Contrasting Rhizospheric and Heterotrophic Components of Soil Respiration during Growing and Non-Growing Seasons in a Temperate Deciduous Forest
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Field Measurements
2.2. Data Analysis
3. Results
3.1. Seasonal Variations in Soil Respiration and Related Environmental Factors
3.2. Responses of Soil Respiration to Soil Temperature and Water Content
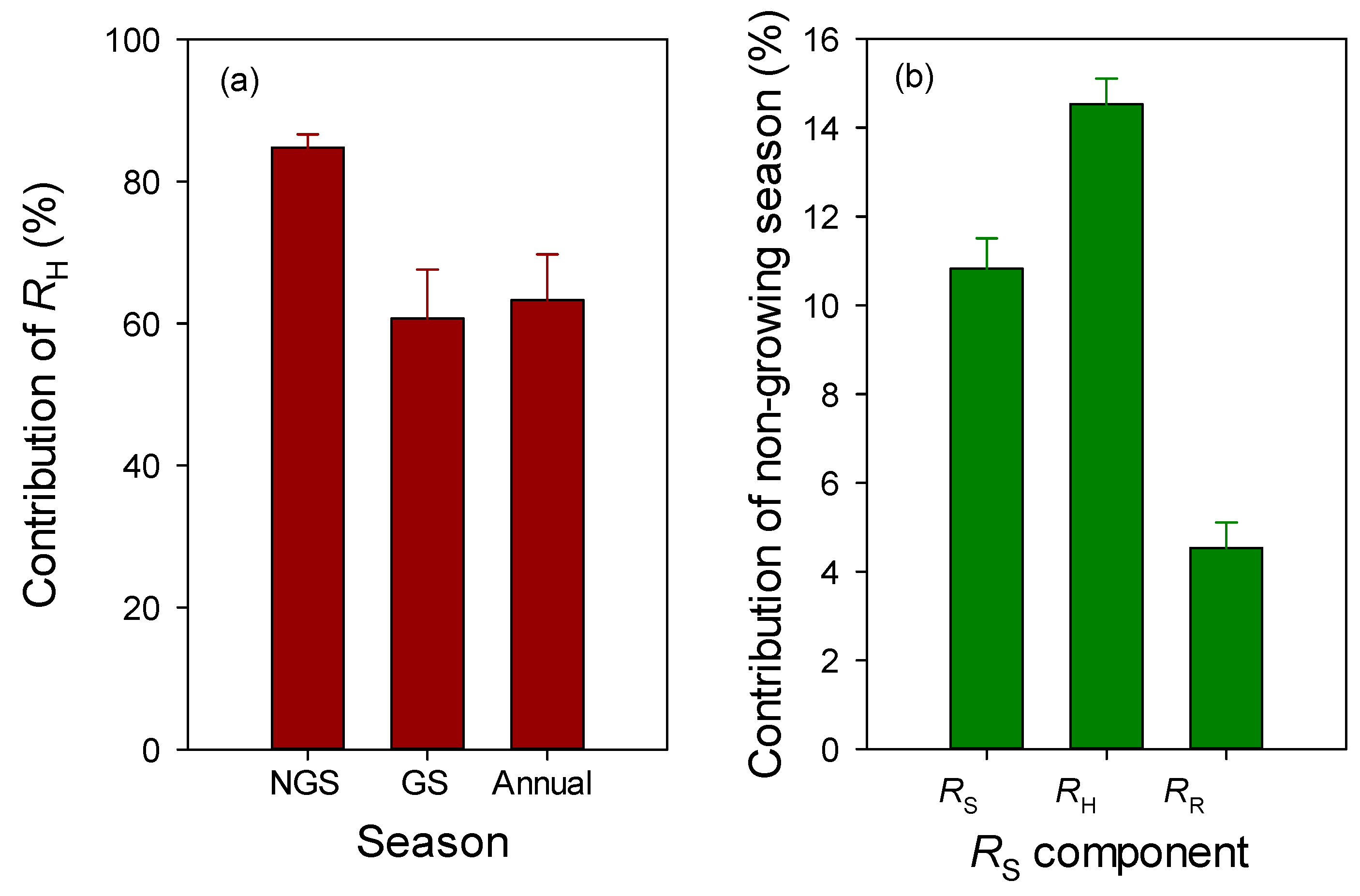
3.3. Contribution of Rhizospheric and Heterotrophic Respiration
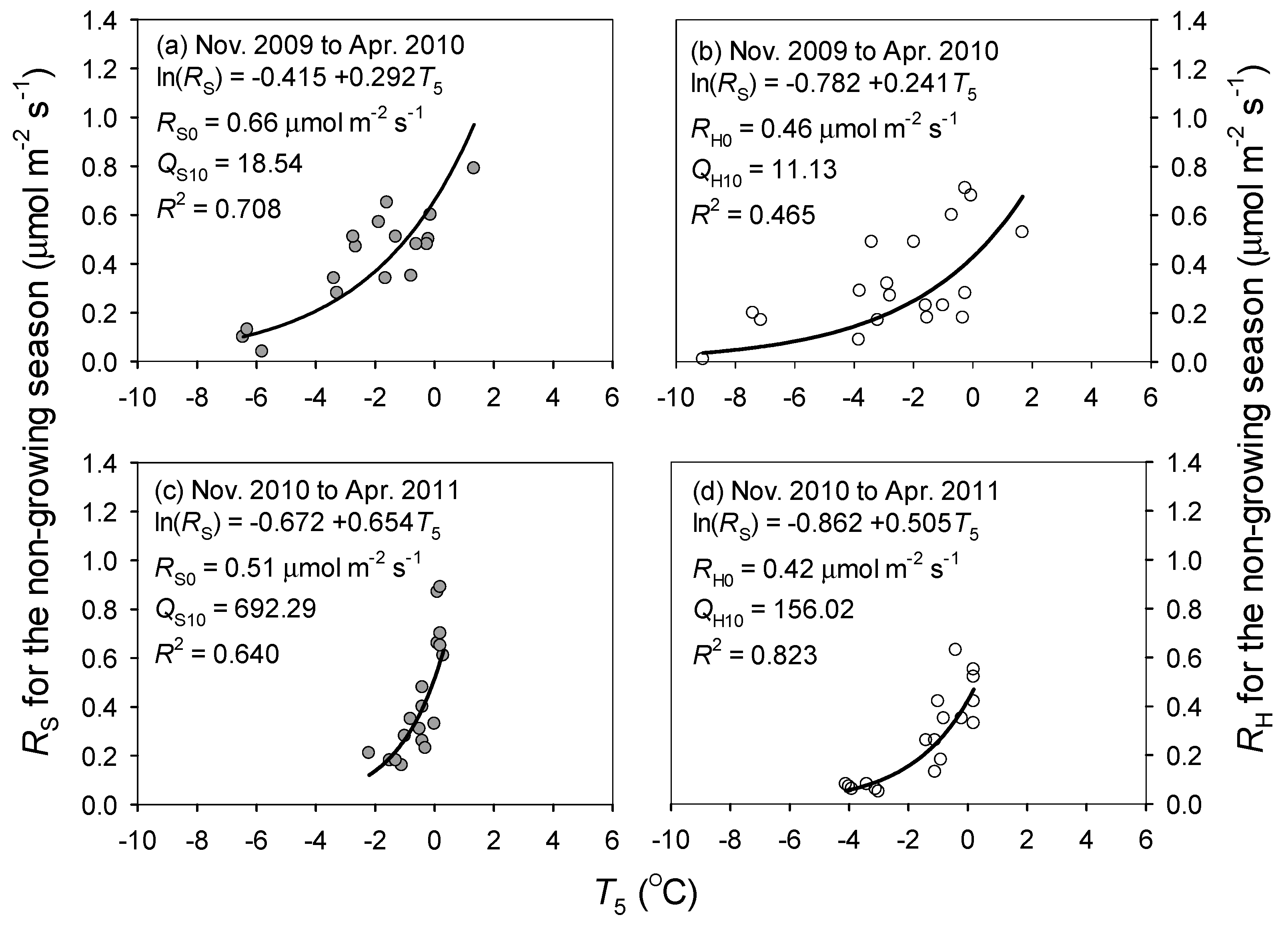
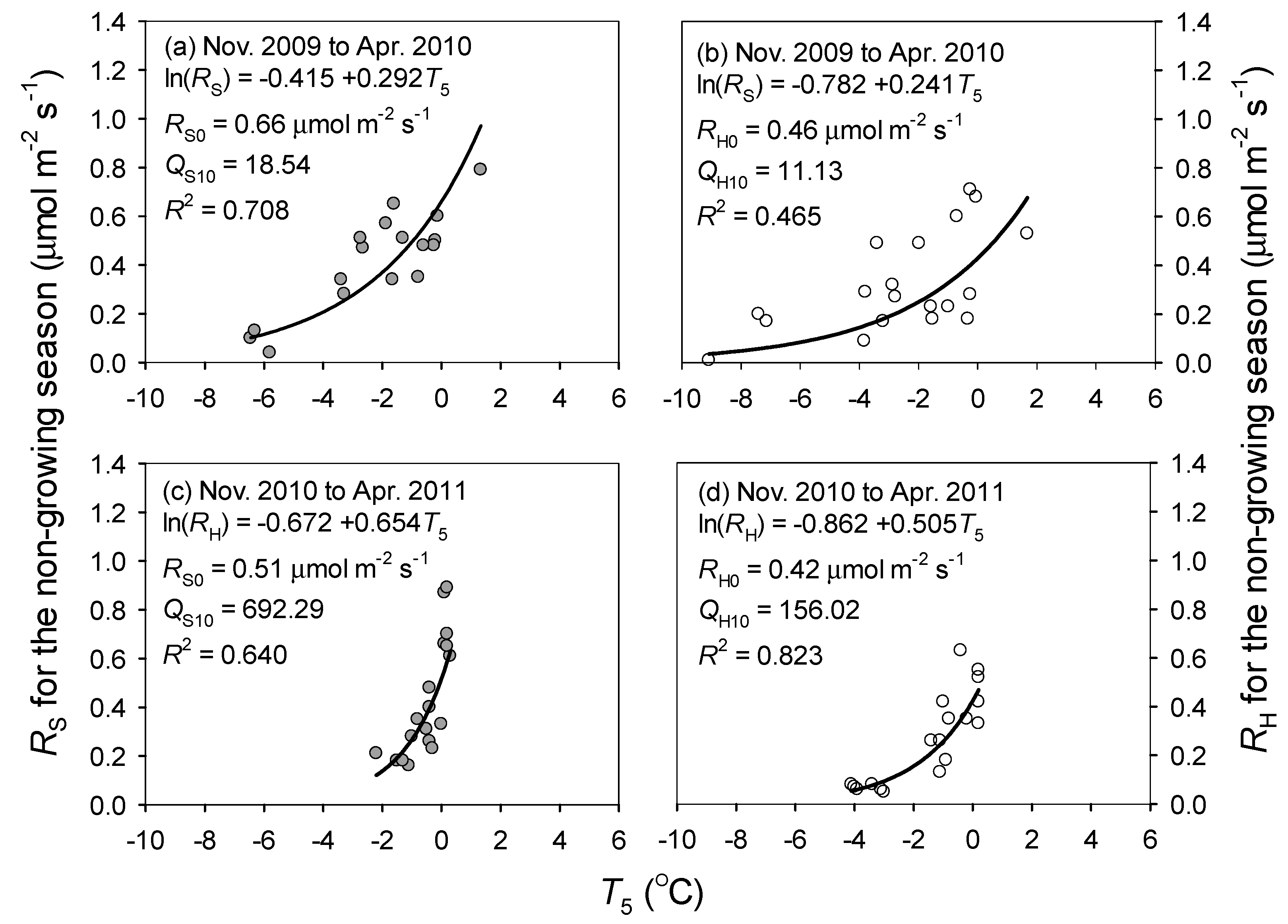
3.4. Contribution of Non-Growing Season Soil Respiration
3.5. Effects of Plot Trenching on Soil Respiration and Related Environmental Factors
4. Discussion
4.1. Response of Soil Respiration to Temperature
4.2. Non-Growing Season and Annual Soil Respiration
4.3. Contribution of Rhizospheric and Heterotrophic Respiration
4.4. Effects of Plot Trenching on Soil Respiration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bond-Lamberty, B.; Thomson, A. Temperature-associated increases in the global soil respiration record. Nature 2010, 464, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Carvalhais, N.; Ito, A.; Migliavacca, M.; Nishina, K.; Reichstein, M. Global spatiotemporal distribution of soil respiration modeled using a global database. Biogeosciences 2015, 12, 4121–4132. [Google Scholar] [CrossRef]
- Hursh, A.; Ballantyne, A.; Cooper, L.; Maneta, M.; Kimball, J.; Watts, J. The sensitivity of soil respiration to soil temperature, moisture, and carbon supply at the global scale. Glob. Chang. Biol. 2017, 23, 2090–2103. [Google Scholar] [CrossRef]
- Beer, C.; Reichstein, M.; Tomelleri, E.; Ciais, P.; Jung, M.; Carvalhais, N.; Rödenbeck, C.; Arain, M.A.; Baldocchi, D.; Bonan, G.B.; et al. Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science 2010, 329, 834–838. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Thomson, A. A global database of soil respiration data. Biogeosciences 2010, 7, 1915–1926. [Google Scholar] [CrossRef]
- Chen, S.; Huang, Y.; Xie, W.; Zou, J.; Lu, Y.; Hu, Z. A new estimate of global soil respiration from 1970 to 2008. Chin. Sci. Bull. 2013, 58, 4153–4160. [Google Scholar] [CrossRef]
- Zhao, Z.; Peng, C.; Yang, Q.; Meng, F.-R.; Song, X.; Chen, S.; Epule, T.E.; Li, P.; Zhu, Q. Model prediction of biome-specific global soil respiration from 1960 to 2012. Earth’s Future 2017, 5, 715–729. [Google Scholar] [CrossRef]
- Scott-Denton, L.E.; Rosenstiel, T.N.; Monson, R.K. Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Glob. Chang. Biol. 2006, 12, 205–216. [Google Scholar] [CrossRef]
