Improving the Efficiency of Oncological Diagnosis of the Breast Based on the Combined Use of Simulation Modeling and Artificial Intelligence Algorithms
Abstract
1. Introduction
- ↗
- Non-invasive method;
- ↗
- Very fast temperature measurement;
- ↗
- Inexpensive method;
- ↗
- No contraindications;
- ↗
- No restrictions on the procedure frequency;
- ↗
- It is possible to measure both the thermodynamic temperature T and local changes in the electromagnetic characteristics of the biological tissue (primarily the electrical conductivity), as MWR measures the brightness temperature by the electric field;
- ↗
- The device for measuring brightness temperature is a portable system.
- ↘
- Low accuracy of building temperature fields compared to the resolution of structures when using ultrasound, tomography, mammography, or magnetic resonance elastography;
- ↘
- Poor spatial error in measuring the brightness temperature in the plane and along the depth of the tissue;
- ↘
- The MWR method determines only the brightness temperature , which requires additional data processing to relate to the real thermodynamic temperature T and is model-dependent;
- ↘
- Restrictions on the air temperature in the room where measurements are taken.
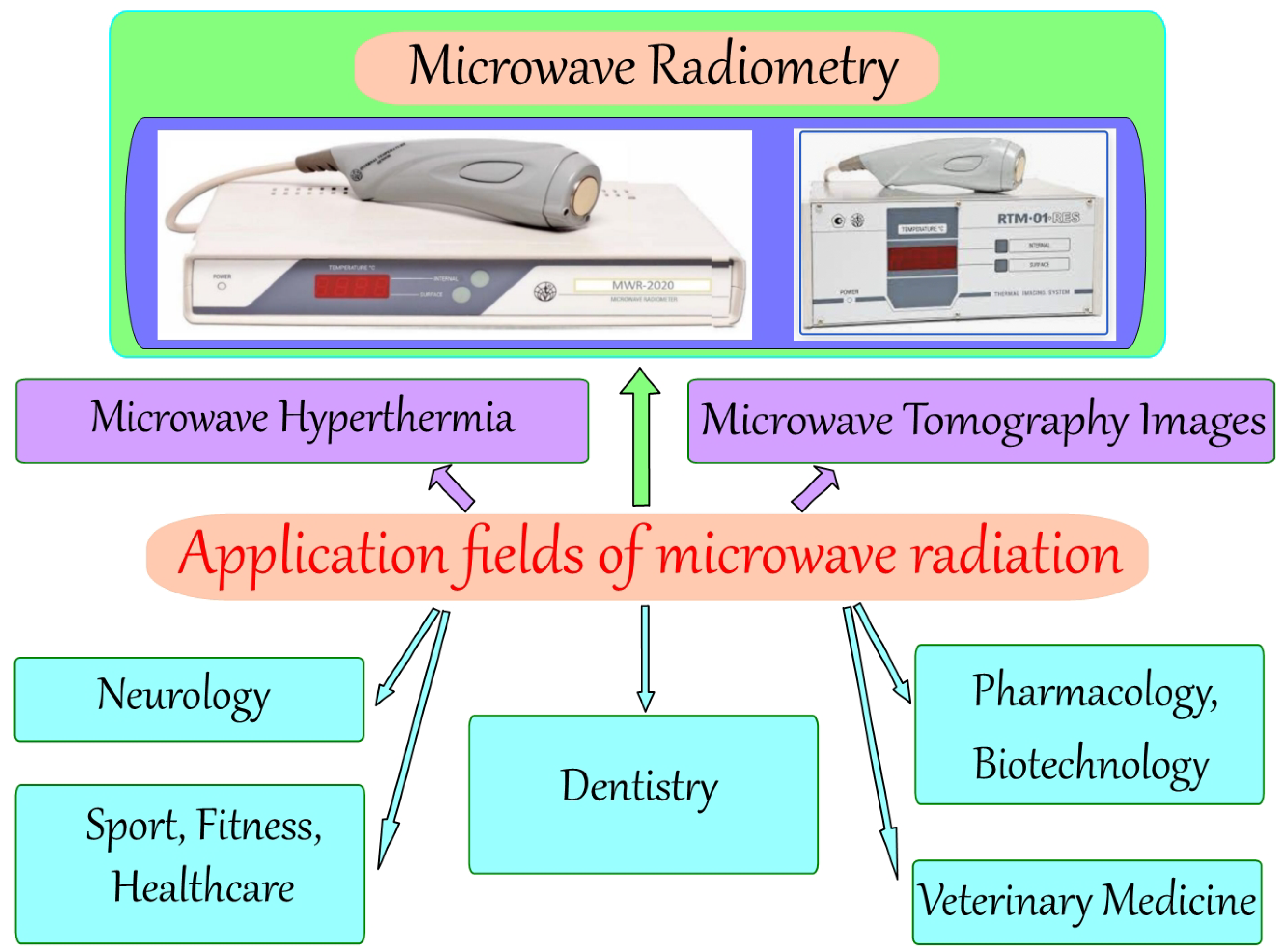
1.1. Application Fields of Microwave Radiation in Medicine
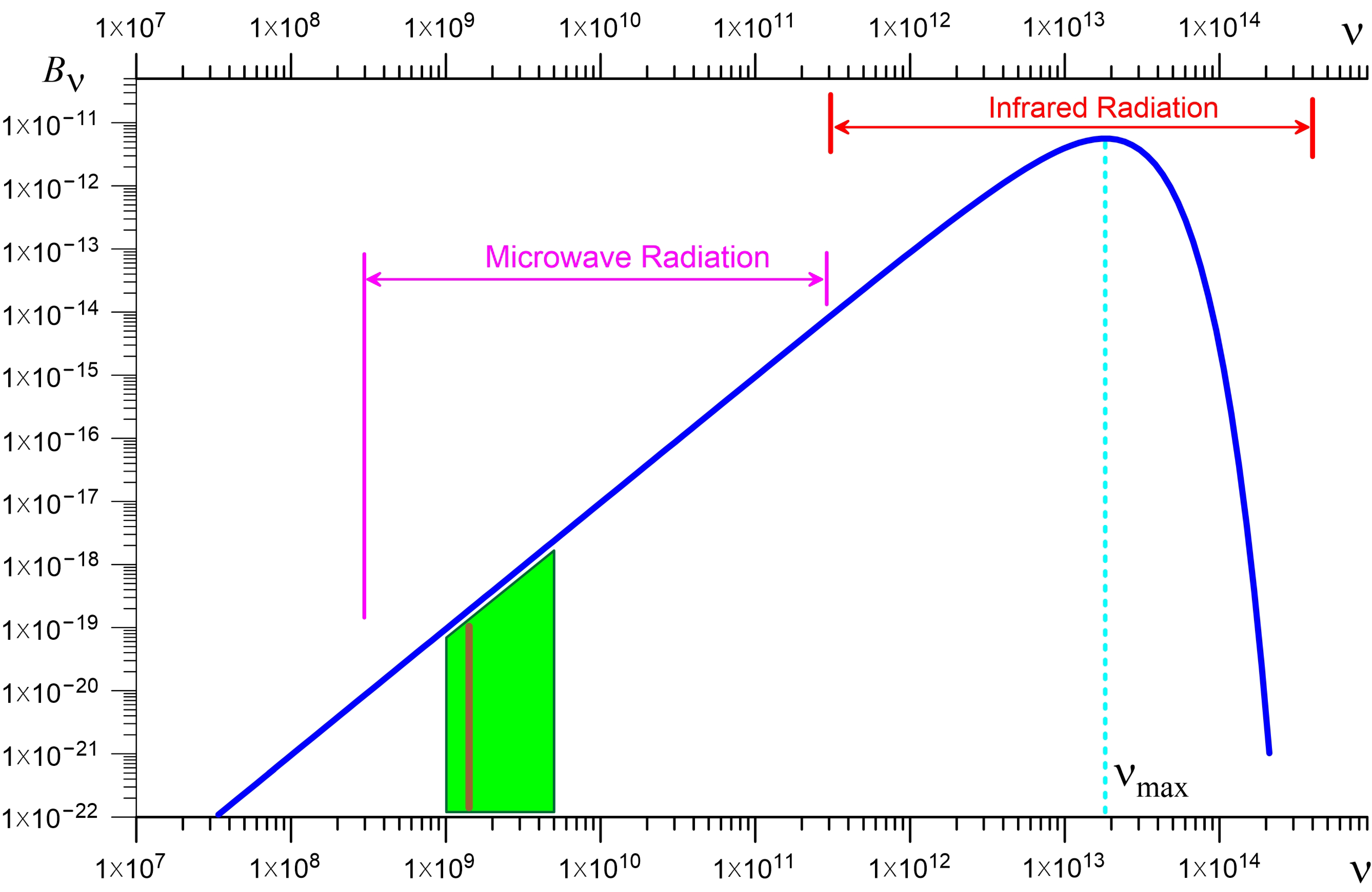
1.2. Diagnostics Based on Microwave Radiometry
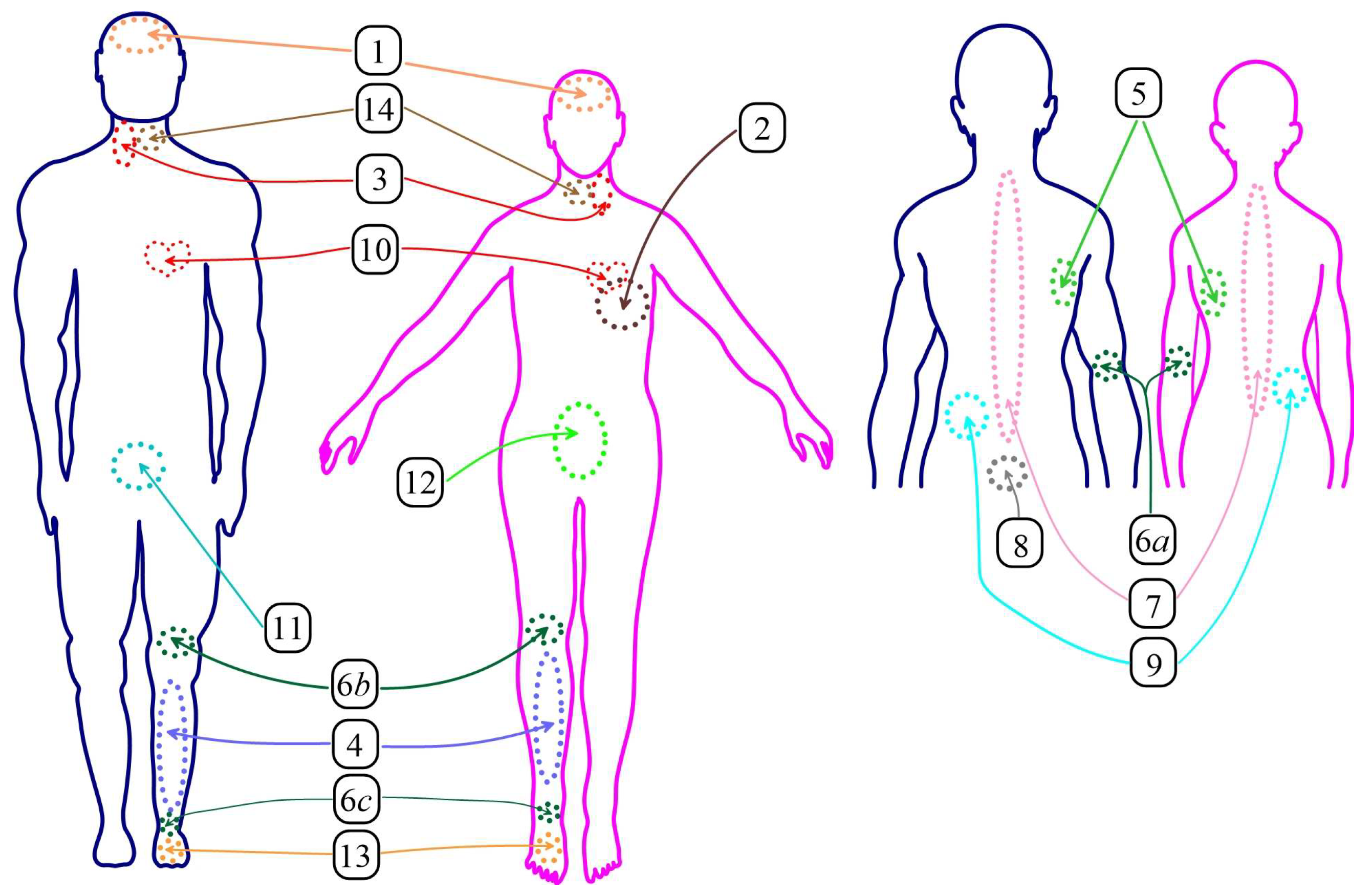
1.3. MWR Method for Detecting Breast Cancer
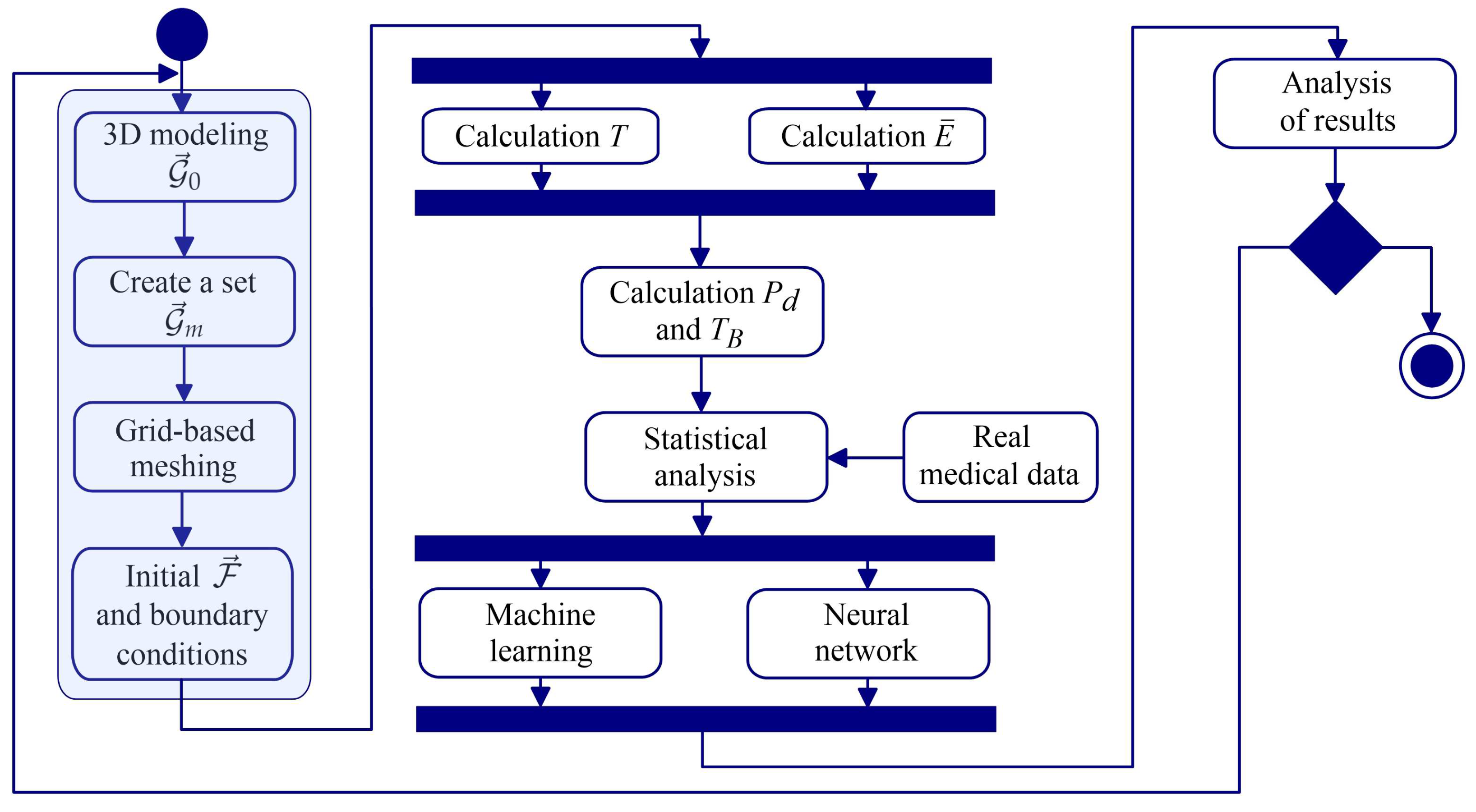
2. Materials and Methods
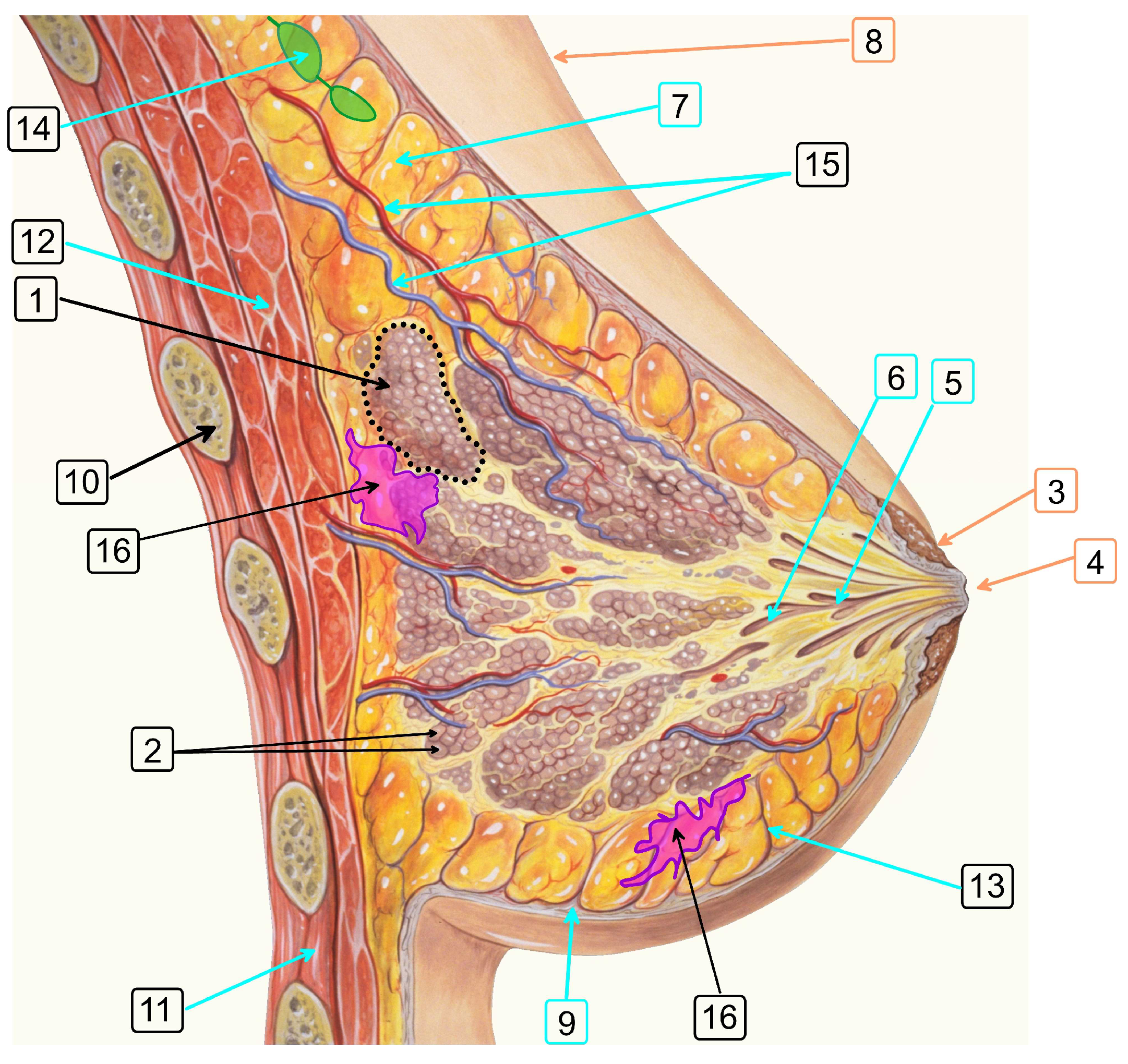
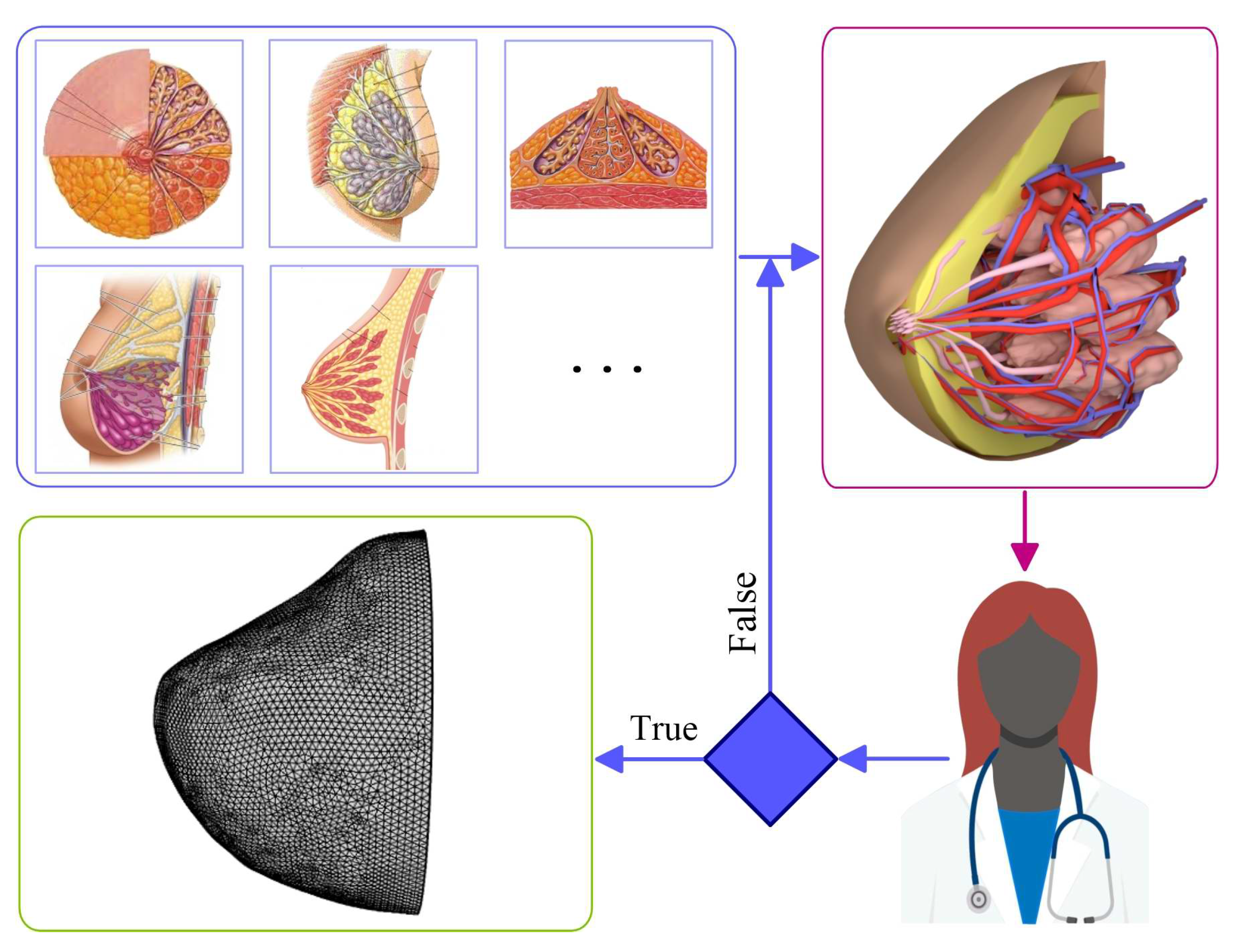
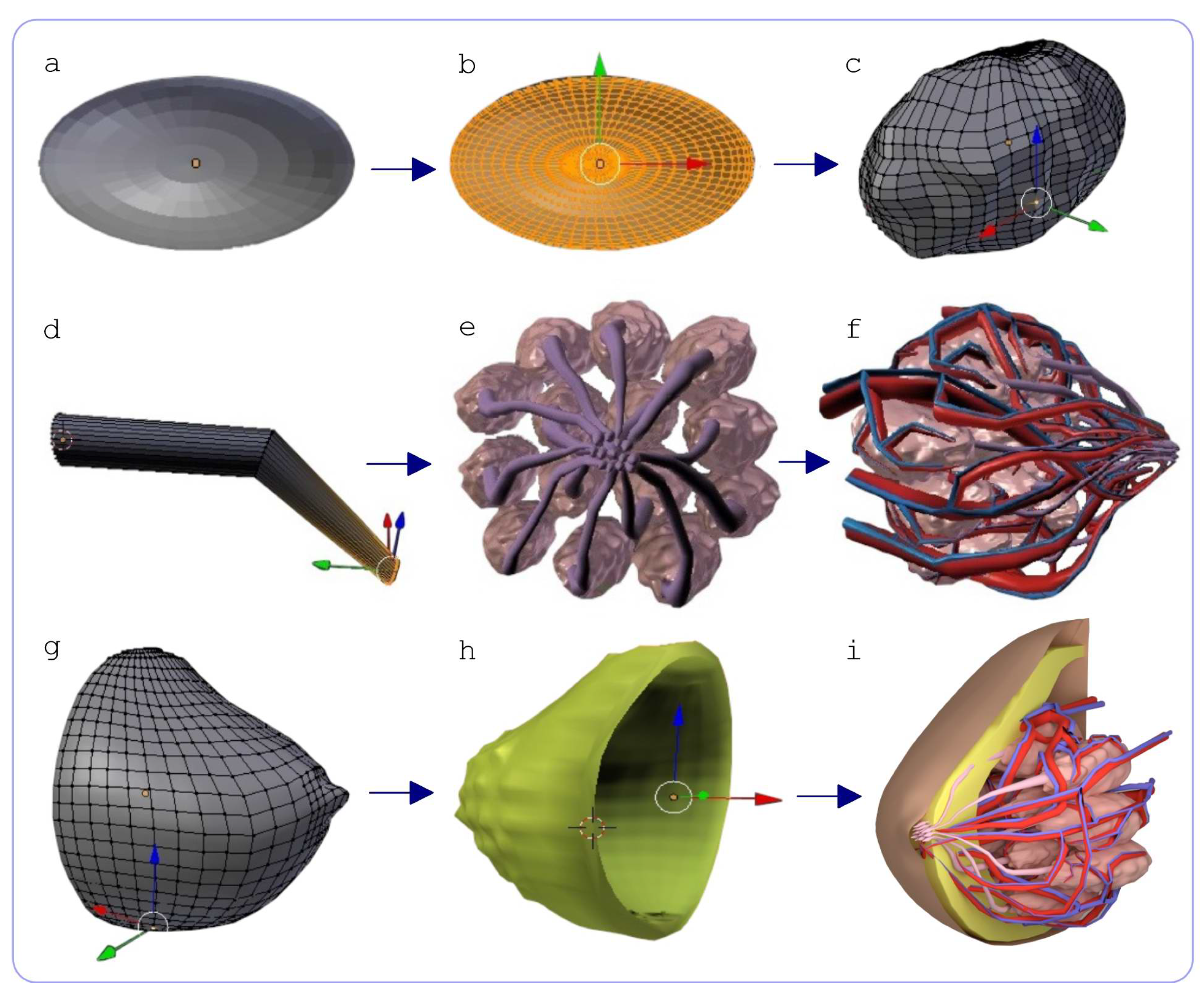
2.1. Method for 3D Reconstruction of Multicomponent Tissue
2.2. Electrical and Thermal Characteristics of Biological Tissues
