Similar Impacts of Alien and Native Tree Species on Understory Light Availability in a Temperate Forest
Abstract
1. Introduction
2. Materials and Methods
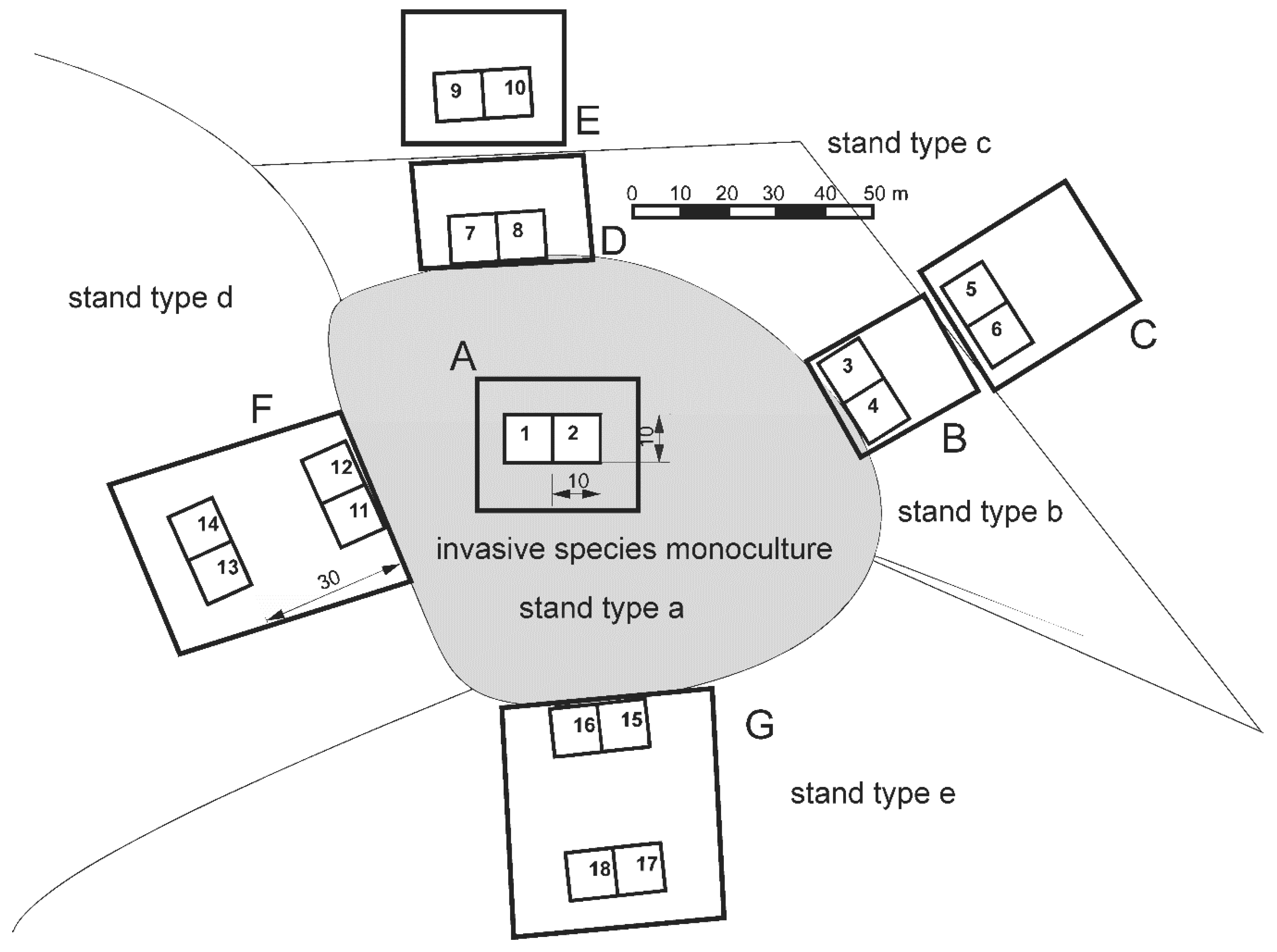
2.1. Study Design
2.2. Data Collection
2.3. Data Analysis
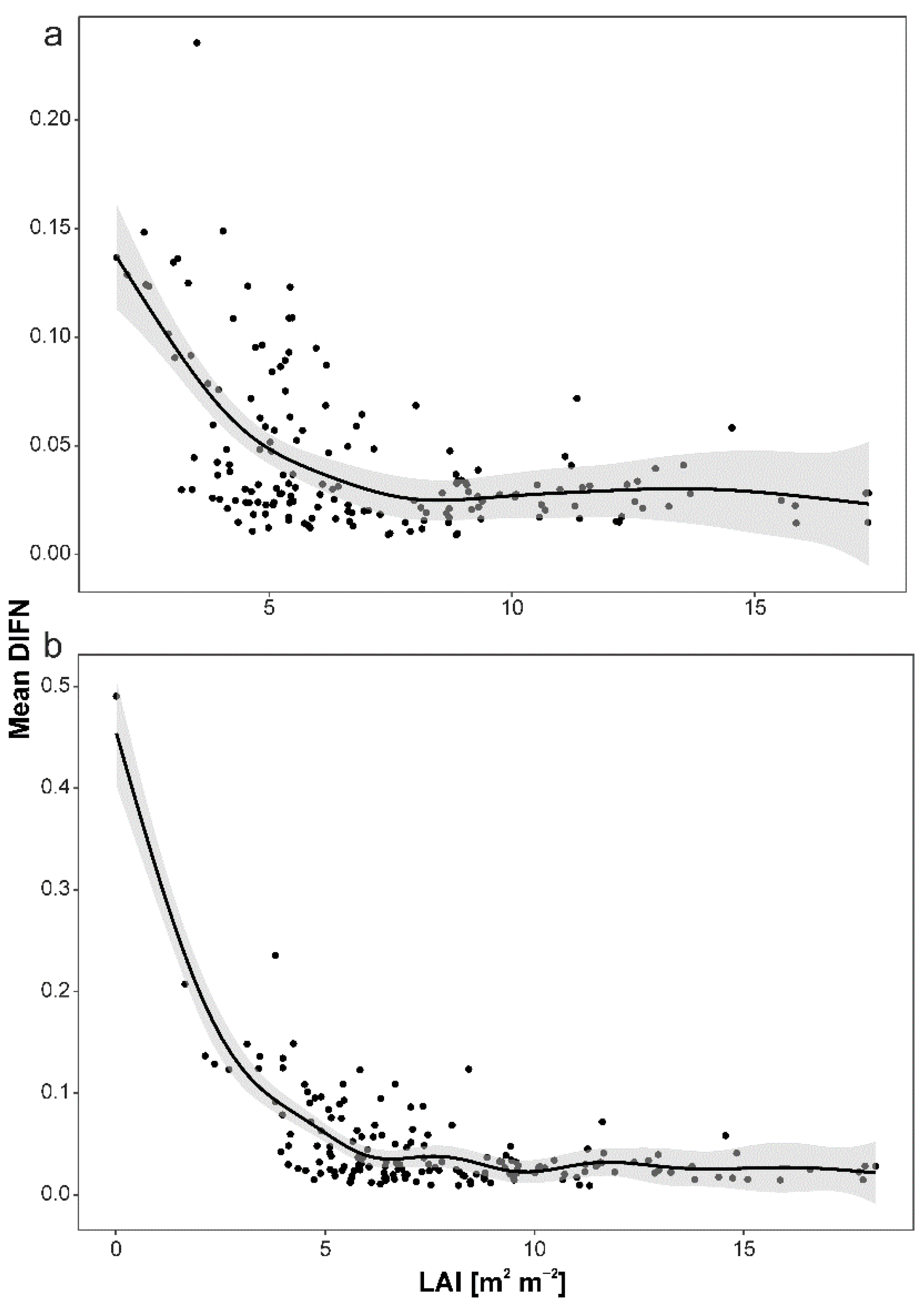
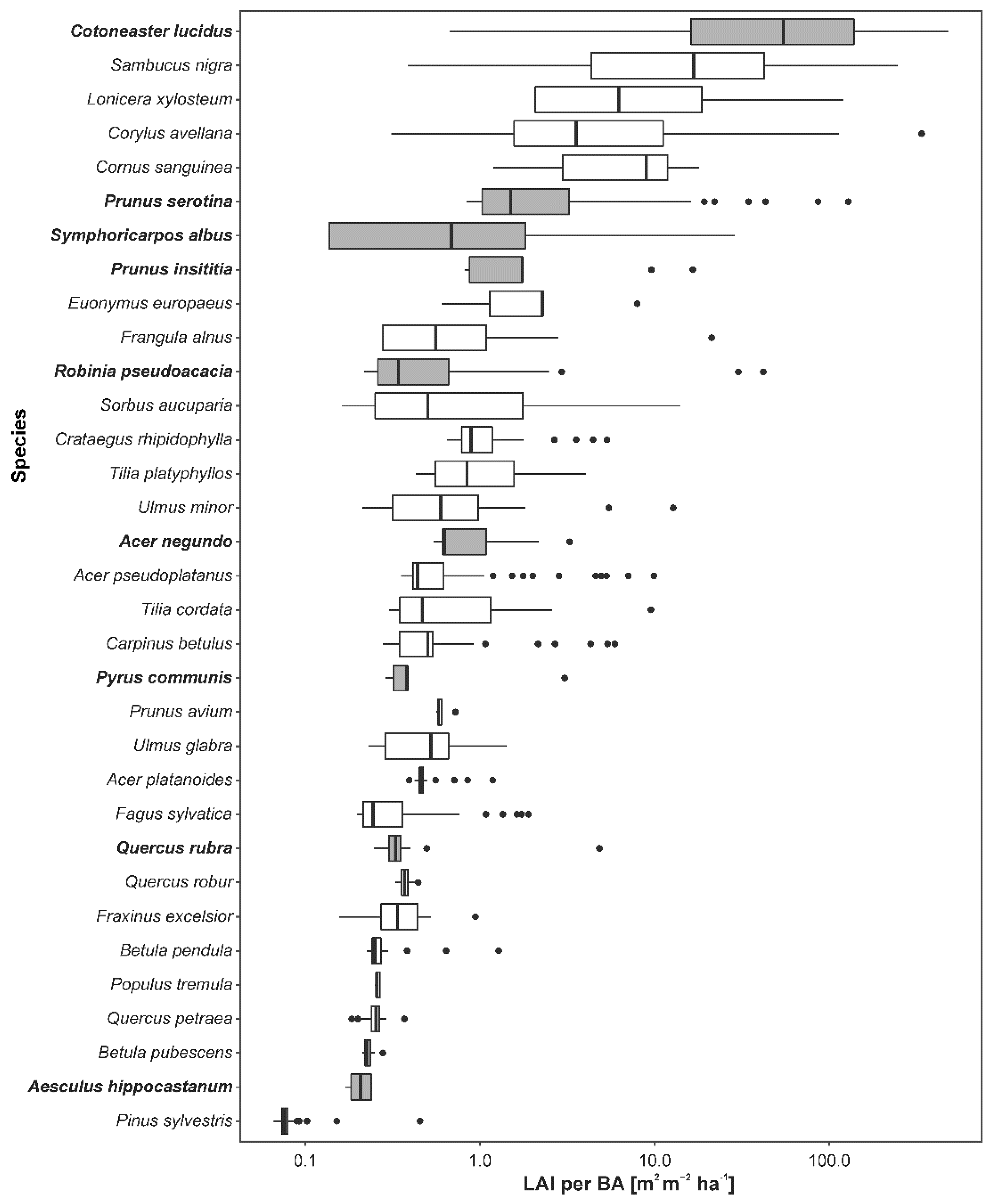
3. Results
4. Discussion
4.1. Drivers of Light Interception by Canopy
4.2. Impacts of Alien Species
4.3. Sources of Uncertainty
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hulme, P.E.; Pyšek, P.; Pergl, J.; Jarošík, V.; Schaffner, U.; Vilà, M. Greater Focus Needed on Alien Plant Impacts in Protected Areas: Plant invasion impacts in protected areas. Conserv. Lett. 2014, 7, 459–466. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Bacher, S.; Essl, F.; Hulme, P.E.; Jeschke, J.M.; Kühn, I.; Kumschick, S.; Nentwig, W.; Pergl, J.; Pyšek, P.; et al. Framework and guidelines for implementing the proposed IUCN Environmental Impact Classification for Alien Taxa (EICAT). Divers. Distrib. 2015, 21, 1360–1363. [Google Scholar] [CrossRef]
- Essl, F.; Hulme, P.E.; Jeschke, J.M.; Keller, R.; Pyšek, P.; Richardson, D.M.; Saul, W.-C.; Bacher, S.; Dullinger, S.; Estévez, R.A.; et al. Scientific and Normative Foundations for the Valuation of Alien-Species Impacts: Thirteen Core Principles. BioScience 2017, 67, 166–178. [Google Scholar] [CrossRef]
- Rodríguez-Echeverría, S.; Afonso, C.; Correia, M.; Lorenzo, P.; Roiloa, S.R. The effect of soil legacy on competition and invasion by Acacia dealbata Link. Plant Ecol. 2013, 214, 1139–1146. [Google Scholar] [CrossRef]
- Gioria, M.; Osborne, B.A. Resource competition in plant invasions: Emerging patterns and research needs. Front. Plant Sci. 2014, 5, 501. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Liu, Y.; Chu, C.; Xu, H.; Fang, S. Fast seedling root growth leads to competitive superiority of invasive plants. Biol. Invasions 2018, 20, 1821–1832. [Google Scholar] [CrossRef]
- Crooks, J.A. Characterizing ecosystem-level consequences of biological invasions: The role of ecosystem engineers. Oikos 2002, 97, 153–166. [Google Scholar] [CrossRef]
- Rothstein, D.E.; Vitousek, P.M.; Simmons, B.L. An Exotic Tree Alters Decomposition and Nutrient Cycling in A Hawaiian Montane Forest. Ecosystems 2004, 7, 805–814. [Google Scholar] [CrossRef]
- Corenblit, D.; Steiger, J.; Tabacchi, E.; González, E.; Planty-Tabacchi, A.-M. Ecosystem engineers modulate exotic invasions in riparian plant communities by modifying hydrogeomorphic connectivity: Ecosystem engineers modulate exotic invasions. River Res. Appl. 2014, 30, 45–59. [Google Scholar] [CrossRef]
- Richardson, D.M.; Pyšek, P.; Rejmánek, M.; Barbour, M.G.; Panetta, F.D.; West, C.J. Naturalization and invasion of alien plants: Concepts and definitions. Divers. Distrib. 2000, 6, 93–107. [Google Scholar] [CrossRef]
