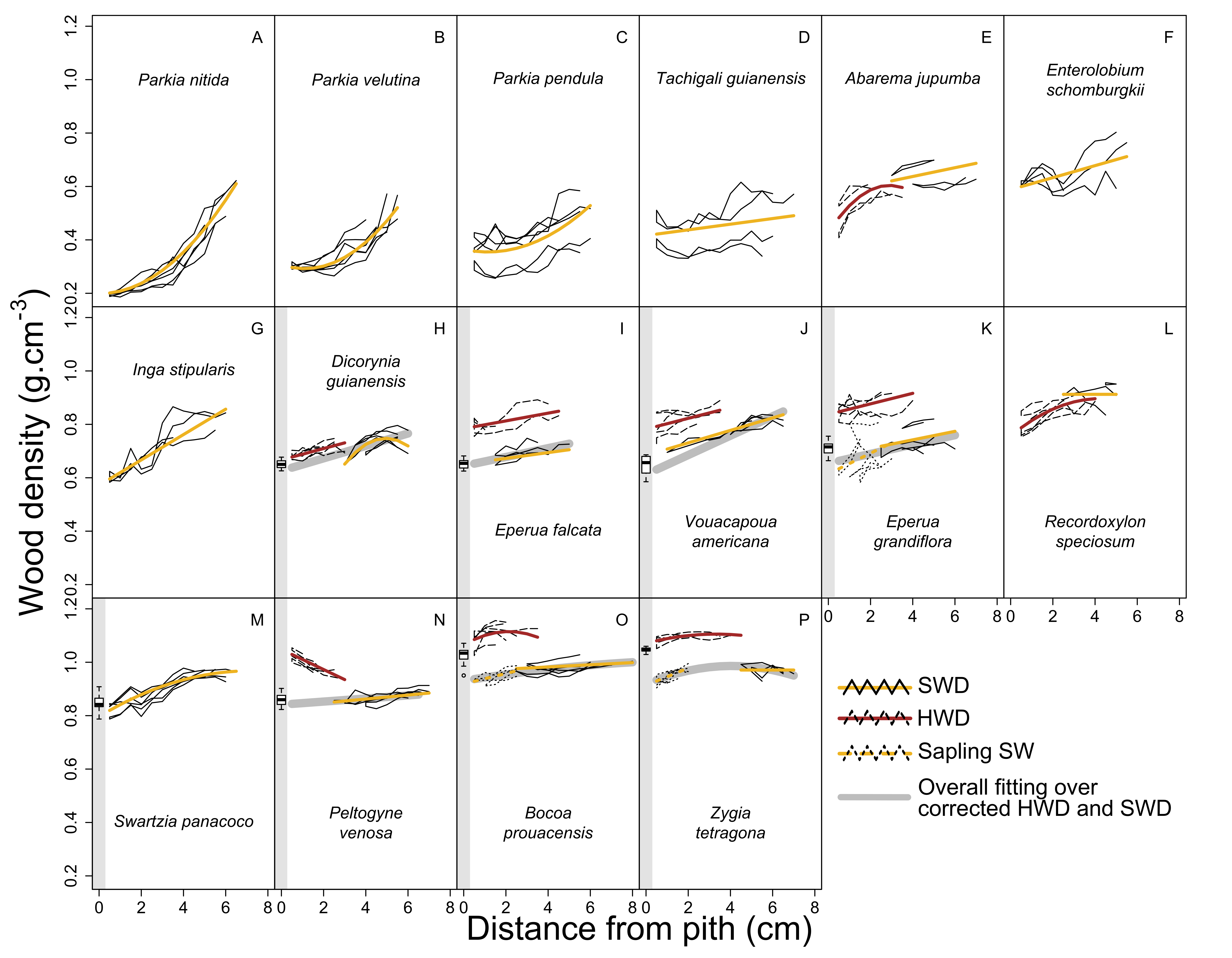
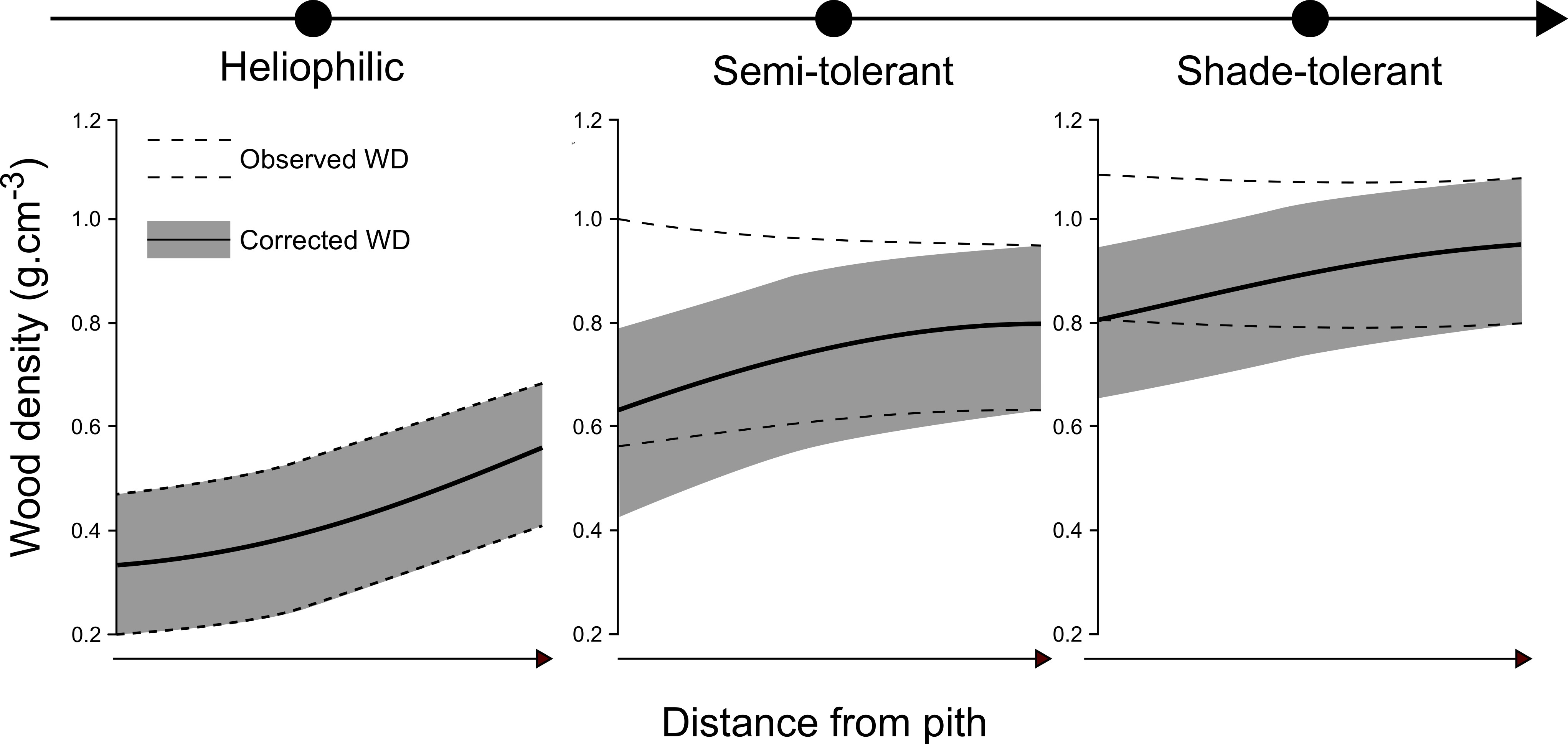
3.3. Modeling of WD Radial Variation in Heartwood and Sapwood
3.4. Effect of Extractives in Bocoa prouacensis, Zygia tetragona, and Eperua falcata
3.5. Observed and Corrected Radial Gradient
3.6. Correlations among WD Variables and between WD Variables and Demographic Variables
3.7. Links between WD Variables, WD Profile, and Successional Status
4. Discussion
4.1. The Effect of HW Extractives Impregnation on Wood Density Radial Gradients and Possible Functional Misinterpretation Due to Uncorrected Wood Density
4.2. On the Corrected Radial Gradient, Patterns and Their Link with Overall, Inner/Outer WD, and the Shade-Tolerance Continuum