2.3. Models of the Dynamics of Thermal and Radiation Fields
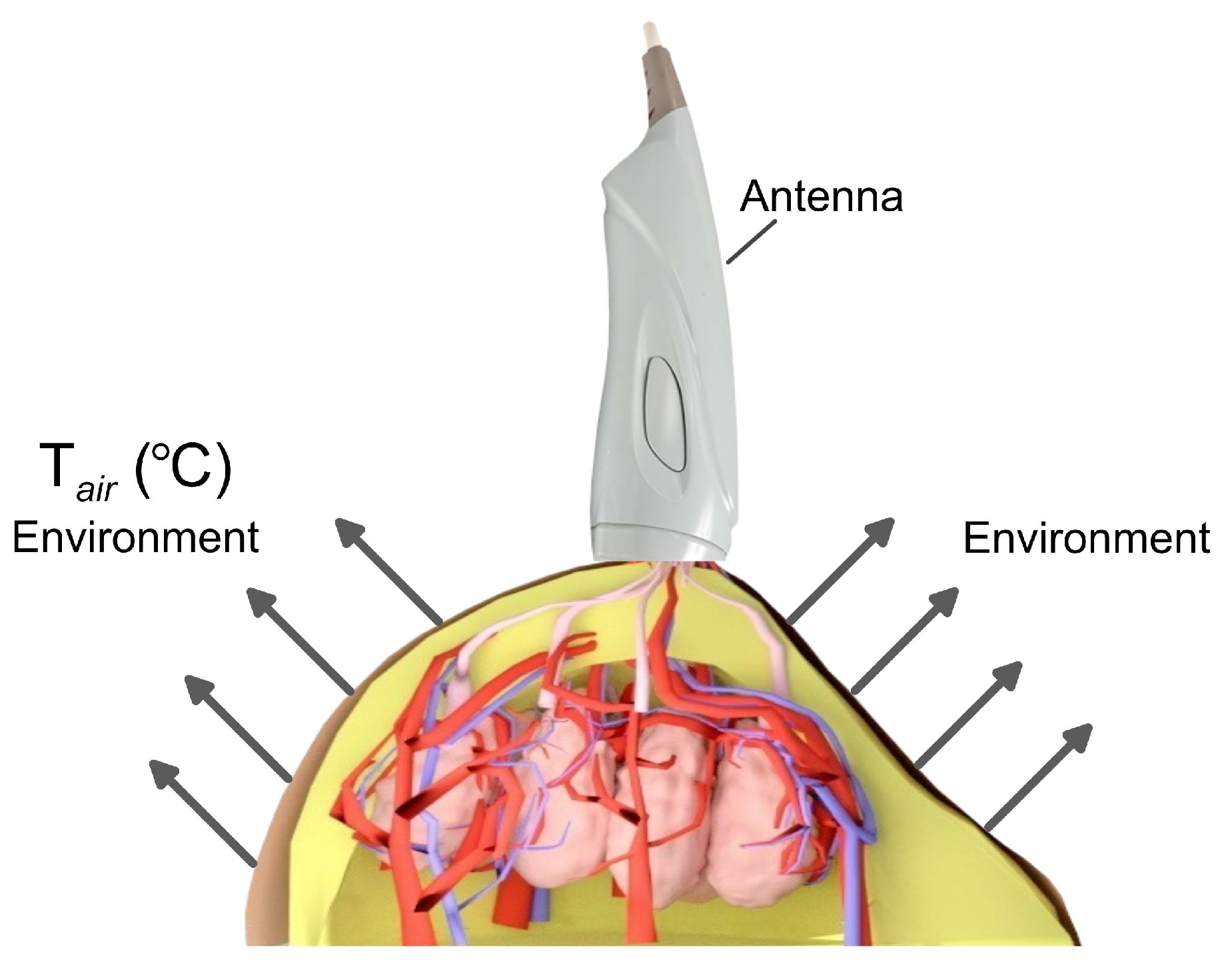
2.4. Calculation of Brightness Temperature in Biological Tissues
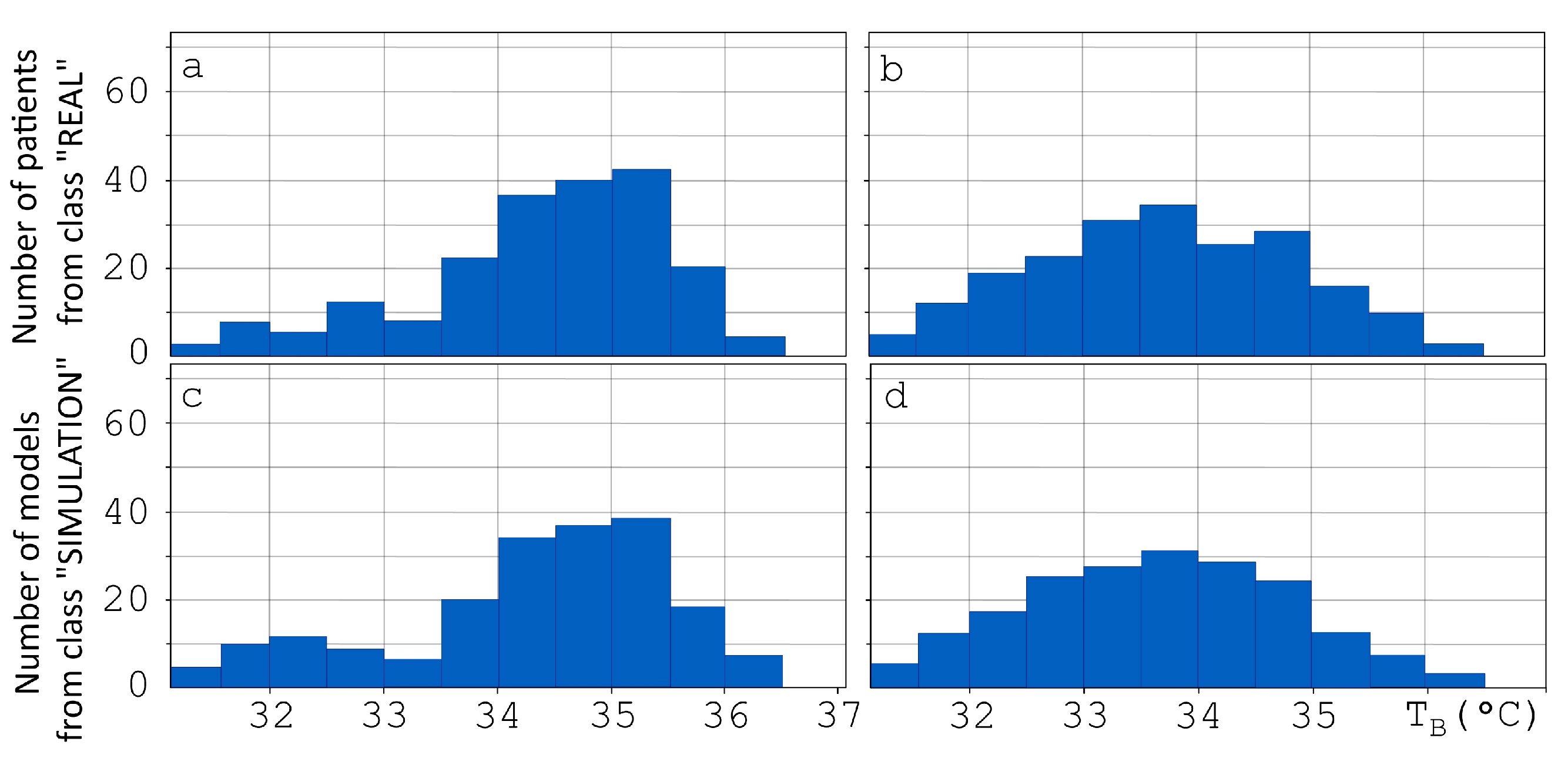
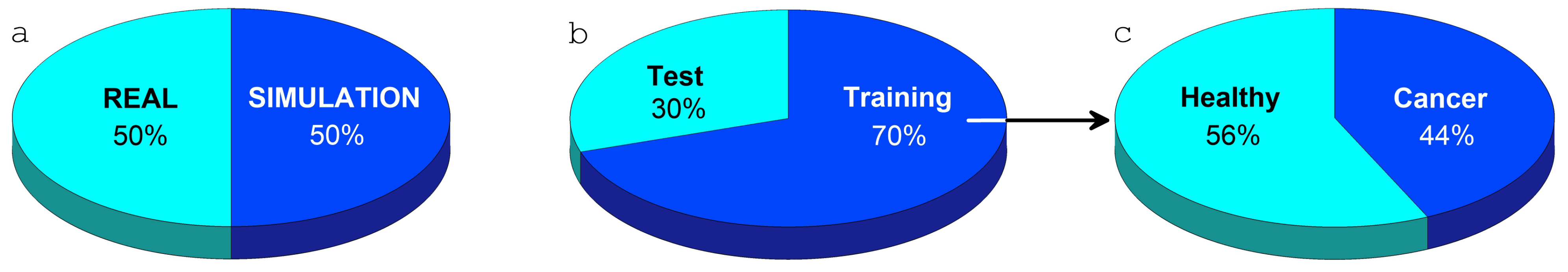
2.5. Breast Thermometric Database
2.6. Validation of Computer Model for Diagnostics of Oncological Diseases Based on Machine Learning
- Building a classifier SVM over a slice with the dataset “REAL” for the class “H” and classifying the dataset “SIMULATION”;
- Building a classifier SVM over a slice with the dataset “SIMULATION” for the class “H” and classifying the dataset “REAL”;
- Analysis of the results and values of characteristics that the classifier considers incorrect;
- The model parameters are changed for the subsequent generation of new data as a result of a series of simulations, with the return to step 1 if necessary.