- Richardson, D.M. Forestry trees as invasive aliens. Conserv. Biol. 1998, 12, 18–26. [Google Scholar] [CrossRef]
- Richardson, D.M.; Rejmánek, M. Trees and shrubs as invasive alien species—A global review. Divers. Distrib. 2011, 17, 788–809. [Google Scholar] [CrossRef]
- Rejmánek, M.; Richardson, D.M. Trees and shrubs as invasive alien species—2013 update of the global database. Divers. Distrib. 2013, 19, 1093–1094. [Google Scholar] [CrossRef]
- Allison, S.D.; Vitousek, P.M. Rapid nutrient cycling in leaf litter from invasive plants in Hawaii. Oecologia 2004, 141, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, E.; Petrillo, M.; Petrella, F.; Tambone, F.; Celi, L. Alien red oak affects soil organic matter cycling and nutrient availability in low-fertility well-developed soils. Plant Soil 2015, 395, 215–229. [Google Scholar] [CrossRef]
- Horodecki, P.; Jagodziński, A.M. Tree species effects on litter decomposition in pure stands on afforested post-mining sites. For. Ecol. Manag. 2017, 406, 1–11. [Google Scholar] [CrossRef]
- Wang, S.; Niu, S. Do biological invasions by Eupatorium adenophorum increase forest fire severity? Biol. Invasions 2015, 18, 717–729. [Google Scholar] [CrossRef]
- Taylor, K.T.; Maxwell, B.D.; McWethy, D.B.; Pauchard, A.; Nuñez, M.A.; Whitlock, C. Pinus contorta invasions increase wildfire fuel loads and may create a positive feedback with fire. Ecology 2017, 98, 678–687. [Google Scholar] [CrossRef]
- Davis, K.T.; Maxwell, B.D.; Caplat, P.; Pauchard, A.; Nuñez, M.A. Simulation model suggests that fire promotes lodgepole pine (Pinus contorta) invasion in Patagonia. Biol. Invasions 2019, 21, 1–14. [Google Scholar] [CrossRef]
- Trocha, L.K.; Kałucka, I.; Stasińska, M.; Nowak, W.; Dabert, M.; Leski, T.; Rudawska, M.; Oleksyn, J. Ectomycorrhizal fungal communities of native and non-native Pinus and Quercus species in a common garden of 35-year-old trees. Mycorrhiza 2012, 22, 121–134. [Google Scholar] [CrossRef]
- Mueller, K.E.; Eisenhauer, N.; Reich, P.B.; Hobbie, S.E.; Chadwick, O.A.; Chorover, J.; Dobies, T.; Hale, C.M.; Jagodziński, A.M.; Kałucka, I.; et al. Light, earthworms, and soil resources as predictors of diversity of 10 soil invertebrate groups across monocultures of 14 tree species. Soil Biol. Biochem. 2016, 92, 184–198. [Google Scholar] [CrossRef]
- Kamczyc, J.; Dyderski, M.K.; Horodecki, P.; Jagodziński, A.M. Mite Communities (Acari, Mesostigmata) in the Initially Decomposed ‘Litter Islands’ of 11 Tree Species in Scots Pine (Pinus sylvestris L.) Forest. Forests 2019, 10, 403. [Google Scholar] [CrossRef]
- Gorchov, D.L.; Trisel, D.E. Competitive effects of the invasive shrub, Lonicera maackii (Rupr.) Herder (Caprifoliaceae), on the growth and survival of native tree seedlings. Plant Ecol. 2003, 166, 13–24. [Google Scholar] [CrossRef]