4.3. Do Low WD Gradients Only Illustrate Late-Successional Species?
4.4. Limitations to the Correction of WD by Extractive Content
4.5. WD Variations and Demographic Variables
4.6. What Can We Expect for Wood Density Gradients of Canopy Trees?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Methods of Chemical Extraction for Zygia tetragona
References
- Kollmann, F.F.P.; Côté, W.A. Principles of Wood Science and Technology: I Solid Wood; Springer: Berlin, Germany, 1984. [Google Scholar]
- Muller-Landau, H.C. Interspecific and inter-site variation in wood specific gravity of tropical trees. Biotropica 2004, 36, 20–32. [Google Scholar] [CrossRef]
- Van Gelder, H.A.; Poorter, L.; Sterck, F.J. Wood mechanics, allometry, and life-history variation in a tropical rain forest tree community. New Phyt. 2006, 171, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Chave, J.; Coomes, D.; Jansen, S.; Lewis, S.L.; Swenson, N.G.; Zanne, A.E. Towards a worldwide wood economics spectrum. Ecol. Lett. 2009, 12, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.J.; Kitajima, K.; Kraft, N.J.B.; Reich, P.B.; Wright, I.J.; Bunker, D.E.; Condit, R.; Dalling, J.W.; Davies, S.J.; Díaz, S.; et al. Functional traits and the growth–mortality trade-off in tropical trees. Ecology 2010, 91, 3664–3674. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J. Influence of tissue density-specific mechanical properties on the scaling of plant height. Ann. Bot. 1993, 72, 173–179. [Google Scholar] [CrossRef]
- Niklas, K.J.; Spatz, H.-C. Worldwide correlations of mechanical properties and green wood density. Am. J. Bot. 2010, 97, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Pratt, R.B.; Jacobsen, A.L.; Ewers, F.W.; Davis, S.D. Relationships among xylem transport, biomechanics and storage in stems and roots of nine Rhamnaceae species of the California chaparral. New Phyt. 2007, 174, 787–798. [Google Scholar] [CrossRef]
- Lachenbruch, B.; Moore, J.; Evans, R. Radial Variation in Wood Structure and Function in Woody Plants, and Hypotheses for Its Occurrence. In Size- and Age-Related Changes in Tree Structure and Function; Meinzer, F.C., Lachenbruch, B., Dawson, T.E., Eds.; Springer: Berlin, Germany, 2011; Volume 4, pp. 121–164. [Google Scholar]
- Hacke, U.G.; Sperry, J.S.; Pockman, W.T.; Davis, S.D.; McCulloh, K.A. Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 2001, 126, 457–461. [Google Scholar] [CrossRef]
- Markesteijn, L.; Poorter, L.; Paz, H.; Sack, L.; Bongers, F. Ecological differentiation in xylem cavitation resistance is associated with stem and leaf structural traits. Plant Cell Environ. 2011, 34, 137–148. [Google Scholar] [CrossRef]
- Rosner, S. Wood density as a proxy for vulnerability to cavitation: Size matters. J. Plant Hydraul. 2017, 4, 1–10. [Google Scholar] [CrossRef]
- Zanne, A.E.; Westoby, M.; Falster, D.S.; Ackerly, D.D.; Loarie, S.R.; Arnold, S.E.J.; Coomes, D.A. Angiosperm wood structure: Global patterns in vessel anatomy and their relation to wood density and potential conductivity. Am. J. Bot. 2010, 97, 207–215. [Google Scholar] [CrossRef]
- King, D.A.; Davies, S.J.; Tan, S.; Noor, N.S.M. The role of wood density and stem support costs in the growth and mortality of tropical trees. J. Ecol. 2006, 94, 670–680. [Google Scholar] [CrossRef]
- Poorter, L.; Wright, S.J.; Paz, H.; Ackerly, D.D.; Condit, R.; Ibarra-Manríquez, G.; Harms, K.E.; Licona, J.C.; Martínez-Ramos, M.; Mazer, S.J.; et al. Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology 2008, 89, 1908–1920. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, H.E.M.; Laurance, W.F.; Condit, R.; Laurance, S.G.; D’Angelo, S.; Andrade, A.C. Demographic and life-history correlates for Amazonian trees. J. Veg. Sci. 2005, 16, 625–634. [Google Scholar] [CrossRef]
