Modelling the Effect of Microsite Influences on the Growth and Survival of Juvenile Eucalyptus globoidea (Blakely) and Eucalyptus bosistoana (F. Muell) in New Zealand †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sites
2.2. Data Collection and Preparation
2.2.1. Tree Data
2.2.2. Topographic Data
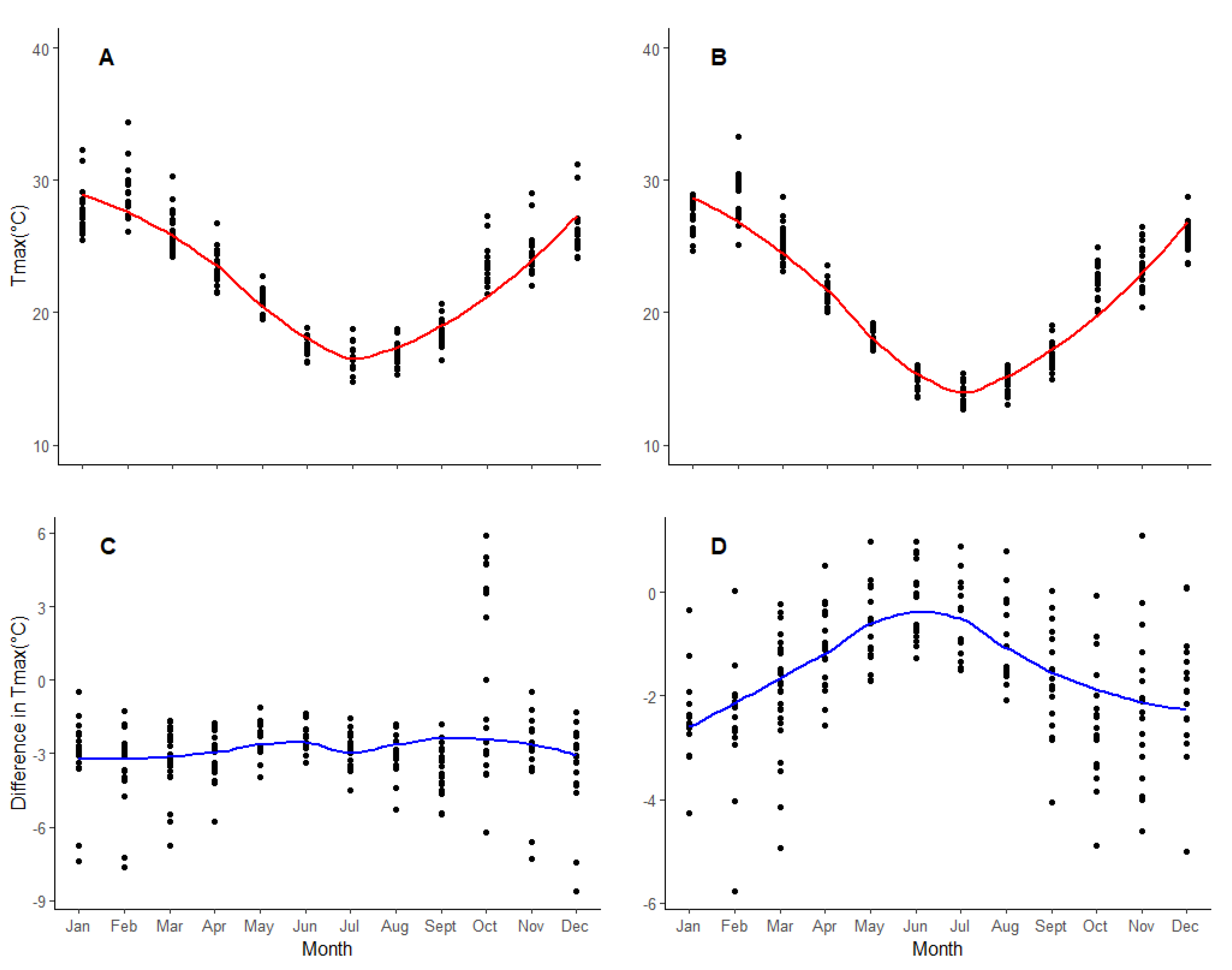
2.2.3. Climatic Data
2.3. Modelling Strategy
2.4. Model Testing and Validation
2.5. Statistical Analysis
3. Results
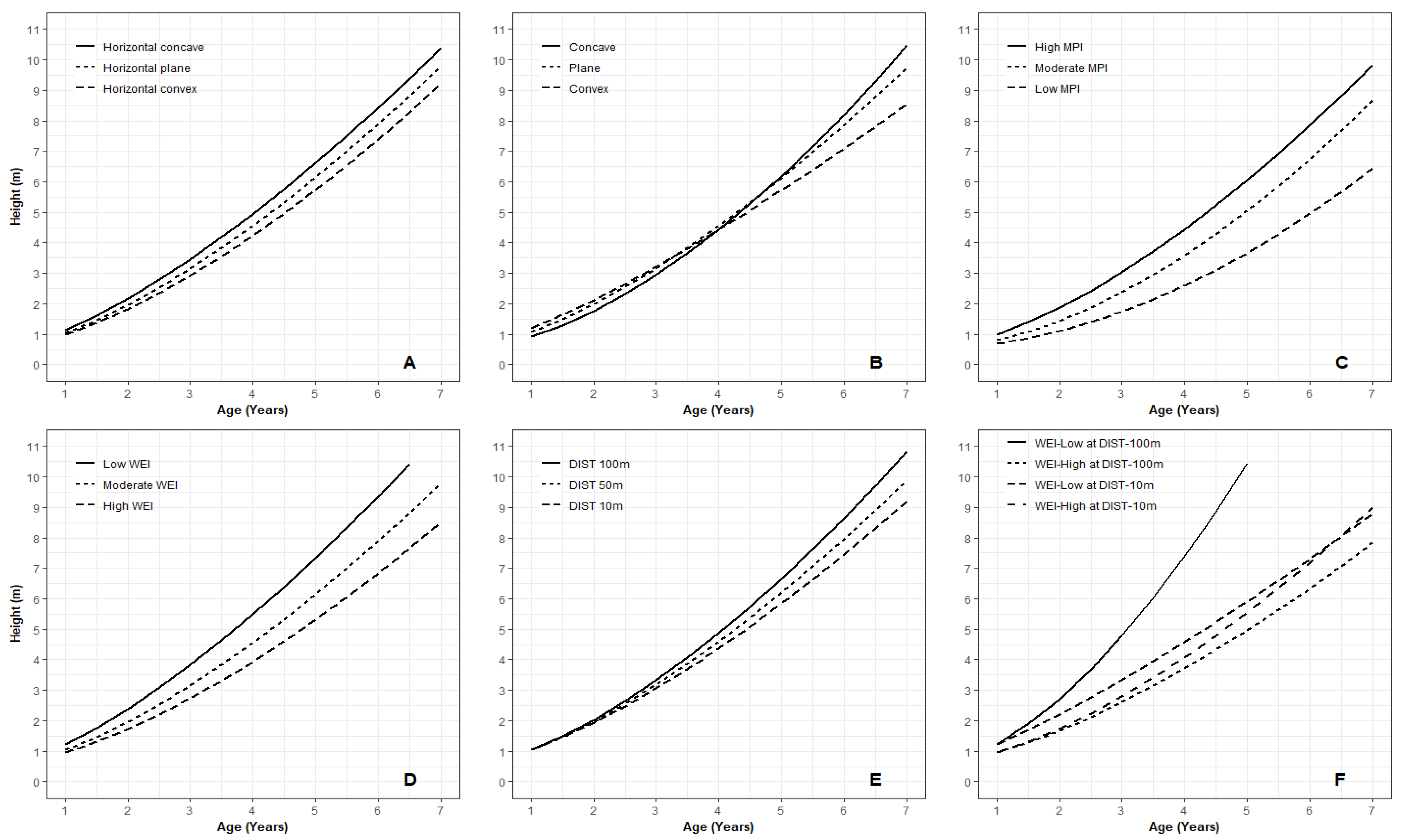
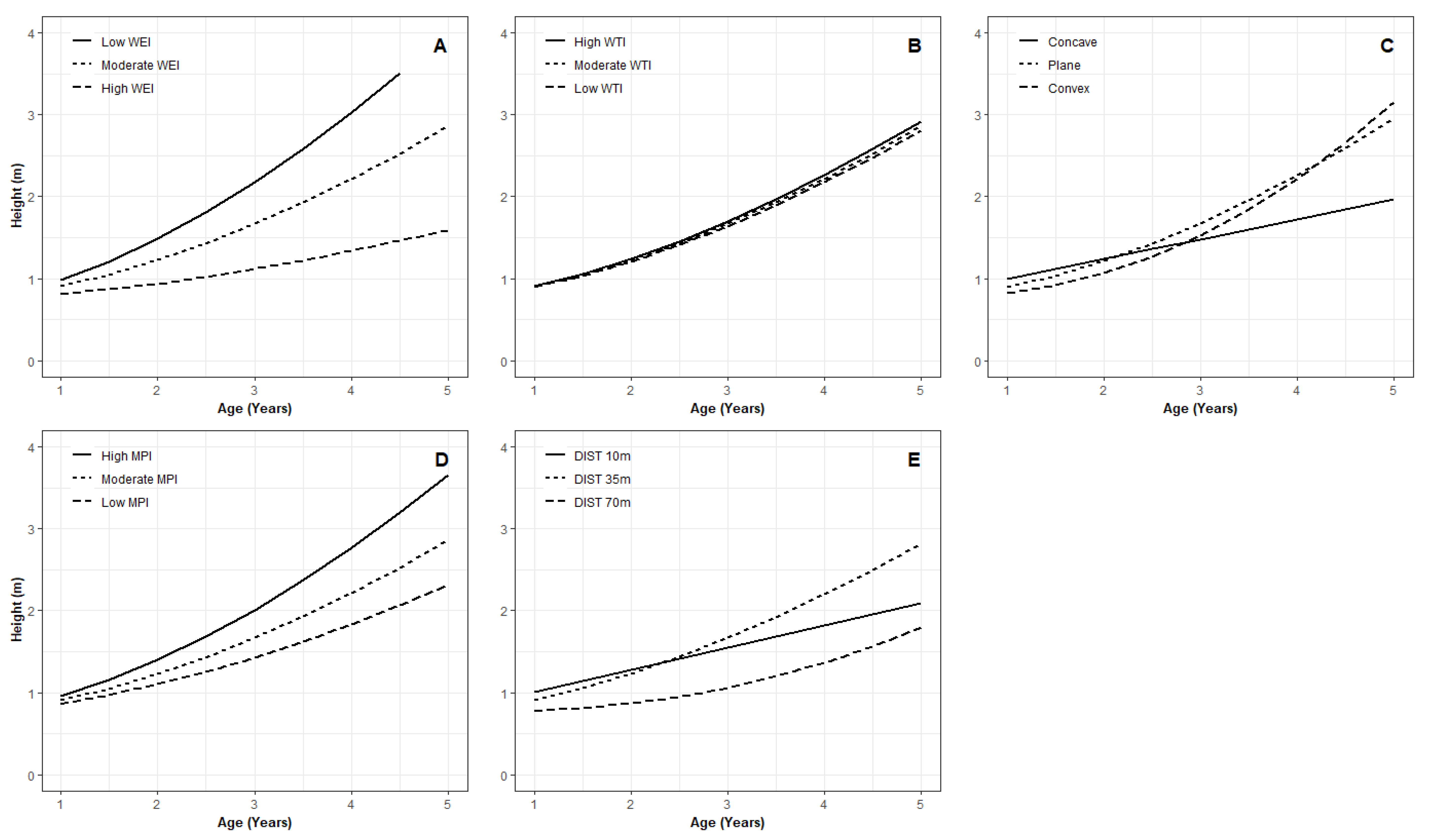
3.1. Juvenile Height Models
3.2. Key Variables for Microsite Height Growth
3.3. Juvenile Survival Model
3.4. Key Variables Influencing Juvenile Microsite Survival
4. Discussion
4.1. Juvenile Microsite Models
4.2. Microsite Variables Affect Juvenile Tree Height Growth
4.3. Microsite Variability on Juvenile Tree Survival
4.4. Data Constraints
5. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Radford, I.J.; Nicholas, M.; Tiver, F.; Brown, J.; Kriticos, D. Seedling establishment, mortality, tree growth rates and vigour of Acacia nilotica in different astrebla grassland habitats: Implications for invasion. Austral Ecol. 2002, 27, 258–268. [Google Scholar] [CrossRef]
- Bailey, R.G.; Pfister, R.D.; Henderson, J.A. Nature of land and resource classification—A review. J. For. 1978, 76, 650–655. [Google Scholar]
- Grey, D. On the concept of site in forestry. S. Afr. For. J. 1980, 113, 81–83. [Google Scholar] [CrossRef]
- Louw, J.H. A review of site-growth studies in south africa. S. Afr. For. J. 1999, 185, 57–65. [Google Scholar] [CrossRef]
- Skovsgaard, J.P.; Vanclay, J.K. Forest site productivity: A review of the evolution of dendrometric concepts for even-aged stands. Forestry 2008, 81, 13–31. [Google Scholar] [CrossRef]
- Skovsgaard, J.P.; Vanclay, J.K. Forest site productivity: A review of spatial and temporal variability in natural site conditions. Forestry 2013, 86, 305–315. [Google Scholar] [CrossRef]
- Dungey, H.S.; Dash, J.P.; Pont, D.; Clinton, P.W.; Watt, M.S.; Telfer, E.J. Phenotyping whole forests will help to track genetic performance. Trends Plant Sci. 2018, 23, 854–864. [Google Scholar] [CrossRef]
- Koch, G.W.; Sillett, S.C.; Jennings, G.M.; Davis, S.D. The limits to tree height. Nature 2004, 428, 851–854. [Google Scholar] [CrossRef]
- Forrester, D.I. Linking forest growth with stand structure: Tree size inequality, tree growth or resource partitioning and the asymmetry of competition. For. Ecol. Manag. 2019, 447, 139–157. [Google Scholar] [CrossRef]