- Knight, K.S.; Oleksyn, J.; Jagodziński, A.M.; Reich, P.B.; Kasprowicz, M. Overstorey tree species regulate colonization by native and exotic plants: A source of positive relationships between understorey diversity and invasibility. Divers. Distrib. 2008, 14, 666–675. [Google Scholar] [CrossRef]
- Niinemets, Ü. A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol. Res. 2010, 25, 693–714. [Google Scholar] [CrossRef]
- Gilliam, F.S.; Roberts, M.R. Interactions between the Herbaceous Layer and Overstory Canopy of Eastern Forests. In The Herbaceous Layer in Forests of Eastern North America; Gilliam, F.S., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 233–254. [Google Scholar]
- Landuyt, D.; Lombaerde, E.D.; Perring, M.P.; Hertzog, L.R.; Ampoorter, E.; Maes, S.L.; Frenne, P.D.; Ma, S.; Proesmans, W.; Blondeel, H.; et al. The functional role of temperate forest understorey vegetation in a changing world. Glob. Chang. Biol. 2019, 25, 3625–3641. [Google Scholar] [CrossRef]
- Czapiewska, N.; Dyderski, M.K.; Jagodziński, A.M. Seasonal Dynamics of Floodplain Forest Understory–Impacts of Degradation, Light Availability and Temperature on Biomass and Species Composition. Forests 2019, 10, 22. [Google Scholar] [CrossRef]
- Canham, C.D.; Finzi, A.C.; Pacala, S.W.; Burbank, D.H. Causes and consequences of resource heterogeneity in forests: Interspecific variation in light transmission by canopy trees. Can. J. For. Res. 1994, 24, 337–349. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved—A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Modrý, M.; Hubený, D.; Rejšek, K. Differential response of naturally regenerated European shade tolerant tree species to soil type and light availability. For. Ecol. Manag. 2004, 188, 185–195. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A. Seedling survival of Prunus serotina Ehrh., Quercus rubra L. and Robinia pseudoacacia L. in temperate forests of Western Poland. For. Ecol. Manag. 2019, 450, 117498. [Google Scholar] [CrossRef]
- Knüsel, S.; Conedera, M.; Bugmann, H.; Wunder, J. Low litter cover, high light availability and rock cover favour the establishment of Ailanthus altissima in forests in southern Switzerland. NeoBiota 2019, 46, 91–116. [Google Scholar] [CrossRef]
- Emborg, J. Understorey light conditions and regeneration with respect to the structural dynamics of a near-natural temperate deciduous forest in Denmark. For. Ecol. Manag. 1998, 106, 83–95. [Google Scholar] [CrossRef]
- Beckage, B.; Clark, J.S. Seedling Survival and Growth of Three Forest Tree Species: The Role of Spatial Heterogeneity. Ecology 2003, 84, 1849–1861. [Google Scholar] [CrossRef]
