Herbivory and Competing Vegetation Interact as Site Limiting Factors in Maritime Forest Restoration
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design and Treatments
2.3. Plant Material
2.4. Measurements
2.5. Statistical Analyses
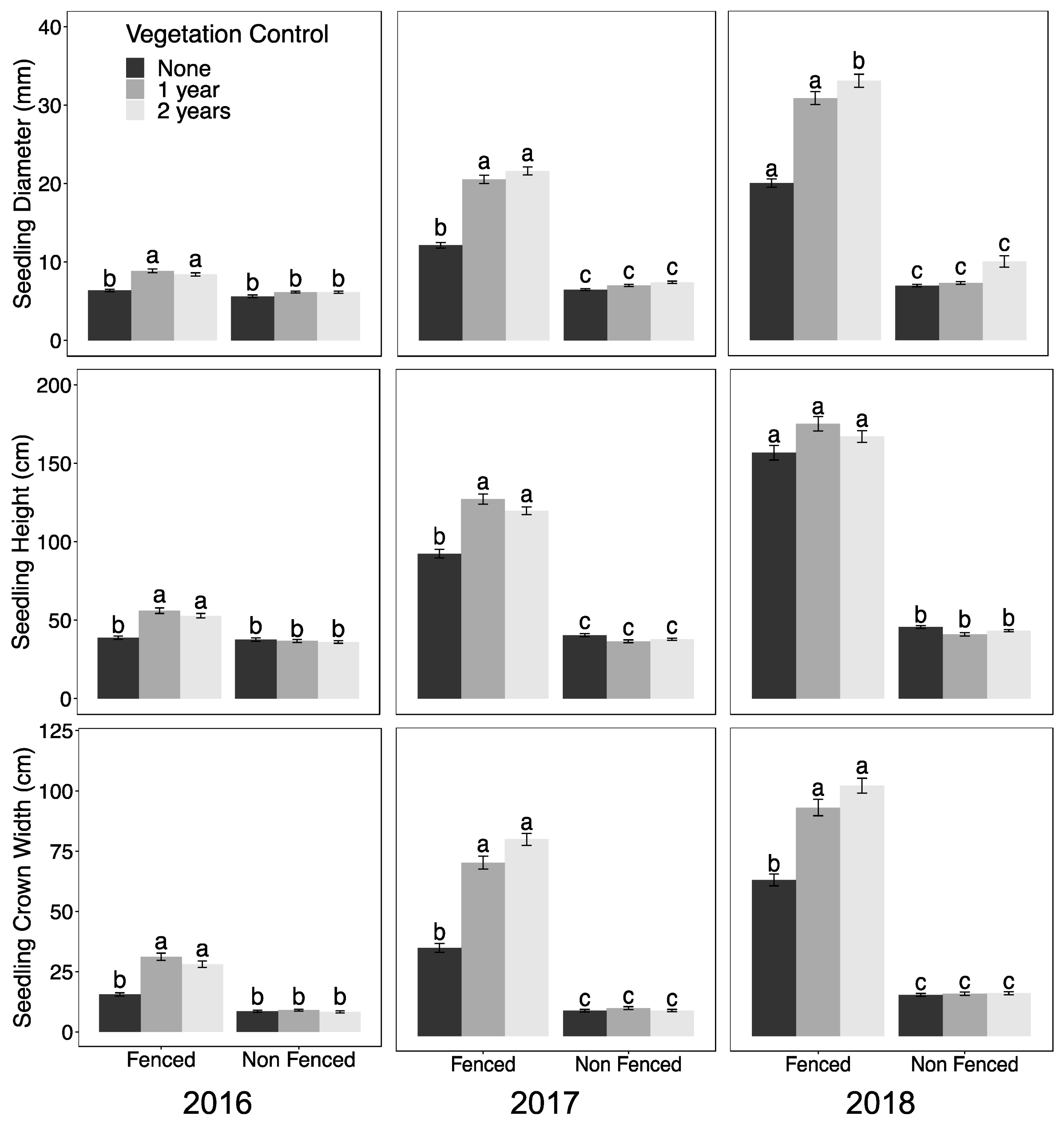
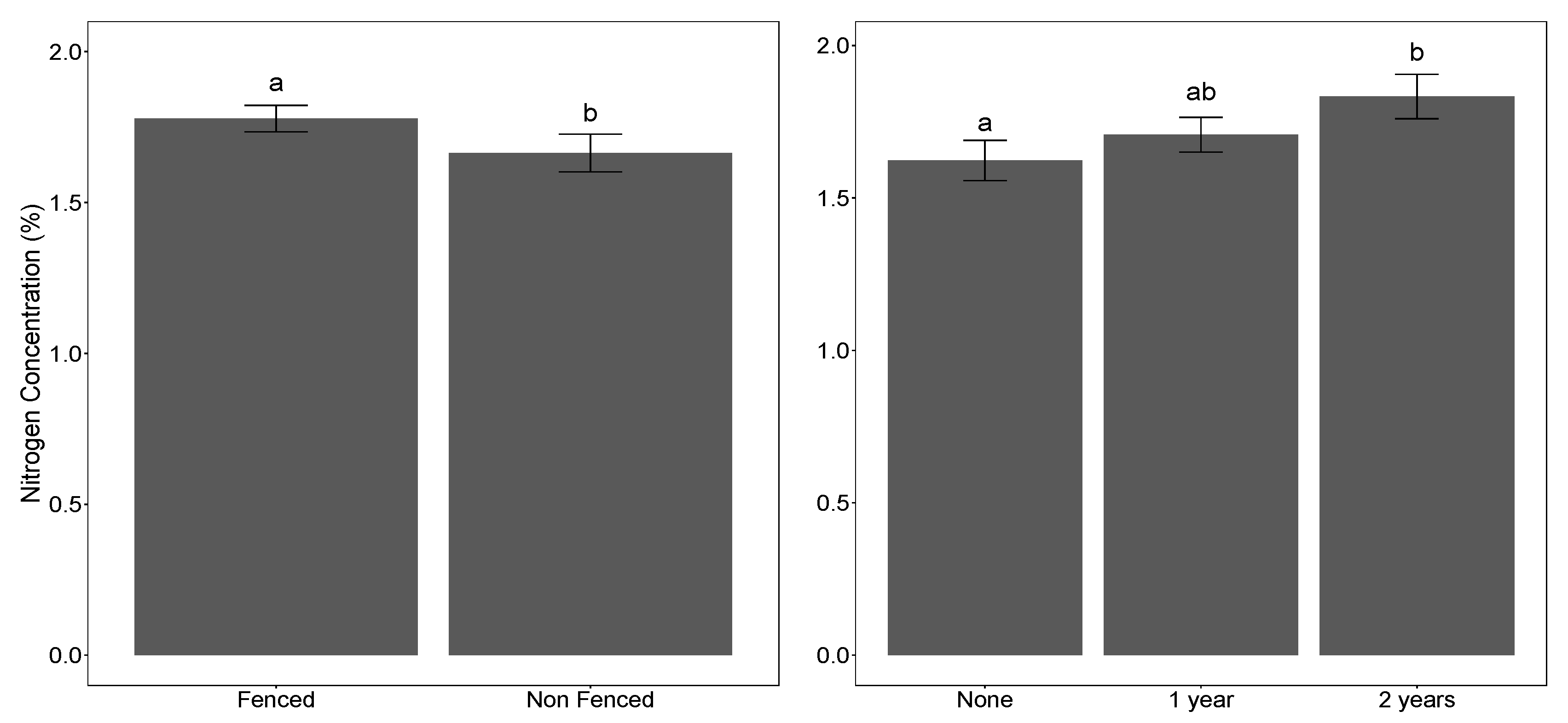
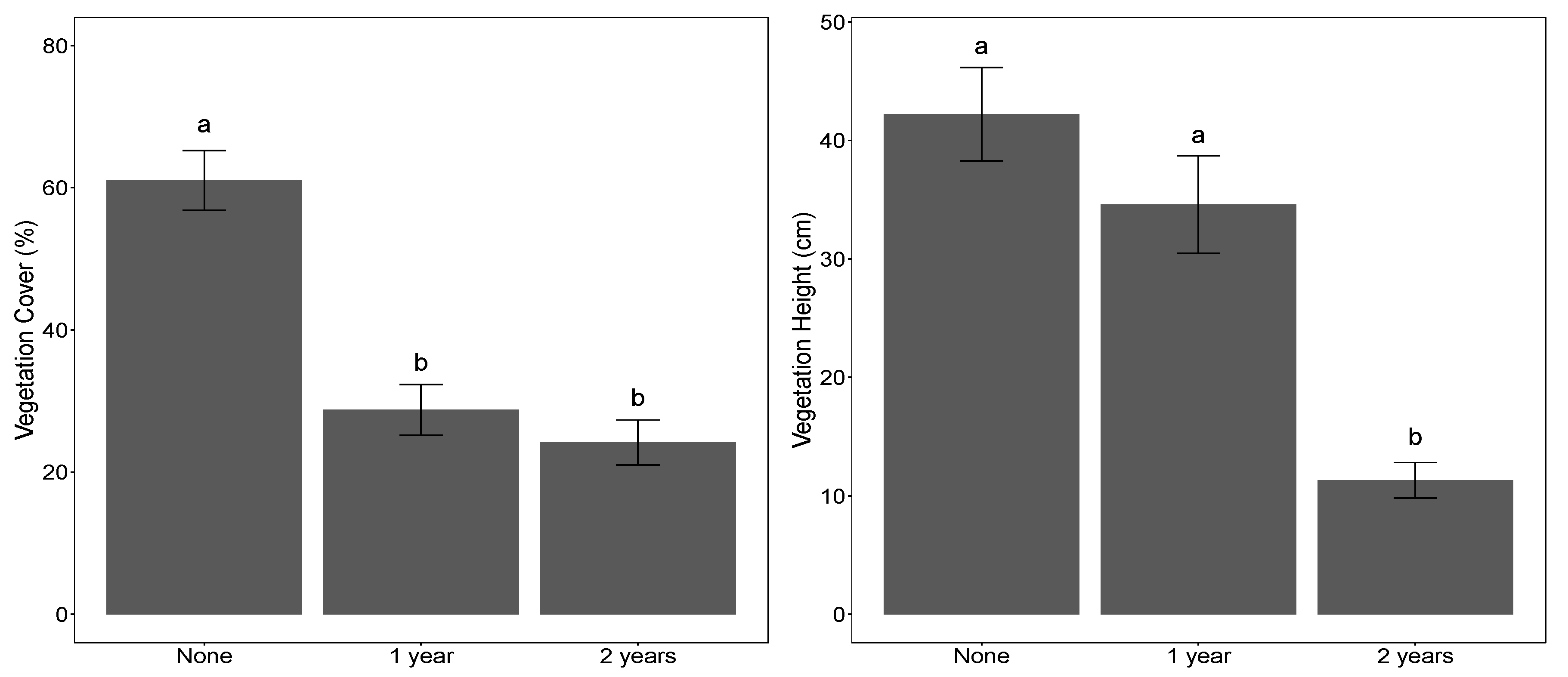
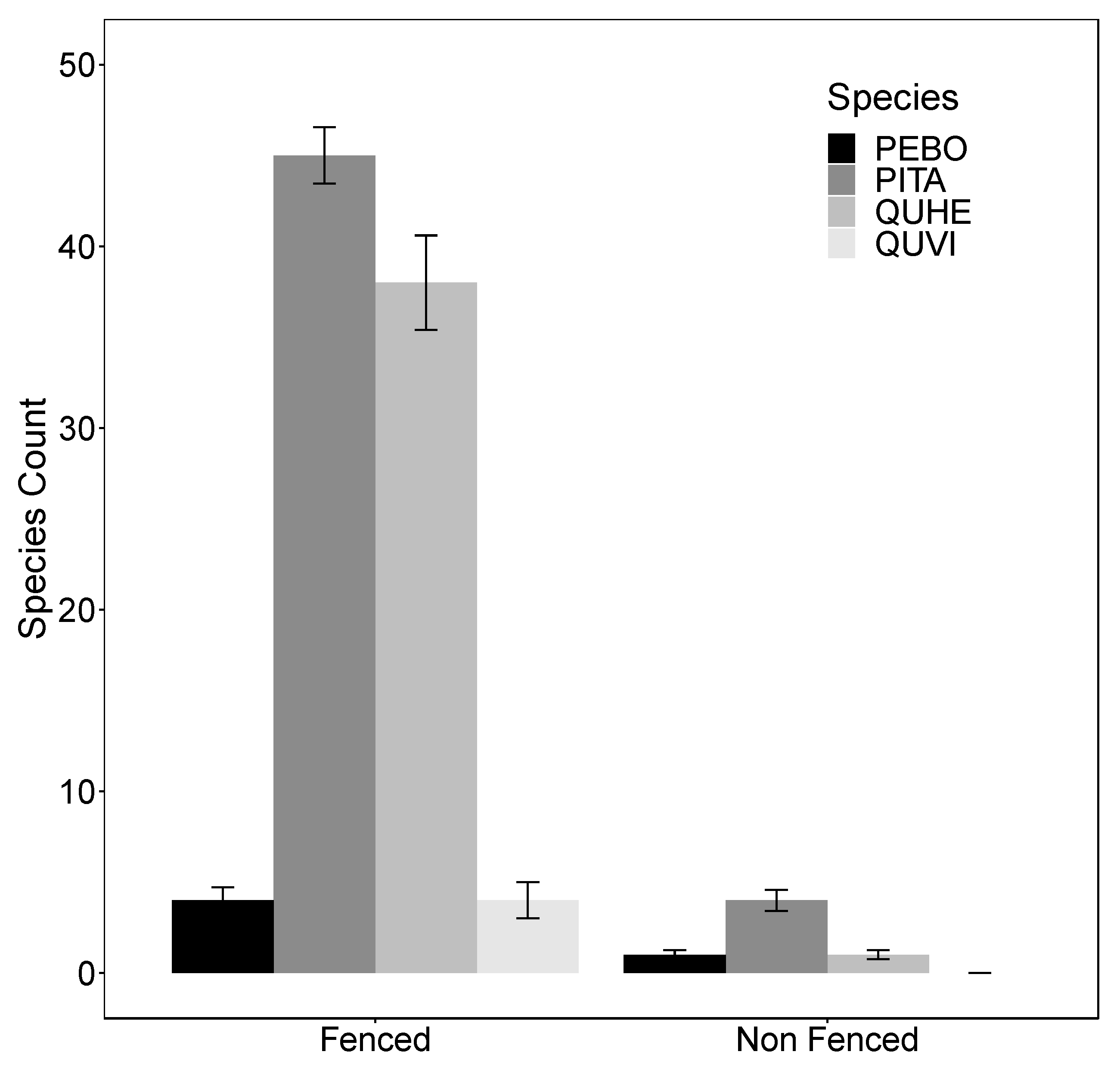
3. Results
3.1. Seedling Performance
3.2. Vegetation Surveys and Natural Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beguin, J.; Tremblay, J.P.; Thiffault, N.; Pothier, D.; Côté, S.D. Management of forest regeneration in boreal and temperate deer-forest systems: Challenges, guidelines, and research gaps. Ecosphere 2016, 7, 1–16. [Google Scholar] [CrossRef]
- Maltoni, A.; Mariotti, B.; Tani, A.; Martini, S.; Jacobs, D.F.; Tognetti, R. Natural regeneration of Pinus pinaster facilitates Quercus ilex survival and growth under severe deer browsing pressure. For. Ecol. Manag. 2019, 432, 356–364. [Google Scholar] [CrossRef]
- Leiva, M.J.; Pérez-Romero, J.A.; Mateos-Naranjo, E. The effect of simulated damage by weevils on Quercus ilex subsp. Ballota acorns germination, seedling growth and tolerance to experimentally induced drought. For. Ecol. Manag. 2018, 409, 740–748. [Google Scholar] [CrossRef]
- Takatsuki, S. Effects of sika deer on vegetation in Japan: A review. Biol. Conserv. 2009, 142, 1922–1929. [Google Scholar] [CrossRef]