- Berrill, J.P.; O’Hara, K.L. How do biophysical factors contribute to height and basal area development in a mixed multiaged coast redwood stand? Forestry 2016, 89, 170–181. [Google Scholar] [CrossRef]
- Bravo-Oviedo, A.; Tomé, M.; Bravo, F.; Montero, G.; del Río, M. Dominant height growth equations including site attributes in the generalized algebraic difference approach. Can. J. For. Res. 2008, 38, 2348–2358. [Google Scholar] [CrossRef]
- Landsberg, J. Physiology in forest models: History and the future. FBMIS 2003, 1, 49–63. [Google Scholar]
- Wiens, J.A. Spatial scaling in ecology. Funct. Ecol. 1989, 3, 385–397. [Google Scholar] [CrossRef]
- Chen, J.; Saunders, S.C.; Crow, T.R.; Naiman, R.J.; Brosofske, K.D.; Mroz, G.D.; Brookshire, B.L.; Franklin, J.F. Microclimate in forest ecosystem and landscape ecology variations in local climate can be used to monitor and compare the effects of different management regimes. BioScience 1999, 49, 288–297. [Google Scholar] [CrossRef]
- Lilja-Rothsten, S.; Chantal, M.D.; Peterson, C.; Kuuluvainen, T.; Vanha-Majamaa, I.; Puttonen, P. Microsites before and after restoration in managed Picea abies stands in southern finland: Effects of fire and partial cutting with dead wood creation. Silva Fenn. 2008, 42, 165–176. [Google Scholar] [CrossRef]
- Coates, K.D. Tree recruitment in gaps of various size, clearcuts and undisturbed mixed forest of interior British Columbia, Canada. For. Ecol. Manag. 2002, 155, 387–398. [Google Scholar] [CrossRef]
- Kuuluvainen, T. Natural variability of forests as a reference for restoring and managing biological diversity in boreal fennoscandia. Silva Fenn. 2002, 36, 97–125. [Google Scholar] [CrossRef]
- Martín-Alcón, S.; Coll, L.; Salekin, S. Stand-level drivers of tree-species diversification in mediterranean pine forests after abandonment of traditional practices. For. Ecol. Manag. 2015, 353, 107–117. [Google Scholar] [CrossRef]
- Narukawa, Y.; Yamamoto, S.I. Gap formation, microsite variation and the conifer seedling occurrence in a subalpine old-growth forest, central japan. Ecol. Res. 2001, 16, 617–625. [Google Scholar] [CrossRef]
- Ahtikoski, A.; Siipilehto, J.; Salminen, H.; Lehtonen, M.; Hynynen, J. Effect of stand structure and number of sample trees on optimal management for scots pine: A model-based study. Forests 2018, 9, 750. [Google Scholar] [CrossRef]