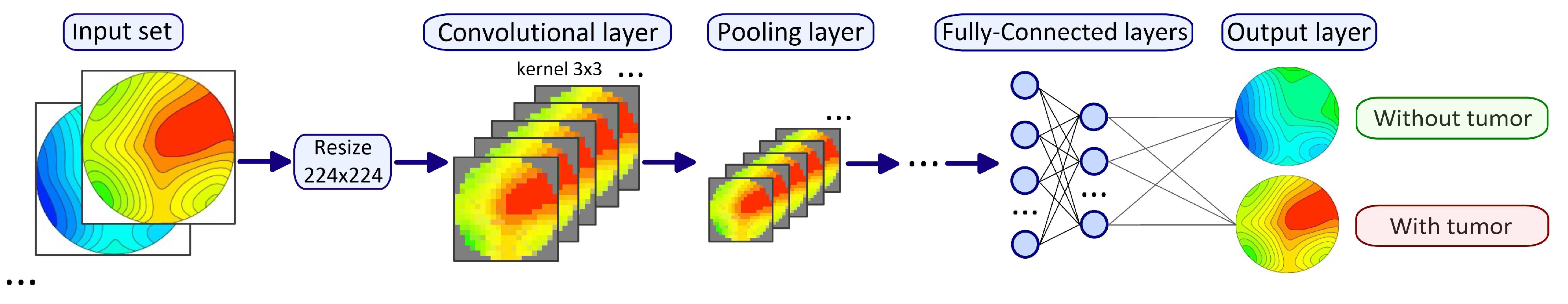
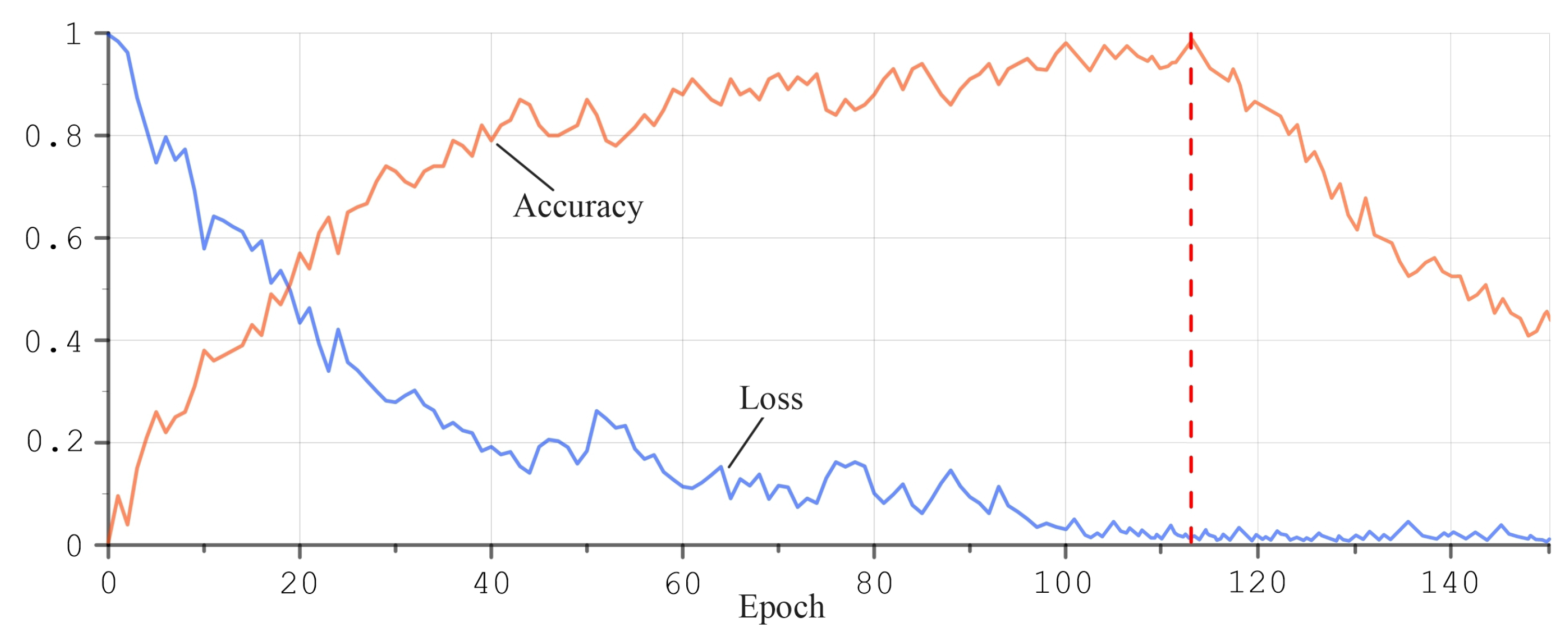
2.7. Artificial Intelligence Algorithms for Temperature Data Processing
3. Results
3.1. Conditions for Detecting Weak Tumors
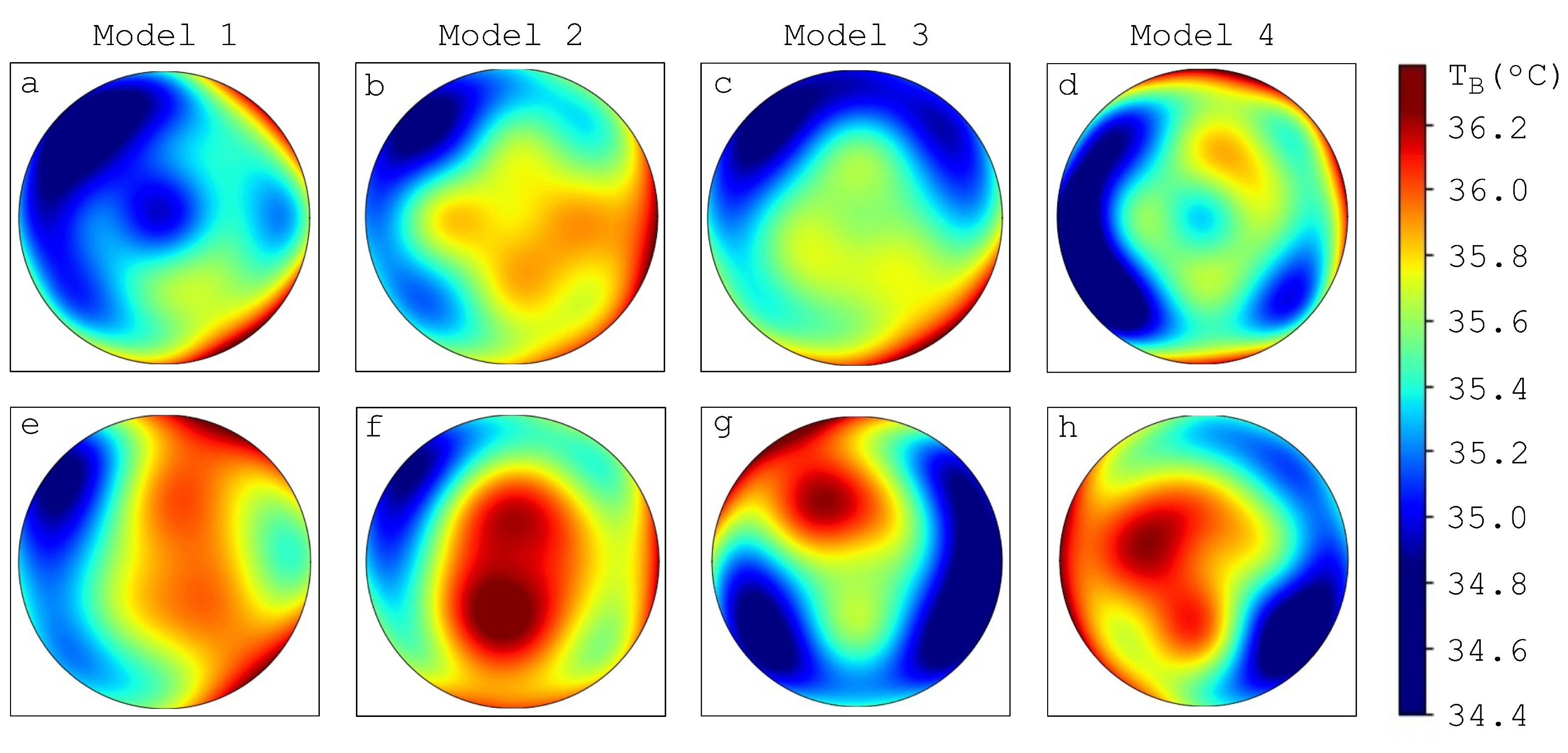
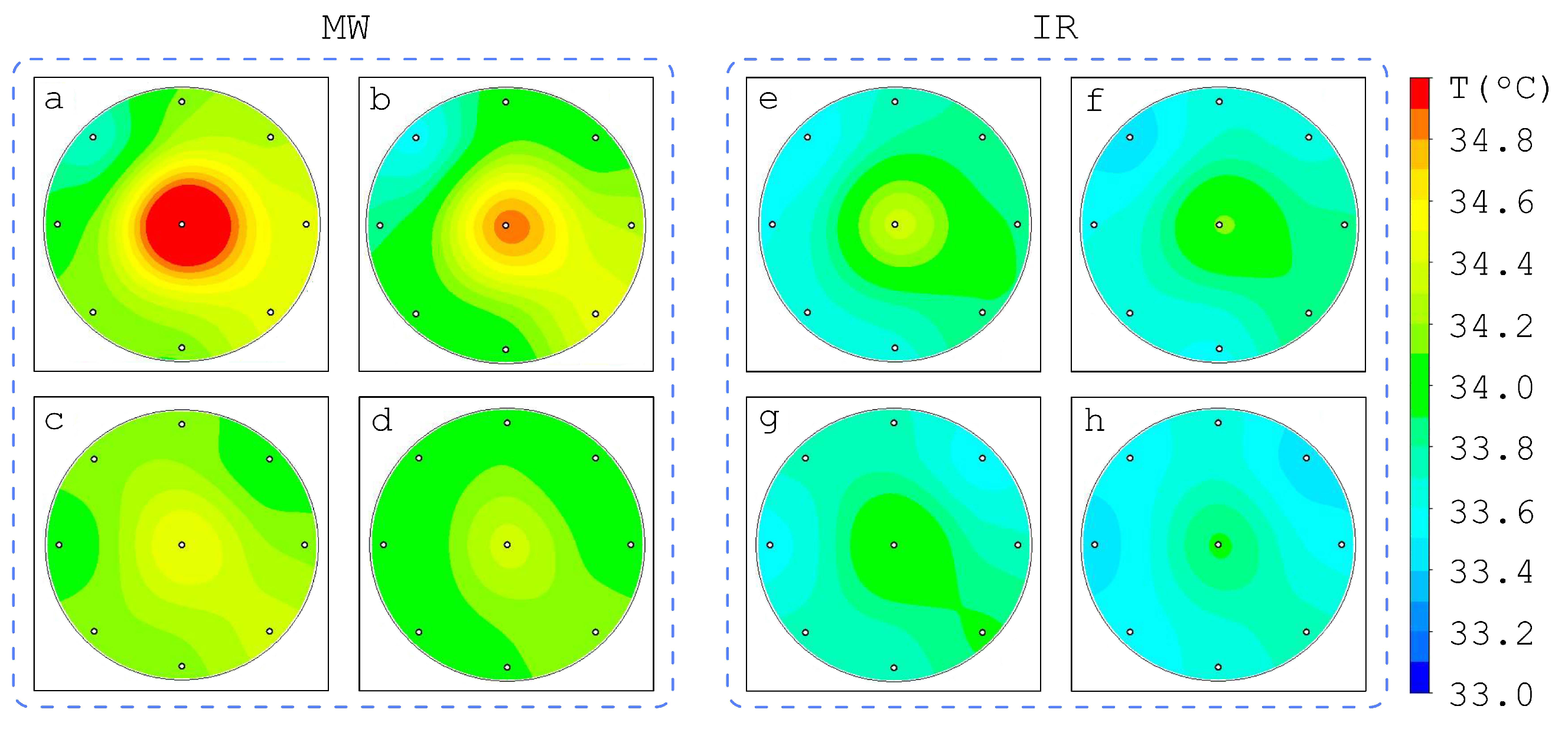
3.2. Influence of Tumor Spatial Location on the Brightness Temperature
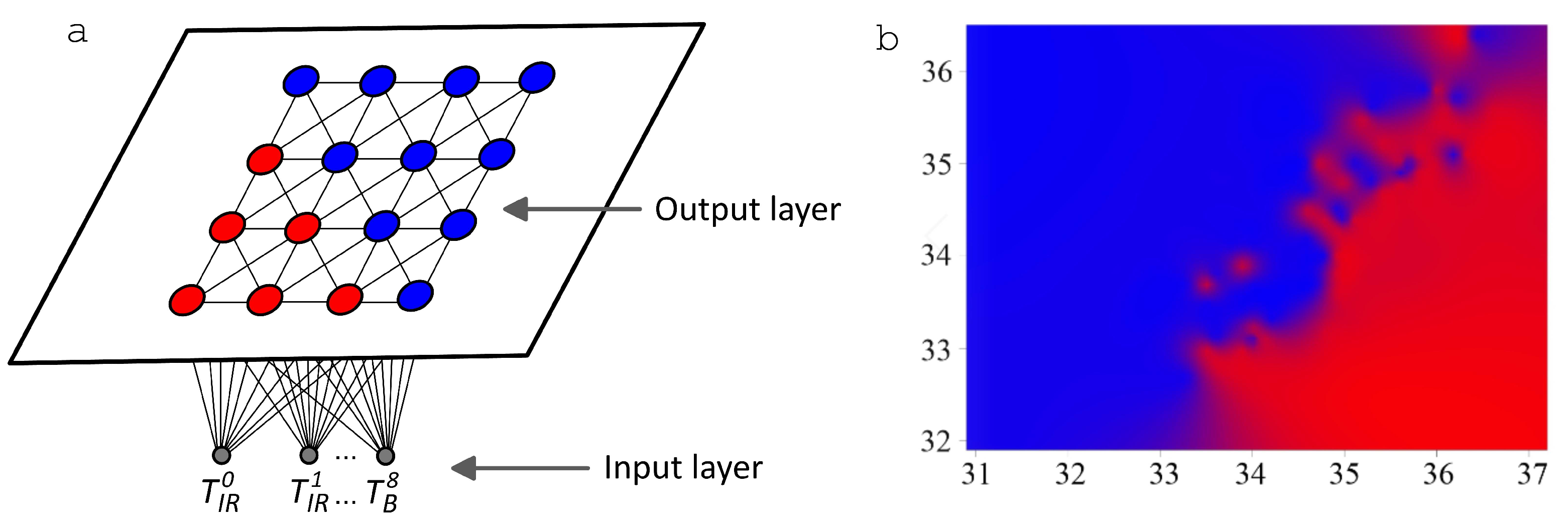
3.3. Application of Artificial Neural Networks for MWR Data
4. Conclusions and Discussion
- (1)
- We propose a dataset formation method based on combining two samples. One contains the results of real temperature measurements (“REAL”). The second sample is based on simulations of thermal and radiation processes inside breast models (“SIMULATION”). The sample “SIMULATION” must satisfy the requirement of statistical closeness to the data “REAL”. This combination of data can significantly increase the amount of data to be processed. The method provides a unique opportunity to evaluate the parameters of the tumor, primarily the size and power of heat generated by the tumor.