- Suzuki, M.; Ito, E. Combined effects of gap creation and deer exclusion on restoration of belowground systems of secondary woodlands: A field experiment in warm-temperate monsoon Asia. For. Ecol. Manag. 2014, 329, 227–236. [Google Scholar] [CrossRef]
- Ashton, M.S.; Goodale, U.M.; Bawa, K.S.; Ashton, P.S.; David Neidel, J. Restoring working forests in human dominated landscapes of tropical South Asia: An introduction. For. Ecol. Manag. 2014, 329, 335–339. [Google Scholar] [CrossRef]
- Griscom, H.P.; Griscom, B.W.; Ashton, M.S. Forest regeneration from pasture in the dry tropics of Panama: Effects of cattle, exotic grass, and forested riparia. Restor. Ecol. 2009, 17, 117–126. [Google Scholar] [CrossRef]
- Cole, R.J.; Litton, C.M.; Koontz, M.J.; Loh, R.K. Vegetation recovery 16 years after feral pig removal from a wet Hawaiian forest. Biotropica 2012, 44, 463–471. [Google Scholar] [CrossRef]
- Russell, F.L.; Zippin, D.B.; Fowler, N.L. Effects of white-tailed deer (Odocoileus virginianus) on Plants, Plant Populations and Communities: A Review. Am. Midl. Nat. 2001, 146, 1–26. [Google Scholar] [CrossRef]
- Abrams, M.D.; Mostoller, S.A. Gas exchange, leaf structure and nitrogen in contrasting successional tree species growing in open and understory sites during a drought. Tree Physiol. 1995, 15, 361–370. [Google Scholar] [CrossRef]
- McEwan, R.W.; Dyer, J.M.; Pederson, N. Multiple interacting ecosystem drivers: Toward an encompassing hypothesis of oak forest dynamics across eastern North America. Ecography 2011, 34, 244–256. [Google Scholar] [CrossRef]