- Hanson, P.; Edwards, N.; Garten, C.; Andrews, J. Separating root and soil microbial contributions to soil respiration: A review of methods and observations. Biogeochemistry 2000, 48, 115–146. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. A global relationship between the heterotrophic and autotrophic components of soil respiration? Glob. Chang. Biol. 2004, 10, 1756–1766. [Google Scholar] [CrossRef]
- Högberg, P.; Nordgren, A.; Buchmann, N.; Taylor, A.F.; Ekblad, A.; Högberg, M.N.; Nyberg, G.; Ottosson-Löfvenius, M.; Read, D.J. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 2001, 411, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Savage, K.; Davidson, E.; Tang, J. Diel patterns of autotrophic and heterotrophic respiration among phenological stages. Glob. Chang. Biol. 2013, 19, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Luan, J.; Liu, S.; Wang, J.; Zhu, X.; Shi, Z. Rhizospheric and heterotrophic respiration of a warm-temperate oak chronosequence in China. Soil Biol. Biochem. 2011, 43, 503–512. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Wang, S. Forest soil respiration and its heterotrophic and autotrophic components: Global patterns and responses to temperature and precipitation. Soil Biol. Biochem. 2010, 42, 1236–1244. [Google Scholar]
- Bond-Lamberty, B.; Bailey, V.L.; Chen, M.; Gough, C.M.; Vargas, R. Globally rising soil heterotrophic respiration over recent decades. Nature 2018, 560, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Trumbore, S. Carbon respired by terrestrial ecosystems-recent progress and challenges. Glob. Chang. Biol. 2006, 12, 141–153. [Google Scholar] [CrossRef]
- Ryan, M.G.; Law, B.E. Interpreting, measuring, and modeling soil respiration. Biogeochemistry 2005, 73, 3–27. [Google Scholar] [CrossRef]
- Wang, W.; Peng, S.; Wang, T.; Fang, J. Winter soil CO2 efflux and its contribution to annual soil respiration in different ecosystems of a forest-steppe ecotone, north China. Soil Biol. Biochem. 2010, 42, 451–458. [Google Scholar] [CrossRef]
- Campbell, J.L.; Mitchell, M.J.; Groffman, P.M.; Christenson, L.M.; Hardy, J.P. Winter in northeastern North America: A critical period for ecological processes. Front. Ecol. Environ. 2005, 3, 314–322. [Google Scholar] [CrossRef]
- Sullivan, P.F.; Welker, J.M.; Arens, S.J.T.; Sveinbjörnsson, B. Continuous estimates of CO2 efflux from arctic and boreal soils during the snow-covered season in Alaska. J. Geophys. Res. 2008, 113, G04009. [Google Scholar] [CrossRef]
- Nobrega, S.; Grogan, P. Deeper snow enhances winter respiration from both plant-associated and bulk soil carbon pools in birch hummock tundra. Ecosystems 2007, 10, 419–431. [Google Scholar] [CrossRef]
