Ecophysiological Responses of Carpinus turczaninowii L. to Various Salinity Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Salt Stress Treatments
2.3. Determination of Plant Growth Parameters
2.4. Measurement of Photosynthetic Parameters and Chlorophyll Fluorescence
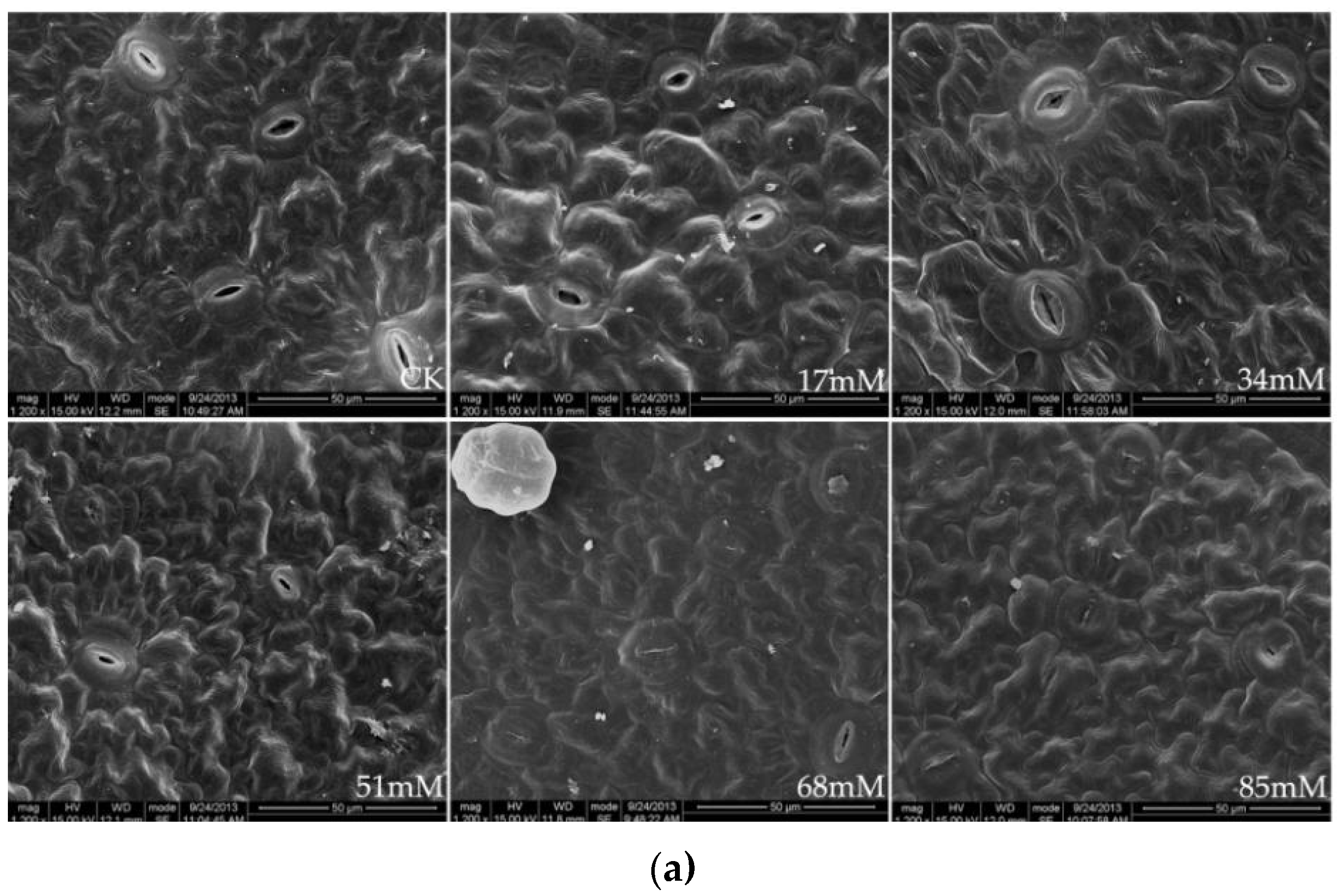
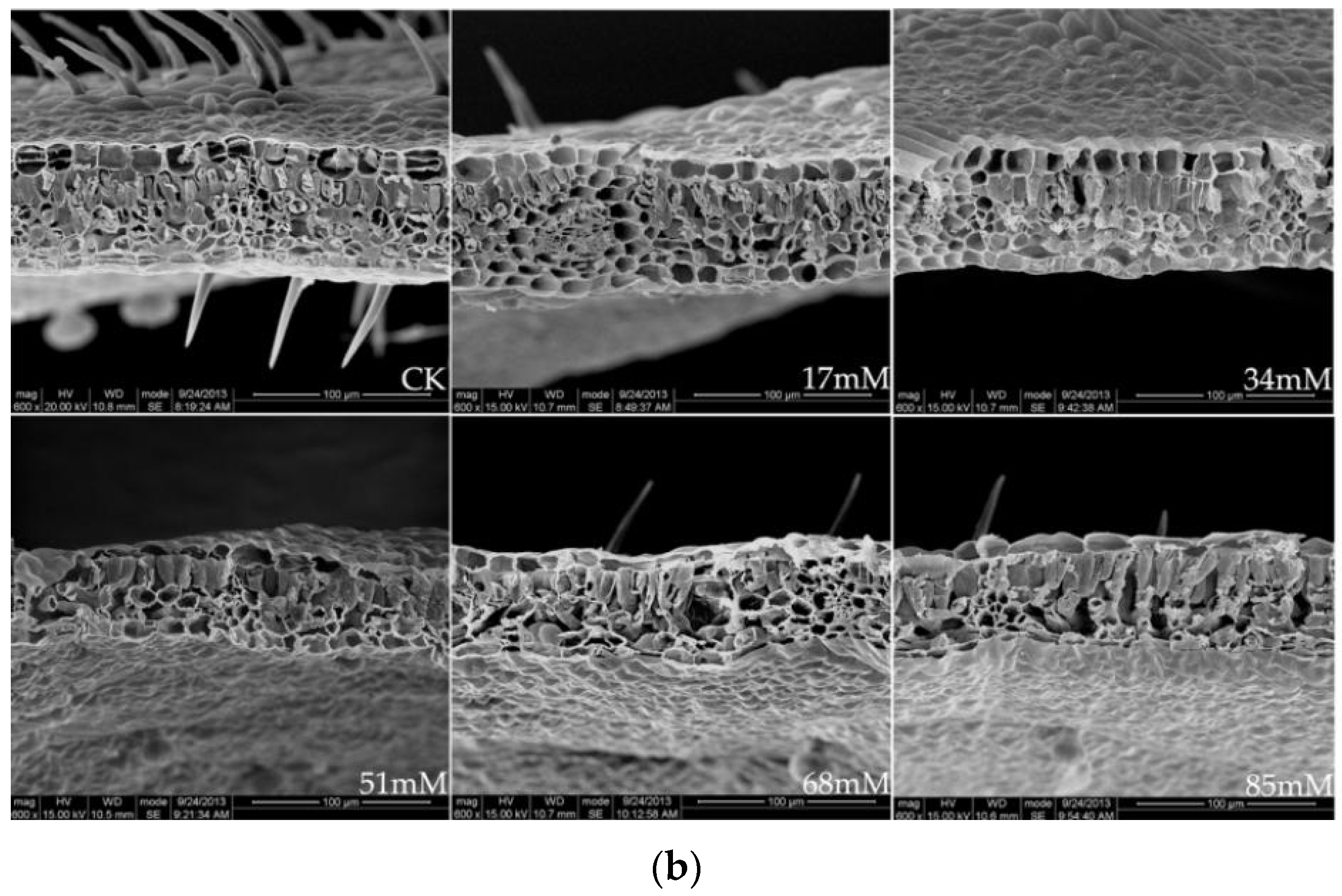
2.5. Scanning Electron Microscopy
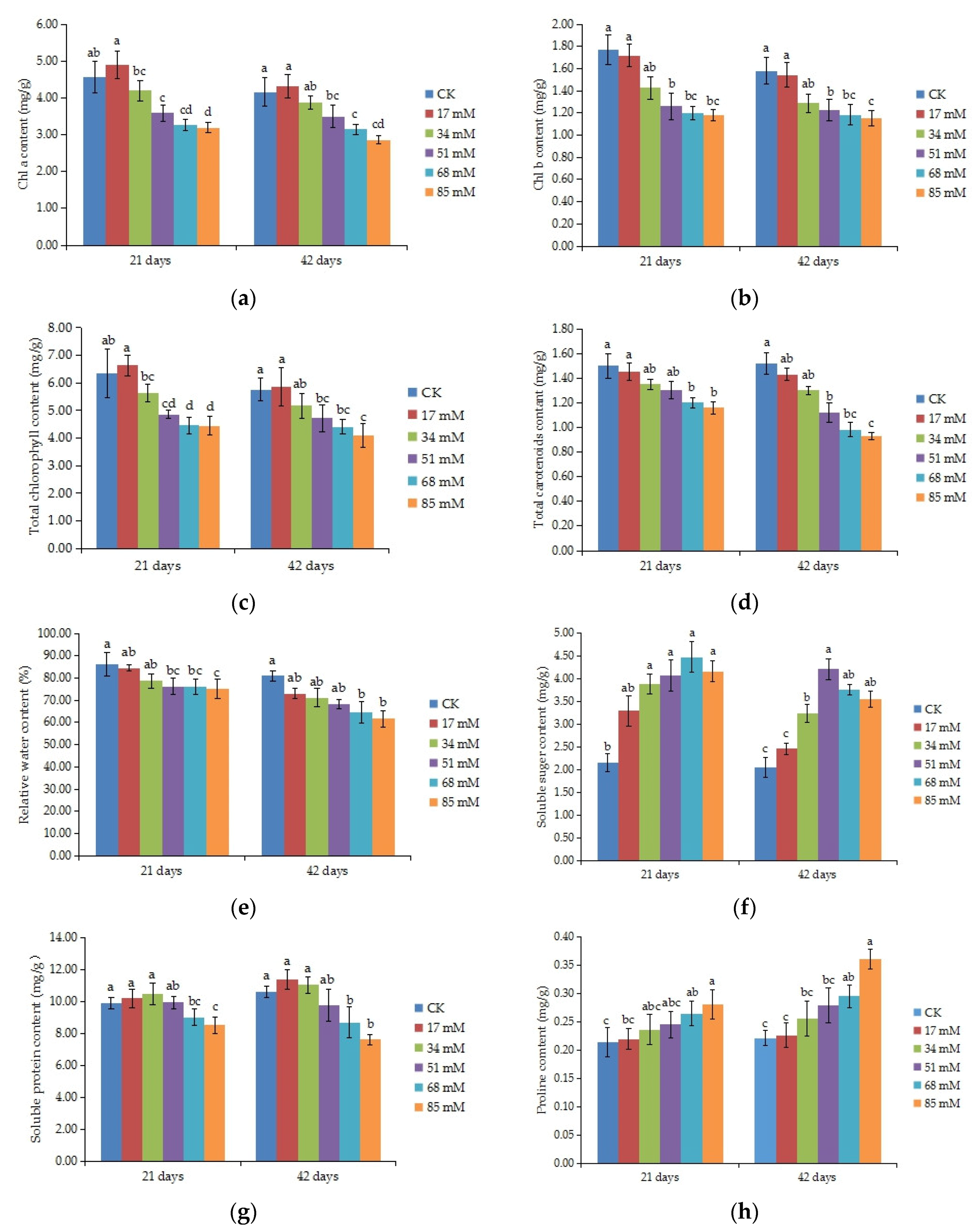
2.6. Photosynthetic Pigments
2.7. Relative Water Content (RWC)
2.8. Organic Osmolytes
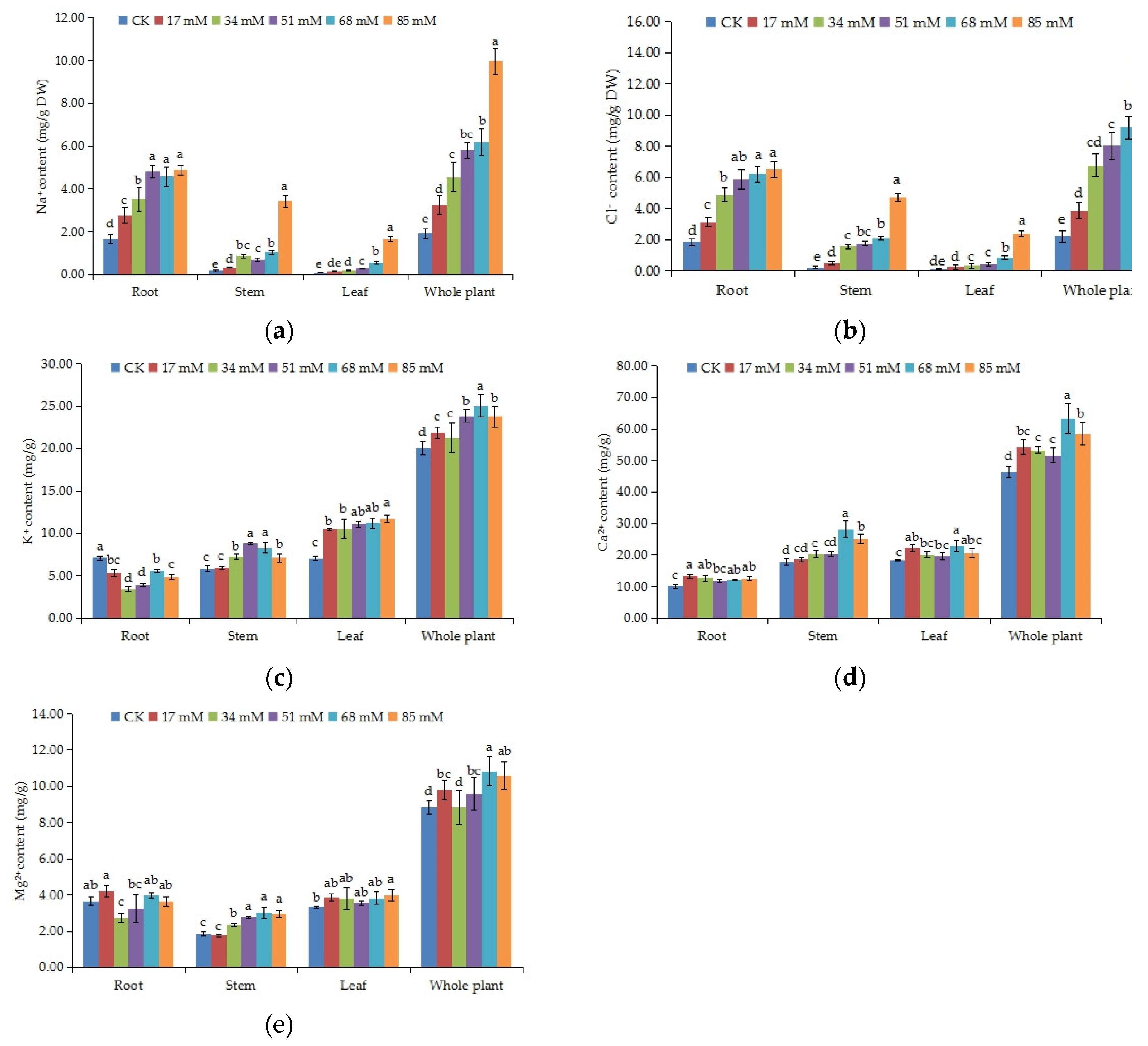
2.9. Measurements of the Na+, Cl−, K+, Ca2+, and Mg2+ Contents of Different Organs
2.10. Malondialdehyde Concentration (MDA) and Cell Membrane Stability
2.11. Antioxidant Enzyme Activities
2.12. Statistical Analysis
3. Results
3.1. Plant Growth
3.2. Photosynthesis, Chlorophyll Fluorescence, and Stomatal Behavior
3.3. Photosynthetic Pigments
3.4. Relative Water Content (RWC)
3.5. Organic Osmolytes
3.6. Inorganic Ion Content of Various Organs
3.7. Malondialdehyde Content (MDA) and Cell Membrane Stability
3.8. Antioxidant Enzyme Activities
4. Discussion
4.1. Seedling Growth
4.2. Physiological Parameters
4.3. Mineral Content and Uptake
4.4. Oxidative Stress and Antioxidant Mechanisms
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dobbertin, M. Tree growth as indicator of tree vitality and of tree reaction to environmental stress: A review. Eur. J. For. Res. 2006, 125, 89. [Google Scholar] [CrossRef]
- Amira, M.S.; Abdul, Q. Effect of salt stress on plant growth and metabolism of bean plant Vicia faba (L.). J. Saudi Soc. Agric. Sci. 2011, 10, 7–15. [Google Scholar] [CrossRef]
- Dashti, A.; Khan, A.A.; Collins, J.C. Effects of salinity on growth, ionic relations and solute content of Sorghum Bicolor (L.) Monench. J. Plant Nutr. 2009, 32, 1219–1236. [Google Scholar] [CrossRef]
- Houska, C. Deicing Salt-Recognizing the Corrosion Threat; TMR Consulting: International Molybdenum Association: Pittsburgh, PA, USA, 2007; pp. 1–11. [Google Scholar]
- Cunningham, M.A.; Snyder, E.; Yonkin, D.; Ross, M.; Elsen, T. Accumulation of deicing salts in soils in an urban environment. Urban Ecosyst. 2008, 11, 17–31. [Google Scholar] [CrossRef]
- Lundmark, A.; Olofsson, B. Chloride Deposition and distribution in soils along a deiced highway- assessment using different methods of measurement. Water Air Soil Pollut. 2007, 182, 173–185. [Google Scholar] [CrossRef]
