Low Water Availability Increases Necrosis in Picea abies after Artificial Inoculation with Fungal Root Rot Pathogens Heterobasidion parviporum and Heterobasidion annosum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Fungal Material
2.2. Pre-Experiment Detection of Heterobasidion Species
2.3. Fungal Material
2.4. DNA Extractions, PCR and Sequencing
2.5. Experimental Design
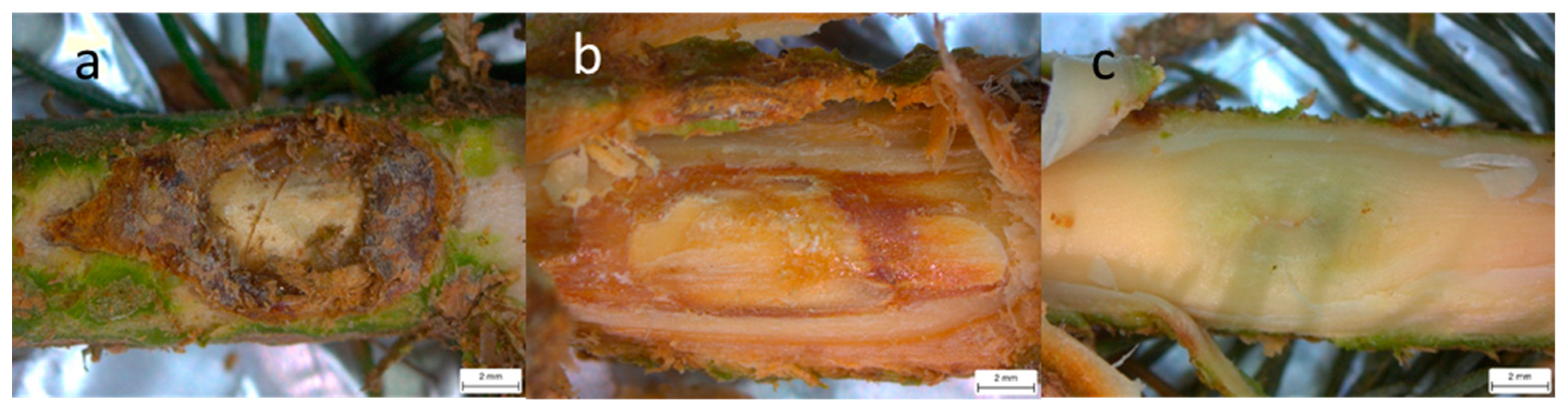
2.6. Data Collection and Post-Experiment Detection of Heterobasidion Species