- Brooks, P.D.; McKnight, D.; Elder, K. Carbon limitation of soil respiration under winter snowpacks: Potential feedbacks between growing season and winter carbon fluxes. Glob. Chang. Biol. 2004, 11, 231–238. [Google Scholar] [CrossRef]
- Monson, R.K.; Lipson, D.L.; Burns, S.P.; Turnipseed, A.A.; Delany, A.C.; Williams, M.W.; Schmidt, S.K. Winter forest soil respiration controlled by climate and microbial community composition. Nature 2006, 439, 711–714. [Google Scholar] [CrossRef]
- Wang, C.; Han, Y.; Chen, J.; Wang, X.; Zhang, Q.; Bond-Lamberty, B. Seasonality of soil CO2 efflux in a temperate forest: Biophysical effects of snowpack and spring freeze–thaw cycles. Agric. For. Meteorol. 2013, 177, 83–92. [Google Scholar] [CrossRef]
- Gaumont-Guay, D.; Black, T.A.; Barr, A.G.; Jassal, R.S.; Nesic, Z. Biophysical controls on rhizospheric and heterotrophic components of soil respiration in a boreal black spruce stand. Tree Physiol. 2008, 28, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Nakane, K.; Nakatsubo, T.; Koizumi, H. Seasonal changes in the contribution of root respiration to total soil respiration in a cool-temperate deciduous forest. Plant Soil 2003, 255, 311–318. [Google Scholar] [CrossRef]
- Ruehr, N.K.; Buchmann, N. Soil respiration fluxes in a temperate mixed forest: Seasonality and temperature sensitivities differ among microbial and root–rhizosphere respiration. Tree Physiol. 2010, 30, 165–176. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J. Rhizospheric and heterotrophic components of soil respiration in six Chinese temperate forests. Glob. Chang. Biol. 2007, 13, 123–131. [Google Scholar] [CrossRef]
- Shi, W.Y.; Zhang, J.G.; Yan, M.J.; Yamanaka, N.; Du, S. Seasonal and diurnal dynamics of soil respiration fluxes in two typical forests on the semiarid Loess Plateau of China: Temperature sensitivities of autotrophs and heterotrophs and analyses of integrated driving factors. Soil Biol. Biochem. 2012, 52, 99–107. [Google Scholar] [CrossRef]
- Brown, R.; Markewitz, D. Soil heterotrophic respiration: Measuring and modeling seasonal variation and silvicultural impacts. For. Ecol. Manag. 2018, 430, 594–608. [Google Scholar] [CrossRef]
- Carbone, M.S.; Richardson, A.D.; Chen, M.; Davidson, E.A.; Hughes, H.; Savage, K.E.; Hollinger, D.Y. Constrained partitioning of autotrophic and heterotrophic respiration reduces model uncertainties of forest ecosystem carbon fluxes but not stocks. J. Geophys. Res. Biogeosci. 2016, 121, 2476–2492. [Google Scholar] [CrossRef]
- Tucker, C.; Young, J.; Williams, D.; Ogle, K. Process-based isotope partitioning of winter soil respiration in a subalpine ecosystem reveals importance of rhizospheric respiration. Biogeochemistry 2014, 121, 389–408. [Google Scholar] [CrossRef]