- Weiskittel, A.; Temesgen, H.; Wilson, D.; Maguire, D. Sources of within- and between-stand variability in specific leaf area of three ecologically distinct conifer species. Ann. For. Sci. 2008, 65, 103. [Google Scholar] [CrossRef]
- Mummery, D.; Battaglia, M. Data input quality and resolution effects on regional and local scale Eucalyptus globulus productivity predictions in north-east tasmania. Ecol. Model. 2002, 156, 13–25. [Google Scholar] [CrossRef]
- Gallart, M.; Love, J.; Meason, D.F.; Coker, G.; Clinton, P.W.; Xue, J.; Jameson, P.E.; Klápště, J.; Turnbull, M.H. Field-scale variability in site conditions explain phenotypic plasticity in response to nitrogen source in Pinus radiata d. Don. Plant Soil 2019. [Google Scholar] [CrossRef]
- Spiecker, H.; Mieläikinen, K.; Köhl, M.; Skovsgaard, J.P. Discussion. In Growth Trends in European Forests; Spiecker, H., Mielikäinen, K., Köhl, M., Skovsgaard, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 355–367. [Google Scholar]
- Zhang, S.; Burkhart, H.E.; Amateis, R.L. Modeling individual tree growth for juvenile loblolly pine plantations. For. Ecol. Manag. 1996, 89, 157–172. [Google Scholar] [CrossRef]
- Clutter, J.L. Compatible growth and yield models for loblolly pine. For. Sci. 1963, 9, 354–371. [Google Scholar]
- Garcia, O. New class of growth models for even-aged stands: Pinus radiata in golden downs forest. N. Z. J. For. Sci. 1984, 14, 65–88. [Google Scholar]
- Burkhart, H.E.; Tomé, M. Modeling Forest Trees and Stands; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Weiskittel, A.R.; Hann, D.W.; Kershaw, J.A.; Vanclay, J.K. Forest Growth and Yield Modeling; Wiley: Hoboken, NJ, USA, 2011. [Google Scholar]
- Avila, O.B. Modeling Growth Dynamics of Juvenile Loblolly Pine Plantations; Virginia Polytechnic Institute and State Univeristy: Blacksburg, VA, USA, 1993. [Google Scholar]
- Mason, E.G.; Whyte, A.G.D.; Woollons, R.C.; Richardson, B. A model of the growth of juvenile Radiata pine in the central north island of New Zealand: Links with older models and rotation-length analyses of the effects of site preparation. For. Ecol. Manag. 1997, 97, 187–195. [Google Scholar] [CrossRef]
- Mason, E.G.; Whyte, A.G.D. Modelling initial survival and growth of Radiata pine in New Zealand. Acta For. Fenn. 1997, 2, 1–38. [Google Scholar] [CrossRef]
- Casnati, A.C.R. Hybrid Mensurational-Physiological Models for Pinus Taeda and Eucalyptus Grandis in Uruguay. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 2016. [Google Scholar]