- Kayama, M.; Quoreshi, A.M.; Kitaoka, S.; Kitahashi, Y.; Sakamoto, Y.; Maruyama, Y. Effects of deicing salt on the vitality and health of two spruce species, Picea abies Karst., and Picea glehnii Masters planted along roadsides in northern Japan. Environ. Pollut. 2003, 124, 127–137. [Google Scholar] [CrossRef]
- Randrup, T.B. Differences in salt sensitivity of four deciduous tree species to soil or airborne salt. Physiol. Plant. 2010, 114, 223–230. [Google Scholar] [CrossRef]
- Rozentsvet, O.A.; Nesterov, V.N.; Bogdanova, E.S. Membrane-forming lipids of wild halophytes growing under the conditions of Prieltonie of South Russia. Phytochemistry 2014, 105, 37–42. [Google Scholar] [CrossRef]
- Jinbiao, X.I.; Zhang, F.; Chen, Y.; Mao, D.; Yin, C.; Tian, C. A preliminary study on salt contents of soil in root-canopy area of halophytes. Chin. J. Appl. Ecol. 2004, 15, 53–58. (In Chinese) [Google Scholar] [CrossRef]
- Ashraf, M.; Orooj, A. Salt stress effects on growth, ion accumulation and seed oil concentration in an arid zone traditional medicinal plant ajwain (Trachyspermum ammi [L.] Sprague). J. Arid Environ. 2006, 64, 209–220. [Google Scholar] [CrossRef]
- Schiop, S.T.; Hassan, M.A.; Sestras, A.F.; Boscaiu, N.; Mónica, T.; Vicente, M.; Óscar. Identification of salt stress biomarkers in Romanian Carpathian populations of Picea abies (L.) Karst. PLoS ONE 2015, 10, 980–981. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.A. Diagnosis and improvement of saline and alkaline soils. Soil Sci. 1954, 78, 154. [Google Scholar] [CrossRef]
- Volkmar, K.M.; Hu, Y.; Steppuhn, H. Physiological responses of plants to salinity: A review. Can. J. Plant Sci. 1998, 78, 19–27. [Google Scholar] [CrossRef]
- Ashraf, M.; Ozturk, M.; Ahmad, M.S.A. Structural and Functional Adaptations in Plants for Salinity Tolerance; Springer: Dordrecht, The Netherlands; Berlin, Germany, 2010; pp. 151–170. [Google Scholar] [CrossRef]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Bio/Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Fardus, J.; Matin, M.A.; Hasanuzzaman, M.; Hossain, M.S.; Nath, S.D.; Hossain, M.A. Exogenous salicylic acid-mediated physiological responses and improvement in yield by modulating antioxidant defense system of wheat under salinity. Not. Sci. Biol. 2017, 9, 219–232. [Google Scholar] [CrossRef]