- Meinzer, F.C.; Lachenbruch, B.; Dawson, T.E. Size- and Age-Related Changes in Tree Structure and Function; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Wiemann, M.; Williamson, G. Extreme radial changes in wood specific gravity in some tropical pioneers. Wood Fiber Sci. 1988, 20, 344–349. [Google Scholar]
- Rueda, R.; Williamson, G.B. Radial and vertical wood specific gravity in Ochroma pyramidale (Cav. ex Lam.) Urb. (Bombacaceae). Biotropica 1992, 24, 512–518. [Google Scholar] [CrossRef]
- Williamson, G.B.; Wiemann, M.C.; Geaghan, J.P. Radial wood allocation in Schizolobium parahyba. Am. J. Bot. 2012, 99, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- Bastin, J.-F.; Fayolle, A.; Tarelkin, Y.; Van den Bulcke, J.; de Haulleville, T.; Mortier, F.; Beeckman, H.; Van Acker, J.; Serckx, A.; Bogaert, J.; et al. Wood specific gravity variations and biomass of central African tree species: The simple choice of the outer wood. PLoS ONE 2015, 10, e0142146. [Google Scholar] [CrossRef] [PubMed]
- Longuetaud, F.; Mothe, F.; Santenoise, P.; Diop, N.; Dlouha, J.; Fournier, M.; Deleuze, C. Patterns of within-stem variations in wood specific gravity and water content for five temperate tree species. Ann. For. Sci. 2017, 74, 64. [Google Scholar] [CrossRef]
- Wiemann, M.C.; Williamson, B. Testing a novel method to approximate wood specific gravity of trees. For. Sci. 2012, 58, 577–591. [Google Scholar] [CrossRef]
- Wiemann, M.C.; Williamson, G.B. Wood specific gravity gradients in tropical dry and montane rain forest trees. Am. J. Bot. 1989, 76, 924–928. [Google Scholar] [CrossRef]
- Wiemann, M.C.; Williamson, G.B. Radial gradients in the specific gravity of wood in some tropical and temperate trees. For. Sci. 1989, 35, 197–210. [Google Scholar]
- Parolin, P. Radial gradients in wood specific gravity in trees of central amazonian floodplains. IAWA J. 2002, 23, 449–457. [Google Scholar] [CrossRef]
- Abe, H.; Kuroda, K.; Yamashita, K.; Yazaki, K.; Noshiro, S.; Fujiwara, T. Radial variation of wood density of Quercus spp. (Fagaceae) in Japan. Mokuzai Gakkaishi 2012, 58, 329–338. [Google Scholar] [CrossRef]
- Lei, H.; Milota, M.R.; Gartner, B.L. Between- and within-tree variation in the anatomy and specific gravity of wood in oregon White Oak (Quercus garryana Dougl.). IAWA J. 1996, 17, 445–461. [Google Scholar] [CrossRef]
- Woodcock, D.; Shier, A. Wood specific gravity and its radial variations: The many ways to make a tree. Trees 2002, 16, 437–443. [Google Scholar] [CrossRef]
- Hérault, B.; Beauchêne, J.; Muller, F.; Wagner, F.; Baraloto, C.; Blanc, L.; Martin, J.-M. Modeling decay rates of dead wood in a neotropical forest. Oecologia 2010, 164, 243–251. [Google Scholar] [CrossRef]
- Thibaut, B.; Baillères, H.; Chanson, B.; Fournier-Djimbi, M. Plantations d’arbres à croissance rapide et qualité des produits forestiers sous les tropiques. Bois For. Trop. 1997, 252, 49–54. [Google Scholar]
- Nock, C.A.; Geihofer, D.; Grabner, M.; Baker, P.J.; Bunyavejchewin, S.; Hietz, P. Wood density and its radial variation in six canopy tree species differing in shade-tolerance in western Thailand. Ann. Bot. 2009, 104, 297–306. [Google Scholar] [CrossRef]
- Hietz, P.; Valencia, R.; Joseph Wright, S. Strong radial variation in wood density follows a uniform pattern in two neotropical rain forests. Funct. Ecol. 2013, 27, 684–692. [Google Scholar] [CrossRef]
- Osazuwa-Peters, O.L.; Wright, S.J.; Zanne, A.E. Radial variation in wood specific gravity of tropical tree species differing in growth–mortality strategies. Am. J. Bot. 2014, 101, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Plourde, B.T.; Boukili, V.K.; Chazdon, R.L. Radial changes in wood specific gravity of tropical trees: Inter- and intraspecific variation during secondary succession. Funct. Ecol. 2015, 29, 111–120. [Google Scholar] [CrossRef]