- Rossell, C.R.; Gorsira, B.; Patch, S. Effects of white-tailed deer on vegetation structure and woody seedling composition in three forest types on the Piedmont Plateau. For. Ecol. Manag. 2005, 210, 415–424. [Google Scholar] [CrossRef]
- Miller, G.W.; Brose, P.H.; Gottschalk, K.W. Advanced Oak Seedling Development as Influenced by Shelterwood Treatments, Competition Control, Deer Fencing, and Prescribed Fire. J. For. 2016, 115, 179–189. [Google Scholar] [CrossRef]
- Sweeney, B.W.; Czapka, S.J.; Yerkes, T. Riparian forest restoration: Increasing success by reducing plant competition and herbivory. Restor. Ecol. 2002, 10, 392–400. [Google Scholar] [CrossRef]
- Menges, E.S. Restoration demography and genetics of plants: When is a translocation successful? Aust. J. Bot. 2008, 56, 187–196. [Google Scholar] [CrossRef]
- Owings, C.F.; Jacobs, D.F.; Shields, J.M.; Saunders, M.R.; Jenkins, M.A. Individual and interactive effects of white-tailed deer and an exotic shrub on artificial and natural regeneration in mixed hardwood forests. AoB Plants 2017, 9, 1–14. [Google Scholar] [CrossRef]
- Bergquist, J.; Löf, M.; Örlander, G. Effects of roe deer browsing and site preparation on performance of planted broadleaved and conifer seedlings when using temporary fences. Scand. J. For. Res. 2009, 24, 308–317. [Google Scholar] [CrossRef]
- Laurent, L.; Mårell, A.; Balandier, P.; Holveck, H.; Saïd, S. Understory vegetation dynamics and tree regeneration as affected by deer herbivory in temperate hardwood forests. iForest 2017, 10, 837–844. [Google Scholar] [CrossRef]