- Ma, P.; Han, X.H.; Lin, Y.; Moore, J.; Guo, Y.X.; Yue, M. Exploring the relative importance of biotic and abiotic factors that alter the self-thinning rule: Insights from individual-based modelling and machine-learning. Ecol. Model. 2019, 397, 16–24. [Google Scholar] [CrossRef]
- Mason, E.G. A model of the juvenile growth and survival of Pinus radiata d. Don; adding the effects of initial seedling diameter and plant handling. New For. 2001, 22, 133–158. [Google Scholar] [CrossRef]
- Woollons, R.C.; Snowdon, P.; Mitchell, N.D. Augmenting empirical stand projection equations with edaphic and climatic variables. For. Ecol. Manag. 1997, 98, 267–275. [Google Scholar] [CrossRef]
- Snowdon, P.; Jovanovic, T.; Booth, T.H. Incorporation of indices of annual climatic variation into growth models for Pinus radiata. For. Ecol. Manag. 1999, 117, 187–197. [Google Scholar] [CrossRef]
- Mäkelä, A.; Landsberg, J.; Ek, A.R.; Burk, T.E.; Ter-Mikaelian, M.; Ågren, G.I.; Oliver, C.D.; Puttonen, P. Process-based models for forest ecosystem management: Current state of the art and challenges for practical implementation. Tree Physiol. 2000, 20, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Watt, M.S.; Kimberley, M.O.; Richardson, B.; Whitehead, D.; Mason, E.G. Testing a juvenile tree growth model sensitive to competition from weeds, using Pinus radiata at two contrasting sites in New Zealand. Can. J. For. Res. 2004, 34, 1985–1992. [Google Scholar] [CrossRef]
- Dyck, B. Precision forestry—The path to increased profitability. In Proceedings of the 2nd International Precision Forestry Symposium, Seattle, WA, USA, 15–17 June 2003; pp. 3–8. [Google Scholar]
- Adão, T.; Hruška, J.; Pádua, L.; Bessa, J.; Peres, E.; Morais, R.; Sousa, J. Hyperspectral imaging: A review on uav-based sensors, data processing and applications for agriculture and forestry. Remote Sens. 2017, 9, 1110. [Google Scholar] [CrossRef]
- Akay, A.E.; Oǧuz, H.; Karas, I.R.; Aruga, K. Using lidar technology in forestry activities. Environ. Monit. Assess. 2009, 151, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Salekin, S.; Burgess, J.; Morgenroth, J.; Mason, E.; Meason, D. A comparative study of three non-geostatistical methods for optimising digital elevation model interpolation. ISPRS Int. J. Geo Inf. 2018, 7, 300. [Google Scholar] [CrossRef]
- NZFOA. New Zealand Plantation Forest Industry: Facts and Figures 2016/17; Ministry for Primary Industries: Wellington, New Zealand, 2017.
