Photosynthetic Performance in Pinus canariensis at Semiarid Treeline: Phenotype Variability to Cope with Stressful Environment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Meteorological Conditions
2.2. Measurements of Gas Exchange at Field Site
2.3. Chlorophyll Fluorescence Measurements
2.4. Pigments and Tocopherol Contents
2.5. SLA and Needles Water Potential Determinations
2.6. Statistical Analysis
3. Results
3.1. Meteorological Conditions
3.2. Broad Analysis of Gas Exchange Parameters: Detecting Optimal Values
3.3. Seasonal Pattern in Gas Exchange Parameters
3.4. SLA and Needle Water Potential
3.5. Seasonal Pattern in Chlorophyll Fluorescence
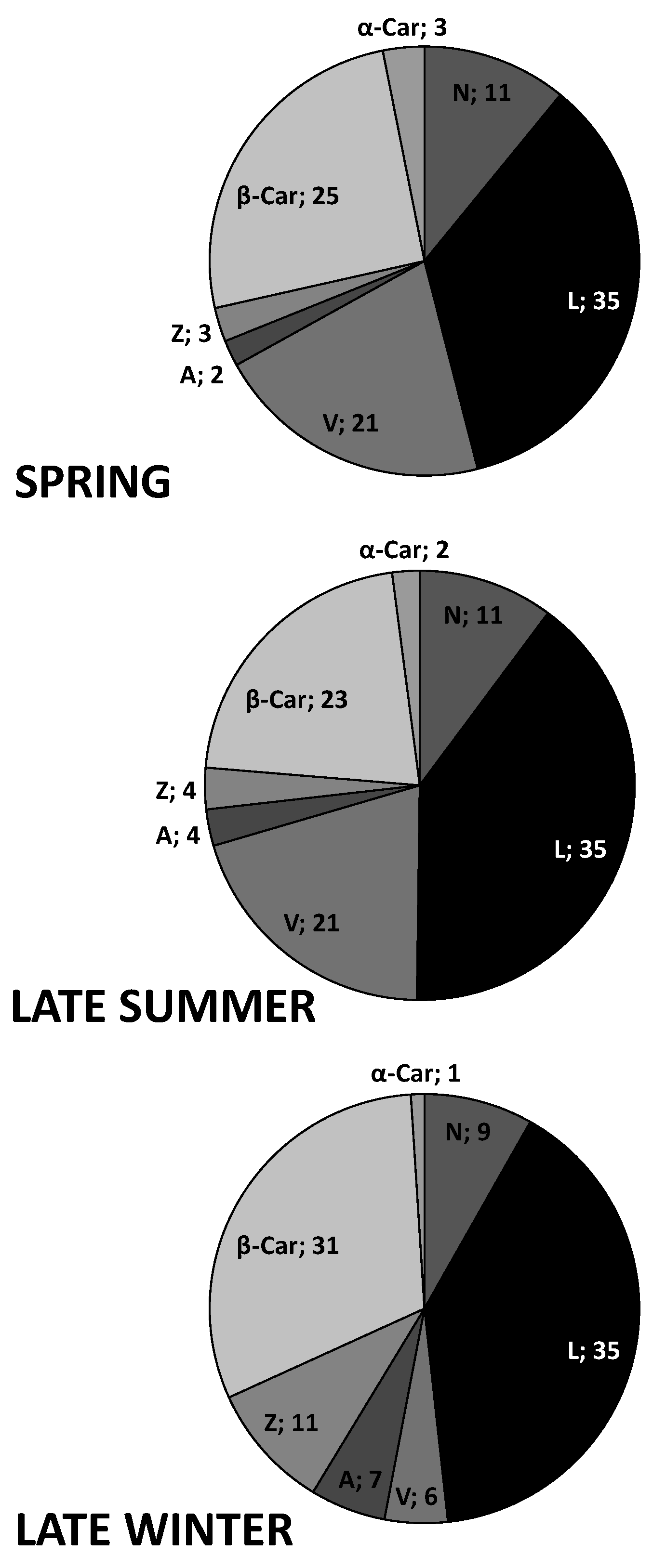
3.6. Seasonal Changes in Pigments and α-tocopherol
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Körner, C. Alpine Treelines. Functional Ecology of the Global High Elevation Tree Limits; Springer: Basel, Switzerland, 2012; p. 219. [Google Scholar]
- Silva-Cancino, M.C.; Esteban, R.; Artetxe, U.; García-Plazaola, J.I. Patterns of spatio-temporal distribution of winter chronic photoinhibition in leaves of three evergreen Mediterranean species with contrasting acclimation responses. Physiol. Plant. 2012, 144, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ferri, E.; Manrique, E.; Valladares, F.; Balaguer, L. Winter photoinhibition in the field involves different processes in four co-occurring Mediterranean tree species. Tree Physiol. 2004, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Diaz-Espejo, A.; Gagoa, J.; Galléa, A.; Galmés, J.; Gulías, J.; Medrano, H. Photosynthetic limitations in Mediterranean plants: A review. Env. Exp. Bot. 2014, 103, 12–23. [Google Scholar] [CrossRef]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Dall’Osto, L.; Cazzaniga, S.; North, H.; Marion-Poll, A.; Bassi, R. The Arabidopsis aba4-1 mutant reveals a specific function for neoxanthin in protection against photooxidative stress. Plant Cell 2007, 19, 1048–1064. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef] [PubMed]
- Havaux, M.; Eymery, F.; Porfirova, S.; Rey, P.; Dörmann, P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 2005, 17, 3451–3469. [Google Scholar] [CrossRef]