- Esfandiari, E.; Gohari, G. Response of ROS-scavenging systems to salinity stress in two different wheat (Triticum aestivum L.) cultivars. Not. Bot. Horti Agrobot. 2017, 45, 287–291. [Google Scholar] [CrossRef]
- Chen, Z.D.; Xing, S.P.; Liang, H.X.; Lu, A.M. Morphogenesis of female reproductive organs in Carpinus turczaninowii and Ostryopsis davidiana (Betulaceae). Acta Bot. Sin. 2001, 43, 1110–1114. (In Chinese) [Google Scholar] [CrossRef]
- Cheng, L.X.; Jin, C.Z.; Zhu, Z.L. Growth characteristics of Carpinus turczaninowii and chlorophyll fluorescence annual variation. J. Shanghai Jiaotong Univ. 2014, 32, 21–26. (In Chinese) [Google Scholar] [CrossRef]
- Ko, H.N.; Kim, J.M.; Bu, H.J.; Lee, N.H. Chemical constituents from the branches of Carpinus turczaninowii with antioxidative activities. J. Korean Chem. Soc. 2013, 57, 520–524. [Google Scholar] [CrossRef]
- Kang, J.M.; Kim, J.E.; Lee, N.H. Anti-melanogenesis active constituents from the extracts of Carpinus turczaninowii leaves. J. Soc. Cosmet. Sci. Korea 2017, 43, 35–41. [Google Scholar] [CrossRef]
- Yeo, J.H.; Son, Y.K.; Bang, W.Y.; Kim, O.Y. Carpinus truczaninowii extract showed anti-inflammatory response on human aortic vascular smooth muscle cells. Planta Med. 2016, 82, S1–S381. [Google Scholar] [CrossRef]
- Hothem, S.D.; Marley, K.A.; Larson, R.A. Photochemistry in Hoagland’s Nutrient Solution. J. Plant Nutr. 2003, 26, 845–854. [Google Scholar] [CrossRef]
- Camposeo, S.; Palasciano, M.; Vivaldi, G.A.; Godini, A. Effect of increasing climatic water deficit on some leaf and stomatal parameters of wild and cultivated almonds under Mediterranean conditions. Sci. Hortic. 2011, 127, 234–241. [Google Scholar] [CrossRef]
- Lichenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Vivekanandan, M. Drought-induced responses of photosynthesis and antioxidant metabolism in higher plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef]
- Magné, C.; Saladin, G.; Clément, C. Transient effect of the herbicide flazasulfuron on carbohydrate physiology in Vitis vinifera L. Chemosphere 2006, 62, 650–657. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Steinert, A.; Bielka, S. Determination of free proline in stressed plants. Arch. Züchtungsforsch. 1990, 20, 199–204. [Google Scholar]