2.7. Data Analysis
3. Results
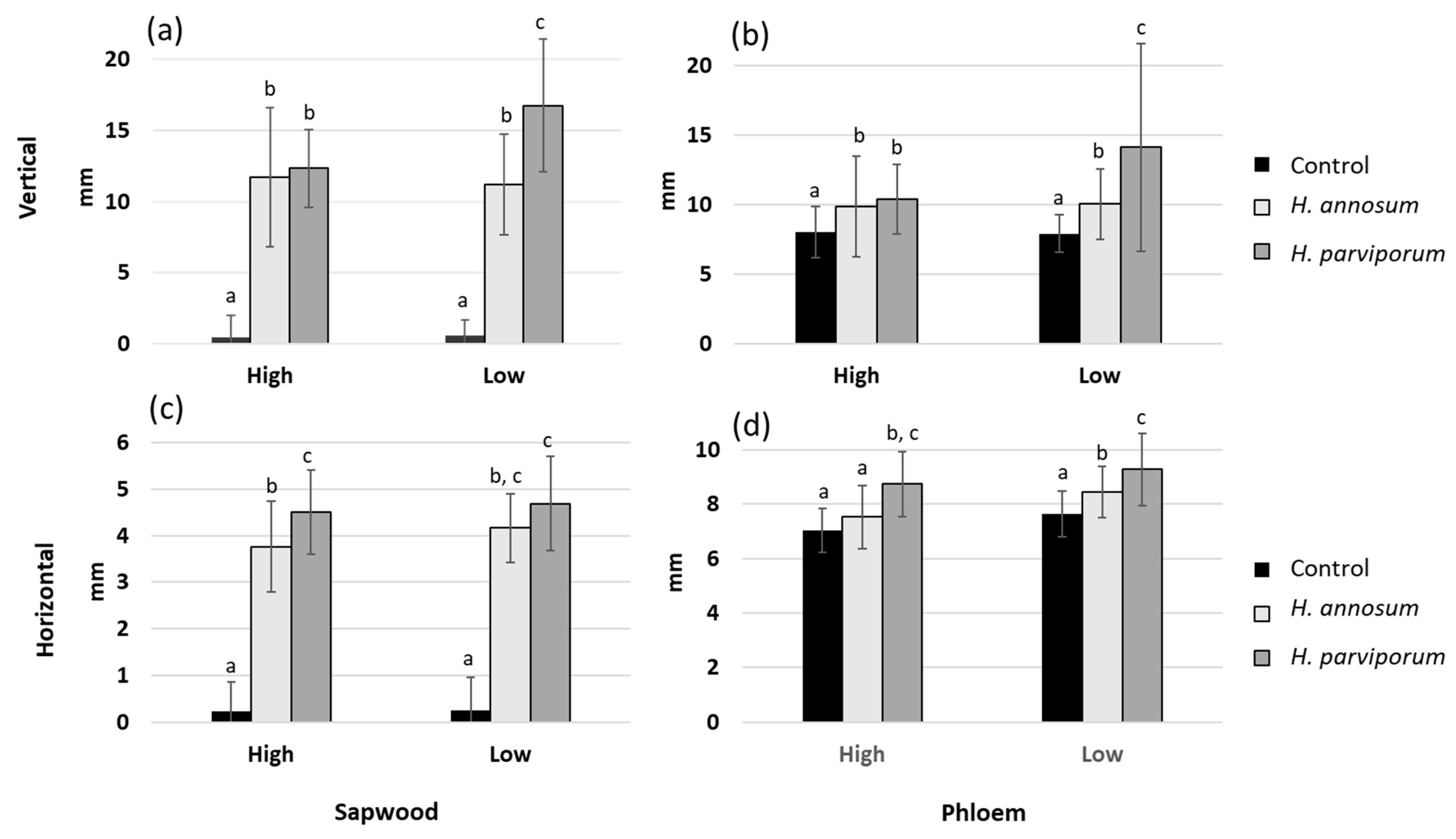
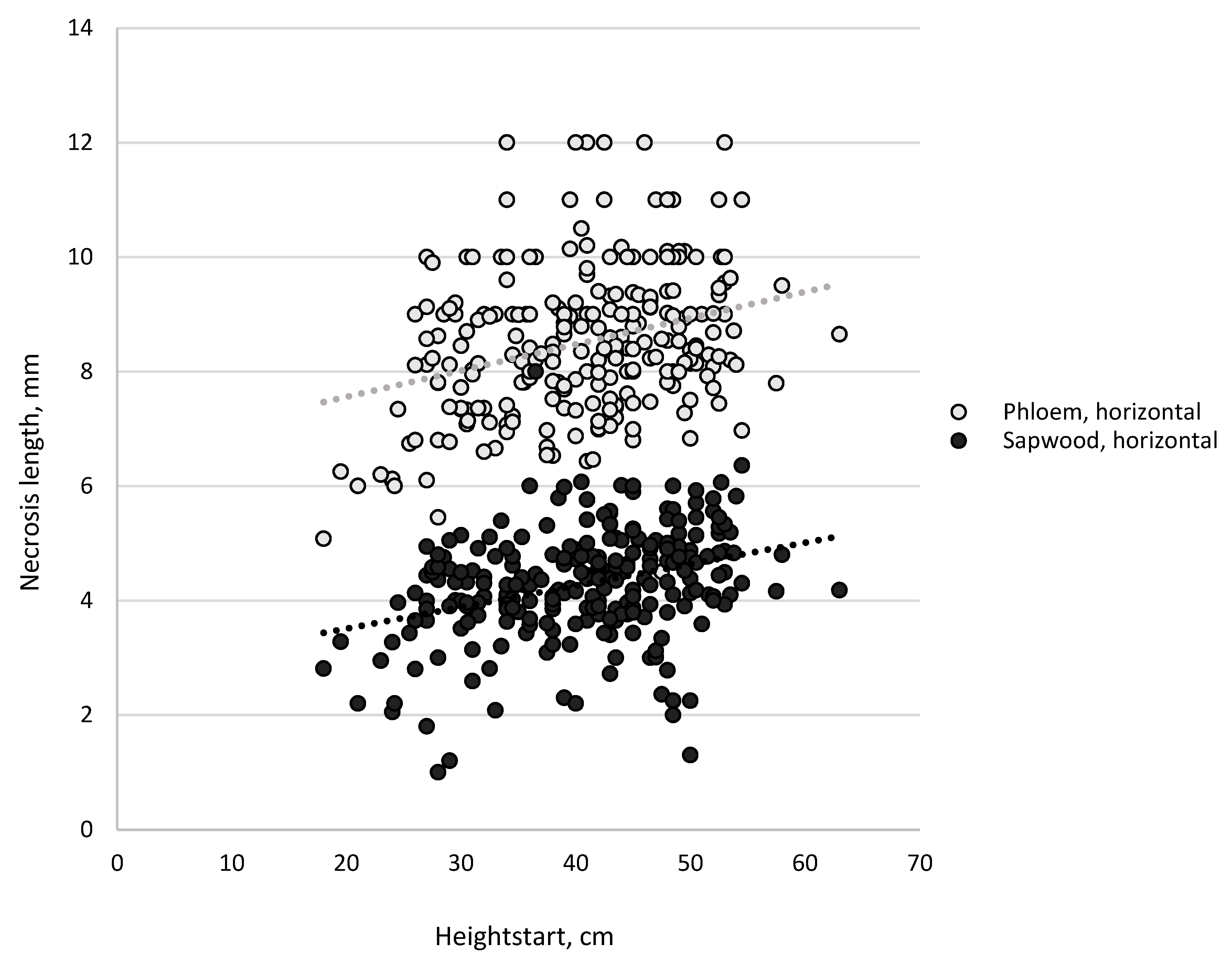
3.1. Necrosis Length
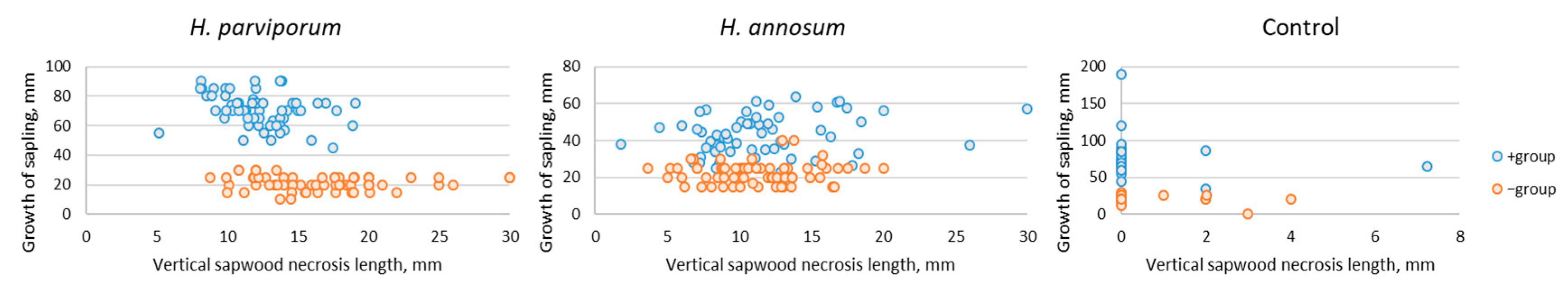
3.2. Saplings Growth
3.3. Plant and Fungal Material
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seidl, R.; Thom, D.; Kautz, M.; Martin-Benito, D.; Peltoniemi, M.; Vacchiano, G.; Wild, J.; Ascoli, D.; Petr, M.; Honkaniemi, J.; et al. Forest disturbances under climate change. Nat. Clim. Chang. 2017, 7, 395–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnakoski, R.; Sugano, J.; Junttila, S.; Pulkkinen, P.; Asiegbu, F.O.; Forbes, K.M. Effects of water availability on a forestry pathosysstem: Fungal strain-specific variation in disease severity. Sci. Rep. 2017, 7, 13501. [Google Scholar] [CrossRef] [PubMed]
- Thünen-Institut, Dritte Bundeswaldinventur—Ergebnisdatenbank. Available online: https://bwi.info (accessed on 3 December 2018).