- Peng, C.L.; Lin, Z.-F.; Su, Y.-Z.; Lin, G.-Z.; Dou, H.-Y.; Zhao, C.-X. The antioxidative function of lutein: Electron spin resonance studies and chemical detection. Funct. Plant Biol. 2006, 33, 839–846. [Google Scholar] [CrossRef]
- Wieser, G.; Tausz, M. Trees at Their Upper Limit: Treelife Limitation at the Alpine Timberline; Springer: Dordrecht, The Netherlands, 2007; p. 225. [Google Scholar]
- Piersma, T.; Drent, J. Phenotypic flexibility and the evolution of organismal design. Trends Ecol. Evol. 2003, 18, 228–233. [Google Scholar] [CrossRef]
- Fernández-Palacios, F.M.; de Nicolás, J.P. Altitudinal pattern of vegetation variation on Tenerife. J. Veg. Sci. 1995, 6, 183–190. [Google Scholar] [CrossRef]
- Sperling, F.N.; Washington, R.; Whittaker, R.J. Future Climate Change of the Subtropical North Atlantic: Implications for the Cloud Forests of Tenerife. Clim. Chang. 2004, 65, 103–123. [Google Scholar] [CrossRef]
- IPCC. Intergovernmental Panel of Climate Change (IPCC) Synthesis Report, Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change 2014; IPCC: Geneva, Switzerland, 2014. [Google Scholar] [CrossRef]
- Expósito, F.J.; González, A.; Pérez, J.C.; Díaz, J.P.; Taima, D. High-resolution future projections of temperature and precipitation in the Canary Islands. J. Clim. 2015, 28, 7846–7856. [Google Scholar] [CrossRef]
- Severin, D.H.I.; Anthelme, F.; Harter, D.E.V.; Jentsch, A.; Lotter, E.; Steinbauer, M.J.; Beierkuhnlein, C. Patterns of island treeline elevation—A global perspective. Ecography 2015, 38, 001–010. [Google Scholar]
- Brito, P.; Morales, D.; Wieser, G.; Jiménez, M.S. Spatial and seasonal variations in stem CO2 efflux of Pinus canariensis at their upper distribution limit. Trees 2010, 24, 523–531. [Google Scholar] [CrossRef]
- Brito, P.; Jiménez, M.S.; Morales, D.; Wieser, G. Assessment of ecosystem CO2 efflux and its components in a Pinus canariensis forest at the treeline. Trees 2013, 27, 999–1009. [Google Scholar] [CrossRef]
- Brito, P.; Lorenzo, J.R.; González-Rodríguez, A.M.; Morales, D.; Wieser, G.; Jiménez, M.S. Canopy transpiration of a Pinus canariensis forest at the tree line: Implications for its distribution under predicted climate warming. Eur. J. Res. 2014, 133, 491–500. [Google Scholar] [CrossRef]