- Hodges, D.M.; Delong, J.M.; Forney, C.F.; Prange, P.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Civello, P.M.; Martinez, G.A.; Chaves, A.R.; Anon, M.C. Peroxidase from strawberry fruit (Fragaria ananassa Duch.): Partial purification and determination of some properties. J. Agric. Food Chem. 1995, 43, 2596–2601. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Ma, F.; Cheng, L. The sun-exposed peel of apple fruit has higher xanthophyll cycle-dependent thermal dissipation and antioxidants of the ascorbate–glutathione pathway than the shaded peel. Plant Sci. 2003, 165, 819–827. [Google Scholar] [CrossRef]
- Niu, G.H.; Cabrera, R.I. Growth and physiological responses of landscape plants to saline water irrigation: A review. Hortscience 2010, 45, 1605–1609. [Google Scholar] [CrossRef]
- Laffray, X.; Alaoui-Sehmer, L.; Bourioug, M.; Bourgeade, P.; Alaoui-Sossé, B.; Aleya, L. Effects of sodium chloride salinity on ecophysiological and biochemical parameters of oak seedlings (Quercus robur L.) from use of de-icing salts for winter road maintenance. Environ. Monit. Assess. 2018, 190, 266. [Google Scholar] [CrossRef]
- Lu, N.; Luo, Z.; Ke, Y.; Dai, L.; Duan, H.D.; Hou, R.X.; Cui, B.B.; Dou, S.H.; Zhang, Y.D.; Sun, Y.H. Growth, physiological, biochemical, and ionic responses of Morus alba L. seedlings to various salinity levels. Forests 2017, 8, 488. [Google Scholar] [CrossRef]
- Christian, Z.R.; Mühling, K.H.; Ulrich, K.; Christoph-Martin, G. Salinity stiffens the epidermal cell walls of salt-stressed maize leaves: Is the epidermis growth-restricting? PLoS ONE 2015, 10, e118406. [Google Scholar] [CrossRef]
- Wang, B.X.; Zeng, Y.H.; Wang, D.Y.; Zhao, R.; Xu, X. Responses of leaf stomata to environmental stresses in distribution and physiological characteristics. Agric. Res. Arid Areas 2010, 28, 112–122. (In Chinese) [Google Scholar]
- Zhou, Q.; Zhu, Z.L.; Shi, M.; Cheng, L.X. Growth and physicochemical changes of Carpinus betulus L. influenced by salinity treatments. Forests 2018, 9, 354. [Google Scholar] [CrossRef]
- Kharusi, L.A.; Assaha, D.; Al-Yahyai, R.; Yaish, M.W. Screening of Date Palm (Phoenix dactylifera L.) cultivars for salinity tolerance. Forests 2017, 8, 136. [Google Scholar] [CrossRef]
- Tuteja, N. Mechanisms of high salinity tolerance in plants. Methods Enzymol. 2007, 428, 419–438. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, P.M.; Bressan, R.A.; Zhu, J.K.; Bohnert, H.J. Plant cellular and molecular responses to high salinity. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Barazani, O.; Golan-Goldhirsh, A. Salt-driven interactions between Pistacia lentiscus and Salsola inermis. Environ. Sci. Pollut. Res. Int. 2009, 16, 855. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [PubMed]