- Asiegbu, F.O.; Adomas, A.; Stenlid, J. Conifer root and butt rot caused by Heterobasidion annosum (Fr.) Bref. s.l. Mol. Plant Pathol. 2005, 6, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Garbelotto, M.; Gonthier, P. Biology, epidemiology, and control of Heterobasidion species worldwide. Annu. Rev. Phytopathol. 2013, 51, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Deutscher Wetterdienst. Available online: https://www.dwd.de/DE/leistungen/klimakartendeutschland/klimakartendeutschland.html?nn=480164 (accessed on 9 January 2019).
- Langer, G.J.; Northwest German Forest Research Institute, Göttingen, Germany. Personal communication, 2018.
- Rishbeth, J. Observations on the Biology of Fomes annosus, with particular reference to East Anglian pine plantations: II. Spore production, stump infection, and saprophytic activity in stumps. Ann. Bot. 1951, 15, 1–22. [Google Scholar] [CrossRef]
- Langer, G.J.; Bressem, U.; Haberman, M. Vermehrt Pilzkrankheiten an Bergahorn in Nordwestdeutschland. AFZ/Der Wald 2013, 6, 22–26. [Google Scholar]
- Rishbeth, J. Dispersal of Fomes annosus Fr. and Peniophora gigantea (Fr.) Massee. Trans. Br. Mycol. Soc. 1959, 42, 243–260. [Google Scholar] [CrossRef]
- Isomäki, A.; Kallio, T. Consequences of injury caused by timber harvesting machines on the growth and decay of spruce (Picea abies (L.) Karst.). Acta For. Fennica 1974, 136, 1–25. [Google Scholar] [CrossRef]
- Redfern, D.B.; Stenlid, J. Spore dispersal and infection. In Heterobasidion annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK; New York, NY, USA, 1998; pp. 105–124. [Google Scholar]
- Korhonen, K. Intersterility groups of Heterobasidion annosum. Commun. Inst. For. Fenn. 1978, 94, 1–25. [Google Scholar]
- Capretti, P.; Korhonen, K.; Mugnai, L.; Romagnioli, C. An intersterility group of Heterobasidion annosum, specialized to Abies alba. Eur. J. For. Pathol. 1990, 20, 257–262. [Google Scholar] [CrossRef]
- Metzler, B.; Langer, G.J.; Heydeck, P.; Peters, F.; Scham, J.; Langer, E. Survey on Heterobasidion species and perspectives of butt rot control in Germany. In XIII Conference Root and Butt Rot of Forest Trees IUFRO; Firenze University Press: Firenze, Italy, 2011; pp. 206–208. [Google Scholar]
- Langer, G.J.; Bressem, U. Phlebiopsis gigantea als Antagonist des Wurzelschwamms. AFZ/Der Wald 2017, 72, 39–43. [Google Scholar]
- Hantula, J.; Vainio, E. Specific primers for the differentiation of Heterobasidion annosum (s.str.) and H. parviporum infected stumps in northern Europe. Silva Fenn. 2003, 37, 181–187. [Google Scholar] [CrossRef]
- Rishbeth, J. Stump protection against Fomes annosus. Treatment with substances other than creosote. Ann. Appl. Biol. 1959, 47, 529–541. [Google Scholar] [CrossRef]
- Oliva, J.; Bendz-Hellgren, M.; Stenlid, J. Spread of Heterobasidion annosum s.s. and Heterobasidion parviporum in Picea abies 15 years after stump inoculation. FEMS Microbiol. Ecol. 2011, 75, 414–429. [Google Scholar] [CrossRef] [PubMed]
- NW-FVA 2018 Gemeiner Wurzelschwamm (Heterobasidion annosum s.l.) Praxis Information Nr. 5—Oktober 2018. Available online: https://www.nw-fva.de/index.php?id=173 (accessed on 1 December 2018).