- Turner, J.A.; West, G.; Dungey, H.; Wakelin, S.; Maclaren, P.; Adams, T.; Silcock, P.J. Managing New Zealand Planted Forests for Carbon: A Review of Selected Scenarios Identification of Knowledge Gaps; The Ministry of Agriculture Forestry: Wellington, New Zealand, 2008; p. 130.
- Millen, P.; van Ballekom, S.; Altaner, C.; Apiolaza, L.; Mason, E.; McConnochie, R.; Morgenroth, J.; Murray, T. Durable eucalypt forests–a multi-regional opportunity for investment in New Zealand drylands. N. Z. J. For. 2018, 63, 11–23. [Google Scholar]
- Liu, C.L.C.; Kuchma, O.; Krutovsky, K.V. Mixed-species versus monocultures in plantation forestry: Development, benefits, ecosystem services and perspectives for the future. Glob. Ecol. Conserv. 2018, 15, e00419. [Google Scholar] [CrossRef]
- van der Plas, F.; Manning, P.; Allan, E.; Scherer-Lorenzen, M.; Verheyen, K.; Wirth, C.; Zavala, M.A.; Hector, A.; Ampoorter, E.; Baeten, L.; et al. Jack-of-all-trades effects drive biodiversity–ecosystem multifunctionality relationships in european forests. Nat. Commun. 2016, 7, 11109. [Google Scholar] [CrossRef]
- Menzies, H. Eucalypts show potential. Farm For. Rev. 1995, 33–34. [Google Scholar]
- Nicholas, I.; Millen, P. Durable Eucalypt Leaflet Series: Eucalyptus Bosistoana; NZDFI, Ed.; NZDFI: Blenheim, New Zealand, 2012. [Google Scholar]
- Nicholas, I.; Millen, P. Durable Eucalypt Leaflet Series: Eucalyptus Globoidea; NZDFI, Ed.; NZDFI: Blenheim, New Zealand, 2012. [Google Scholar]
- Kakitani, T. The global timberlization movement and the potential for durable eucalyts: Downstream opportunities. In Durable Eucalypts on Drylands: Protecting and Enhanching Value; Altaner, C.M., Murray, T.J., Morgenroth, J., Eds.; NZDFI, Marlborough Research Centre: Blenheim, New Zealand, 2017. [Google Scholar]
- Satchell, D.; Turner, J. Solid Timber Recovery and Economics of Short-Rotation Small Diameter Eucalypt Forestry Using Novel Sawmilling Strategy Applied to Eucalyptus Regnans; SCION Report No. FFR-DS028; New Zealand Forest Research Institute Limited: Rotorua, New Zealand, 2010. [Google Scholar]
- Nicholas, I.D. Best Practice with Farm Forestry Timber Species: No. 2 Eucalypts; Farm Forestry Association: Wellington, New Zealand, 2009. [Google Scholar]
- Millner, J.P.; Kemp, P.D. Seasonal growth of eucalyptus species in New Zealand hill country. New For. 2012, 43, 31–44. [Google Scholar] [CrossRef]
- NIWA. Overview of New Zealand Climate. Available online: https://www.niwa.co.nz/education-and-training/schools/resources/climate/overview (accessed on 26 August 2015).
- New Zealand Department of Scientific and Industrial Research. General survey of the soils of South Island New Zealnd. N. Z. Soil Bur. Bull. 1968. [Google Scholar] [CrossRef]
- Hewitt, A.E. New Zealand Soil Classification, 3rd ed.; Manaaki Whenua Press: Lincoln, New Zealand, 2010; p. 136. [Google Scholar]
- Travis, M.R.; Elsner, G.H.; Iverson, W.D.; Johnson, C.G. Viewit: Computation of Seen Areas, Slope, and Aspect for Land-Use Planning; General Technical Report PSW-GTR-11; Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture: Berkeley, CA, USA, 1975; p. 70.