- Hillis, W.E. Secondary Changes in Wood. In The Structure, Biosynthesis, and Degradation of Wood; Loewus, F., Runeckles, V.C., Eds.; Plenum Press: New York, NY, USA, 1977; Volume 11, pp. 247–309. [Google Scholar]
- Hillis, W.E. Heartwood and Tree Exudates; Springer-Verlag: Berlin, Germany, 1987. [Google Scholar]
- Yang, K.C. The Ageing Process of Sapwood Ray Parenchyma Cells in Four Woody Species. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 1990. [Google Scholar]
- Royer, M.; Stien, D.; Beauchêne, J.; Herbette, G.; McLean, J.P.; Thibaut, A.; Thibaut, B. Extractives of the tropical wood wallaba (Eperua falcata Aubl.) as natural anti-swelling agents. Holzforschung 2010, 64, 211–215. [Google Scholar] [CrossRef]
- Amusant, N.; Moretti, C.; Richard, B.; Prost, E.; Nuzillard, J.M.; Thévenon, M.F. Chemical compounds from Eperua falcata and Eperua grandiflora heartwood and their biological activities against wood destroying fungus (Coriolus versicolor). Holz Roh Werkst. 2006, 65, 23–28. [Google Scholar] [CrossRef]
- Lehnebach, R. Variabilité Ontogénique du Profil Ligneux chez les Légumineuses de Guyane Française. Ph.D. Thesis, Université de Montpellier, Montpellier, France, 2015. [Google Scholar]
- Sabatier, D.; Prévost, M.F. Quelques données sur la composition floristique, et la diversite des peuplements forestiers de guyane francaise. Bois For. Trop. 1990, 219, 31–55. [Google Scholar]
- Ter Steege, H.; Pitman, N.C.A.; Phillips, O.L.; Chave, J.; Sabatier, D.; Duque, A.; Molino, J.-F.; Prevost, M.-F.; Spichiger, R.; Castellanos, H.; et al. Continental-scale patterns of canopy tree composition and function across Amazonia. Nature 2006, 443, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Ter Steege, H.; Vaessen, R.W.; Cárdenas-López, D.; Sabatier, D.; Antonelli, A.; de Oliveira, S.M.; Pitman, N.C.A.; Jørgensen, P.M.; Salomão, R.P. The discovery of the Amazonian tree flora with an updated checklist of all known tree taxa. Sci. Rep. 2016, 6, 29549. [Google Scholar] [CrossRef]
- Woodcock, D.W.; Shier, A.D. Does canopy position affect wood specific gravity in temperate forest trees? Ann. Bot. 2003, 91, 529–537. [Google Scholar] [CrossRef]
- Osazuwa-Peters, O.L.; Wright, S.J.; Zanne, A.E. Linking wood traits to vital rates in tropical rainforest trees: Insights from comparing sapling and adult wood. Am. J. Bot. 2017, 104, 1464–1473. [Google Scholar] [CrossRef]
- Favrichon, V. Classification des espèces arborées en groupes fonctionnels en vue de la réalisation d'un modèle de dynamique de peuplement en forêt guyanaise. Rev. Ecol. Terre Vie 1994, 49, 379–403. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Taylor, A.M.; Gartner, B.L.; Morrell, J.J. Heartwood formation and natural durability—A review. Wood Fiber Sci. 2002, 34, 587–611. [Google Scholar]
- Molino, J.F.; Sabatier, D. Tree diversity in tropical rain forests: A validation of the intermediate disturbance hypothesis. Science 2001, 294, 1702–1704. [Google Scholar] [CrossRef] [PubMed]
- Vincent, G.; Molino, J.-F.; Marescot, L.; Barkaoui, K.; Sabatier, D.; Freycon, V.; Roelens, J.B. The relative importance of dispersal limitation and habitat preference in shaping spatial distribution of saplings in a tropical moist forest: A case study along a combination of hydromorphic and canopy disturbance gradients. Ann. For. Sci. 2011, 68, 357–370. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D. Mixed-Effects Models in S and S-PLUS; Springer-Verlag: New York, NY, USA, 2000. [Google Scholar]
- Hurvich, C.M.; Tsai, C.-L. Bias of the corrected AIC criterion for underfitted regression and time series models. Biometrika 1991, 78, 499–509. [Google Scholar] [CrossRef]
- Mazerolle, M.J. AICcmodavg: Model Selection and Multimodel Inference Based on (Q)AIC(c). R Package Version 2.1-0. 2016. Available online: https://cran.r-project.org/package=AICcmodavg (accessed on 1 December 2018).