- (2)
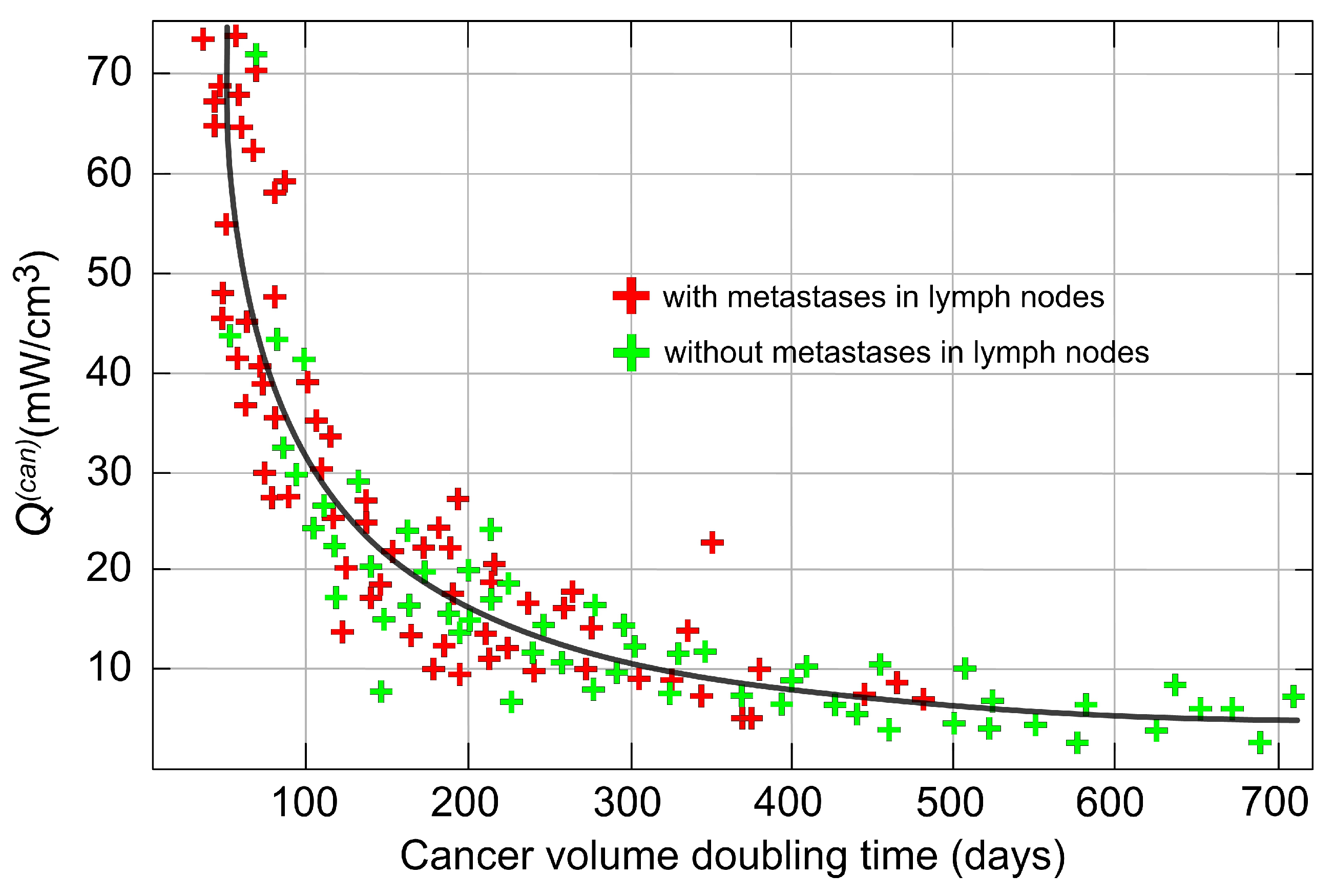
- Using the combined dataset, tumors as small as 0.5 cm can be detected if they are in the rapid growth stage [62], when volume doubling occurs in approximately 100 days or fewer.
- (3)
- Convolutional neural networks for the “SIMULATION” sample give 71.5 percent accuracy in determining the location of the tumor based on the criterion of being in a given breast sector. We note the good agreement of this result with the estimates when using a multilayer perceptron network within 62–64 percent [108].
- (4)
- An important feature of the MWR diagnostics is the ability to simultaneously have high values of sensitivity and specificity. As a rule, mammography, ultrasound and MRI methods are better at detecting cancer patients (high sensitivity), but poorer at recognizing healthy people (low specificity). This is due to the difference in physical methods, when mammography, ultrasound and MRI are based on the determination of structural changes in tissues. The MWR method detects temperature anomalies caused by inflammatory processes due to disease.
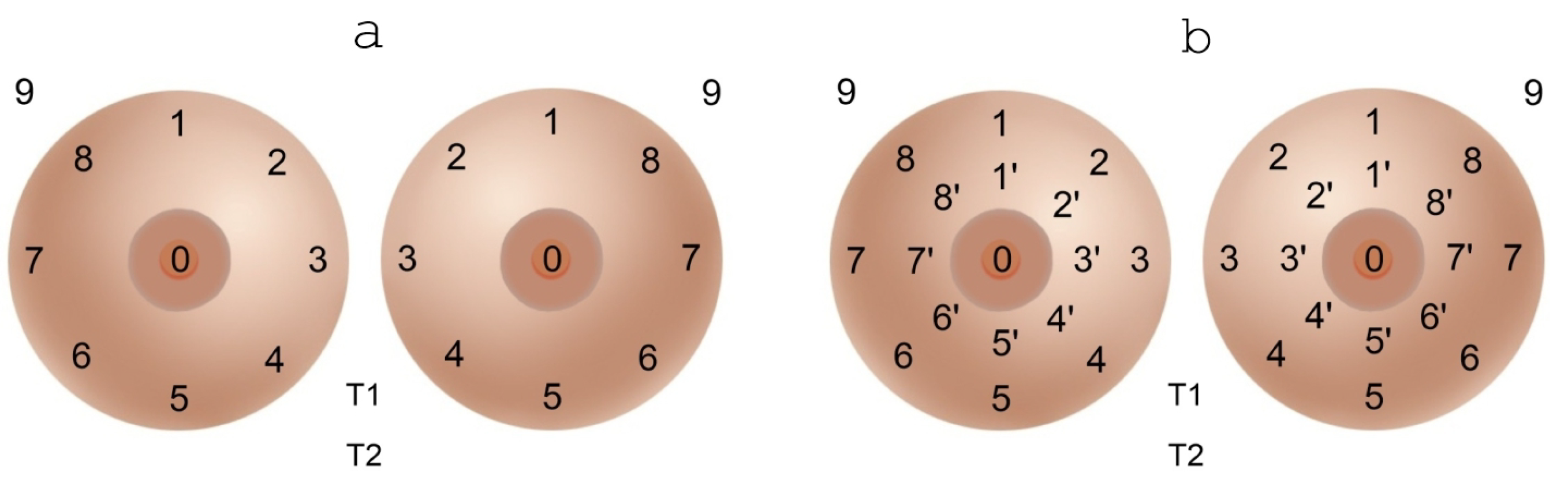
- (5)
- We propose a new 17-point breast examination scheme (instead of the traditional 9-point scheme), which allows us to build a better picture of temperature fields. Our analysis showed an increase in both sensitivity and specificity for this modified diagnostic algorithm.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moloney, B.M.; McAnena, P.F.; Abd Elwahab, S.M.; Fasoula, A.; Duchesne, L.; Cano, J.D.G.; Glynn, C.; O’Connell, A.; Ennis, R.; Lowery, A.J.; et al. Microwave Imaging in Breast Cancer—Results from the First-In-Human Clinical Investigation of the Wavelia System. Acad. Radiol. 2022, 29, S211–S222. [Google Scholar] [CrossRef]
- Levshinskii, V.; Polyakov, M.; Losev, A.; Khoperskov, A. Verification and Validation of Computer Models for Diagnosing Breast Cancer Based on Machine Learning for Medical Data Analysis. Commun. Comput. Inf. Sci. 2019, 1084, 447–460. [Google Scholar] [CrossRef]
- Stauffer, P.R.; Snow, B.W.; Rodrigues, D.B.; Salahi, S.; Oliveira, T.R.; Reudink, D.; Maccarini, P.F. Non-invasive measurement of brain temperature with microwave radiometry: Demonstration in a head phantom and clinical case. Neuroradiol. J. 2014, 27, 3–12. [Google Scholar] [CrossRef]
- Osmonov, B.; Ovchinnikov, L.; Galazis, C.; Emilov, B.; Karaibragimov, M.; Seitov, M.; Vesnin, S.; Losev, A.; Levshinskii, V.; Popov, I.; et al. Passive microwave radiometry for the diagnosis of coronavirus disease 2019 lung complications in Kyrgyzstan. Diagnostics 2021, 11, 259. [Google Scholar] [CrossRef]
- Fasoula, A.; Duchesne, L.; Gil Cano, J.D.; Moloney, B.M.; Abd Elwahab, S.M.; Kerin, M.J. Automated Breast Lesion Detection and Characterization with the Wavelia Microwave Breast Imaging System: Methodological Proof-of-Concept on First-in-Human Patient Data. Appl. Sci. 2021, 11, 9998. [Google Scholar] [CrossRef]
- Moloney, B.M.; O’Loughlin, D.; Abd Elwahab, S.; Kerin, M.J. Breast Cancer Detection—A Synopsis of Conventional Modalities and the Potential Role of Microwave Imaging. Diagnostics 2020, 10, 103. [Google Scholar] [CrossRef]
- Robert, J.; Edrich, J.; Thouvenot, P.; Gautherie, M.; Escanye, J.M. Millimeter-wave thermography: Preliminary clinical findings in head and neck diseases. J. Microw. Power 1979, 14, 131–134. [Google Scholar] [CrossRef]
- Levshinskii, V.; Galazis, C.; Losev, A.; Zamechnik, T.; Kharybina, T.; Vesnin, S.; Goryanin, I. Using AI and passive medical radiometry for diagnostics (MWR) of venous diseases. Comput. Methods Programs Biomed. 2022, 215, 106611. [Google Scholar] [CrossRef]
- Barrett, A.H.; Myers, P.C. Subcutaneous temperatures: A method of noninvasive sensing. Science 1975, 190, 669–671. [Google Scholar] [CrossRef]
- Barrett, A.H.; Myers, P.C.; Sadowsky, N.L. Detection of breast cancer by microwave radiometry. Radio Sci. 1977, 12, 167–171. [Google Scholar] [CrossRef]
- Edrich, J.; Smyth, C.J. Arthritis inflammation monitored by subcutaneous millimeter wave thermography. J. Rheumatol. 1978, 5, 59–67. [Google Scholar]
- Polyakov, M.V.; Popov, I.E.; Losev, A.G.; Khoperskov, A.V. Application of computer simulation results and machine learning in analysis of microwave radiothermometry data. Math. Phys. Comput. Simul. 2021, 24, 27–37. [Google Scholar] [CrossRef]
- Germashev, I.; Dubovskaya, V.; Losev, A. Substantiation of the Result of the Work of the Cyber-Physical System for Breast Cancer Diagnostics. Stud. Syst. Decis. Control 2022, 416, 3–13. [Google Scholar] [CrossRef]
- Ekici, S.; Jawzal, H. Breast cancer diagnosis using thermography and convolutional neural networks. Med. Hypotheses 2020, 137, 109542. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Losev, A.G.; Svetlov, A.V. Artificial Intelligence Algorithms in Diagnosis of Breast Cancer. Smart Innov. Syst. Technol. 2022, 287, 175–182. [Google Scholar] [CrossRef]
- Paulides, M.M.; Dobsicek Trefna, H.; Curto, S.; Rodrigues, D.B. Recent technological advancements in radiofrequency-andmicrowave-mediated hyperthermia for enhancing drug delivery. Adv. Drug Deliv. Rev. 2020, 163–164, 3–18. [Google Scholar] [CrossRef]
- Schiavon, G.; Capone, G.; Frize, M.; Zaffagnini, S.; Candrian, C.; Filardo, G. Infrared Thermography for the Evaluation of Inflammatory and Degenerative Joint Diseases: A Systematic Review. Cartilage 2021, 13, 1790S. [Google Scholar] [CrossRef]
- Hassan, A.M.; El-Shenawee, M. Review of electromagnetic techniques for breast cancer detection. IEEE Rev. Biomed. Eng. 2011, 4, 103–118. [Google Scholar] [CrossRef]
- Sowmya, S.; Raghavendra Swamy, K.N.; Vardhini, H.; Keerthi, R.; Alla, R.K.; Aradya, A. Effect of Microwave Sterilisation on the Dimensional Property of Complete Denture: A Review. Trends Biomater. Artif. Organs 2022, 36, 54–57. [Google Scholar]
- Liang, X.; Yu, X.; Gao, T. Machine learning with magnetic resonance imaging for prediction of response to neoadjuvant chemotherapy in breast cancer: A systematic review and meta-analysis. Eur. J. Radiol. 2022, 150, 110247. [Google Scholar] [CrossRef] [PubMed]
- Losev, A.G.; Popov, I.E.; Petrenko, A.Y.; Gudkov, A.G.; Vesnin, S.G.; Chizhikov, S.V. Some Methods for Substantiating Diagnostic Decisions Made Using Machine Learning Algorithms. Biomed. Eng. 2022, 55, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Ragab, D.A.; Attallah, O.; Sharkas, M.; Ren, J.; Marshall, S. A framework for breast cancer classification using Multi-DCNNs. Comput. Biol. Med. 2021, 131, 104245. [Google Scholar] [CrossRef]
- Ullah, R.; Arslan, T. Parallel Delay Multiply and Sum Algorithm for Microwave Medical Imaging Using Spark Big Data Framework. Algorithms 2021, 14, 157. [Google Scholar] [CrossRef]
- Scapaticci, R.; Di Donato, L.; Catapano, I.; Crocco, L. A feasibility study on microwave imaging for brain stroke monitoring. Prog. Electromagn. Res. B 2012, 40, 305–324. [Google Scholar] [CrossRef]
- Huang, C.F.; Tien, Y.W.; Chen, C.Y.; Lin, X.Z. Design techniques for antenna needles used in microwave hyperthermia therapy for tumor treatment. In Proceedings of the 2014 IEEE-APS Topical Conference on Antennas and Propagation in Wireless Communications (APWC), Palm Beach, Aruba, 3–9 August 2014; pp. 37–39. [Google Scholar] [CrossRef]
- Witte, R.S.; Tamimi, E.A. Emerging photoacoustic and thermoacoustic imaging technologies for detecting primary and metastatic cancer and guiding therapy. Clin. Exp. Metastasis 2022, 39, 213–217. [Google Scholar] [CrossRef]
- Moon, T.J.; Brace, C.L. Design of a dual slot antenna for small animal microwave ablation studies. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 348–351. [Google Scholar] [CrossRef]
- Goryanin, I.; Karbainov, S.; Shevelev, O.; Tarakanov, A.; Redpath, K.; Vesnin, S.; Ivanov, Y. Passive microwave radiometry in biomedical studies. Drug Discov. Today 2020, 25, 757–763. [Google Scholar] [CrossRef]
- Groumpas, E.; Koutsoupidou, M.; Karanasiou, I.S.; Papageorgiou, C.; Uzunoglu, N. Real-time passive brain monitoring system using near-field microwave radiometry. IEEE Trans. Biomed. Eng. 2020, 67, 158–165. [Google Scholar] [CrossRef]
- Available online: http://www.resltd.ru/eng/radiometry/ (accessed on 11 August 2022).