- Brito, P.; Lorenzo, J.R.; González-Rodríguez, A.M.; Morales, D.; Wieser, G.; Jiménez, M.S. Canopy transpiration of a semi arid Pinus canariensis forest at a treeline ecotone in two hydrologically contrasting years. Agric. For. Meteorol. 2015, 201, 120–127. [Google Scholar] [CrossRef]
- Gieger, T.; Leuschner, C. Altitudinal change in needle water relations of Pinus canariensis and possible evidence of a drought-induced alpine timberline on Mt. Teide, Tenerife. Flora 2004, 199, 100–109. [Google Scholar] [CrossRef]
- Paulsen, J.; Körner, C. A climate-based model to predict potential treeline position around the globe. Alp. Bot. 2014, 124, 1–12. [Google Scholar] [CrossRef]
- Peters, J.; Morales, D.; Jiménez, M.S. Gas exchange characteristics of Pinus canariensis needles in a forest stand on Tenerife, Canary Islands. Trees 2003, 17, 492–500. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Farquhar, G.D. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 1981, 153, 376–397. [Google Scholar] [CrossRef] [PubMed]
- Gratani, L. Plant Phenotypic Plasticity in Response to Environmental Factors. Adv. Bot. 2014, 2018, 17. [Google Scholar] [CrossRef]
- Prioul, J.L.; Chartier, P. Partitioning of transfer and carboxylation components of intracellular resistance to photosynthetic CO2 fixation: A critical analysis of the methods used. Ann. Bot. 1977, 41, 789–800. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron-transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Werner, C.; Correia, O.; Beyschlag, W. Characteristic patterns of chronic and dynamic photoinhibition of different functional groups in a Mediterranean ecosystem. Funct. Plant Biol. 2002, 29, 999–1011. [Google Scholar] [CrossRef]
- Osmond, C.B. What is photoinhibition? Some insights from comparison of shade and sun plants. In Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field; Baker, N.R., Bowyer, J.R., Eds.; BIOS Scientific Publishers: Lancaster, PA, USA, 1994; pp. 1–24. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, R., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Pflug, E.; Brüggemann, W. Frost-acclimation of photosynthesis in overwintering Mediterranean holm oak, grown in Central Europe. Int. J. Plant Biol. 2012, 3. [Google Scholar] [CrossRef]