- Dech, J.P.; Robinson, L.M.; Nosko, P. Understorey plant community characteristics and natural hardwood regeneration under three partial harvest treatments applied in a northern red oak (Quercus rubra L.) stand in the Great Lakes-St. Lawrence forest region of Canada. For. Ecol. Manag. 2008, 256, 760–773. [Google Scholar] [CrossRef]
- Kolb, T.E.; Steiner, K.C.; McCormick, L.H.; Bowersox, T.W. Growth response of northern red-oak and yellow-poplar seedlings to light, soil moisture and nutrients in relation to ecological strategy. For. Ecol. Manag. 1990, 38, 65–78. [Google Scholar] [CrossRef]
- Kuehne, C.; Nosko, P.; Horwath, T.; Bauhus, J. A comparative study of physiological and morphological seedling traits associated with shade tolerance in introduced red oak (Quercus rubra) and native hardwood tree species in southwestern Germany. Tree Physiol. 2014, 34, 184–193. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Jagodziński, A.M. Drivers of invasive tree and shrub natural regeneration in temperate forests. Biol. Invasions 2018, 20, 2363–2379. [Google Scholar] [CrossRef]
- Nowak, G.; Kara, M.; Bernat, Z.; Cykowiak, Z. Wybrane zagadnienia z planu ochrony ekosystemów leśnych Wielkopolskiego Parku Narodowego. Morena 2000, 7, 85–129. [Google Scholar]
- Purcel, A. Obce gatunki drzew i krzewów w Wielkopolskim Parku Narodowym—Ich występowanie i rola w biocenozach Parku. Morena 2009, 14, 35–191. [Google Scholar]
- Gazda, A.; Szwagrzyk, J. Introduced species in Polish National Parks: Distribution, abundance and management approaches. In Introduced Tree Species in European Forests: Opportunities and Challenges; Krumm, F., Vítková, L., Eds.; European Forest Institute: Freiburg, Germany, 2016; pp. 168–175. [Google Scholar]
- Dyderski, M.K.; Jagodziński, A.M. Functional traits of acquisitive invasive woody species differ from conservative invasive and native species. NeoBiota 2019, 41, 91–113. [Google Scholar] [CrossRef]
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. Rośliny Obcego Pochodzenia w Polsce ze Szczególnym Uwzględnieniem Gatunków Inwazyjnych; Generalna Dyrekcja Ochrony Środowiska: Warszawa, Poland, 2012; ISBN 978-83-62940-33-2.
- Ellenberg, H. Vegetation Ecology of Central Europe; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Wagner, V.; Chytrý, M.; Jiménez-Alfaro, B.; Pergl, J.; Hennekens, S.; Biurrun, I.; Knollová, I.; Berg, C.; Vassilev, K.; Rodwell, J.S.; et al. Alien plant invasions in European woodlands. Divers. Distrib. 2017, 23, 969–981. [Google Scholar] [CrossRef]
- GBIF Global Biodiversity Information Facility. Available online: http://www.gbif.org/ (accessed on 24 October 2019).
- Machado, J.-L.; Reich, P.B. Evaluation of several measures of canopy openness as predictors of photosynthetic photon flux density in deeply shaded conifer-dominated forest understory. Can. J. For. Res. 1999, 29, 1438–1444. [Google Scholar] [CrossRef]