- Heerdegen, R.G.; Beran, M.A. Quantifying source areas through land surface curvature and shape. J. Hydrol. 1982, 57, 359–373. [Google Scholar] [CrossRef]
- Zevenbergen, L.W.; Thorne, C.R. Quantitative analysis of land surface topography. Earth Surf. Process. Landf. 1987, 12, 47–56. [Google Scholar] [CrossRef]
- Riley, S.J.; de Gloria, S.D.; Elliot, R. A terrain ruggedness that quantifies topographic heterogeneity. Int. J. Sci. 1999, 5, 23–27. [Google Scholar]
- Weiss, A.D. Topographic position and landforms analysis (Poster presentation). In Proceedings of the ESRI User Conference, San Diego, CA, USA, 9–13 July 2001. [Google Scholar]
- Beven, K.J.; Kirkby, M.J. A physically based, variable contributing area model of basin hydrology/un modèle à base physique de zone d’appel variable de l’hydrologie du bassin versant. Hydrol. Sci. Bull. 1979, 24, 43–69. [Google Scholar] [CrossRef]
- Moore, I.D.; Grayson, R.B.; Ladson, A.R. Digital terrain modelling: A review of hydrological, geomorphological, and biological applications. Hydrol. Process. 1991, 5, 3–30. [Google Scholar] [CrossRef]
- Gerlitz, L.; Conrad, O.; Böhner, J. Large-scale atmospheric forcing and topographic modification of precipitation rates over high asia & ndash; a neural-network-based approach. Earth Syst. Dyn. 2015, 6, 61–81. [Google Scholar]
- Yokoyama, R.; Shlrasawa, M.; Richard, I.P. Visualizing topography by openness: A new application of image processing to digital elevation models. Photogramm. Eng. Remote Sens. 2005, 68, 257–266. [Google Scholar]
- ESRI. ArcGIS 10.1; ESRI: Redlands, CA, USA, 2012. [Google Scholar]
- Conrad, O.; Bechtel, B.; Bock, M.; Dietrich, H.; Fischer, E.; Gerlitz, L.; Wehberg, J.; Wichmann, V.; Böhner, J. System for automated geoscientific analyses (saga) v. 2.1.4. Geosci. Model Dev. 2015, 8, 1991–2007. [Google Scholar] [CrossRef]
- Belli, K.L.; Ek, A.R. Growth and survival modeling for planted conifers in the great lakes region. For. Sci. 1988, 34, 458–473. [Google Scholar]
- Mason, E.G. Decision-Support Systems for Establishing Radiata Pine Plantations in the Central North Island of New Zealand. Ph.D. Thesis, University of Canterbury, Christchurch, New Zealand, 1992. [Google Scholar]
- Amateis, R.L.; Burkhart, H.E.; Liu, J. Modeling survival in juvenile and mature Loblolly pine plantations. For. Ecol. Manag. 1997, 90, 51–58. [Google Scholar] [CrossRef]
- Kozak, A.; Kozak, R. Does cross validation provide additional information in the evaluation of regression models? Can. J. For. Res. 2003, 33, 976–987. [Google Scholar] [CrossRef]
- Sargent, R.G. Verification and validation of simulation models. J. Simul. 2013, 7, 12–24. [Google Scholar] [CrossRef] [Green Version]
- Uzoh, F.C.C.; Mori, S.R. Applying survival analysis to managed even-aged stands of Ponderosa pine for assessment of tree mortality in the western united states. For. Ecol. Manag. 2012, 285, 101–122. [Google Scholar] [CrossRef]
- Dobbin, K.K.; Simon, R.M. Optimally splitting cases for training and testing high dimensional classifiers. BMC Med. Genom. 2011, 4, 31. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016; Available online: http://www. R-project.org (accessed on 7 June 2017).
- Fox, J.A.; Weisberg, S. An R Companion to Applied Regression; SAGE Publications: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Cook, R.D.; Weisberg, S. Applied Regression Including Computing and Graphics; John Wiley & Sons: Hoboken, NJ, USA, 2009; Volume 488. [Google Scholar]
- Hamner, B.; Frasco, M. Metrics: Evaluation Metrics for Machine Learning, R package version 0.1.4; R-CRAN: Vienna, Austria, 2018. [Google Scholar]
- Spiess, A.; Ritz, C. qpcR: Modelling and Analysis of Real-Time PCR Data, R package version 1.4-0; R-CRAN: Vienna, Austria, 2014. [Google Scholar]
- Nyström, K.; Kexi, M. Individual tree basal area growth models for young stands of norway spruce in sweden. For. Ecol. Manag. 1997, 97, 173–185. [Google Scholar] [CrossRef]
- Ritchie, M.W.; Hamann, J.D. Modeling dynamics of competing vegetation in young conifer plantations of northern california and southern oregon, USA. Can. J. For. Res. 2006, 36, 2523–2532. [Google Scholar] [CrossRef]