- Schindlbacher, A.; Zechmeister-Boltenstern, S.; Glatzel, G.; Jandl, R. Winter soil respiration from an Austrian mountain forest. Agric. For. Meteorol. 2007, 146, 205–215. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Wang, C.; Gower, S.T. Contribution of root respiration to soil surface CO2 flux in a boreal black spruce chronosequence. Tree Physiol. 2004, 24, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Subke, J.A.; Inglima, I.; Francesca Cotrufo, M. Trends and methodological impacts in soil CO2 efflux partitioning: A metaanalytical review. Glob. Chang. Biol. 2006, 12, 921–943. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol. Biochem. 2006, 38, 425–448. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.; Wang, X.; Zhang, J.; Zhang, Z.; Wang, J. Spatial patterns of biomass in the temperate broadleaved deciduous forest within the fetch of the Maoershan flux tower. Acta Ecol. Sin. 2016, 36, 6506–6519. [Google Scholar]
- Liu, Z.; Wang, C.; Chen, J.M.; Wang, X.; Jin, G. Empirical models for tracing seasonal changes in leaf area index in deciduous broadleaf forests by digital hemispherical photography. For. Ecol. Manag. 2015, 351, 67–77. [Google Scholar] [CrossRef]
- Liu, F.; Wang, C.; Wang, X. Monitoring temporal dynamics in leaf area index of the temperate broadleaved deciduous forest in Maoershan region with tower-based radiation measurements. Chin. J. Appl. Ecol. 2016, 27, 2409–2419. [Google Scholar]
- Kong, Q.; Wang, C.; Wang, X. Effects of detritus removal on soil carbon, nitrogen and phosphorus stoichiometry and related factors in a temperate deciduous forest in the Maoershan Mountain, China. Chin. J. Appl. Ecol. 2018, 29, 2173–2182. [Google Scholar]
- Jassal, R.S.; Black, T.A. Estimating heterotrophic and autotrophic soil respiration using small-area trenched plot technique: Theory and practice. Agric. For. Meteorol. 2006, 140, 193–202. [Google Scholar] [CrossRef]
- Wang, C.; Yang, J.; Zhang, Q. Soil respiration in six temperate forests in China. Glob. Chang. Biol. 2006, 12, 2103–2114. [Google Scholar] [CrossRef]
- Davidson, E.; Savage, K.; Verchot, L.; Navarro, R. Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agric. For. Meteorol. 2002, 113, 21–37. [Google Scholar] [CrossRef]
- Groffman, P.M.; Hardy, J.P.; Driscoll, C.T.; Fahey, T.J. Snow depth, soil freezing, and fluxes of carbon dioxide, nitrous oxide and methane in a northern hardwood forest. Glob. Chang. Biol. 2006, 12, 1748–1760. [Google Scholar] [CrossRef]
- Xu, M.; Qi, Y. Soil-surface CO2 efflux and its spatial and temporal variations in a young ponderosa pine plantation in northern California. Glob. Chang. Biol. 2001, 7, 667–677. [Google Scholar] [CrossRef]