- Piri, T. The spreading of the S type of Heterobasidion annosum from Norway spruce stumps to the subsequent tree stand. Eur. J. For. Pathol. 1996, 26, 193–204. [Google Scholar] [CrossRef]
- Stenlid, J.; Redfern, D. Spread within the tree and stand. In Heterobasidion annosum: Biology, Ecology, Impact and Control; Woodward, S., Stenlid, J., Karjalainen, R., Hüttermann, A., Eds.; CAB International: Wallingford, UK; New York, NY, USA, 1998; pp. 125–142. [Google Scholar]
- Piri, T.; Korhonen, K. Spatial distribution and persistence of Heterobasidion parviporum genets on a Norway spruce site. For. Pathol. 2007, 37, 1–8. [Google Scholar] [CrossRef]
- Piri, T. Early development of root rot in young Norway spruce planted on sites infected by Heterobasidion in southern Finland. Can. J. For. Res. 2003, 33, 604–611. [Google Scholar] [CrossRef]
- La Porta, N.; Capretti, P.; Thomsen, I.M.; Kasanen, R.; Hietala, A.M.; Von Weissenberg, K. Forest pathogens with higher damage potential due to climate change in Europe. Can. J. Plant Pathol. 2008, 30, 177–195. [Google Scholar] [CrossRef]
- Trishkin, M.; Lopatin, E.; Gavrilova, O. The potential impact of climate change and forest management practices on Heterobasidion spp. infection distribution in northwestern Russia—A case study in the Republic of Karelia. J. For. Sci. 2016, 62, 529–536. [Google Scholar] [CrossRef]
- Gori, Y.; Cherubini, P.; Camin, F.; La Porta, N. Fungal root pathogen (Heterobasidion parviporum) increases drought stress in Norway spruce stand at low elevation in the Alps. Eur. J. For. Res. 2013, 132, 607. [Google Scholar] [CrossRef]
- Dimitri, L.; Schumann, G. Further experiments on the host-parasite relationship between Norway spruce and Heterobasidion annosum. In Proceedings of the 7th International Conference on Root and Butt Rots, Vernon and Victoria, BC, Canada, 9–16 August 1988; pp. 171–179. [Google Scholar]
- Langer, G. Die Gattung Botryobasidium Donk (Corticiaceae, Basidiomycetes). Bibl. Mycol. 1994, 158, 459. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for higher fungi and basidiomycetes: Application to identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols—A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Madmony, A.; Tognetti, R.; Zamponi, L.; Capretti, P.; Michelozzi, M. Monoterpene responses to interacting effects of drought stress and infection by the fungus Heterobasidion parviporum in two clones of Norway spruce (Picea abies). Environ. Exp. Bot. 2018, 152, 137–148. [Google Scholar] [CrossRef]
- Karlsson, B.; Tsopelas, P.; Zamponi, L.; Capretti, P.; Soulioti, N.; Swedjemark, G. Susceptibility to Heterobasidion parviporum in Picea abies clones grown in different environments. For. Pathol. 2008, 38, 83–89. [Google Scholar] [CrossRef]
- Mukrimin, M.; Kovalchuk, A.; Neves, L.G.; Jaber, E.H.A.; Haapanen, M.; Kirst, M.; Asiegbu, F.O. Genome-wide exon-capture approach identifies genetic variants of Norway spruce genes associated with susceptibility to Heterobasidion parviporum infection. Front. Plant. Sci. 2018, 9, 793. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, M.; Saurer, M.; Siegwolf, R.; Eilmann, B.; Brang, P.; Bugmann, H.; Rigling, A. Drought response of five conifer species under contrasting water availability suggests high vulnerability of Norway spruce and European larch. Glob. Chang. Biol. 2013, 19, 3184–3199. [Google Scholar] [CrossRef]