- Ioana, M.P.; Sara, G.O.; Mohamad, A.H.; Adriana, F.S.; Oscar, V.; Jaime, P.; Radu, E.S.; Monica, B. Effects of drought and salinity on European Larch (Larix decidua Mill.) Seedlings. Forests 2018, 9, 320. [Google Scholar] [CrossRef]
- Li, S.H.; Ge, Z.M.; Xie, L.N.; Chen, W.; Yuan, L.; Wang, D.Q. Ecophysiological response of native and exotic salt marsh vegetation to waterlogging and salinity: Implications for the effects of sea-level rise. Sci. Rep. 2018, 8, 2441. [Google Scholar] [CrossRef]
- Silveira, J.A.G.; Araujo, S.A.M. Roots and leaves display contrasting osmotic adjustment mechanisms in response to NaCl-salinity in Atriplex nummularia. Environ. Exp. Bot. 2009, 66, 1–8. [Google Scholar] [CrossRef]
- Abdullakasim, S.; Kongpaisan, P.; Thongjang, P.; Saradhuldhat, P. Physiological responses of potted Dendrobium orchid to salinity stress. Hortic. Environ. Biotechnol. 2018, 59, 491–498. [Google Scholar] [CrossRef]
- Sotiropoulos, T.E. Effect of NaCl and CaCl2 on growth and contents of minerals, chlorophyll, proline and sugars in the apple rootstock M 4 cultured in vitro. Biol. Plant. 2007, 51, 177–180. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef]
- Zidan, M.A.; Elewa, M.A. Effect of salinity on germination, seedling growth and some metabolic changes in four plant species (Umbelliferae). Indian J. Plant Physiol. 1995, 38, 57–61. [Google Scholar] [CrossRef]
- Fatemeh, Z.; Mohammad, E.A.; Alireza, N.; Parviz, A. Physiological and morphological responses of the ‘Dargazi’ pear (Pyrus communis) to in vitro salinity. Agric. Conspec. Sci. 2018, 2, 169–174. [Google Scholar]
- Cristiano, G.; Camposeo, S.; Fracchiolla, M.; Vivaldi, G.A.; Lucia, B.D.; Cazzato, E. Salinity differentially affects growth and ecophysiology of two Mastic tree (Pistacia lentiscus L.) accessions. Forests 2016, 7, 156. [Google Scholar] [CrossRef]
- Ramoliya, P.J.; Pandey, A.N. Effect of increasing salt concentration on emergence, growth and survival of seedlings of Salvadora oleoides (Salvadoraceae). J. Arid Environ. 2002, 51, 121–132. [Google Scholar] [CrossRef]