- Pumpanen, J.; Kolari, P.; Ilvesniemi, H.; Minkkinen, K.; Vesala, T.; Niinistö, S.; Lohila, A.; Larmola, T.; Morero, M.; Pihlatie, M.; et al. Comparison of different chamber techniques for measuring soil CO2 efflux. Agric. For. Meteorol. 2004, 123, 159–176. [Google Scholar] [CrossRef]
- Madsen, R.A.; Demetriades-Shah, T.H.; Garcia, R.L.; McDermitt, D.K. Soil CO2 Flux Measurements: Comparisons between the LI-COR LI-6400 and LI-8100; Technical Note; LI-COR Biosciences: Lincoln, NE, USA, 2004. [Google Scholar]
- Reinmann, A.B.; Templer, P.H. Increased soil respiration in response to experimentally reduced snow cover and increased soil freezing in a temperate deciduous forest. Biogeochemistry 2018, 140, 359–371. [Google Scholar] [CrossRef]
- Ngao, J.; Longdoz, B.; Granier, A.; Epron, D. Estimation of autotrophic and heterotrophic components of soil respiration by trenching is sensitive to corrections for root decomposition and changes in soil water content. Plant Soil 2007, 301, 99–110. [Google Scholar] [CrossRef]
- Moyano, F.E.; Kutsch, W.L.; Rebmann, C. Soil respiration fluxes in relation to photosynthetic activity in broad-leaf and needle-leaf forest stands. Agric. For. Meteorol. 2008, 148, 135–143. [Google Scholar] [CrossRef]
- Sampson, D.A.; Janssens, I.A.; Curiel yuste, J.; Ceulemans, R. Basal rates of soil respiration are correlated with photosynthesis in a mixed temperate forest. Glob. Chang. Biol. 2007, 13, 2008–2017. [Google Scholar] [CrossRef]
- Zhang, Q.; Phillips, R.P.; Manzoni, S.; Scott, R.L.; Oishi, A.C.; Finzi, A.; Daly, E.; Vargas, R.; Novick, K.A. Changes in photosynthesis and soil moisture drive the seasonal soil respiration-temperature hysteresis relationship. Agric. For. Meteorol. 2018, 259, 184–195. [Google Scholar] [CrossRef]
- Suzuki, S.; Ishizuka, S.; Kitamura, K.; Yamanoi, K.; Nakai, Y. Continuous estimation of winter carbon dioxide efflux from the snow surface in a deciduous broadleaf forest. J. Geophys. Res. 2006, 111, D17101. [Google Scholar] [CrossRef]
- Gao, D.; Hagedorn, F.; Zhang, L.; Liu, J.; Qu, G.; Sun, J.; Peng, B.; Fan, Z.; Zheng, J.; Jiang, P.; et al. Small and transient response of winter soil respiration and microbial communities to altered snow depth in a mid-temperate forest. Appl. Soil Ecol. 2018, 130, 40–49. [Google Scholar] [CrossRef]
- Hubbard, R.M.; Ryan, M.G.; Elder, K.; Rhoades, C.C. Seasonal patterns in soil surface CO2 flux under snow cover in 50 and 300 year old subalpine forests. Biogeochemistry 2005, 73, 93–107. [Google Scholar] [CrossRef]
- Du, E.; Zhou, Z.; Li, P.; Jiang, L.; Hu, X.; Fang, J. Winter soil respiration during soil-freezing process in a boreal forest in Northeast China. J. Plant Ecol. 2013, 6, 349–357. [Google Scholar] [CrossRef]