- Preece, N.D.; Lawes, M.J.; Rossman, A.K.; Curran, T.J.; van Oosterzee, P. Modelling the growth of young rainforest trees for biomass estimates and carbon sequestration accounting. For. Ecol. Manag. 2015, 351, 57–66. [Google Scholar] [CrossRef]
- Richardson, B.; Watt, M.S.; Mason, E.G.; Kriticos, D.J. Advances in modelling and decision support systems for vegetation management in young forest plantations. Forestry 2006, 79, 29–42. [Google Scholar] [CrossRef]
- Kohama, T.; Mizoue, N.; Ito, S.; Inoue, A.; Sakuta, K.; Okada, H. Effects of light and microsite conditions on tree size of 6-year-old cryptomeria japonica planted in a group selection opening. J. For. Res. 2006, 11, 235–242. [Google Scholar] [CrossRef]
- Mason, E.G.; Milne, P.G. Effects of weed control, fertilization, and soil cultivation on the growth of Pinus radiata at midrotation in canterbury, New Zealand. Can. J. For. Res. 1999, 29, 985–992. [Google Scholar] [CrossRef]
- Mason, E.G. Effects of soil cultivation, fertilisation, initial seedling diameter and plant handling on the development of maturing Pinus radiata d. Don on kaingaroa gravelly sand in the central north island of New Zealand. Bosque 2004, 25, 43–55. [Google Scholar] [CrossRef]
- Ares, A.; Marlats, R.M. Site factors related to growth of coniferous plantations in a temperate, hilly zone of argentina. Aust. For. 1995, 58, 118–128. [Google Scholar] [CrossRef]
- Adams, H.R.; Barnard, H.R.; Loomis, A.K. Topography alters tree growth–climate relationships in a semi-arid forested catchment. Ecosphere 2014, 5, art148. [Google Scholar] [CrossRef]
- Brüchert, F.; Gardiner, B. The effect of wind exposure on the tree aerial architecture and biomechanics of sitka spruce (Picea sitchensis, pinaceae). Am. J. Bot. 2006, 93, 1512–1521. [Google Scholar] [CrossRef]
- Swanson, F.J.; Kratz, T.K.; Caine, N.; Woodmansee, R.G. Landform effects on ecosystem patterns and processes. BioScience 1988, 38, 92–98. [Google Scholar] [CrossRef]
- Whiteman, C.D. Breakup of temperature inversions in deep mountain valleys: Part I. Observations 1982, 21, 270–289. [Google Scholar] [CrossRef]
- Leopold, L.B.; Wolman, M.G.; Miller, J.P. Fluvial Processes in Geomorphology; Courier Corporation: New York, NY, USA, 2012. [Google Scholar]
- Rohner, B.; Waldner, P.; Lischke, H.; Ferretti, M.; Thürig, E. Predicting individual-tree growth of central european tree species as a function of site, stand, management, nutrient, and climate effects. Eur. J. For. Res. 2018, 137, 29–44. [Google Scholar] [CrossRef]
- Monserud, R.A.; Sterba, H. A basal area increment model for individual trees growing in even- and uneven-aged forest stands in austria. For. Ecol. Manag. 1996, 80, 57–80. [Google Scholar] [CrossRef]
- Michael, J.R.; Noah, O.B.; Thomas, R.S.; David, B.L.; Wolfram, S. Comparing and combining process-based crop models and statistical models with some implications for climate change. Environ. Res. Lett. 2017, 12, 095010. [Google Scholar]
- Jame, Y.; Cutforth, H. Crop growth models for decision support systems. Can. J. Plant Sci. 1996, 76, 9–19. [Google Scholar] [CrossRef]



| Site | A | B | C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Est. (Year) | 2011 | 2009 | 2012 | |||||||||
| Area (ha) | 4.7 | 3.7 | 2.2 | |||||||||
| Trees/ha | 2243 | 1460 | 1767 | |||||||||
| Age (year) | 6 | 8 | 5 | |||||||||
| Variable | Height | Survival | Height | Survival | Height | Survival | ||||||
| Fitting | Validation | Fitting | Validation | Fitting | Validation | Fitting | Validation | Fitting | Validation | Fitting | Validation | |
| Plots (n) | 217 | 65 | 217 | 65 | 112 | 38 | - | - | 81 | 27 | 81 | 27 |
| Mean | 1.54 | 1.48 | 0.75 | 0.74 | 4.88 | 4.99 | - | - | 2.11 | 2.04 | 0.99 | 0.99 |
| Min | 0.33 | 0.46 | 0.19 | 0.33 | 0.98 | 1.07 | - | - | 1.29 | 1.26 | 0.89 | 0.92 |
| Max | 4.58 | 3.67 | 1.00 | 1.00 | 13.47 | 13.66 | - | - | 3.74 | 2.93 | 1.00 | 1.00 |
| SD | 0.84 | 0.73 | 0.18 | 0.19 | 2.60 | 2.69 | - | - | 0.52 | 0.44 | 0.02 | 0.02 |
| Site | Soil Series | Dominant Soil Type | Class Name | Comments |
|---|---|---|---|---|
| A | Flaxbourne | Hill soils | Typic argillic pallic | Argillic pallic soils have a clay accumulation in the sub-soils |
| B | Flaxbourne | Hill soils | Typic argillic pallic | |