- Kleyer, M.; Bekker, R.M.; Knevel, I.C.; Bakker, J.P.; Thompson, K.; Sonnenschein, M.; Poschlod, P.; Van Groenendael, J.M.; Klimeš, L.; Klimešová, J.; et al. The LEDA Traitbase: A database of life-history traits of the Northwest European flora. J. Ecol. 2008, 96, 1266–1274. [Google Scholar] [CrossRef]
- Enquist, B.J.; Condit, R.; Peet, R.K.; Schildhauer, M.; Thiers, B.M. Cyberinfrastructure for an integrated botanical information network to investigate the ecological impacts of global climate change on plant biodiversity. PeerJ Prepr. 2016, 4, e2615v2. [Google Scholar]
- Forrester, D.I.; Tachauer, I.H.H.; Annighoefer, P.; Barbeito, I.; Pretzsch, H.; Ruiz-Peinado, R.; Stark, H.; Vacchiano, G.; Zlatanov, T.; Chakraborty, T.; et al. Generalized biomass and leaf area allometric equations for European tree species incorporating stand structure, tree age and climate. For. Ecol. Manag. 2017, 396, 160–175. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Karolewski, P.; Giertych, M.J.; Żmuda, M.; Jagodziński, A.M.; Oleksyn, J. Season and light affect constitutive defenses of understory shrub species against folivorous insects. Acta Oecolo 2013, 53, 19–32. [Google Scholar] [CrossRef]
- Paź-Dyderska, S.; Dyderski, M.K.; Szwaczka, P.; Brzezicha, M.; Bigos, K.; Jagodziński, A.M. Leaf Traits and Aboveground Biomass Variability of Forest Understory Herbaceous Plant Species. Ecosystems 2019, 1–15. [Google Scholar] [CrossRef]
- Schepaschenko, D.; Moltchanova, E.; Shvidenko, A.; Blyshchyk, V.; Dmitriev, E.; Martynenko, O.; See, L.; Kraxner, F. Improved Estimates of Biomass Expansion Factors for Russian Forests. Forests 2018, 9, 312. [Google Scholar] [CrossRef]
- Jagodziński, A.M.; Dyderski, M.K.; Gęsikiewicz, K.; Horodecki, P. Tree- and Stand-Level Biomass Estimation in a Larix decidua Mill. Chronosequence. Forests 2018, 9, 587. [Google Scholar] [CrossRef]
- Annighöfer, P.; Mölder, I.; Zerbe, S.; Kawaletz, H.; Terwei, A.; Ammer, C. Biomass functions for the two alien tree species Prunus serotina Ehrh. and Robinia pseudoacacia L. in floodplain forests of Northern Italy. Eur. J. For. Res. 2012, 131, 1619–1635. [Google Scholar] [CrossRef]
- Starfinger, U.; Kowarik, I.; Rode, M.; Schepker, H. From desirable ornamental plant to pest to accepted addition to the flora? – the perception of an alien tree species through the centuries. Biol. Invasions 2003, 5, 323–335. [Google Scholar] [CrossRef]
- Blujdea, V.N.B.; Pilli, R.; Dutca, I.; Ciuvat, L.; Abrudan, I.V. Allometric biomass equations for young broadleaved trees in plantations in Romania. For. Ecol. Manag. 2012, 264, 172–184. [Google Scholar] [CrossRef]
- Brown, J.K. Estimating shrub biomass from basal stem diameters. Can. J. For. Res. 1976, 6, 153–158. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019.