- Hajlaoui, H.; Ayeb, N.E.; Garrec, J.P.; Denden, M. Differential effects of salt stress on osmotic adjustment and solutes allocation on the basis of root and leaf tissue senescence of two silage maize (Zea mays L.) varieties. Ind. Crop. Prod. 2010, 31, 122–130. [Google Scholar] [CrossRef]
- Aragüés, R.; Puy, J.; Royo, A.; Espada, J.L. Three-year field response of young olive trees (Olea europaea L. cv. Arbequina) to soil salinity: Trunk growth and leaf ion accumulation. Plant Soil 2005, 271, 265–273. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Michailidi, E.; Tzortzakis, N. Physiological and biochemical responses of Lavandula angustifolia to salinity under mineral foliar application. Front. Plant Sci. 2018, 9, 1–23. [Google Scholar] [CrossRef]
- Ashraf, M.; Ali, Q. Relative membrane permeability and activities of some antioxidant enzymes as the key determinants of salt tolerance in canola (Brassica napus L.). Environ. Exp. Bot. 2008, 63, 266–273. [Google Scholar] [CrossRef]
- Tarchoune, I.; Sgherri, C.; Izzo, R.; Lachaal, M.; Navari-Izzo, F.; Ouerghi, Z. Changes in the antioxidative systems of Ocimum basilicum L. (cv. Fine) under different sodium salts. Acta Physiol. Plant. 2012, 34, 1873–1881. [Google Scholar] [CrossRef]
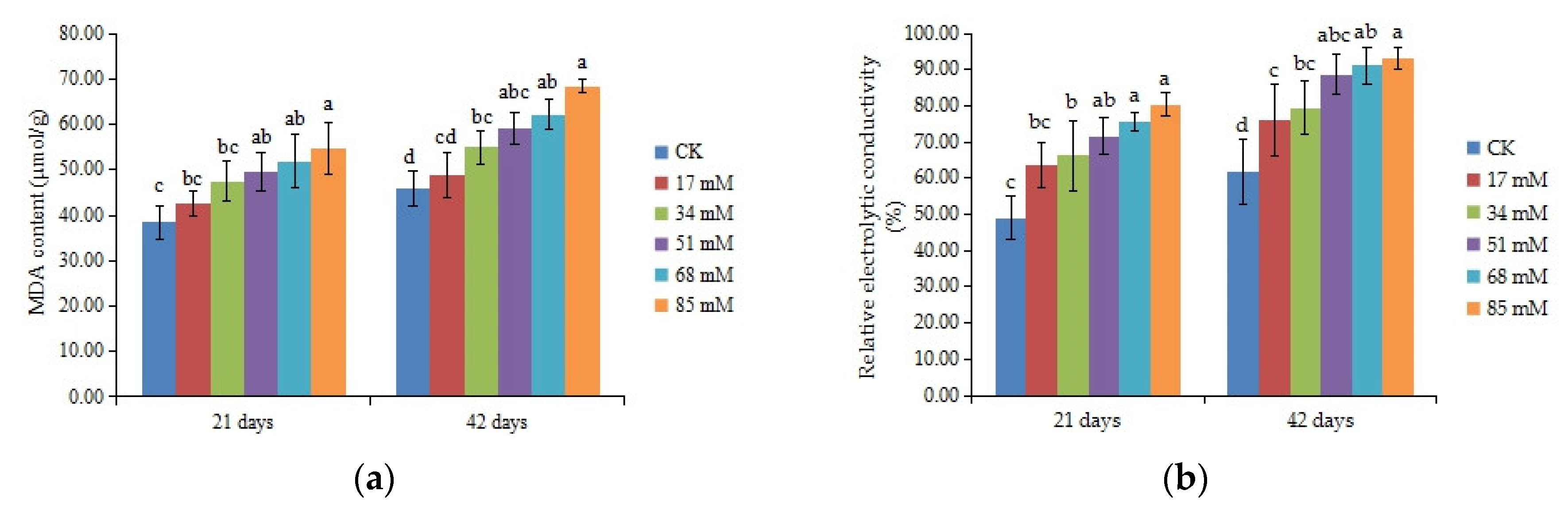
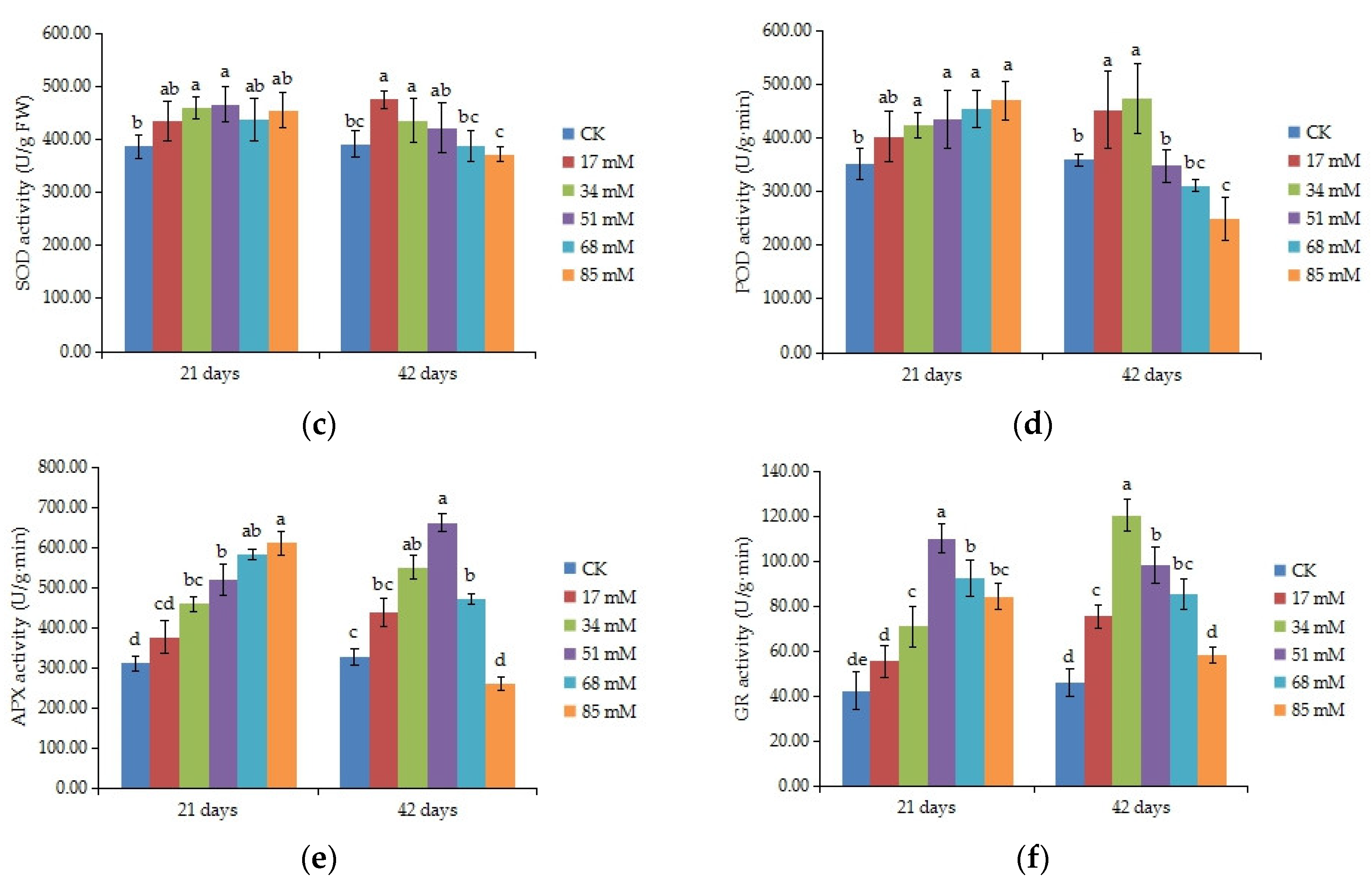
| Salinity Level (mM) | Survival Rate (%) | Stem Length Increase (cm) | Diameter Increase (cm) | Stem Biomass (DW, g) | Taproot Length (cm) |
| CK | 100 ± 0.00 a | 9.03 ± 0.76 a | 0.103 ± 0.10 a | 2.21 ± 0.22 a | 22.33± 1.86 a |
| 17 | 100 ± 0.00 a | 8.90 ± 0.79 a | 0.095 ± 0.04 ab | 2.02 ± 0.09 ab | 21.24 ± 1.54 a |
| 34 | 100 ± 0.00 a | 8.57 ± 0.60 a | 0.089 ± 0.07 ab | 1.72 ± 0.04 bc | 20.01 ± 1.06 ab |
| 51 | 92 ± 2.00 ab | 7.87 ± 0.78 a | 0.079 ± 0.09 b | 1.51 ± 0.15 cd | 18.65 ± 0.25 b |
| 68 | 75 ± 1.67 b | 5.07 ± 0.74 b | 0.060 ± 0.06 c | 1.38 ± 0.22 cd | 15.58 ± 0.88 bc |
| 85 | 45 ± 2.33 c | 4.67 ± 0.76 b | 0.048 ± 0.04 c | 1.18 ± 0.20 d | 12.91 ± 0.75 c |
| Salinity Level (mM) | Root Biomass (DW, g) | Leaf Area (cm2) | Leaf Biomass (DW, g) | Root/Shoot Ratio (R/S) | Total Biomass (DW, g) |
| CK | 1.63 ±0.10 a | 9.82 ± 0.48 a | 2.13 ± 0.24 a | 0.38 ± 0.02 b | 5.96 ± 0.55 a |
| 17 | 1.41 ± 0.09 ab | 9.32 ± 1.22 a | 1.88 ± 0.07 ab | 0.36 ± 0.03 c | 5.31 ± 0.15 ab |
| 34 | 1.27 ± 0.02 b | 8.89 ± 1.56 ab | 1.60 ± 0.04 bc | 0.38 ± 0.01 b | 4.60 ± 0.09 bc |