- Loomis, J.D.; Matter, S.F.; Cameron, G.N. Effects of Invasive Amur Honeysuckle (Lonicera maackii) and White-Tailed Deer (Odocoileus virginianus) on Survival of Sugar Maple Seedlings in a Southwestern Ohio Forest. Am. Midl. Nat. 2015, 174, 65–73. [Google Scholar] [CrossRef]
- Meiners, S.J.; Handel, S.N. Additive and nonadditive effects of Herbivory and competition on tree seedling mortality, growth, and allocation. Am. J. Bot. 2000, 87, 1821–1826. [Google Scholar] [CrossRef]
- Saunders, M.R.; Puettmann, K.J. Effects of overstory and understory competition and simulated herbivory on growth and survival of white pine seedlings. Can. J. For. Res. 1999, 29, 536–546. [Google Scholar] [CrossRef]
- Wagner, R.G.; Zasada, J.C. Integrating plant autecology and silvicultural activities to prevent forest vegetation management problems. For. Chron. 1991, 67, 506–513. [Google Scholar] [CrossRef]
- Salifu, K.F.; Jacobs, D.F.; Birge, Z.K.D. Nursery nitrogen loading improves field performance of bareroot oak seedlings planted on abandoned mine lands. Restor. Ecol. 2009, 17, 339–349. [Google Scholar] [CrossRef]
- Grossnickle, S.C. Why seedlings survive: Influence of plant attributes. New For. 2012, 43, 711–738. [Google Scholar] [CrossRef]
- Dey, D.C.; Jacobs, D.; McNabb, K.; Miller, G.; Baldwin, V.; Foster, G. Artificial regeneration of major oak (Quercus) species in the Eastern United States—A review of the literature. For. Sci. 2008, 54, 77–106. [Google Scholar]