- Tausz, M.; Wonisch, A.; Grill, D.; Morales, D.; Jiménez, M.S. Measuring antioxidants in tree species in the natural environment: From sampling to data evaluation. J. Exp. Bot. 2003, 387, 1505–1510. [Google Scholar] [CrossRef]
- Morales, D.; Peters, J.; Jiménez, M.S.; Tausz, M.; Wonisch, A.; Grill, D. Gas exchange of irrigated and non-irrigated Pinus canariensis seedlings growing outdoors in La Laguna, Canary Islands, Spain. Naturforsch 1999, 54, 693–697. [Google Scholar] [CrossRef][Green Version]
- González-Rodríguez, A.M.; Morales, D.; Jimenez, M.S. Leaf gas exchange characteristics of a Canarian laurel forest tree species (Persea indica (L.) Spreng) under natural conditions. J. Plant Phys. 2002, 159, 695–704. [Google Scholar]
- Peters, J.; González-Rodríguez, A.M.; Jiménez, M.S.; Morales, D.; Wieser, G. Influence of canopy position, needle age and season on the foliar gas exchange of Pinus canariensis. Eur. J. Res. 2008, 127, 293–299. [Google Scholar] [CrossRef]
- Rundel, P.W.; Yoder, B.J. Ecophysiology of Pinus. In Ecology and Biogeography of Pinus; Richardson, D.M., Ed.; Cambridge University Press: Cambridge, UK, 2000; pp. 296–323. [Google Scholar]
- Larcher, W. Physiological Plant Ecology. Ecophysiology and Stress Physiology of Functional Groups, 4th ed.; Springer: Basel, Switzerland, 2003; p. 488. [Google Scholar]
- Peters, J. Ecofisiología del pino canario. Ph.D. Dissertation, University of La Laguna, Santa Cruz de Tenerife, Spain, 2000. [Google Scholar]
- Gulías, J.; Flexas, J.; Mus, M.; Cifre, J.; Lefi, E.; Medrano, H. Relationship between maximum leaf photosynthesis, nitrogen content and specific leaf area in Balearic endemic and non endemic Mediterranean species. Ann. Bot. 2003, 92, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Zellnig, G.; Peters, J.; Jiménez, M.S.; Morales, D.; Grill, D.; Perktold, A. Three-Dimensional reconstruction of the stomatal complex in Pinus canariensis needles using serial sections. Plant Biol. 2002, 4, 70–76. [Google Scholar] [CrossRef]