- Kovalchuk, A.; Keriö, S.; Oghenekaro, A.O.; Jaber, E.; Raffaello, T.; Asiegbu, F.O. Antimicrobial defenses and resistance in forest trees: Challenges and perspectives in a genomic era. Annu. Rev. Phytopathol. 2013, 51, 221–244. [Google Scholar] [CrossRef]
- Neilson, E.H.; Goodger, J.Q.; Woodrow, I.E.; Møller, B.L. Plant chemical defense: At what cost? Trends Plant Sci. 2013, 5, 250–258. [Google Scholar] [CrossRef]
- Allen, C.D.; Macalady, A.K.; Chenchouni, H.; Bachelet, D.; McDowell, N.; Vennetier, M.; Kitzberger, T.; Rigling, A.; Breshears, D.D.; Hogg, E.H.; et al. A global overview of drought and heat induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010, 259, 660–684. [Google Scholar] [CrossRef]
- Gooding, G.V.; Hodges, C.S., Jr.; Ross, E.W., Jr. Effect of temperature on growth and survival of Fomes annosus. For. Sci. 1966, 12, 325–333. [Google Scholar] [CrossRef]
- Brown, A.V.; Webber, J.F. Biocontrol of decay in seasoning utility poles. I. Growth rate and colonizing ability of bluestain and decay fungi in vivo and in vitro. For. Pathol. 2009, 39, 145–156. [Google Scholar] [CrossRef]
- Scirè, M.; Motta, E.; D’Amico, L. Behaviour of Heterobasidion annosum and Heterobasidion irregulare isolates from central Italy in inoculated Pinus pinea seedlings. Mycol. Prog. 2011, 10, 85–91. [Google Scholar] [CrossRef]
- Müller, M.M.; Sievänen, R.; Beuker, E.; Meesenburg, H.; Kuuskeri, J.; Hamberg, L.; Korhonen, K. Predicting the activity of Heterobasidion parviporum on Norway spruce in warming climate from its respiration rate at different temperatures. For. Pathol. 2014, 44, 325–336. [Google Scholar] [CrossRef]
- Bendz-Hellgren, M.; Stenlid, J. Effects of clear-cutting, thinning, and wood moisture content on the susceptibility of Norway spruce stumps to Heterobasidion annosum. Can. J. For. Res. 1998, 28, 759–765. [Google Scholar] [CrossRef]
- Linares, J.C.; Camarero, J.J.; Bowker, M.A.; Ochoa, V.; Carreira, J.A. Stand-structural effects on Heterobasidion abietinum-related mortality following drought events in Abies pinsapo. Oecologia 2010, 164, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Ruosteenoja, K.; Tuomenvirta, H.; Jylhä, K. GCM-based regional temperature and precipitation change estimates for Europe under four SRES scenarios applying a super-ensemble pattern-scaling method. Clim. Chang. 2007, 81, 193–208. [Google Scholar] [CrossRef]
- Swedjemark, G.; Stenlid, J. Variation in spread of Heterobasidion annosum in clones of Picea abies grown at different vegetation phases under greenhouse conditions. Scand. J. For. Res. 1996, 11, 137–144. [Google Scholar] [CrossRef]
- Swedjemark, G.; Stenlid, J.; Karlsson, B. Variation in growth of Heterobasidion annosum among clones of Picea abies incubated for different periods of time. For. Pathol. 2001, 31, 163–175. [Google Scholar] [CrossRef]
- Gaul, D.; Hertel, D.; Borken, W.; Matzner, E.; Leuschner, C. Effects of experimental drought on the fine root system of mature Norway spruce. For. Ecol. Manag. 2008, 256, 1151–1159. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Subramanian, N.; Bergh, J.; Johansson, U.; Nilsson, U.; Sallnäs, O. Adaptation of forest management regimes in southern Sweden to increased risks associated with climate change. Forests 2016, 7, 8. [Google Scholar] [CrossRef]