- Gardiner, E.S.; Dey, D.C.; Stanturf, J.A.; Lockhart, B.R. Approaches to restoration of oak forests on farmed lowlands of the Mississippi River and its tributaries. Rev. Columbia For. 2010, 13, 223–236. [Google Scholar]
- Paquette, A.; Bouchard, A.; Cogliastro, A. Successful under-planting of red oak and black cherry in early-successional deciduous shelterwoods of North America. Ann. For. Sci. 2006, 63, 823–831. [Google Scholar] [CrossRef]
- Borkowski, J.; Dobrowolska, D.; Dąbrowski, W.; Banul, R.; Załuski, D. Young conifer stands form a deer browsing refuge for an oak admixture: Silvicultural implications for forest regeneration under herbivore pressure. Eur. J. For. Res. 2017, 136, 787–800. [Google Scholar] [CrossRef]
- Oliver, L.B.; Stovall, J.P.; Comer, C.E.; Williams, H.M.; Symmank, M.E. Weed control and overstory reduction improve survival and growth of under-planted oak and hickory seedlings. Restor. Ecol. 2018, 27, 70–81. [Google Scholar] [CrossRef]
- Albers, G.; Alber, M. A vegetative survey of back-barrier islands near Sapelo Island, Georgia. In Proceedings of the Georgia Water Resources Conference, Athens, GA, USA, 23–24 April 2003; pp. 1–4. [Google Scholar]
- Bellis, V.J. Ecology of Maritime Forests of the Southern Atlantic Coast: A Community Profile; U.S. Department of the Interior, National Biological Service, Southern Science Center: Lafayette, LA, USA, 1995; pp. 1–95.