- Harrel, F.E.J. Hmisc: Harrell Miscellaneous. R Package Version 3.14-3. 2016. Available online: https://CRAN.R-project.org/package=Hmisc (accessed on 1 December 2018).
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.2-4. 2016. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 1 December 2018).
- Morel, H.; Lehnebach, R.; Cigna, J.; Ruelle, J.; Nicolini, E.; Beauchêne, J. Basic wood density variations of Parkia velutina Benoist, a long-lived heliophilic Neotropical rainforest tree. Bois For. Trop. 2018, 335, 59–69. [Google Scholar] [CrossRef]
- Bossu, J. Potentiel de Bagassa guianensis et Cordia alliodora pour la Plantation en Zone Tropicale: Description d’une Stratégie de Croissance Optimale Alliant Vitesse de Croissance et Qualité du Bois. Ph.D. Thesis, Université de Guyane, Kourou, French Guiana, 2015. [Google Scholar]
- Oldeman, R.A.A. L’Architecture de la Forêt Guyanaise; Office de la Recherche Scientifique et Technique Outre-Mer: Paris, France, 1974. [Google Scholar]
- Anten, N.P.R.; Schieving, F. The role of wood mass density and mechanical constraints in the economy of tree architecture. Am. Nat. 2010, 175, 11. [Google Scholar] [CrossRef] [PubMed]
- Larjavaara, M.; Muller-Landau, H.C. Rethinking the value of high wood density. Funct. Ecol. 2010, 24, 701–705. [Google Scholar] [CrossRef]
- Lachenbruch, B.; McCulloh, K.A. Traits, properties, and performance: How woody plants combine hydraulic and mechanical functions in a cell, tissue, or whole plant. New Phyt. 2014, 204, 747–764. [Google Scholar] [CrossRef]
- Chapotin, S.M.; Razanameharizaka, J.H.; Holbrook, N.M. A biomechanical perspective on the role of large stem volume and high water content in baobab trees (Adansonia spp.; Bombacaceae). Am. J. Bot. 2006, 93, 1251–1264. [Google Scholar] [CrossRef]
- Kuo, M.-L.; Arganbright, D.G. Cellular distribution of extractives in redwood and incense cedar—Part II. Microscopic observation of the location of cell wall and cell cavity extractives. Holzforschung 1980, 34, 41–47. [Google Scholar] [CrossRef]
- Olson, J.R.; Carpenter, S.B. Specific gravity, fibre length, and extractive content of young Paulownia. Wood Fiber Sci. 1985, 17, 428–438. [Google Scholar]
- Stringer, J.W.; Olson, J.R. Radial and vertical variations in stem properties of juvenile black locust (Robinia pseudoacacia). Wood Fiber Sci. 1987, 19, 59–67. [Google Scholar]
- Gierlinger, N.; Wimmer, R. Radial distribution of heartwood extractives and lignin in mature European larch. Wood Fiber Sci. 2004, 36, 387–394. [Google Scholar]
- Bossu, J.; Beauchêne, J.; Estevez, Y.; Duplais, C.; Clair, B. New insights on wood dimensional stability influenced by secondary metabolites: The case of a fast-growing tropical species Bagassa guianensis Aubl. PLoS ONE 2016, 11, e0150777. [Google Scholar] [CrossRef] [PubMed]
- Amusant, N.; Beauchene, J.; Fournier, M.; Janin, G.; Thevenon, M.-F. Decay resistance in Dicorynia guianensis Amsh.: Analysis of inter-tree and intra-tree variability and relations with wood colour. Ann. For. Sci. 2004, 61, 373–380. [Google Scholar] [CrossRef]
- Hillis, W.E. Distribution, properties and formation of some wood extractives. Wood Sci. Technol. 1971, 5, 272–289. [Google Scholar] [CrossRef]
- Taylor, A.; Freitag, C.; Cadot, E.; Morrell, J. Potential of near infrared spectroscopy to assess hot-water-soluble extractive content and decay resistance of a tropical hardwood. Holz Roh Werkst. 2008, 66, 107–111. [Google Scholar] [CrossRef]