| 51 | 1.19 ± 0.12 b | 8.52 ± 1.01 ab | 1.33 ± 0.09 cd | 0.42 ± 0.01 a | 4.03 ± 0.35 cd |
| 68 | 1.12 ± 0.18 bc | 7.14 ± 1.33 b | 1.23 ± 0.09 cd | 0.43 ± 0.02 a | 3.72 ± 0.49 cd |
| 85 | 0.83 ± 0.15 c | 6.53 ± 0.68 c | 1.05 ± 0.21 d | 0.37 ± 0.01 bc | 3.05 ± 0.55 d |
| Salinity Level (mM) | Pn (μmol m−2s−1) | Tr (mmol m−2s−1) | Gs (μmol m−2s−1) | Ci (μmol m−2s−1) | WUEi |
| CK | 7.50 ± 0.70 a | 2.21 ± 0.09 a | 86.33 ± 6.03 a | 212.00 ± 19.67 d | 3.40 ± 0.39 a |
| 17 | 7.20 ± 0.66 a | 2.47 ± 0.08 a | 82.33 ± 8.08 ab | 227.67 ± 11.93 cd | 2.92 ± 0.33 a |
| 34 | 6.10 ± 0.46 ab | 2.31 ± 0.07 a | 70.00 ± 11.14 abc | 257.00 ± 14.93 bc | 2.64 ± 0.17 ab |
| 51 | 4.90 ± 0.50 b | 2.15 ± 0.11 a | 61.67 ± 6.11 bc | 264.33 ± 20.13 bc | 2.28 ± 0.19 b |
| 68 | 3.40 ± 0.40 c | 1.67 ± 0.11 b | 58.00 ± 8.54 c | 277.33 ± 10.07 ab | 2.05 ± 0.41 b |
| 85 | 1.20 ± 0.20 d | 1.47 ± 0.12 c | 51.33 ± 7.10 c | 312.00 ± 6.56 a | 0.82 ± 0.16 c |
| Salinity Level (mM) | ΦPSII | Fv/Fm | qP | ETR (μmol m−2s−1) | NPQ |
| CK | 0.50 ± 0.08 a | 0.64 ± 0.04 a | 0.91 ± 0.06 a | 274.99 ± 19.11 a | 0.81 ± 0.06 c |
| 17 | 0.47 ± 0.03 a | 0.66 ± 0.05 ab | 0.94 ± 0.08 a | 287.89 ± 25.37 a | 0.95 ± 0.08 b |
| 34 | 0.52 ± 0.04 a | 0.58 ± 0.03 abc | 0.96 ± 0.06 a | 266.40 ± 21.18 ab | 1.12 ± 0.13 ab |
| 51 | 0.45 ± 0.04 a | 0.53 ± 0.04 abc | 0.87 ± 0.07 ab | 256.81 ± 15.50 ab | 1.25 ± 0.07 ab |
| 68 | 0.41 ± 0.03 ab | 0.49 ± 0.10 bc | 0.81 ± 0.09 ab | 225.57 ± 22.38 b | 1.34 ± 0.10 a |
| 85 | 0.38 ± 0.06 b | 0.47 ± 0.06 c | 0.72 ± 0.06 b | 217.86 ± 25.25 b | 1.42 ± 0.12 a |
| Treatment (mM) | Stomatal Density (number mm−2) | Stomatal Length (μm) | Stomatal Width (μm) | Leaf Thickness (μm) | Palisade Tissue Thickness (μm) | Palisade Tissue Thickness/Leaf Thickness (%) |
|---|---|---|---|---|---|---|
| CK | 242.40 ± 22.12 ab | 15.54 ± 1.21 a | 12.69 ± 1.98 a | 69.23 ± 5.02 ab | 18.46 ± 3.21 c | 26.67 ± 3.68 c |
| 17 | 264.58 ± 25.38 a | 13.23 ± 1.43 a | 11.02 ± 2.06 a | 84.62 ± 6.87 a | 22.08 ± 2.60 bc | 26.09 ± 2.02 c |
| 34 | 230.06 ± 20.08 ab | 11.15 ± 1.51 ab | 9.68 ± 1.32 ab | 73.85± 6.93 ab | 24.34 ± 2.88 bc | 32.96 ± 4.41 bc |
| 51 | 190.65 ± 17.33 b | 9.87 ± 1.06 bc | 7.12 ± 1.58 b | 64.62 ± 7.11 bc | 25.69 ± 3.21 ab | 39.76 ± 4.12 ab |
| 68 | 162.58 ± 15.11 c | 8.23 ± 1.22 bc | 5.33 ± 1.82 c | 61.54 ± 5.04 bc | 27.54 ± 4.08 a | 44.75 ± 5.05 ab |
| 85 | 135.62 ± 12.49 d | 7.85 ± 1.18 c | 4.58 ± 0.95 c | 59.61 ± 5.36 c | 28.77 ± 4.65 a | 48.26 ± 3.82 a |