- Galmés, J.; Flexas, J.; Medrano, H.; Niinemets, Ü.; Valladares, F. Ecophysiology of photosynthesis in semi-arid cenvironments. In Terrestrial Photosynthesis in a Changing Environment. A Molecular, Physiological and Ecological Approach; Flexas, J., Loreto, F., Medrano, H., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 448–464. [Google Scholar]
- Niinemets, Ü. Climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs at the global scale. Ecology 2001, 82, 453–469. [Google Scholar] [CrossRef]
- Paula, S.; Pausas, J.G. Leaf traits and resprouting ability in the Mediterranean basin. Funct. Ecol. 2006, 20, 941–947. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Zunzunegui, M.; Barradas, M.C.D.; Ain-Lhout, F.; Alvarez-Cansino, L.; Esquivias, M.P.; Novo, F.G. Seasonal physiological plasticity and recovery capacity after summer stress in Mediterranean scrub communities. Plant Ecol. 2011, 212, 127–142. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 403, 1607–1621. [Google Scholar] [CrossRef]
- Nippert, J.B.; Duursma, R.A.; Marshall, J.D. Seasonal variation in photosynthetic capacity of montane conifers. Funct. Ecol. 2004, 18, 876–886. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Juurola, E.; Ensminger, I.; Berninger, F.; Hari, P.; Nikinmaa, E. Seasonal acclimation of photosystem II in Pinus sylvestris. II. Using the rate constants of sustained thermal energy dissipation and photochemistry to study the effect of the light environment. Tree Physiol. 2008, 28, 1483–1491. [Google Scholar] [CrossRef] [PubMed]
- Robakowski, P.; Wyka, T. Winter photoinhibition in needles of Taxus baccata seedlings acclimated to different light levels. Photosynthetica 2009, 47, 527–535. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Olano, J.M.; Hernández, A.; Becerril, J.M. Photoprotection in evergreen Mediterranean plants during sudden periods of intense cold weather. Trees-Struct. Funct. 2003, 17, 285–291. [Google Scholar]