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Kellomäki, S.; Hari, P.; Kanninen, M.; Pirkko, I. Eco-physiological studies on young Scots pine stands: II. Distribution of needle biomass and its application in approximating light conditions inside the canopy. Silva Fenn. 1980, 14, 243–257. [Google Scholar] [CrossRef][Green Version]
- Jagodziński, A.M.; Dyderski, M.K.; Horodecki, P.; Rawlik, K. Limited dispersal prevents Quercus rubra invasion in a 14-species common garden experiment. Divers. Distrib. 2018, 24, 403–414. [Google Scholar] [CrossRef]
- Bauer, I.E.; Gignac, L.D.; Vitt, D.H. Development of a peatland complex in boreal western Canada: Lateral site expansion and local variability in vegetation succession and long-term peat accumulation. Can. J. Bot. 2003, 81, 833–847. [Google Scholar] [CrossRef]
- Zerbe, S.; Wirth, P. Non-indigenous plant species and their ecological range in Central European pine (Pinus sylvestris L.) forests. Ann. For. Sci. 2006, 63, 189–203. [Google Scholar] [CrossRef]
- Enquist, B.J.; Niklas, K.J. Global allocation rules for patterns of biomass partitioning in seed plants. Science 2002, 295, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Niklas, K.J.; Enquist, B.J. Canonical rules for plant organ biomass partitioning and annual allocation. Am. J. Bot. 2002, 89, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Jagodziński, A.M.; Ruiz-Peinado, R.; Kuyah, S.; Luo, Y.; Oleksyn, J.; Usoltsev, V.A.; Buckley, T.N.; Reich, P.B.; Sack, L. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol. 2015, 208, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Jagodziński, A.M.; Dyderski, M.K.; Gęsikiewicz, K.; Horodecki, P.; Cysewska, A.; Wierczyńska, S.; Maciejczyk, K. How do tree stand parameters affect young Scots pine biomass?—Allometric equations and biomass conversion and expansion factors. For. Ecol. Manag. 2018, 409, 74–83. [Google Scholar] [CrossRef]
- Doyle, T.W. The Role of Disturbance in the Gap Dynamics of a Montane Rain Forest: An Application of a Tropical Forest Succession Model. In Forest Succession; West, D.C., Shugart, H.H., Botkin, D.B., Eds.; Springer Advanced Texts in Life Sciences; Springer: New York, NY, USA, 1981; pp. 56–73. [Google Scholar]
- Pretzsch, H. Forest Dynamics, Growth, and Yield. From Measurement to Model; Springer: Berlin, Germany, 2009; ISBN 978-3-540-88306-7. [Google Scholar]
- Conti, G.; Gorné, L.D.; Zeballos, S.R.; Lipoma, M.L.; Gatica, G.; Kowaljow, E.; Whitworth-Hulse, J.I.; Cuchietti, A.; Poca, M.; Pestoni, S.; et al. Developing allometric models to predict the individual aboveground biomass of shrubs worldwide. Glob. Ecol. Biogeogr. 2019, 28, 961–975. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Maire, V.; Wright, I.J.; Prentice, I.C.; Batjes, N.H.; Bhaskar, R.; van Bodegom, P.M.; Cornwell, W.K.; Ellsworth, D.; Niinemets, Ü.; Ordonez, A.; et al. Global effects of soil and climate on leaf photosynthetic traits and rates. Glob. Ecol. Biogeogr. 2015, 24, 706–717. [Google Scholar] [CrossRef]
- Midolo, G.; Frenne, P.D.; Hölzel, N.; Wellstein, C. Global patterns of intraspecific leaf trait responses to elevation. Glob. Chang. Biol. 2019, 25, 2485–2498. [Google Scholar] [CrossRef]
- Wyka, T.P.; Oleksyn, J.; Żytkowiak, R.; Karolewski, P.; Jagodziński, A.M.; Reich, P.B. Responses of leaf structure and photosynthetic properties to intra-canopy light gradients: A common garden test with four broadleaf deciduous angiosperm and seven evergreen conifer tree species. Oecologia 2012, 170, 11–24. [Google Scholar] [CrossRef]
- Jagodziński, A.M.; Dyderski, M.K.; Rawlik, K.; Kątna, B. Seasonal variability of biomass, total leaf area and specific leaf area of forest understory herbs reflects their life strategies. For. Ecol. Manag. 2016, 374, 71–81. [Google Scholar] [CrossRef]