- Schmidt, M.; Hanewinkel, M.; Kändler, G.; Kublin, E.; Kohnle, U. An inventory-based approach for modelling single tree storm damage—Experiences with the winter storm 1999 in south-western Germany. Can. J. For. Res. 2010, 40, 1636–1652. [Google Scholar] [CrossRef]
- Overbeck, M.; Schmidt, M. Modelling infestation risk of Norway spruce by Ips typographus (L.) in the Lower Saxon Harz Mountains (Germany). For. Ecol. Manag. 2012, 266, 115–125. [Google Scholar] [CrossRef]
- Spellmann, H.; Sutmöller, J.; Meesenburg, H. Risikovorsorge im Zeichen des Klimawandels. Vorläufige Empfehlungen der NW-FVA am Beispiel des Fichtenanbaus. AFZ/Der Wald 2007, 62, 1246–1249. [Google Scholar]
- Spellmann, H.; Albert, M.; Schmidt, M.; Sutmöller, J.; Overbeck, M. Waldbauliche Anpassungsstrategien für veränderte Klimaverhältnisse. AFZ/Der Wald 2011, 11, 19–23. [Google Scholar]


| Month | Average, °C | Min, °C | Max, °C |
|---|---|---|---|
| April | 17.9 | 13.3 | 20.7 |
| May | 21.8 | 15.6 | 25.7 |
| June | 24.8 | 20.4 | 31.1 |
| July | 24.2 | 17.4 | 30.9 |
| August | 32.5 | 32.5 | 32.8 |
| Variable | Fixed Effects | Std. Error | F | Sig. |
|---|---|---|---|---|
| Seedling growth | Water treatment | 0.281 | 341.586 | <0.001 |
| Inoculation treatment | 0.529 | 0.751 | 0.603 | |
| Phloem, vertical | 0.027 | 1.032 | 0.311 | |
| Phloem, horizontal | 0.084 | 5.004 | 0.026 | |
| Sapwood, vertical | 0.032 | 3.866 | 0.05 | |
| Sapwood, horizontal | 0.114 | 2.156 | 0.143 | |
| Sapwood, vertical | Inoculation × water treatment | 1.117 | 106.57 | <0.001 |
| Heightstart | 0.028 | 1.802 | 0.181 | |
| Seedling growth | 0.151 | 2.236 | 0.136 | |
| Sapwood, horizontal | Inoculation × water treatment | 0.36 | 186.452 | <0.001 |
| Heightstart | 0.007 | 13.592 | <0.001 | |
| Seedling growth | 0.035 | 0.009 | 0.926 | |
| Phloem, vertical | Inoculation × water treatment | 1.56 | 11.542 | <0.001 |
| Heightstart | 0.032 | 0.569 | 0.451 | |
| Seedling growth | 0.172 | 0.03 | 0.863 | |
| Phloem, horizontal | Inoculation × water treatment | 0.426 | 15.252 | <0.001 |
| Heightstart | 0.008 | 11.164 | <0.001 | |
| Seedling growth | 0.044 | 2.012 | 0.157 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terhonen, E.; Langer, G.J.; Bußkamp, J.; Rӑscuţoi, D.R.; Blumenstein, K. Low Water Availability Increases Necrosis in Picea abies after Artificial Inoculation with Fungal Root Rot Pathogens Heterobasidion parviporum and Heterobasidion annosum. Forests 2019, 10, 55. https://doi.org/10.3390/f10010055
Terhonen E, Langer GJ, Bußkamp J, Rӑscuţoi DR, Blumenstein K. Low Water Availability Increases Necrosis in Picea abies after Artificial Inoculation with Fungal Root Rot Pathogens Heterobasidion parviporum and Heterobasidion annosum. Forests. 2019; 10(1):55. https://doi.org/10.3390/f10010055
Chicago/Turabian StyleTerhonen, Eeva, Gitta Jutta Langer, Johanna Bußkamp, David Robert Rӑscuţoi, and Kathrin Blumenstein. 2019. "Low Water Availability Increases Necrosis in Picea abies after Artificial Inoculation with Fungal Root Rot Pathogens Heterobasidion parviporum and Heterobasidion annosum" Forests 10, no. 1: 55. https://doi.org/10.3390/f10010055
APA StyleTerhonen, E., Langer, G. J., Bußkamp, J., Rӑscuţoi, D. R., & Blumenstein, K. (2019). Low Water Availability Increases Necrosis in Picea abies after Artificial Inoculation with Fungal Root Rot Pathogens Heterobasidion parviporum and Heterobasidion annosum. Forests, 10(1), 55. https://doi.org/10.3390/f10010055