- Müller, M.; Hernández, I.; Alegre, L.; Munné-Bosch, S. Enhanced alpha-tocopherol quinone levels and xanthophyll cycle de-epoxidation in rosemary plants exposed to water deficit during a Mediterranean winter. J. Plant Physiol. 2006, 163, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Somersalo, S.; Krause, H.G. Photoinhibition at chilling temperatures and effects of freezing stress on cold acclimated spinach leaves in the field. A fluorescence study. Physiol. Plant. 1990, 79, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Robakowski, P. Susceptibility to low-temperature photoinhibition in three conifers differing in successional status. Tree Physiol. 2005, 25, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Ottander, C.; Öquist, G. Recovery of photosynthesis in winter-stressed Scots pine. Plant Cell Env. 1991, 14, 345–349. [Google Scholar] [CrossRef]
- Westin, J.; Sundblad, L.G.; Hällgren, J.E. Seasonal variation in photochemical activity and hardiness in clones of Norway spruce (Picea abies). Tree Physiol. 1995, 15, 685–689. [Google Scholar] [CrossRef][Green Version]
- Oleksyn, J.; Reich, P.B.; Tjoelker, M.G.; Chalupka, W. Biogeographic differences in shoot elongation pattern among European Scots pine populations. Ecol. Man. 2001, 148, 207–220. [Google Scholar] [CrossRef]
- Sheffield, M.C.P.; Gagnon, J.L.; Jack, S.B.; McConville, D.J. Phenological patterns of mature longleaf pine (Pinus palustris Miller) under two different soil moisture regimes. For. Ecol. Manag. 2003, 179, 157–167. [Google Scholar] [CrossRef]
- Gasulla, F.; Gómez de Nova, O.; Barreno, E. Relaciones entre la variabilidad fenológica de Pinus canariensis, el clima y la concentración foliar de los nutrientes. Cuad. Soc. Esp. Cienc. 2004, 20, 141–146. [Google Scholar]
- Esteban, R.; Barrutia, O.; Artetxe, U.; Fernández-Marín, B.; Hernández, A.; García-Plazaola, J.I. Internal and external factors affecting photosynthetic pigment composition in plants: A meta-analytical approach. New Phytol. 2015, 206, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Elvira, S.; Alonso, R.; Castillo, F.J.; Gimeno, B.S. On the response of pigments and antioxidants of Pinus halepensis seedlings to Mediterranean climatic factors and long-term ozone exposure. New Phytol. 1998, 138, 419–432. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Babani, F. Light adaptation and senescence of the photosynthetic apparatus. Changes in pigment composition, chlorophyll fluorescence parameters and photosynthetic activity. Adv. Photosynth. Respir. 2004, 19, 713–736. [Google Scholar]