| Salinity Level (mM) | K/Na | Ca/Na | Mg/Na | |
|---|---|---|---|---|
| Root | CK | 4.34 ± 0.56 a | 6.20 ± 0.99 a | 2.23 ± 0.35 a |
| 17 | 1.97 ± 0.36 b | 4.89 ± 0.74 a | 1.53 ± 0.27 b | |
| 34 | 0.98 ± 0.13 c | 3.62 ± 0.35 b | 0.77 ± 0.10 c | |
| 51 | 0.82 ± 0.01 c | 2.45 ± 0.04 b | 0.67 ± 0.12 c | |
| 68 | 1.23 ± 0.15 bc | 2.70 ± 0.31 b | 0.87 ± 0.11 c | |
| 85 | 1.00 ± 0.10 c | 2.58 ± 0.13 b | 0.75 ± 0.04 c | |
| Stem | CK | 33.77 ± 4.87 a | 101.68 ± 14.97 a | 10.52 ±1.44 a |
| 17 | 18.52 ±0.88 b | 57.57 ± 2.65 b | 5.41 ± 0.20 b | |
| 34 | 8.45 ± 0.50 c | 23.59 ± 1.54 bc | 2.70 ± 0.16 c | |
| 51 | 12.54 ± 0.72 c | 28.79 ± 0.94 c | 3.94 ± 0.23 bc | |
| 68 | 7.92 ± 0.24 c | 26.93 ± 0.27 c | 2.89 ± 0.01 c | |
| 85 | 2.09 ± 0.03 d | 7.34 ± 0.16 d | 0.86 ± 0.04 d | |
| Leaf | CK | 90.77 ± 15.49 a | 236.54 ± 39.71 a | 42.86 ± 6.34 a |
| 17 | 71.10 ± 12.75 ab | 150.01 ± 24.05 b | 26.05 ± 4.08 b | |
| 34 | 59.19 ± 3.42 bc | 112.56 ± 3.62 bc | 21.23 ± 0.87 b | |
| 51 | 37.55 ± 2.41 cd | 66.32 ± 3.21 cd | 12.02 ± 0.74 c | |
| 68 | 19.81 ± 1.04 de | 40.44 ± 2.13E de | 6.75 ± 0.22 dc | |
| 85 | 7.10 ± 0.19 e | 12.52 ± 0.10 e | 2.40 ± 0.03 d | |
| Whole plant | CK | 128.88 ± 20.92 a | 344.43 ± 55.67 a | 55.61 ± 8.13 a |
| 17 | 91.58 ± 13.99 b | 212.47 ± 27.45 b | 33.00 ± 4.55 b | |
| 34 | 68.63 ± 4.05 c | 139.77 ± 5.51 c | 24.70 ± 1.12 bc | |
| 51 | 50.91 ± 3.13 c | 97.56 ± 4.19 cd | 16.64 ± 1.09 cd | |
| 68 | 28.96 ± 1.43 d | 70.07 ± 2.70 d | 10.52 ± 0.35 d | |
| 85 | 10.19 ± 0.32 d | 22.44 ± 0.39 e | 4.01 ± 0.11 e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Shi, M.; Zhu, Z.; Cheng, L. Ecophysiological Responses of Carpinus turczaninowii L. to Various Salinity Treatments. Forests 2019, 10, 96. https://doi.org/10.3390/f10020096
Zhou Q, Shi M, Zhu Z, Cheng L. Ecophysiological Responses of Carpinus turczaninowii L. to Various Salinity Treatments. Forests. 2019; 10(2):96. https://doi.org/10.3390/f10020096
Chicago/Turabian StyleZhou, Qi, Man Shi, Zunling Zhu, and Longxia Cheng. 2019. "Ecophysiological Responses of Carpinus turczaninowii L. to Various Salinity Treatments" Forests 10, no. 2: 96. https://doi.org/10.3390/f10020096
APA StyleZhou, Q., Shi, M., Zhu, Z., & Cheng, L. (2019). Ecophysiological Responses of Carpinus turczaninowii L. to Various Salinity Treatments. Forests, 10(2), 96. https://doi.org/10.3390/f10020096