- Amusant, N.; Nigg, M.; Thibaut, B.; Beauchene, J. Diversity of decay resistance strategies of durable tropical woods species: Bocoa prouacensis Aublet, Vouacapoua americana Aublet, Inga alba (Sw.) Wild. Int. Biodeterior. Biodegrad. 2014, 94, 103–108. [Google Scholar] [CrossRef]
- Falster, D.S.; Westoby, M. Tradeoffs between height growth rate, stem persistence and maximum height among plant species in a post-fire succession. Oikos 2005, 111, 57–66. [Google Scholar] [CrossRef]
- Panshin, A.J.; de Zeeuw, C. Textbook of Wood Technology: Structure, Identification, Properties, and Uses of the Commercial Woods of the United States and Canada; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Hernández, R.E. Influence of accessory substances, wood density and interlocked grain on the compressive properties of hardwoods. Wood Sci. Technol. 2007, 41, 249–265. [Google Scholar] [CrossRef]
- Gherardi Hein, P.R.; Tarcísio Lima, J. Relationships between microfibril angle, modulus of elasticity and compressive strength in Eucalyptus wood. Maderas. Cienc. Tecnol. 2012, 14, 267–274. [Google Scholar] [CrossRef]
- Cave, I.D.; Walker, J.C.F. Stiffness of wood in fast-grown plantation softwoods: Theinfluence of microfibril angle. For. Prod. J. 1994, 44, 43–48. [Google Scholar]
- Bossu, J.; Lehnebach, R.; Corn, S.; Regazzi, A.; Beauchêne, J.; Clair, B. Interlocked grain and density patterns in Bagassa guianensis: Changes with ontogeny and mechanical consequences for trees. Trees 2018, 32, 1643–1655. [Google Scholar] [CrossRef]
- Hart, J.; Johnson, K. Production of decay-resistant sapwood in response to injury. Wood Sci. Technol. 1970, 4, 267–272. [Google Scholar] [CrossRef]
- Boddy, L. Microenvironmental Aspects of Xylem Defenses to Wood Decay Fungi. In Defense Mechanisms of Woody Plants Against Fungi; Blanchette, R.A., Biggs, A.R., Eds.; Springer: Berlin, Germany, 1992; pp. 96–132. [Google Scholar]
- Roszaini, K.; Hale, M.D.; Salmiah, U. In-vitro decay resistance of 12 malaysian broadleaf hardwood trees as a function of wood density and extractives compounds. J. Trop. For. Sci. 2016, 28, 533–540. [Google Scholar]
- Stamm, A.J. Density of wood substance, adsorption by wood, and permeability of wood. J. Phys. Chem. 1929, 33, 398–414. [Google Scholar] [CrossRef]
| Genus species Author | n = 43 | DBH (cm) | Tree Height (m) | Trunk Height (m) | Shade-Tolerance | |||
|---|---|---|---|---|---|---|---|---|
| Abarema jupunba (Willd.) Britton & Killip | 2 | 14.2 | (13.5–14) | 16.5 | (14.5–18.6) | 11.5 | (8.1–14) | S-T |
| Bocoa prouacensis Aubl. | 3 | 13.1 | (11–14.4) | 18.7 | (10–25) | 10.9 | (9–14.8) | Sh-T |
| Dicorynia guianensis Amshoff | 3 | 12.9 | (12.3–13.5) | 19.3 | (17.5–22.8) | 11.6 | (9.2–15) | S-T |
| Enterolobiun schomburgkii (Benth.) Benth. | 2 | 11.5 | (10–13) | 9.6 | (8–11.2) | 6.1 | (3.2–9.1) | S-T |
| Eperua falcata Aubl. | 3 | 11.8 | (10.5–14.5) | 16.4 | (13.5–20) | 7.7 | (6.7–8.2) | S-T |
| Eperua grandiflora (Aubl.) Benth. | 3 | 11.8 | (11–13) | 16.2 | (14.5–19) | 11.8 | (9–13.7) | S-T |
| Inga stipularis DC. | 2 | 12.4 | (11.5–13.4) | 12 | (12–12) | 7.4 | (6.5–8.3) | Helio |
| Parkia nitida Miq. | 3 | 13 | (11.5–15) | 14.3 | (10.5–22) | 11.4 | (7.4–18.3) | Helio |