- Telfer, A. Singlet Oxygen Production by PSII Under light stress: Mechanism, detection and the protective role of β-Carotene. Plant Cell Physiol. 2014, 55, 1216–1223. [Google Scholar] [CrossRef]
- Triantaphylidès, C.; Krischke, M.; Hoeberichts, F.A.; Ksas, B.; Gresser, G.; Havaux, M.; Van Breusegem, F.; Martin, J.M. Singlet oxygen is the major reactive oxygen species involved in photooxidative damage to plants. Plant Physiol. 2008, 148, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S. The role of a-tocopherol in plant stress tolerance. J. Plant Phys. 2005, 162, 743–748. [Google Scholar] [CrossRef]
- Falk, J.; Munné-Bosch, S. Tocochromanol functions in plants: Antioxidation and beyond. J. Exp. Bot. 2010, 61, 1549–1566. [Google Scholar] [CrossRef]
- Maeda, H.; Song, W.; Sage, S.L.; Della Pennaa, D. Tocopherols play a crucial role in low-temperature adaptation and phloem loading in Arabidopsis. Plant Cell 2006, 18, 2710–2732. [Google Scholar] [CrossRef]
- Flexas, J.; Gulías, J.; Jonasson, S.; Medrano, H.; Mus, M. Seasonal patterns and control of gas exchange in local populations of the Mediterranean evergreen shrub Pistacia lentiscus L. Acta Oecol. 2001, 22, 33–43. [Google Scholar] [CrossRef]
- Martín, J.L.; Bethencourt, J.; Cuevas-Agulló, E. Assessment of global warming on the island of Tenerife, Canary Islands (Spain). Trends in minimum, maximum and mean temperatures since 1944. Clim. Chang. 2012, 114, 343–355. [Google Scholar] [CrossRef]

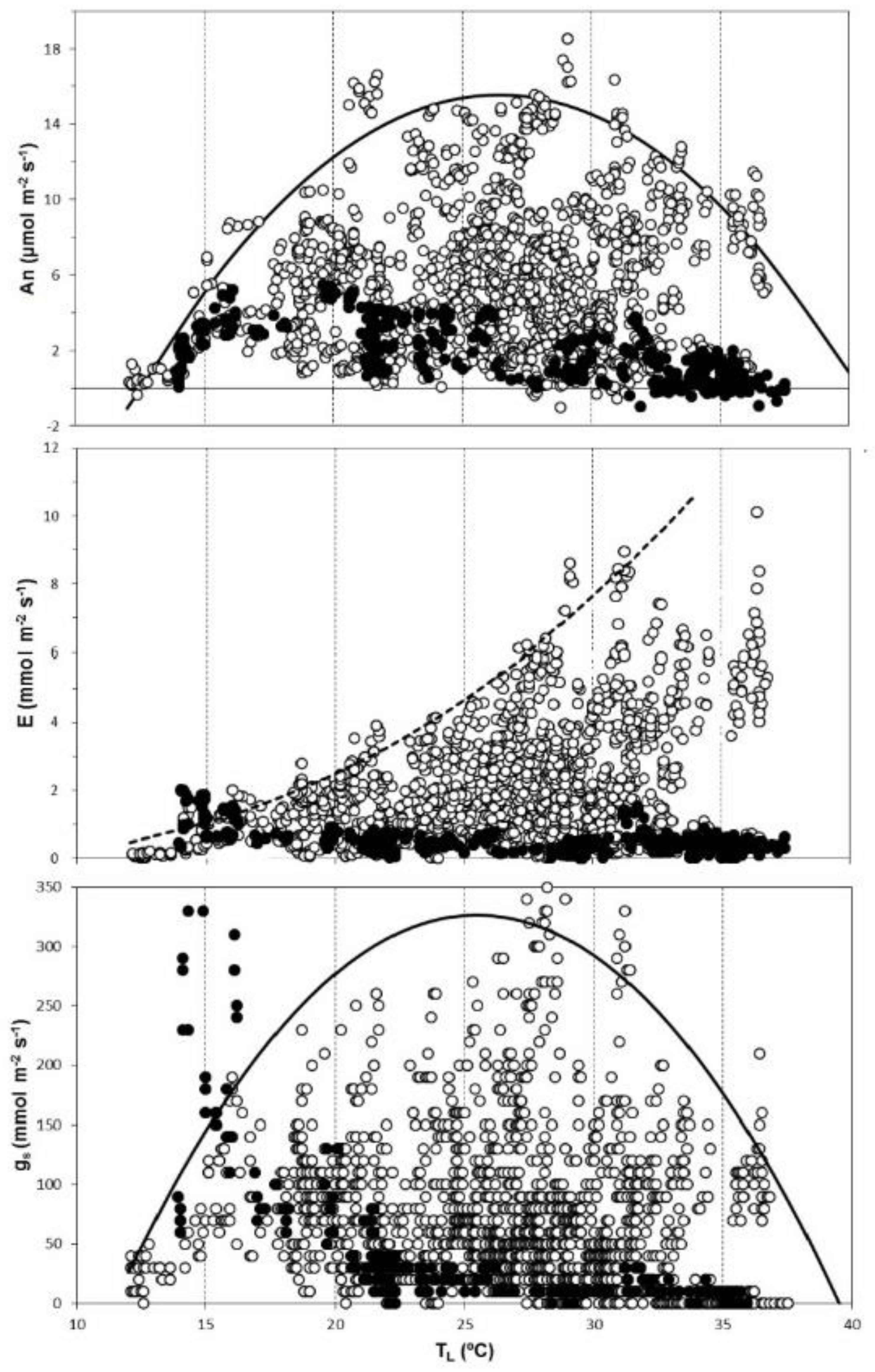
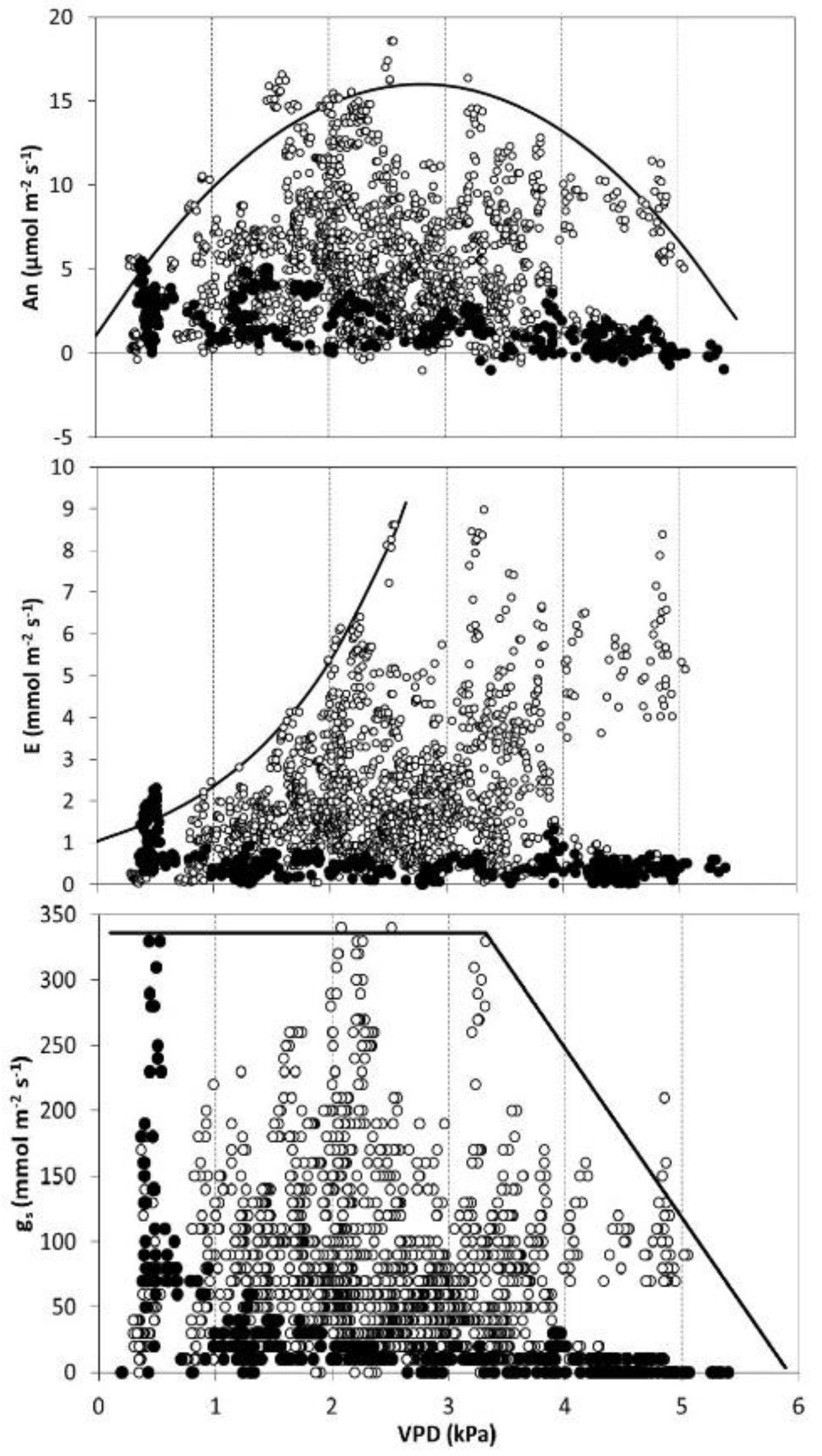

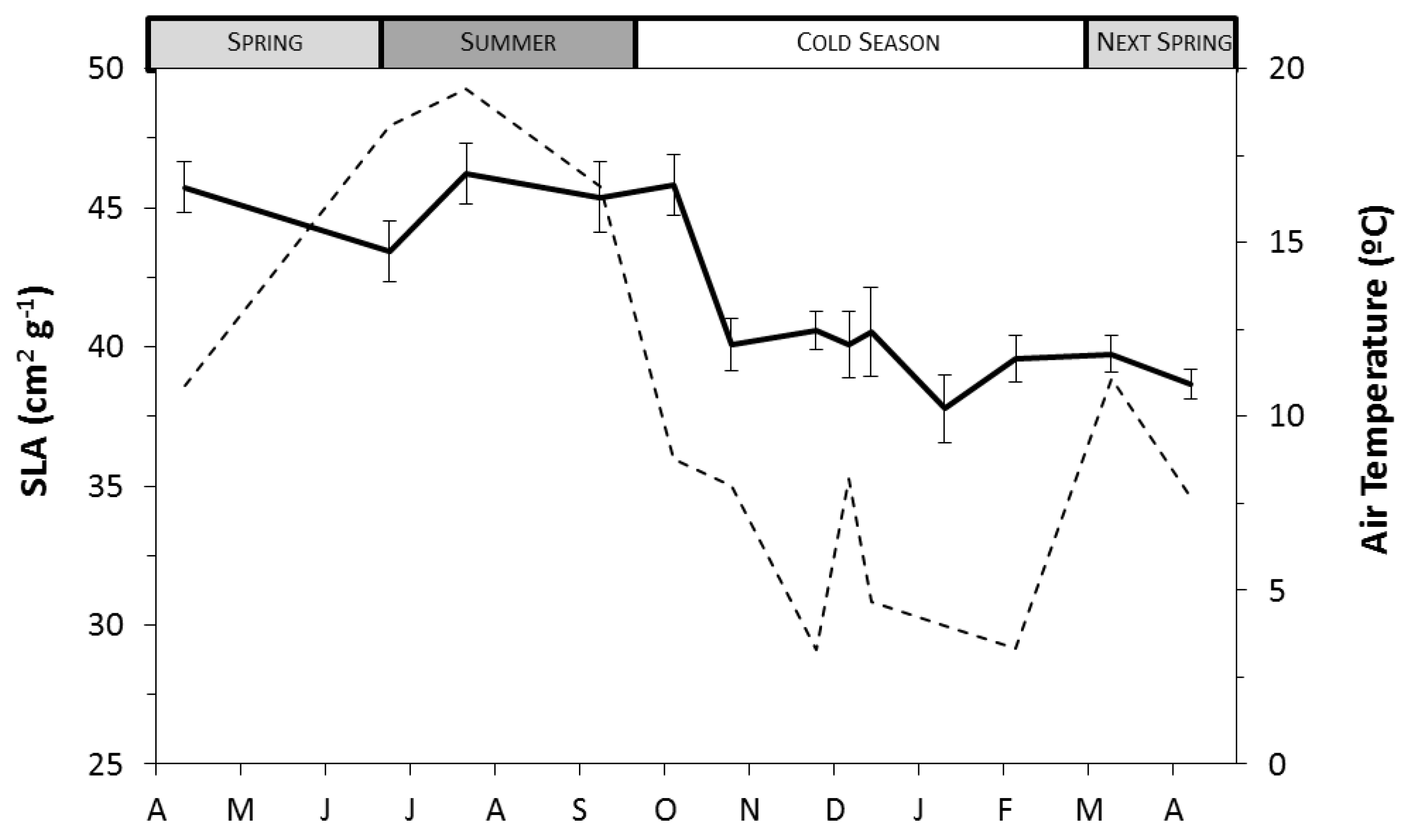
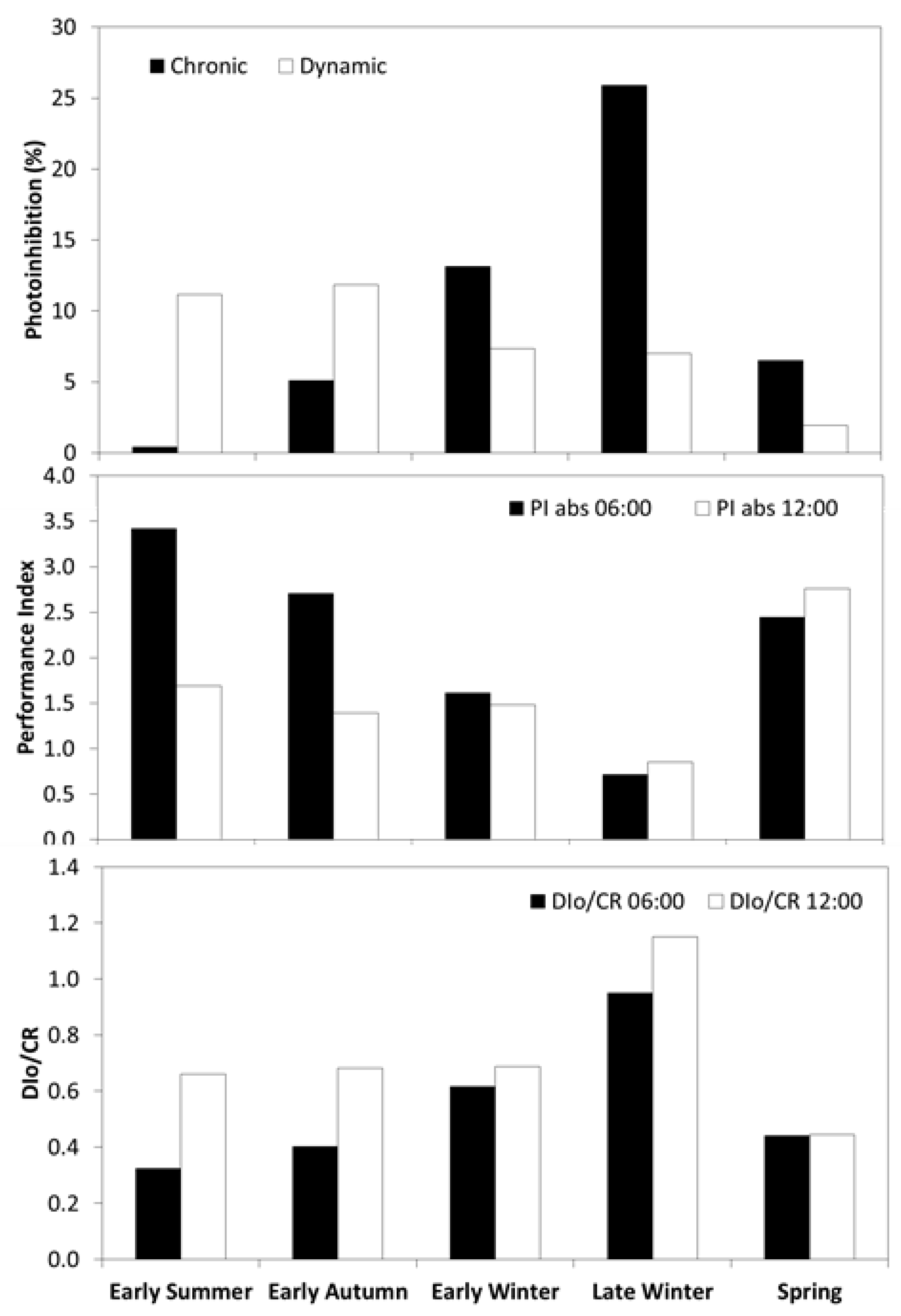
| Season | VPD (kPa) | GR (W m−2) | Tair (°C) | SWP (MPa) |
|---|---|---|---|---|
| Spring | 0.85 ± 0.54 | 645 ± 90 | 12.33 ± 3.45 | −0.0662 ± 0.0742 |
| Summer | 2.16 ± 1.27 | 665 ± 111 | 21.63 ± 5.91 | −1.1002 ± 0.0000 |
| Cold season (Autumn-Winter) | 0.82 ± 0.33 | 484 ± 77 | 10.56 ± 3.82 | −0.0293 ± 0.0295 |
| Next Spring | 1.10 ± 0.34 | 713 ± 25 | 12.96 ± 2.29 | −0.0202 ± 0.0012 |
| Pigments and Tocopherol | Spring | Summer | Autumn | Winter | Next Spring | Annual Mean |