- Sedankin, M.; Chupina, D.; Vesnin, S.; Nelin, I.; Skuratov, V. Development of a miniature microwave radiothermograph for monitoring the internal brain temperature. East.-Eur. J. Enterp. Technol. 2018, 3, 26–36. [Google Scholar] [CrossRef][Green Version]
- Pennes, H.H. Analysis of Tissue and Arterial Blood Temperatures in the Resting Human Forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef]
- Gastounioti, A.; Desai, S.; Ahluwalia, V.S.; Conant, E.F.; Kontos, D. Artificial intelligence in mammographic phenotyping of breast cancer risk: A narrative review. Breast Cancer Res. 2022, 24, 14. [Google Scholar] [CrossRef] [PubMed]
- Akatsuka, J.; Numata, Y.; Morikawa, H.; Sekine, T.; Kayama, S.; Mikami, H.; Yanagi, M.; Endo, Y.; Takeda, H.; Toyama, Y.; et al. A data-driven ultrasound approach discriminates pathological high grade prostate cancer. Sci. Rep. 2022, 12, 860. [Google Scholar] [CrossRef]
- Chi, Z.; Huang, L.; Wu, D.; Long, X.; Xu, X.; Jiang, H. First assessment of thermoacoustic tomography for in vivo detection of rheumatoid arthritis in the finger joints detection of rheumatoid arthritis in the finger joints. Med. Phys. 2022, 49, 84–92. [Google Scholar] [CrossRef]
- Bohte, A.E.; Nelissen, J.L.; Runge, J.H.; Holub, O.; Lambert, S.A.; de Graaf, L.; Kolkman, S.; van der Meij, S.; Stoker, J.; Strijkers, G.J.; et al. Breast magnetic resonance elastography: A review of clinical work and future perspectives. NMR Biomed. 2018, 31, e3932. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.J.; Lim, H.J.; Ni, M.; Yan, W.H.; Wong, D.W.; Cheung, J.C. Breast Tumour Classification Using Ultrasound Elastography with Machine Learning: A Systematic Scoping Review. Cancers 2022, 14, 367. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Scardo, S.; Method, M.; Schlauch, D.; Misch, A.; Picard, S.; Hamilton, E.; Burris, H.; Spigel, D. A real-world retrospective study of the use of Ki-67 testing and treatment patterns in patients with HR+, HER2- early breast cancer in the United States. BMC Cancer 2022, 22, 502. [Google Scholar] [CrossRef]
- Hong, R.; Sun, H.; Li, D.; Yang, W.; Fan, K.; Liu, C.; Dong, L.; Wang, G. A Review of Biosensors for Detecting Tumor Markers in Breast Cancer. Life 2022, 12, 342. [Google Scholar] [CrossRef]
- Yazdandoost, K.Y. Design and simulation of an antenna for non-invasive temperature detection using microwave radiometry. Prog. Electromagn. Res. C 2021, 111, 109–118. [Google Scholar] [CrossRef]
- Kaprin, A.D.; Kostin, A.A.; Andryukhin, M.I.; Ivanenko, K.V.; Popov, S.V.; Shegai, P.V.; Kruglov, D.P.; Mangutov, F.S.; Leushin, V.Y.; Agasieva, S.V. Microwave Radiometry in the Diagnosis of Various Urological Diseases. Biomed. Eng. 2019, 53, 87–91. [Google Scholar] [CrossRef]
- Shevelev, O.; Petrova, M.; Smolensky, A.; Osmonov, B.; Toimatov, S.; Kharybina, T.; Karbainov, S.; Ovchinnikov, L.; Vesnin, S.; Tarakanov, A.; et al. Using medical microwave radiometry for brain temperature measurements. Drug Discov. Today 2022, 27, 881–889. [Google Scholar] [CrossRef]
- Hand, J.W.; VanLeeuwen, G.M.J.; Mizushina, S.; Van de Kamer, J.B.; Maruyama, K.; Sugiura, T.; Azzopardi, D.V.; Edwards, A.D. Monitoring of deep brain temperature in infants using multi-frequency microwave radiometry and thermal modelling. Phys. Med. Biol. 2001, 46, 1885–1903. [Google Scholar] [CrossRef] [PubMed]
- Laskari, K.; Pitsilka, D.A.; Pentazos, G.; Siores, E.; Tektonidou, M.G.; Sfikakis, P.P. vSAT0657 microwave radiometry-derived thermal changes of sacroiliac joints as a biomarker of sacroiliitis in patients with spondyloarthropathy. Ann. Rheum. Dis. 2018, 77, 1178. [Google Scholar]
- Laskari, K.; Pentazos, G.; Pitsilka, D.; Raftakis, J.; Konstantonis, G.; Toutouzas, K.; Siores, E.; Tektonidou, M.; Sfikakis, P.P. Joint microwave radiometry for inflammatory arthritis assessment. Rheumatology 2020, 59, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Sidorov, I.A.; Gudkov, A.G.; Leushin, V.Y.; Gorlacheva, E.N.; Novichikhin, E.P.; Agasieva, S.V. Measurement and 3D Visualization of the Human Internal Heat Field by Means of Microwave Radiometry. Sensors 2021, 21, 4005. [Google Scholar] [CrossRef]
- Sedankin, M.K.; Gudkov, A.G.; Vesnin, S.G.; Konovalova, A.A.; Leushin, V.Y.; Solov’ev, Y.V.; Sidorov, I.A.; Agasieva, S.V.; Chizhikov, S.V.; Gorlacheva, E.N. Intracavity Thermometry in Medicine. Biomed. Eng. 2021, 55, 224–228. [Google Scholar] [CrossRef]
- Athanasiadi, E.; Bonou, M.; Basoulis, D.; Kapelios, C.J.; Masoura, C.; Skouloudi, M.; Mavrogeni, S.; Aggeli, C.; Psichogiou, M.; Barbetseas, J. Subclinical Left Ventricular Systolic Dysfunction in HIV Patients: Prevalence and Associations with Carotid Atherosclerosis and Increased Adiposity. J. Clin. Med. 2022, 11, 1804. [Google Scholar] [CrossRef]
- Benetos, G.; Galanakos, S.; Koutagiar, I.; Skoumas, I.; Oikonomou, G.; Drakopoulou, M.; Karmpalioti, M.; Katsi, V.; Tsioufis, C.; Toutouzas, K. Carotid artery temperature reduction with statin therapy in patients with familial hyperlipidemia syndromes. J. Clin. Med. 2021, 10, 5008. [Google Scholar] [CrossRef]
- Sedankin, M.K.; Gudkov, A.G. Microwave Radiometry of the Pelvic Organs. Biomed. Eng. 2019, 53, 288–292. [Google Scholar] [CrossRef]
- Law, J.; Chalmers, J.; Morris, D.E.; Robinson, L.; Budge, H.; Symonds, M.E. The use of infrared thermography in the measurement and characterization of brown adipose tissue activation. Temperature 2018, 5, 147–161. [Google Scholar] [CrossRef]
- Zaretsky, D.; Romanovsky, A.; Zaretskaia, M.; Molkov, Y. Tissue oxidative metabolism can increase the difference between local temperature and arterial blood temperature. Temperature 2018, 5, 22–35. [Google Scholar] [CrossRef]
- Chupina, D.N.; Sedankin, M.K.; Vesnin, S.G. Application of modern technologies of mathematical simulation for the development of medical equipment. In Proceedings of the 2017 IEEE 11th International Conference on Application of Information and Communication Technologies (AICT), Moscow, Russia, 20–22 September 2017; pp. 1–5. [Google Scholar] [CrossRef]