- Jones, G.; Snider, A.; Luo, S. Changes in the extent of North Carolina barrier island maritime forests 1988–2011: An assessment of past efforts at protection. J. For. 2013, 111, 186–193. [Google Scholar] [CrossRef]
- Kurtz, C.M.; Savage, J.A.; Huang, I.-Y.; Cavender-Bares, J. Consequences of salinity and freezing stress for two populations of Quercus virginiana Mill. (Fagaceae) grown in a common garden. J. Torrey Bot. Soc. 2013, 140, 145–156. [Google Scholar] [CrossRef]
- Lopazanski, M.J.; Evans, J.P.; Shaw, R.E. An Assessment of Maritime Forest Resources on the North Carolina Coast; North Carolina Department of Natural Resources and Community Development: Raleigh, NC, USA, 1988; pp. 1–104. [Google Scholar]
- Bourdeau, P.F.; Oosting, H.J. The maritime live oak forest in North Carolina. Ecology 1959, 40, 148–152. [Google Scholar] [CrossRef]
- Bratton, S.P. Survivorship of evergreen hardwoods after wildfire in maritime forest, Cumberland Island National Seashore, Georgia. Castanea 1993, 58, 34–44. [Google Scholar]
- Conner, W.H.; Mixon, W.D.; Wood, G.W. Maritime forest habitat dynamics on Bulls Island, Cape Romain National Wildlife Refuge, SC, following Hurricane Hugo. For. Ecol. Manag. 2005, 212, 127–134. [Google Scholar] [CrossRef]
- Mathews, T.D.; Stapor, F.W.; Richter, C.R.; Miglarese, J.V.; McKenzi, M.D.; Barlcay, L.A.; Joseph, E.B. Ecological Characterization of the Sea Island Coastal Region of South Carolina and Georgia: Physical Features of the Characterization Area; U.S. Department of the Interior, Interagency Energy-Environment Research and Development Program, Fish and Wildlife Service, South Carolina Wildlife and Marine Resources: Charleston, SC, USA, 1980; pp. 1–204.
- Asaro, C.; Nowak, J.T.; Elledge, A. Why have southern pine beetle outbreaks declined in the southeastern U.S. with the expansion of intensive pine silviculture? A brief review of hypotheses. For. Ecol. Manag. 2017, 391, 338–348. [Google Scholar] [CrossRef]
- Nowak, J.T.; Meeker, J.R.; Coyle, D.R.; Steiner, C.A.; Brownie, C. Southern pine beetle infestations in relation to forest stand conditions, previous thinning, and prescribed burning: Evaluation of the southern pine beetle prevention program. J. For. 2015, 113, 454–462. [Google Scholar] [CrossRef]
- Pemán, J.; Chirino, E.; Espelta, J.M.; Jacobs, D.F.; Martín-Gómez, P.; Navarro-Cerrillo, R.; Oliet, J.A.; Vilagrosa, A.; Villar-Salvador, P.; Gil-Pelegrín, E. Chapter 14: Physiological keys for natural and artificial regeneration of oaks. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L.; Springer International Publishing: Cham, Switzerland, 2017; pp. 453–511. [Google Scholar]
- Bratton, S.P.; Miller, S.G. Historic field systems and the structure of maritime oak forests, Cumberland Island National Seashore, Georgia. Bull. Torrey Bot. Club 1994, 121, 1–12. [Google Scholar] [CrossRef]
- Taggart, J.; Long, Z. Effects of white-tailed deer (Odocoileus virginianus) on the maritime forest of Bald Head Island, North Carolina. Am. Midl. Nat. 2015, 173, 283–293. [Google Scholar] [CrossRef]
- Löf, M.; Madsen, P.; Metslaid, M.; Witzell, J.; Jacobs, D.F. Restoring forests: Regeneration and ecosystem function for the future. New For. 2019, 50, 139–151. [Google Scholar] [CrossRef]
- Stanturf, J.A.; Palik, B.J.; Dumroese, R.K. Contemporary forest restoration: A review emphasizing function. For. Ecol. Manag. 2014, 331, 292–323. [Google Scholar] [CrossRef]