|---|---|---|---|---|---|---|
| Chl a | 416 ± 94 a | 381 ± 122 a | 361 ± 110 a | 361 ± 95 a | 363 ± 107 a | 368 ± 107 |
| Chl b | 161 ± 40 a | 144 ± 48 ab | 125 ± 40 bc | 118 ± 34 c | 130 ± 37 bc | 130 ± 42 |
| Chl (a + b) | 578 ± 133 a | 525 ± 170 ab | 491 ± 144 ab | 474 ± 132 b | 493 ± 130 ab | 498 ± 148 |
| Chl a/b | 2.60 ± 0.14 a | 2.66 ± 0.21 a | 2.89 ± 0.18 b | 3.07 ± 0.21 c | 2.82 ± 0.22 b | 2.87 ± 0.26 |
| Tot Carot/Chl | 287 ± 32 a | 337 ± 40 b | 374 ± 49 c | 397 ± 54 c | 388 ± 55 c | 370 ± 56 |
| L/Chl | 112 ± 13 a | 127 ± 20 b | 150 ± 16 c | 157 ± 18 c | 154 ± 19 c | 145 ± 22 |
| β-Car/Chl | 69 ± 8 a | 78 ± 11 b | 97 ± 21 c | 115 ± 25 d | 111 ± 22 d | 98 ± 25 |
| VAZ/Chl | 63 ± 16 a | 89 ± 24 b | 87 ± 22 b | 88 ± 23 b | 85 ± 19 b | 87 ± 23 |
| N/Chl | 36.42 ± 1.47 a | 34.65 ± 2.64 b | 34.58 ± 3.08 b | 33.98 ± 3.10 b | 35.25 ± 2.50 ab | 35.52 ± 2.92 |
| A+Z/VAZ | 0.51 ± 0.14 a | 0.21 ± 0.14 b | 0.51 ± 0.24 a | 0.54 ± 0.19 a | 0.49 ± 0.17 a | 0.44 ± 0.23 |
| α-Toc/Chl | 98 ± 82 a | 197 ± 104 b | 341 ± 153 d | 295 ± 117 cd | 259 ± 117 c | 274 ± 141 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Rodríguez, Á.M.; Brito, P.; Lorenzo, J.R.; Jiménez, M.S. Photosynthetic Performance in Pinus canariensis at Semiarid Treeline: Phenotype Variability to Cope with Stressful Environment. Forests 2019, 10, 845. https://doi.org/10.3390/f10100845
González-Rodríguez ÁM, Brito P, Lorenzo JR, Jiménez MS. Photosynthetic Performance in Pinus canariensis at Semiarid Treeline: Phenotype Variability to Cope with Stressful Environment. Forests. 2019; 10(10):845. https://doi.org/10.3390/f10100845
Chicago/Turabian StyleGonzález-Rodríguez, Águeda María, Patricia Brito, Jose Roberto Lorenzo, and María Soledad Jiménez. 2019. "Photosynthetic Performance in Pinus canariensis at Semiarid Treeline: Phenotype Variability to Cope with Stressful Environment" Forests 10, no. 10: 845. https://doi.org/10.3390/f10100845
APA StyleGonzález-Rodríguez, Á. M., Brito, P., Lorenzo, J. R., & Jiménez, M. S. (2019). Photosynthetic Performance in Pinus canariensis at Semiarid Treeline: Phenotype Variability to Cope with Stressful Environment. Forests, 10(10), 845. https://doi.org/10.3390/f10100845