| C | Wither | Hill soils | Argillic-sodic fragic pallic | Fragic pallic soils are predominantly silty and severely restrict root movement |
| Attributes | Site A | Site B | Site C | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | Max | Mean | SD | Min | Max | Mean | SD | Min | Max | Mean | SD | |
| Aspect (°) | 4.57 | 356.20 | 127.01 | 136.8 | 55.7 | 345.9 | 124.5 | 83.6 | 208.7 | 330.1 | 265.7 | 26.44 |
| Slope (°) | 13.9 | 31.70 | 24.60 | 3.54 | 11.95 | 30.37 | 21.35 | 3.22 | 8.56 | 29.38 | 21.70 | 4.56 |
| Elevation (m asl) | 13.4 | 79.22 | 44.87 | 16.93 | 134 | 168.1 | 148.7 | 9.62 | 232.9 | 277.5 | 257.2 | 12.38 |
| Total curvature | −2.40 | 3.81 | 0.11 | 1.15 | −1.83 | 4.93 | 0.20 | 1.17 | −3.34 | 2.78 | 0.30 | 1.31 |
| Prof. curvature | −2.79 | 1.82 | −0.01 | 0.69 | −2.21 | 1.30 | −0.11 | 0.62 | −1.79 | 3.12 | 0.00 | 0.86 |
| Plan curvature | −1.83 | 2.32 | 0.10 | 0.79 | −1.27 | 2.73 | 0.09 | 0.75 | −1.76 | 2.40 | 0.30 | 0.76 |
| TRI | 0.47 | 1.24 | 0.92 | 0.15 | 0.07 | 0.22 | 0.14 | 0.02 | 0.05 | 0.18 | 0.13 | 0.03 |
| TPI | −2.25 | 3.52 | 0.12 | 0.96 | −13.80 | 13.12 | −0.81 | 7.30 | −14.4 | 10.42 | −1.44 | 6.92 |
| TWI | 0 | 3.90 | 0.89 | 0.68 | −0.05 | 2.83 | 0.89 | 0.52 | 0 | 6.91 | 3.45 | 3.70 |
| WEI | 0.98 | 1.10 | 1.02 | 0.02 | 0.88 | 1.20 | 0.99 | 0.08 | 0.96 | 1.10 | 1.03 | 0.03 |
| MPI | 0.07 | 0.19 | 0.14 | 0.02 | 0.06 | 0.18 | 0.12 | 0.02 | 0.05 | 0.18 | 0.13 | 0.03 |
| DIST (m) | 0.38 | 140.58 | 65.40 | 33.22 | 1.64 | 103.55 | 44.76 | 25.53 | 7.58 | 80.98 | 36.93 | 18.64 |
| Species | Site | Action | RMSE | MAE | BIAS | SE |
|---|---|---|---|---|---|---|
| E. globoidea | A | Fitting | 0.453 | 0.338 | 0.009 | 0.435 |
| Validation | 0.348 | 0.273 | 0.011 | 0.350 | ||
| E. bosistoana | B | Fitting | 0.518 | 0.385 | 0.032 | 0.521 |
| Validation | 0.603 | 0.429 | 0.024 | 0.614 | ||
| C | Fitting | 0.342 | 0.274 | 0.001 | 0.347 | |
| Validation | 0.322 | 0.251 | 0.001 | 0.339 |
| Variables | p-Values at Different Sites | ||
|---|---|---|---|
| A | B | C | |
| Maximum daily temperature | NS | NS | NS |
| Prof. curvature | NS | NS | NS |
| Plan curvature | NS | 0.0001** | NS |
| TPI | NS | 2.72e−08*** | 0.0020** |
| WEI | 0.0033** | 0.0002 | 0.0010** |
| TWI | NS | NS | 0.0184* |
| MPI | 0.0008*** | <2e−16*** | 7.43e−05*** |
| Distance from the top ridge (DIST) | <2e−16*** | <2e−16*** | 2.22e−05*** |
| Species | Site | Action | RMSE | MAE | BIAS | SE |
|---|---|---|---|---|---|---|
| E. globoidea | A | Fitting | 0.108 | 0.076 | −0.001 | 0.109 |
| Validation | 0.097 | 0.068 | −2.086e−06 | 0.099 | ||
| E. bosistoana | C | Fitting | 0.019 | 0.013 | −7.951e−06 | 0.020 |
| Validation | 0.021 | 0.015 | 2.980e−05 | 0.022 |
| Variables | p-Values at Different Sites | |
|---|---|---|
| A | C | |
| Maximum daily temperature | NS | NS |
| Prof. curvature | 0.0004*** | 0.0272* |
| Plan curvature | 0.0387* | NS |
| TPI | NS | NS |
| WEI | 4.68e−09*** | NS |
| TWI | NS | NS |
| MPI | NS | NS |
| DIST | 6.81e−09*** | NS |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salekin, S.; Mason, E.G.; Morgenroth, J.; Bloomberg, M.; Meason, D.F. Modelling the Effect of Microsite Influences on the Growth and Survival of Juvenile Eucalyptus globoidea (Blakely) and Eucalyptus bosistoana (F. Muell) in New Zealand. Forests 2019, 10, 857. https://doi.org/10.3390/f10100857
Salekin S, Mason EG, Morgenroth J, Bloomberg M, Meason DF. Modelling the Effect of Microsite Influences on the Growth and Survival of Juvenile Eucalyptus globoidea (Blakely) and Eucalyptus bosistoana (F. Muell) in New Zealand. Forests. 2019; 10(10):857. https://doi.org/10.3390/f10100857
Chicago/Turabian StyleSalekin, Serajis, Euan G. Mason, Justin Morgenroth, Mark Bloomberg, and Dean F. Meason. 2019. "Modelling the Effect of Microsite Influences on the Growth and Survival of Juvenile Eucalyptus globoidea (Blakely) and Eucalyptus bosistoana (F. Muell) in New Zealand" Forests 10, no. 10: 857. https://doi.org/10.3390/f10100857
APA StyleSalekin, S., Mason, E. G., Morgenroth, J., Bloomberg, M., & Meason, D. F. (2019). Modelling the Effect of Microsite Influences on the Growth and Survival of Juvenile Eucalyptus globoidea (Blakely) and Eucalyptus bosistoana (F. Muell) in New Zealand. Forests, 10(10), 857. https://doi.org/10.3390/f10100857