- Rose, R.; Carlson, W.C.; Morgan, P. Chapter 1: The Target Seedling Concept. In Proceedings of the Target Seedling Symposium, Western Forest Nursery Association, Roseburg, OR, USA, 13–17 August 1990; pp. 1–8. [Google Scholar]
- Dumroese, R.K.; Landis, T.D.; Pinto, J.R.; Haase, D.L.; Wilkinson, K.W.; Davis, A.S. Meeting Forest Restoration Challenges: Using the Target Plant Concept. Reforesta 2016, 1, 37–52. [Google Scholar] [CrossRef]
- Stange, E.E.; Shea, K.L. Effects of deer browsing, fabric mats, and tree shelters on Quercus rubra seedlings. Restor. Ecol. 1998, 6, 29–34. [Google Scholar]
- Tripler, C.E.; Canham, C.D.; Inouye, R.S.; Schnurr, J.T. Soil nitrogen availability, plant luxury consumption, and herbivory by white-tailed deer. Oecologia 2002, 133, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Oliet, J.A.; Jacobs, D.F. Restoring forests: Advances in techniques and theory. New For. 2012, 43, 535–541. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Oliet, J.A.; Aronson, J.; Bolte, A.; Bullock, J.M.; Donoso, P.J.; Landhäusser, S.M.; Madsen, P.; Peng, S.; Rey-Benayas, J.M.; et al. Restoring forests: What constitutes success in the twenty-first century? New For. 2015, 46, 601–614. [Google Scholar] [CrossRef]
- Natural Resources Conservation Service, N.R.C.S. United States Soil Survey. Available online: https://websoilsurvey.nrcs.usda.gov (accessed on 11 January 2017).
- Sapelo Island National Estuarine Research Reserve Meterological Monitoring, S.I.N.E.R.R.M.M. Available online: http://cdmo.baruch.sc.edu/ (accessed on 31 December 2018).
- U.S. Climate Data, U.S.C.D. Available online: https://www.usclimatedata.com/ (accessed on 31 December 2018).
- Burney, O.T.; Jacobs, D.F. Species selection—A fundamental silvicultural tool to promote forest regeneration under high animal browsing pressure. For. Ecol. Manag. 2018, 408, 67–74. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Bates, D.; Machler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Karkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models, R Package Version 3.1-131.
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Jacobs, D.F.; Ross-Davis, A.L.; Davis, A.S. Establishment success of conservation tree plantations in relation to silvicultural practices in Indiana, USA. New For. 2004, 28, 23–36. [Google Scholar] [CrossRef]
- Woolery, P.O.; Jacobs, D.F. Planting stock type and seasonality of simulated browsing affect regeneration establishment of Quercus rubra. Can. J. For. Res. 2014, 44, 732–739. [Google Scholar] [CrossRef]
- Close, D.C.; McArthur, C.; Pietrzykowski, E.; Fitzgerald, H.; Paterson, S. Evaluating effects of nursery and post-planting nutrient regimes on leaf chemistry and browsing of eucalypt seedlings in plantations. For. Ecol. Manag. 2004, 200, 101–112. [Google Scholar] [CrossRef]
- Ward, J.S.; Gent, M.P.N.; Stephens, G.R. Effects of planting stock quality and browse protection-type on height growth of northern red oak and eastern white pine. For. Ecol. Manag. 2000, 127, 205–216. [Google Scholar] [CrossRef]
- Horsley, S.B.; Stout, S.L.; DeCalista, D.S. White-tailed deer impact on the vegetation dynamics of a northern hardwood forest. Ecol. Appl. 2003, 13, 98. [Google Scholar] [CrossRef]
- Shelton, A.L.; Henning, J.A.; Schultz, P.; Clay, K. Effects of abundant white-tailed deer on vegetation, animals, mycorrhizal fungi, and soils. For. Ecol. Manag. 2014, 320, 39–49. [Google Scholar] [CrossRef]