- Schmidt, S.; Wilson, K.; Monson, R.; Lipson, D. Exponential growth of “snow molds” at sub-zero temperatures: An explanation for high beneath-snow respiration rates and Q10 values. Biogeochemistry 2009, 95, 13–21. [Google Scholar] [CrossRef]
- Högberg, P. Is tree root respiration more sensitive than heterotrophic respiration to changes in soil temperature? New Phytol. 2010, 188, 9–10. [Google Scholar] [CrossRef]
- Luo, Y.; Zhou, X. Deconvolution analysis to quantify autotrophic and heterotrophic respiration and their temperature sensitivities. New Phytol. 2010, 188, 10–11. [Google Scholar] [CrossRef]
- Bond-Lamberty, B.; Bronson, D.; Bladyka, E.; Gower, S.T. A comparison of trenched plot techniques for partitioning soil respiration. Soil Biol. Biochem. 2011, 43, 2108–2114. [Google Scholar] [CrossRef]
- Boone, R.D.; Nadelhoffer, K.J.; Canary, J.D.; Kaye, J.P. Roots exert a strong influence on the temperature sensitivity of soil respiration. Nature 1998, 396, 570–572. [Google Scholar] [CrossRef]
- Jiang, L.; Shi, F.; Li, B.; Luo, Y.; Chen, J.; Chen, J. Separating rhizosphere respiration from total soil respiration in two larch plantations in northeastern China. Tree Physiol. 2005, 25, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Curiel Yuste, J.; Janssens, I.A.; Carrara, A.; Ceulemans, R. Annual Q10 of soil respiration reflects plant phenological patterns as well as temperature sensitivity. Glob. Chang. Biol. 2004, 10, 161–169. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- McDowell, N.G.; Marshall, J.D.; Hooker, T.D.; Musselman, R. Estimating CO2 flux from snowpacks at three sites in the Rocky Mountains. Tree Physiol. 2000, 20, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Ruehr, N.K.; Knohl, A.; Buchmann, N. Environmental variables controlling soil respiration on diurnal, seasonal and annual time-scales in a mixed mountain forest in Switzerland. Biogeochemistry 2010, 98, 153–170. [Google Scholar] [CrossRef]
- Mo, W.; Lee, M.S.; Uchida, M.; Inatomi, M.; Saigusa, N.; Mariko, S.; Koizumi, H. Seasonal and annual variations in soil respiration in a cool-temperate deciduous broad-leaved forest in Japan. Agric. For. Meteorol. 2005, 134, 81–94. [Google Scholar] [CrossRef]
- Kim, Y.; Ueyama, M.; Nakagawa, F.; Tsunogai, U.; Tanaka, N.; Harazono, Y. Assessment of winter fluxes of CO2 and CH4 in boreal forest soils of central Alaska estimated by the profile method and the chamber method: A diagnosis of methane emission and implications for the regional carbon budget. Tellus B 2007, 59, 223–233. [Google Scholar] [CrossRef]
- Wang, C.; Bond-Lamberty, B.; Gower, S.T. Soil surface CO2 flux in a boreal black spruce fire chronosequence. J. Geophys. Res. 2002, 108, 8224. [Google Scholar] [CrossRef]