- Raiko, J.; Koskensalo, K.; Sainio, T. Imaging-based internal body temperature measurements: The journal Temperature toolbox. Temperature 2020, 7, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Leushin, V.Y.; Gudkov, A.G.; Porokhov, I.O.; Vesnin, S.G.; Sedankin, M.K.; Sidorov, I.A.; Solov’ev, Y.V.; Agasieva, S.V.; Chizhikov, S.V. Possibilities of increasing the interference immunity of radiothermograph applicator antennas for brain diagnostics. Sensors Actuators A Phys. 2022, 337, 113439. [Google Scholar] [CrossRef]
- Zampeli, E.; Raftakis, I.; Michelongona, A.; Nikolaou, C.; Elezoglou, A.; Toutouzas, K.; Siores, E.; Sfikakis, P.P. Detection of Subclinical Synovial Inflammation by Microwave Radiometry. PLoS ONE 2013, 8, e64606. [Google Scholar] [CrossRef] [PubMed]
- Tarakanov, A.V.; Tarakanov, A.A.; Vesnin, S.; Efremov, V.V.; Goryanin, I.; Roberts, N. Microwave Radiometry (MWR) temperature measurement is related to symptom severity in patients with Low Back Pain (LBP). J. Bodyw. Mov. Ther. 2021, 26, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Arunachalam, K.; Maccarini, P.F.; De Luca, V.; Bardati, F.; Snow, B.W.; Stauffer, P.R. Modeling the detectability of vesicoureteral reflux using microwave radiometry. Phys. Med. Biol. 2010, 55, 5417–5435. [Google Scholar] [CrossRef] [PubMed]
- Spiliopoulos, S.; Theodosiadou, V.; Barampoutis, N.; Katsanos, K.; Davlouros, P.; Reppas, L.; Kitrou, P.; Palialexis, K.; Konstantos, C.; Siores, E.; et al. Multi-center feasibility study of microwave radiometry thermometry for non-invasive differential diagnosis of arterial disease in diabetic patients with suspected critical limb ischemia. J. Diabetes Its Complicat. 2017, 31, 1109–1114. [Google Scholar] [CrossRef]
- Vetshev, P.S.; Chilingaridi, K.E.; Zolkin, A.V.; Vesnin, S.G.; Gabaidze, D.I.; Bannyi, D.A. Radiothermometry in diagnosis of thyroid diseases. Khirurgiia 2006, 6, 54–58. [Google Scholar]
- Gautherie, M. Temperature and Blood Flow Patterns in Breast Cancer During Natural Evolution and Following Radiotherapy. Prog. Clin. Biol. Res. 1982, 107, 21–64. [Google Scholar]
- Levshinskii, V.; Galazis, C.; Ovchinnikov, L.; Vesnin, S.; Losev, A.; Goryanin, I. Application of Data Mining and Machine Learning in Microwave Radiometry (MWR). In Proceedings of the 12th International Joint Conference, BIOSTEC 2019, Prague, Czech Republic, 22–24 February 2019; pp. 265–288. [Google Scholar] [CrossRef]
- Losev, A.G.; Levshinskii, V.V. Data mining of microwave radiometry data in the diagnosis of breast cancer. Math. Phys. Comput. Simul. 2017, 20, 49–62. [Google Scholar] [CrossRef]
- Zuluaga-Gomez, J.; Zerhouni, N.; Al Masry, Z.; Devalland, C.; Varnier, C. A survey of breast cancer screening techniques: Thermography and electrical impedance tomography. J. Med. Eng. Technol. 2019, 43, 305–322. [Google Scholar] [CrossRef]
- Vesnin, S.; Turnbull, A.K.; Dixon, J.M.; Goryanin, I. Modern microwave thermometry for breast cancer. J. Mol. Imaging Dyn. 2017, 7, 136. [Google Scholar] [CrossRef]
- Galazis, C.; Vesnin, S.; Goryanin, I. Application of artificial intelligence in microwave radiometry (MWR). In Proceedings of the 12th International Joint Conference on Biomedical Engineering Systems and Technologies, Prague, Czech Republic, 22–24 February 2019; Volume 3, pp. 112–122. [Google Scholar] [CrossRef]
- Schonberger, D. Artificial intelligence in healthcare: A critical analysis of the legal and ethical implications. Int. J. Law Inf. Technol. 2019, 27, 171–203. [Google Scholar] [CrossRef]
- Kobrinskii, B.A. Advisory intelligent medical systems: Classification, principles of construction, efficiency. Inf. Technol. Phys. 2008, 2, 38–47. [Google Scholar]
- Levshinskii, V. Mathematical models for analyzing and interpreting microwave radiometry data in medical diagnosis. J. Comput. Eng. Math. 2021, 8, 3–14. [Google Scholar] [CrossRef]
- Khoperskov, A.V.; Kovalev, M.E.; Astakhov, A.S.; Novochadov, V.V.; Terpilovskiy, A.A.; Tiras, K.P.; Malanin, D.A. Software for Full-Color 3D Reconstruction of the Biological Tissues Internal Structure. Lect. Notes Comput. Sci. 2017, 10594, 1–10. [Google Scholar] [CrossRef]
- Sedankin, M.K.; Leushin, V.Y.; Gudkov, A.G.; Vesnin, S.G.; Sidorov, I.A.; Agasieva, S.V.; Markin, A.V. Mathematical Simulation of Heat Transfer Processes in a Breast with a Malignant Tumor. Biomed. Eng. 2018, 52, 190–194. [Google Scholar] [CrossRef]
- Ferreira, O.J.; Medeiros, V.; Ramalho Costa, G.; Nascimento, J.; Figueiredo, A.; Guimaraes, G. Numerical Analysis of Temperature Field on a Surface of a Breast with a Tumor. In Proceedings of the 25th International Congress of Mechanical Engineering, Uberlandia, Brazil, 20–25 October 2019. [Google Scholar] [CrossRef]
- Mashekova, A.; Zhao, Y.; Ng, E.Y.K.; Zarikas, V.; Fok, S.C.; Mukhmetov, O. Early detection of the breast cancer using infrared technology—A comprehensive review. Therm. Sci. Eng. Prog. 2022, 27, 101142. [Google Scholar] [CrossRef]
- Chen, H.; Wang, K.; Du, Z.; Liu, W.; Liu, Z. Predicting the thermophysical properties of skin tumor based on the surface temperature and deep learning. Int. J. Heat Mass Transf. 2021, 180, 121804. [Google Scholar] [CrossRef]
- Bhowmik, A.; Repaka, R.; Mishra, S.C. Thermographic evaluation of early melanoma within the vascularized skin using combined non-Newtonian blood flow and bioheat models. Comput. Biol. Med. 2014, 53, 206–219. [Google Scholar] [CrossRef]
- Stafford Hatfield, H.; Pugh, L.G.C. Thermal Conductivity of Human Fat and Muscle. Nature 1951, 168, 918–919. [Google Scholar] [CrossRef]
- Ng, E.Y.; Sudharsan, N.M. An improved three-dimensional direct numerical modelling and thermal analysis of a female breast with tumour. Proc. Inst. Mech. Eng. H 2001, 15, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Bowman, H.; Cravalho, M.; Woods, M. Theory, Measurement, and Application of Thermal Properties of Biomaterials. Annu. Rev. Biophys. Bioeng. 1975, 4, 43–80. [Google Scholar] [CrossRef] [PubMed]
- Iljaz, J.; Wrobel, L.C.; Hriberšek, M.; Marn, J. The use of Design of Experiments for steady-state and transient inverse melanoma detection problems. Int. J. Therm. Sci. 2019, 135, 256–275. [Google Scholar] [CrossRef]