| Parkia pendula (Willd.) Walp. | 3 | 11.9 | (11.5–12.6) | 11.9 | (9.6–15) | 7.1 | (1.4–12) | Helio |
| Parkia velutina Benoist | 3 | 10.7 | (10.5–11.2) | 12.8 | (12–13.6) | 10.2 | (7.3–12.7) | Helio |
| Peltogyne venosa (M. Vahl) Benth. | 3 | 13.4 | (12–14.5) | 16.9 | (15.3–19) | 10.2 | (7.8–14) | S-T |
| Recordoxylon speciosum (Benoist) Gazel ex Barneby | 3 | 11.7 | (11.5–12) | 10.1 | (8.9–12.5) | 6.3 | (4–9.4) | S-T |
| Swartzia panacoco (Aubl.) Cowan | 3 | 12.6 | (12–13.5) | 13 | (8–16) | 6.5 | (5.3–8.2) | Sh-T |
| Tachigali guianensis (Benth.) Zarucchi & Herend. | 2 | 14.3 | (14–14.5) | 18.2 | (16.6–19.7) | 12.8 | (9–16.5) | Helio |
| Vouacapoua americana Aubl. | 3 | 13.2 | (13–13.5) | 17.2 | (16–18.5) | 11.8 | (9.2–13.5) | Sh-T |
| Zygia tetragona Barneby & J.W. Grimes | 2 | 12 | (12–12) | 15.8 | (15.6–16) | 8.2 | (6.4–10) | Sh-T |
| WD, HWD, SWD | Wood density, heartwood density and sapwood density (g cm−3) |
| WDmean, corWDmean | Observed and corrected mean wood density (g cm−3) |
| WDctr, corWDctr | Observed and corrected wood density at the stem center (g cm−3) |
| WDgrad, corWDgrad | Observed and corrected wood density radial gradient (g cm−3 m−1) |
| innWD, outWD | Inner and outer wood density distribution (g cm−3) |
| RGR95, sRGR95 | 95th percentile of the radial growth rate distribution of mature tree and sapling (cm year−1) |
| sMR | Sapling mortality rate (Proportion of dead saplings per year) |
| WD Variables | Demographic Variables | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| corWDmean | WDctr | corWDctr | WDgrad | corWDgrad | RGR95 | sRGR95 | sMR | DBHmax | |
| WDmean | 0.99 *** | 0.96 *** | 0.99 *** | −0.66 *** | −0.56 *** | −0.86 *** | −0.61 | −0.47 | −0.34 |
| corWDmean | 0.94 *** | 0.98 *** | −0.61 *** | −0.54 *** | −0.85 *** | −0.57 | −0.45 | −0.38 | |
| WDctr | 0.97 *** | −0.83 *** | −0.69 *** | −0.88 *** | −0.69 *** | −0.42 | −0.22 | ||
| corWDctr | −0.74 *** | −0.66 *** | −0.91 *** | −0.79 *** | −0.71 *** | −0.28 | |||
| WDgrad | 0.91 *** | 0.66 *** | 0.56 | 0.32 | −0.11 | ||||
| corWDgrad | 0.65 *** | 0.69 | 0.41 | −0.16 | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lehnebach, R.; Bossu, J.; Va, S.; Morel, H.; Amusant, N.; Nicolini, E.; Beauchêne, J. Wood Density Variations of Legume Trees in French Guiana along the Shade Tolerance Continuum: Heartwood Effects on Radial Patterns and Gradients. Forests 2019, 10, 80. https://doi.org/10.3390/f10020080
Lehnebach R, Bossu J, Va S, Morel H, Amusant N, Nicolini E, Beauchêne J. Wood Density Variations of Legume Trees in French Guiana along the Shade Tolerance Continuum: Heartwood Effects on Radial Patterns and Gradients. Forests. 2019; 10(2):80. https://doi.org/10.3390/f10020080
Chicago/Turabian StyleLehnebach, Romain, Julie Bossu, Stéphanie Va, Hélène Morel, Nadine Amusant, Eric Nicolini, and Jacques Beauchêne. 2019. "Wood Density Variations of Legume Trees in French Guiana along the Shade Tolerance Continuum: Heartwood Effects on Radial Patterns and Gradients" Forests 10, no. 2: 80. https://doi.org/10.3390/f10020080
APA StyleLehnebach, R., Bossu, J., Va, S., Morel, H., Amusant, N., Nicolini, E., & Beauchêne, J. (2019). Wood Density Variations of Legume Trees in French Guiana along the Shade Tolerance Continuum: Heartwood Effects on Radial Patterns and Gradients. Forests, 10(2), 80. https://doi.org/10.3390/f10020080