- Flores, O.; Garnier, E.; Wright, I.J.; Reich, P.B.; Pierce, S.; Dìaz, S.; Pakeman, R.J.; Rusch, G.M.; Bernard-Verdier, M.; Testi, B.; et al. An evolutionary perspective on leaf economics: Phylogenetics of leaf mass per area in vascular plants. Ecol. Evol. 2014, 4, 2799–2811. [Google Scholar] [CrossRef] [PubMed]
- Pyšek, P.; Danihelka, J.; Sádlo, J.; Chrtek, J.; Chytrỳ, M.; Jarošík, V.; Kaplan, Z.; Krahulec, F.; Moravcová, L.; Pergl, J.; et al. Catalogue of alien plants of the Czech Republic: Checklist update, taxonomic diversity and invasion patterns. Preslia 2012, 84, 155–255. [Google Scholar]
- Van Kleunen, M.; Pyšek, P.; Dawson, W.; Essl, F.; Kreft, H.; Pergl, J.; Weigelt, P.; Stein, A.; Dullinger, S.; König, C.; et al. The Global Naturalized Alien Flora (GloNAF) database. Ecology 2019, 100, e02542. [Google Scholar] [PubMed]
- Straupe, I.; Jankovska, I.; Rusina, S.; Donis, J. The impact of recreational pressure on urban pine forest vegetation in Riga city, Latvia. Int. J. Energy Environ. 2012, 6, 406–414. [Google Scholar]
- Pliszko, A. Spontaneous occurrence of Cotoneaster lucidus Schltdl. in the town of Augustów (NE Poland). Steciana 2014, 18, 33–36. [Google Scholar] [CrossRef]
- Carboneras, C.; Genovesi, P.; Vilà, M.; Blackburn, T.M.; Carrete, M.; Clavero, M.; D′hondt, B.; Orueta, J.F.; Gallardo, B.; Geraldes, P.; et al. A prioritised list of invasive alien species to assist the effective implementation of EU legislation. J. Appl. Ecol. 2018, 22, 539–547. [Google Scholar] [CrossRef]
- Muys, B.; Maddelein, D.; Lust, N. Ecology, practice and policy of black cherry (Prunus serotina Ehrh.) management in Belgium. Silva Gandav. 1992, 57, 28–45. [Google Scholar] [CrossRef]
- Chabrerie, O.; Verheyen, K.; Saguez, R.; Decocq, G. Disentangling relationships between habitat conditions, disturbance history, plant diversity, and American black cherry (Prunus serotina Ehrh.) invasion in a European temperate forest. Divers. Distrib. 2008, 14, 204–212. [Google Scholar] [CrossRef]
- Jagodziński, A.M.; Dyderski, M.K.; Horodecki, P.; Knight, K.S.; Rawlik, K.; Szmyt, J. Light and propagule pressure affect invasion intensity of Prunus serotina in a 14-tree species forest common garden experiment. NeoBiota 2019, 46, 1–21. [Google Scholar] [CrossRef]
- Urban, J.; Tatarinov, F.; Nadezhdina, N.; Čermák, J.; Ceulemans, R. Crown structure and leaf area of the understorey species Prunus serotina. Trees 2009, 23, 391–399. [Google Scholar] [CrossRef]
- Halarewicz, A.; Żołnierz, L. Changes in the understorey of mixed coniferous forest plant communities dominated by the American black cherry (Prunus serotina Ehrh.). For. Ecol. Manag. 2014, 313, 91–97. [Google Scholar] [CrossRef]
- Halarewicz, A.; Pruchniewicz, D. Vegetation and environmental changes in a Scots pine forest invaded by Prunus serotina: What is the threat to terricolous bryophytes? Eur. J. For. Res. 2015, 134, 793–801. [Google Scholar] [CrossRef]
- Closset-Kopp, D.; Chabrerie, O.; Valentin, B.; Delachapelle, H.; Decocq, G. When Oskar meets Alice: Does a lack of trade-off in r/K-strategies make Prunus serotina a successful invader of European forests? For. Ecol. Manag. 2007, 247, 120–130. [Google Scholar] [CrossRef]
- Sebert-Cuvillier, E.; Paccaut, F.; Chabrerie, O.; Endels, P.; Goubet, O.; Decocq, G. Local population dynamics of an invasive tree species with a complex life-history cycle: A stochastic matrix model. Ecol. Model. 2007, 201, 127–143. [Google Scholar] [CrossRef]
- Saccone, P.; Pagès, J.-P.; Girel, J.; Brun, J.-J.; Michalet, R. Acer negundo invasion along a successional gradient: Early direct facilitation by native pioneers and late indirect facilitation by conspecifics. New Phytol. 2010, 187, 831–842. [Google Scholar] [CrossRef]
- Lamarque, L.J.; Porté, A.J.; Eymeric, C.; Lasnier, J.-B.; Lortie, C.J.; Delzon, S. A Test for Pre-Adapted Phenotypic Plasticity in the Invasive Tree Acer negundo L. PLoS ONE 2013, 8, e74239. [Google Scholar] [CrossRef]