- Vogel, J.G.; Valentine, D.W.; Ruess, R.W. Soil and root respiration in mature Alaskan black spruce forests that vary in soil organic matter decomposition rates. Can. J. For. Res. 2005, 35, 161–174. [Google Scholar] [CrossRef]
- Tang, J.; Misson, L.; Gershenson, A.; Cheng, W.; Goldstein, A.H. Continuous measurements of soil respiration with and without roots in a ponderosa pine plantation in the Sierra Nevada Mountains. Agric. For. Meteorol. 2005, 132, 212–227. [Google Scholar] [CrossRef]
- Saiz, G.; Byrne, K.A.; Butterbach-Bahl, K.; Kiese, R.; Blujdea, V.; Farrell, E.P. Stand age-related effects on soil respiration in a first rotation Sitka spruce chronosequence in central Ireland. Glob. Chang. Biol. 2006, 12, 1007–1020. [Google Scholar] [CrossRef]
- Comstedt, D.; Boström, B.; Ekblad, A. Autotrophic and heterotrophic soil respiration in a Norway spruce forest: Estimating the root decomposition and soil moisture effects in a trenching experiment. Biogeochemistry 2011, 104, 121–132. [Google Scholar] [CrossRef]
- Díaz-Pinés, E.; Schindlbacher, A.; Pfeffer, M.; Jandl, R.; Zechmeister-Boltenstern, S.; Rubio, A. Root trenching: A useful tool to estimate autotrophic soil respiration? A case study in an Austrian mountain forest. Eur. J. For. Res. 2010, 129, 101–109. [Google Scholar] [CrossRef]
- Epron, D.; Farque, L.; Lucot, E.; Badot, P.M. Soil CO2 efflux in a beech forest: The contribution of root respiration. Ann. For. Sci. 1999, 56, 289–295. [Google Scholar] [CrossRef]
- Varik, M.; Kukumägi, M.; Aosaar, J.; Becker, H.; Ostonen, I.; Lõhmus, K.; Uri, V. Carbon budgets in fertile silver birch (Betula pendula Roth) chronosequence stands. Ecol. Eng. 2015, 77, 284–296. [Google Scholar] [CrossRef]
- Kukumägi, M.; Ostonen, I.; Uri, V.; Helmisaari, H.S.; Kanal, A.; Kull, O.; Lõhmus, K. Variation of soil respiration and its components in hemiboreal Norway spruce stands of different ages. Plant Soil 2017, 414, 265–280. [Google Scholar] [CrossRef]
- Sulzman, E.W.; Brant, J.B.; Bowden, R.D.; Lajtha, K. Contribution of aboveground litter, belowground litter, and rhizosphere respiration to total soil CO2 efflux in an old growth coniferous forest. Biogeochemistry 2005, 73, 231–256. [Google Scholar] [CrossRef]
- Kuzyakov, Y. Priming effects: Interactions between living and dead organic matter. Soil Biol. Biochem. 2010, 42, 1363–1371. [Google Scholar] [CrossRef]

| Plot Number | BA m2 m−2 | Biomass Mg ha−1 | Litterfall g m−2 yr−1 | FRB g m−2 | FRN g m−2 | SOC mg g−1 | TN mg g−1 | SOC Density Mg ha−1 | TN Density Mg ha−1 |
|---|---|---|---|---|---|---|---|---|---|
| 2 | 19.54 | 155.09 | 459.52 | 330.33 | 253.73 | 97.51 | 9.81 | 92.22 | 9.28 |
| 3 | 26.08 | 147.04 | 469.02 | 237.82 | 267.28 | 56.86 | 7.51 | 68.91 | 9.10 |