- Tanentzap, A.J.; Bazely, D.R.; Koh, S.; Timciska, M.; Haggith, E.G.; Carleton, T.J.; Coomes, D.A. Seeing the forest for the deer: Do reductions in deer-disturbance lead to forest recovery? Biol. Conserv. 2011, 144, 376–382. [Google Scholar] [CrossRef]
- Côté, S.D.; Rooney, T.P.; Tremblay, J.-P.; Dussault, C.; Waller, D.M. Ecological impacts of deer overabundance. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 113–147. [Google Scholar] [CrossRef]
- Wagner, R.G.; Little, K.M.; Richardson, B.; Mcnabb, K. The role of vegetation management for enhancing productivity of the world’s forests. For. Int. J. For. Res. 2006, 79, 57–79. [Google Scholar] [CrossRef]
- Davis, M.A.; Wrage, K.J.; Reich, P.B.; Tjoelker, M.G.; Schaeffer, T.; Muermann, C. Survival, growth, and photosynthesis of tree seedlings competing with herbaceous vegetation along a multiple resource gradient. Plant Ecol. 1999, 145, 341–350. [Google Scholar] [CrossRef]
- Kern, C.C.; Reich, P.B.; Montgomery, R.A.; Strong, T.F. Do deer and shrubs override canopy gap size effects on growth and survival of yellow birch, northern red oak, eastern white pine, and eastern hemlock seedlings? For. Ecol. Manag. 2012, 267, 134–143. [Google Scholar] [CrossRef]
- Soto, D.P.; Jacobs, D.F.; Salas, C.; Donoso, P.J.; Fuentes, C.; Puettmann, K.J. Light and nitrogen interact to influence regeneration in old-growth Nothofagus-dominated forests in south-central Chile. For. Ecol. Manag. 2017, 384, 303–313. [Google Scholar] [CrossRef]
- Uscola, M.; Villar-Salvador, P.; Gross, P.; Maillard, P. Fast growth involves high dependence on stored resources in seedlings of Mediterranean evergreen trees. Ann. Bot. 2015, 115, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Holste, E.K.; Kobe, R.K.; Vriesendorp, C.F. Seedling growth responses to soil resources in the understory of a wet tropical forest. Ecology 2011, 92, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Löf, M.; Dey, D.C.; Navarro, R.M.; Jacobs, D.F. Mechanical site preparation for forest restoration. New For. 2012, 43, 825–848. [Google Scholar] [CrossRef]
- Evans, J.P.; Keen, E.M. Regeneration failure in a remnant stand of pignut hickory (Carya glabra) on a protected barrier island in Georgia, USA. Nat. Areas J. 2013, 33, 171–176. [Google Scholar] [CrossRef]
| Chemical Characteristic | |
|---|---|
| Organic Matter (%) | 4.35 ± 0.20 |
| pH | 5.5 ± 0.7 |
| CEC (ME 100 g−1) | 7.56 ± 3.52 |
| Estimated nitrogen (kg ha−1) | 104.8 ± 2.1 |
| NO3 (ppm) | 13.7 ± 1.4 |
| NH4 (ppm) | 8.1 ± 2.6 |
| Phosphorous (ppm) | 223 ± 4 |
| Potassium (ppm) | 24 ± 3 |
| Magnesium (ppm) | 63 ± 4 |
| Calcium (ppm) | 1367 ± 678 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thyroff, E.C.; Burney, O.T.; Jacobs, D.F. Herbivory and Competing Vegetation Interact as Site Limiting Factors in Maritime Forest Restoration. Forests 2019, 10, 950. https://doi.org/10.3390/f10110950
Thyroff EC, Burney OT, Jacobs DF. Herbivory and Competing Vegetation Interact as Site Limiting Factors in Maritime Forest Restoration. Forests. 2019; 10(11):950. https://doi.org/10.3390/f10110950
Chicago/Turabian StyleThyroff, Emily C., Owen T. Burney, and Douglass F. Jacobs. 2019. "Herbivory and Competing Vegetation Interact as Site Limiting Factors in Maritime Forest Restoration" Forests 10, no. 11: 950. https://doi.org/10.3390/f10110950
APA StyleThyroff, E. C., Burney, O. T., & Jacobs, D. F. (2019). Herbivory and Competing Vegetation Interact as Site Limiting Factors in Maritime Forest Restoration. Forests, 10(11), 950. https://doi.org/10.3390/f10110950