- Dyderski, M.K.; Gdula, A.K.; Jagodziński, A.M. “The rich get richer” concept in riparian woody species – A case study of the Warta River Valley (Poznań, Poland). Urban For. Urban Green. 2015, 14, 107–114. [Google Scholar] [CrossRef]
- Zajdler, M.; Tyborski, J.; Dyderski, M.K.; Jagodzinski, A.M. Analiza dendroklimatologiczna przyrostów radialnych inwazyjnych Acer negundo L. oraz Fraxinus pennsylvanica Marshall z doliny Warty. Sylwan 2018, 162, 547–554. [Google Scholar]
- Hughes, R.F.; Denslow, J.S. Invasion by a N2-Fixing Tree Alters Function and Structure in Wet Lowland Forests of Hawaii. Ecol. Appl. 2005, 15, 1615–1628. [Google Scholar] [CrossRef]
- Cierjacks, A.; Kowarik, I.; Joshi, J.; Hempel, S.; Ristow, M.; von der Lippe, M.; Weber, E. Biological Flora of the British Isles: Robinia pseudoacacia. J. Ecol. 2013, 101, 1623–1640. [Google Scholar] [CrossRef]
- Vítková, M.; Müllerová, J.; Sádlo, J.; Pergl, J.; Pyšek, P. Black locust (Robinia pseudoacacia) beloved and despised: A story of an invasive tree in Central Europe. For. Ecol. Manag. 2017, 384, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Slabejová, D.; Bacigál, T.; Hegedüšová, K.; Májeková, J.; Medvecká, J.; Mikulová, K.; Šibíková, M.; Škodová, I.; Zaliberová, M.; Jarolímek, I. Comparison of the understory vegetation of native forests and adjacent Robinia pseudoacacia plantations in the Carpathian-Pannonian region. For. Ecol. Manag. 2019, 439, 28–40. [Google Scholar] [CrossRef]
- Zianis, D.; Muukkonen, P.; Mäkipää, R.; Mencuccini, M. Biomass and Stem Volume Equations for Tree Species in Europe; Silva Fennica Monographs 4; The Finnish Society of Forest Science The Finnish Forest Research Institute: Helsinki, Finland, 2005. [Google Scholar]
- Cornelissen, J.H.C.; Lavorel, S.; Garnier, E.; Diaz, S.; Buchmann, N.; Gurvich, D.E.; Reich, P.B.; Ter Steege, H.; Morgan, H.D.; Van Der Heijden, M.G.A.; et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 2003, 51, 335–380. [Google Scholar] [CrossRef]
- Roberts, M.R.; Paul, N.D. Seduced by the dark side: Integrating molecular and ecological perspectives on the influence of light on plant defence against pests and pathogens. New Phytol. 2006, 170, 677–699. [Google Scholar] [CrossRef] [PubMed]

| Trees and Shrubs with DBH > 5 cm | ||||
|---|---|---|---|---|
| Parametric Coefficients: | Estimate | SE | t | p |
| (Intercept) | 0.0439 | 0.0023 | 18.7100 | <0.0001 |
| Approximate significance of smooth terms: | edf | Ref.df | F | p |
| LAI | 1.9667 | 1.9990 | 38.2800 | <0.0001 |
| random effect (block) | 0.0004 | 1.0000 | 0.0000 | 0.4760 |
| Model parameters | R2 | Deviance Explained | AIC | AIC of Null Model |
| 0.307 | 31.50% | −692.2 | −523.8 | |
| All Trees and Shrubs | ||||
| Parametric coefficients: | Estimate | SE | t | p |
| (Intercept) | 0.0475 | 0.0022 | 21.8200 | <0.0001 |
| Approximate significance of smooth terms: | edf | Ref.df | F | p |
| LAI | 7.6457 | 8.5370 | 45.3500 | <0.0001 |
| random effect (block) | 0.0000 | 1.0000 | 0.0000 | 0.9650 |
| Model parameters | R2 | Deviance Explained | AIC | AIC of Null Model |
| 0.695 | 70.90% | −717.4 | −523.8 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyderski, M.K.; Jagodziński, A.M. Similar Impacts of Alien and Native Tree Species on Understory Light Availability in a Temperate Forest. Forests 2019, 10, 951. https://doi.org/10.3390/f10110951
Dyderski MK, Jagodziński AM. Similar Impacts of Alien and Native Tree Species on Understory Light Availability in a Temperate Forest. Forests. 2019; 10(11):951. https://doi.org/10.3390/f10110951
Chicago/Turabian StyleDyderski, Marcin K., and Andrzej M. Jagodziński. 2019. "Similar Impacts of Alien and Native Tree Species on Understory Light Availability in a Temperate Forest" Forests 10, no. 11: 951. https://doi.org/10.3390/f10110951
APA StyleDyderski, M. K., & Jagodziński, A. M. (2019). Similar Impacts of Alien and Native Tree Species on Understory Light Availability in a Temperate Forest. Forests, 10(11), 951. https://doi.org/10.3390/f10110951