| 4 | 20.74 | 142.36 | 447.49 | 304.34 | 298.27 | 50.68 | 6.63 | 66.95 | 8.76 |
| Year | Variable | Plot | a | b | c | d | N | adj.R2 | RMSE |
|---|---|---|---|---|---|---|---|---|---|
| 2010 | RS | 2 | 0.44 | 0.044 | −0.837 | 0.104 | 72 | 0.842 | 0.204 |
| 2010 | RS | 3 | −0.03 | 0.091 | 72 | 0.874 | 0.163 | ||
| 2010 | RS | 4 | −0.087 | 0.092 | 0.45 | 72 | 0.842 | 0.176 | |
| 2010 | RH | 2 | 0.281 | 0.077 | −1.181 | 36 | 0.827 | 0.222 | |
| 2010 | RH | 3 | −0.178 | 0.064 | 0.032 | 36 | 0.850 | 0.151 | |
| 2010 | RH | 4 | −0.269 | 0.085 | 36 | 0.833 | 0.181 | ||
| 2011 | RS | 2 | −0.715 | 0.128 | 2.114 | 72 | 0.878 | 0.219 | |
| 2011 | RS | 3 | −0.723 | 0.112 | 2.021 | 72 | 0.825 | 0.242 | |
| 2011 | RS | 4 | −0.648 | 0.118 | 1.976 | 72 | 0.855 | 0.218 | |
| 2011 | RH | 2 | −0.502 | 0.111 | 36 | 0.860 | 0.237 | ||
| 2011 | RH | 3 | −0.873 | 0.104 | 1.654 | 36 | 0.918 | 0.158 | |
| 2011 | RH | 4 | −0.545 | 0.097 | 0.051 | 36 | 0.849 | 0.227 |
| Period | Season | Cumulative RS g C m−2 | Cumulative RH g C m−2 | Cumulative RR g C m−2 |
|---|---|---|---|---|
| November 2009–April 2010 | Non-growing season | 83.96 ± 0.99 | 74.80 ± 5.19 | 9.16 ± 0.82 |
| May 2010–October 2010 | Growing season | 673.35 ± 29.26 | 461.64 ± 136.25 | 211.71 ± 27.75 |
| Nov. 2009–October 2010 | Climatic year | 757.31 ± 29.96 | 536.44 ± 141.44 | 220.87 ± 28.56 |
| November 2010–April 2011 | Non-growing season | 103.47 ± 5.33 | 84.02 ± 9.91 | 19.46 ± 3.42 |
| May 2011–October 2011 | Growing season | 876.52 ± 127.96 | 473.95 ± 211.10 | 402.57 ± 59.49 |
| November 2010–October 2011 | Climatic year | 979.99 ± 133.07 | 557.97 ± 220.15 | 422.02 ± 62.42 |
| Annual mean | 868.65 ± 78.92 | 547.20 ± 179.63 | 307.14 ± 31.97 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Z.; Wang, X. Contrasting Rhizospheric and Heterotrophic Components of Soil Respiration during Growing and Non-Growing Seasons in a Temperate Deciduous Forest. Forests 2019, 10, 8. https://doi.org/10.3390/f10010008
Jiao Z, Wang X. Contrasting Rhizospheric and Heterotrophic Components of Soil Respiration during Growing and Non-Growing Seasons in a Temperate Deciduous Forest. Forests. 2019; 10(1):8. https://doi.org/10.3390/f10010008
Chicago/Turabian StyleJiao, Zhen, and Xingchang Wang. 2019. "Contrasting Rhizospheric and Heterotrophic Components of Soil Respiration during Growing and Non-Growing Seasons in a Temperate Deciduous Forest" Forests 10, no. 1: 8. https://doi.org/10.3390/f10010008
APA StyleJiao, Z., & Wang, X. (2019). Contrasting Rhizospheric and Heterotrophic Components of Soil Respiration during Growing and Non-Growing Seasons in a Temperate Deciduous Forest. Forests, 10(1), 8. https://doi.org/10.3390/f10010008