- Gordon, R.A.; Roemer, R.; Horvath, S. Mathematical Model of the Human Temperature Regulatory System—Transient Cold Exposure Response. IEEE Trans. Biomed. Eng. 1976, 23, 434–444. [Google Scholar] [CrossRef] [PubMed]
- Gomboc, T.; Iljaz, J.; Wrobel, L.C.; Hribersek, M.; Marn, J. Design of constant temperature cooling device for melanoma screening by dynamic thermography. Eng. Anal. Bound. Elem. 2021, 125, 66–79. [Google Scholar] [CrossRef]
- Hensel, H. The Physiology of Thermoreception. Ergeb Physiol. 1952, 47, 166–368. [Google Scholar] [CrossRef]
- Lipkin, M.; Hardy, J. Measurement of Some Thermal Properties of Human Tissues. J. Appl. Physiol. 1954, 7, 212–217. [Google Scholar] [CrossRef]
- Hossain, S.; Mohammadi, F. Thermogram Assessment for tumor parameter estimation considering the body geometry. Comput. Biol. Med. 2016, 39, 219–234. [Google Scholar] [CrossRef]
- Hatwar, R.; Herman, C. Inverse method for quantitative characterization of breast tumors from surface temperature data. Int. J. Hyperth. 2017, 33, 1–56. [Google Scholar] [CrossRef]
- Bezerra, L.; Oliveira, M.M.; Rolim, T.L.; Conci, A.; Santos, F.G.S.; Lyra, P.R.M.; Lima, R.C.F. Estimation of breast tumor thermal properties using infrared images. Signal Process. 2013, 93, 2851–2863. [Google Scholar] [CrossRef]
- Polyakov, M.; Khoperskov, A.; Zamechnic, T. Numerical Modeling of the Internal Temperature in the Mammary Gland. Lect. Notes Comput. Sci. 2017, 10594, 128–135. [Google Scholar] [CrossRef]
- Gonzalez, F.J. Thermal simulation of breast tumors. Rev. Mex. Fis. 2007, 53, 323–326. [Google Scholar]
- Vesnin, S.G.; Sedakin, M.K. Development of antenna-applicator series for tissue temperature non-invasive measurement of a human. Eng. J. Sci. Innov. 2012, 11, 1–18. [Google Scholar] [CrossRef]
- Polyakov, M. Computational modelling to determine the physical characteristics of biological tissues for medical diagnosis. Int. J. Eng. Syst. Model. Simul. 2020, 11, 214–221. [Google Scholar] [CrossRef]
- Wren, J. Microwave Thermotherapy of Prostatic Enlargement-Analysis of Radiometric Thermometry Using a Hybrid Bio-heat Equation. Comput. Methods Biomech. Biomed. Eng. 2004, 7, 177–185. [Google Scholar] [CrossRef]
- Avila-Castro, I.A.; Hernandez-Martinez, A.R.; Estevez, M.; Cruz, M.; Esparza, R.; Perez, R.; Rodriguez, A.L. Thorax thermographic simulator for breast pathologies. J. Appl. Res. Technol. 2017, 15, 143–151. [Google Scholar] [CrossRef]
- Bardati, F.; Iudicello, S. Modeling the Visibility of Breast Malignancy by a Microwave Radiometer. Biomed. Engineering 2008, 55, 214–221. [Google Scholar] [CrossRef]
- Polyakov, M.V.; Khoperskov, A.V.; Svetlov, A.V. Simulation modeling of radiation field in biological tissue of mammary gland. J. Comput. Eng. Math. 2017, 4, 3–10. [Google Scholar] [CrossRef]
- Kohonen, T. Self-Organizing Maps, 3rd Extended ed.; Springer: New York, NY, USA, 2001; p. 501. [Google Scholar]
- Abdel-Jaber, H.; Devassy, D.; Al Salam, A.; Hidaytallah, L.; EL-Amir, M. A Review of Deep Learning Algorithms and Their Applications in Healthcare. Algorithms 2022, 15, 71. [Google Scholar] [CrossRef]
- Lo, S.-H.; Yin, Y. An Interaction-Based Convolutional Neural Network (ICNN) Toward a Better Understanding of COVID-19 X-ray Images. Algorithms 2021, 14, 337. [Google Scholar] [CrossRef]
- Simonyan, K.; Zisserman, A. Very Deep Convolutional Networks for Large-Scale Image Recognition. arXiv 2015, arXiv:1409.1556. [Google Scholar]
- Chen, X.; Ching, W.; Aoki-Kinoshita, K.F.; Furuta, K. Support Vector Machine Methods for the Prediction of Cancer Growth. In Proceedings of the 2012 Fifth International Joint Conference on Computational Sciences and Optimization, Huangshan, China, 28–31 May 2010; pp. 229–232. [Google Scholar] [CrossRef]
- Sutton, C. Nearest-neighbor methods. WIREs Interdiscip. Rev. Comput. Stat. 2012, 4, 307–309. [Google Scholar] [CrossRef]
- Berrar, D. Bayes’ Theorem and Naive Bayes Classifier. Encycl. Bioinform. Comput. Biol. 2019, 1, 403–412. [Google Scholar] [CrossRef]
- Badiger, S.; Moger, J. A comparative study of mammography, sonography and infrared thermography in detection of cancer in breast. Int. Surg. J. 2020, 7, 1886–1892. [Google Scholar] [CrossRef]
- Yahara, T.; Koga, T.; Yoshida, S.; Nakagawa, S.; Deguchi, H.; Shirouzu, K. Relationship between microvessel density and thermographic hot areas in breast cancer. Surg. Today 2003, 33, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Morales-Guadarrama, G.; García-Becerra, R.; Méndez-Pérez, E.A.; García-Quiroz, J.; Avila, E.; Díaz, L. Vasculogenic Mimicry in BreastCancer: Clinical Relevance and Drivers. Cells 2021, 10, 1758. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, K.; Gupta, A.; Dasgupta, S.; Marri, U.; Gupta, A.K.; Hari, S.; Banerjee, S.; Arora, C. Ultra high resolution, multi scale, context aware approach for detection of small cancers on mammography. Sci. Rep. 2022, 12, 11622. [Google Scholar] [CrossRef]
- Ravaioli, S.; Limarzi, F.; Tumedei, M.M.; Palleschi, M.; Maltoni, R.; Bravaccini, S. Are we ready to use TMB in breast cancer clinical practice? Cancer Immunol. Immunother. 2020, 69, 1943–1945. [Google Scholar] [CrossRef]
- Glazunov, V.A.; Polyakov, M.V. Testing the algorithm of tumor localization in breast cancer on the results of temperature field simulation. J. Phys. Conf. Ser. 2021, 1794, 012006. [Google Scholar] [CrossRef]
- Figueiredo, A.A.A.; Menegaz, G.L.; Fernandes, H.C.; Guimaraes, G. Thermographic Computational Analyses of a 3D Model of a Scanned Breast. Lect. Notes Comput. Sci. 2018, 11071, 885–892. [Google Scholar]
- Mukhmetov, O.; Mashekova, A.; Zhao, Y.; Midlenko, A.; Ng, E.Y.K.; Fok, S.C. Patient/Breast-Specific Detection of Breast Tumor Based on Patients’ Thermograms, 3D Breast Scans, and Reverse Thermal Modelling. Appl. Sci. 2021, 11, 6565. [Google Scholar] [CrossRef]
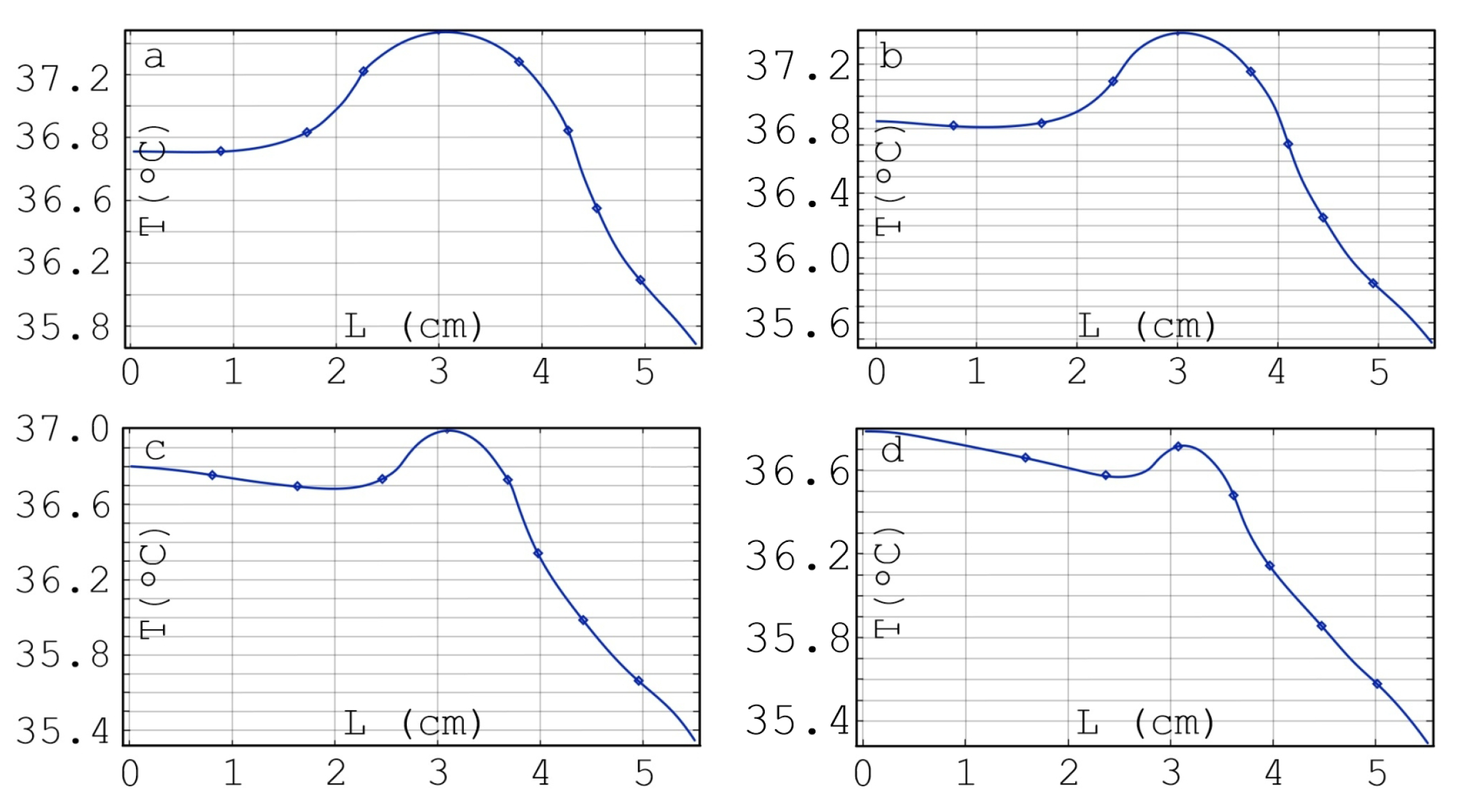
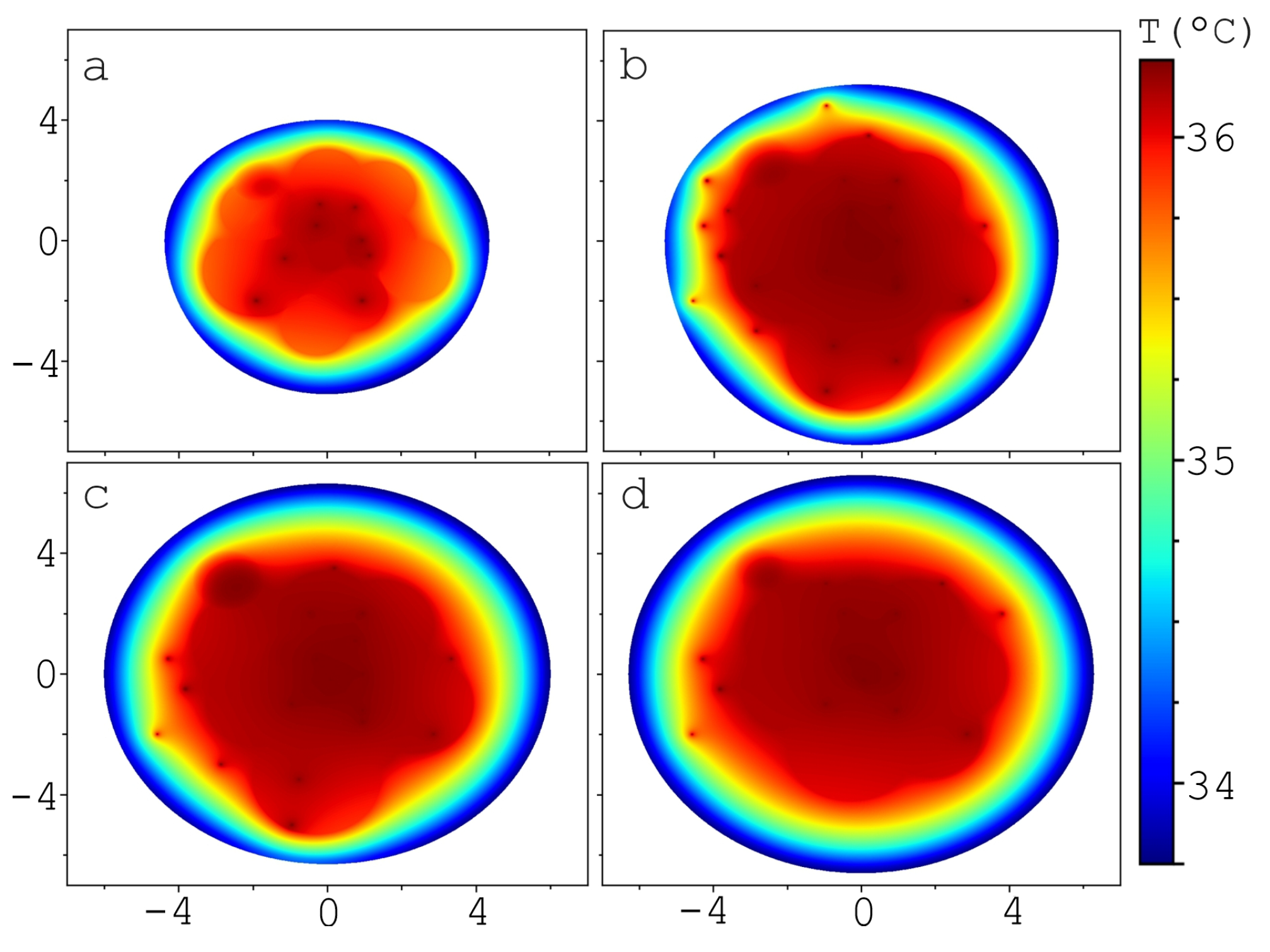
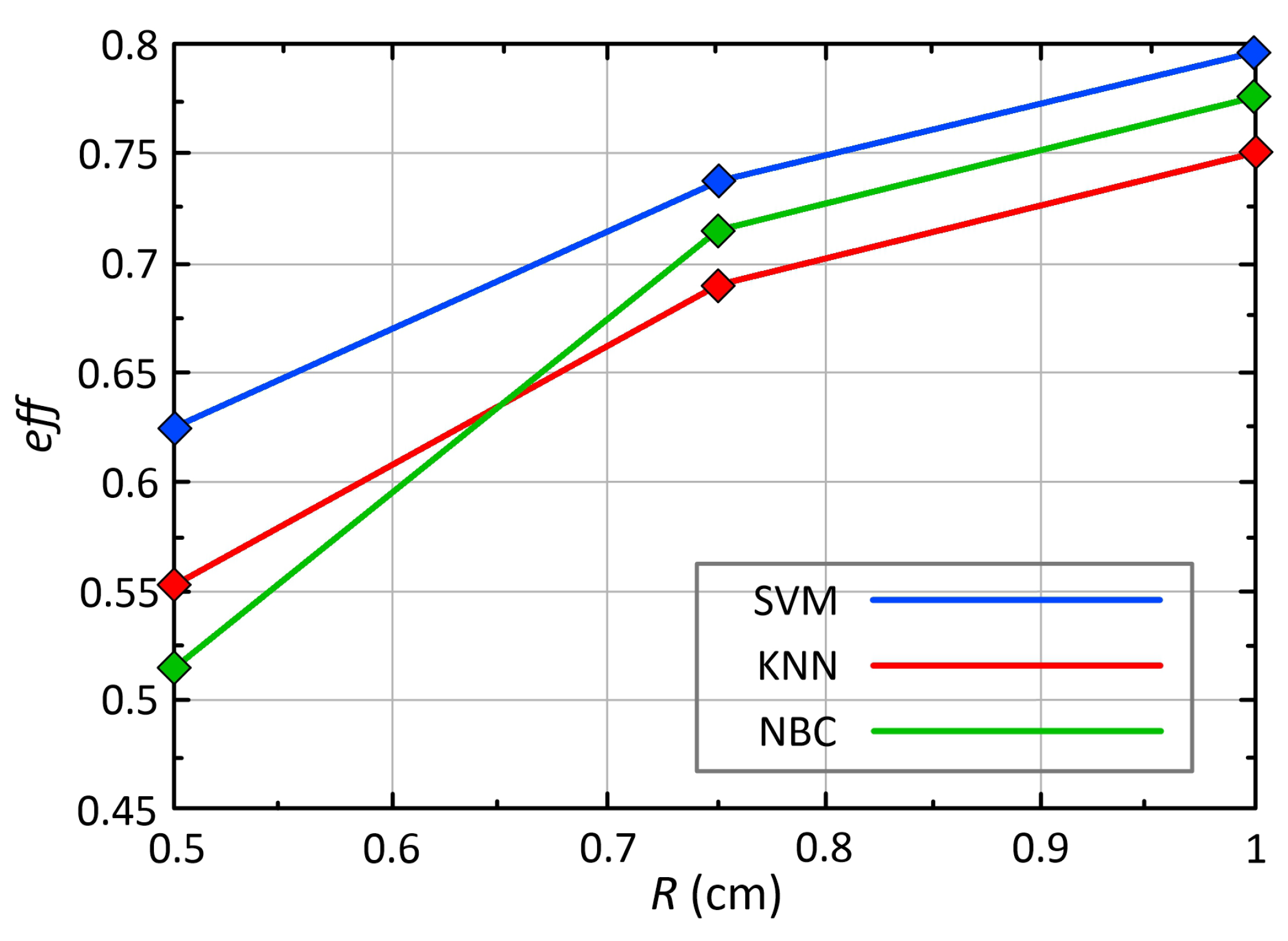
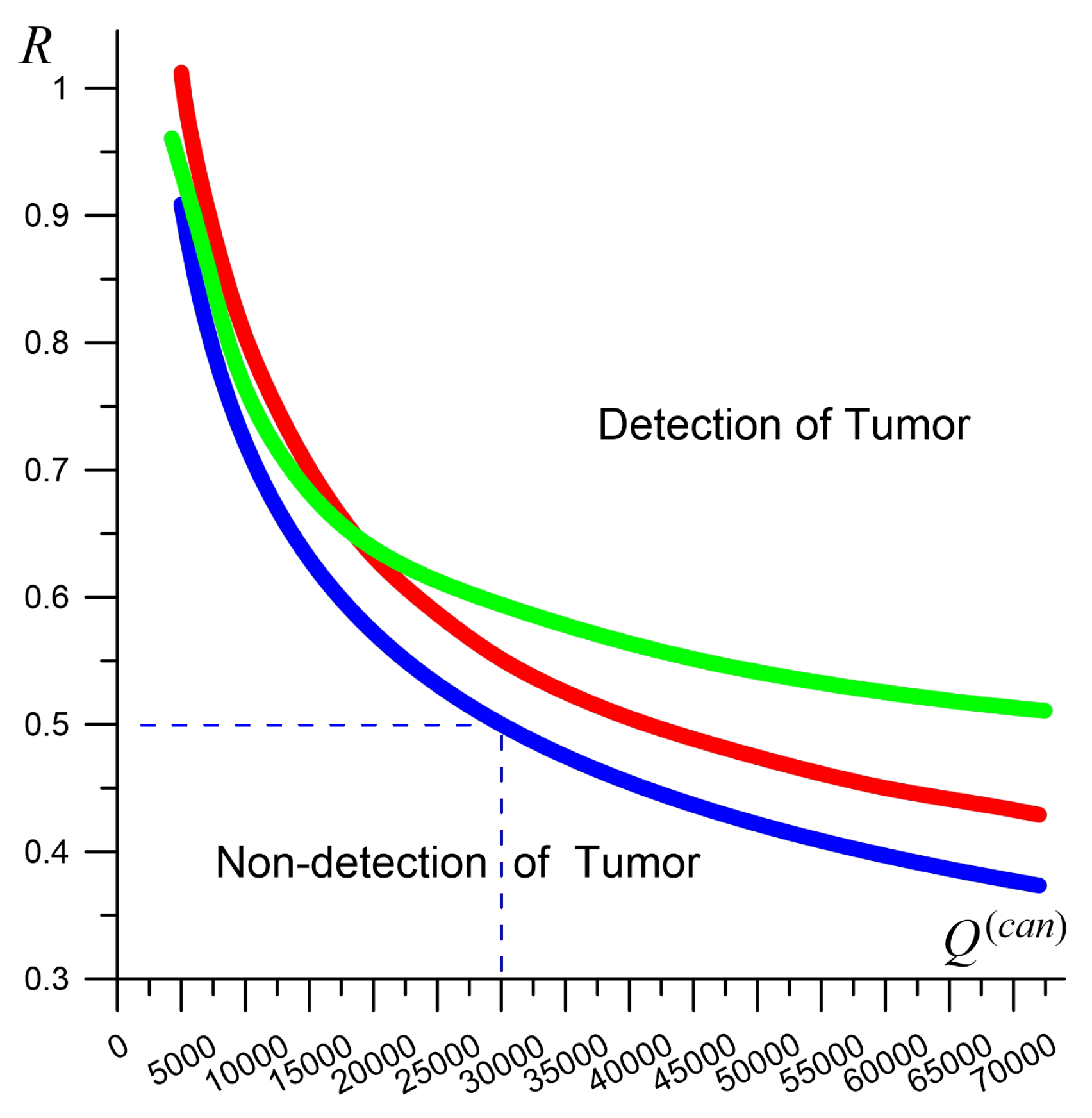
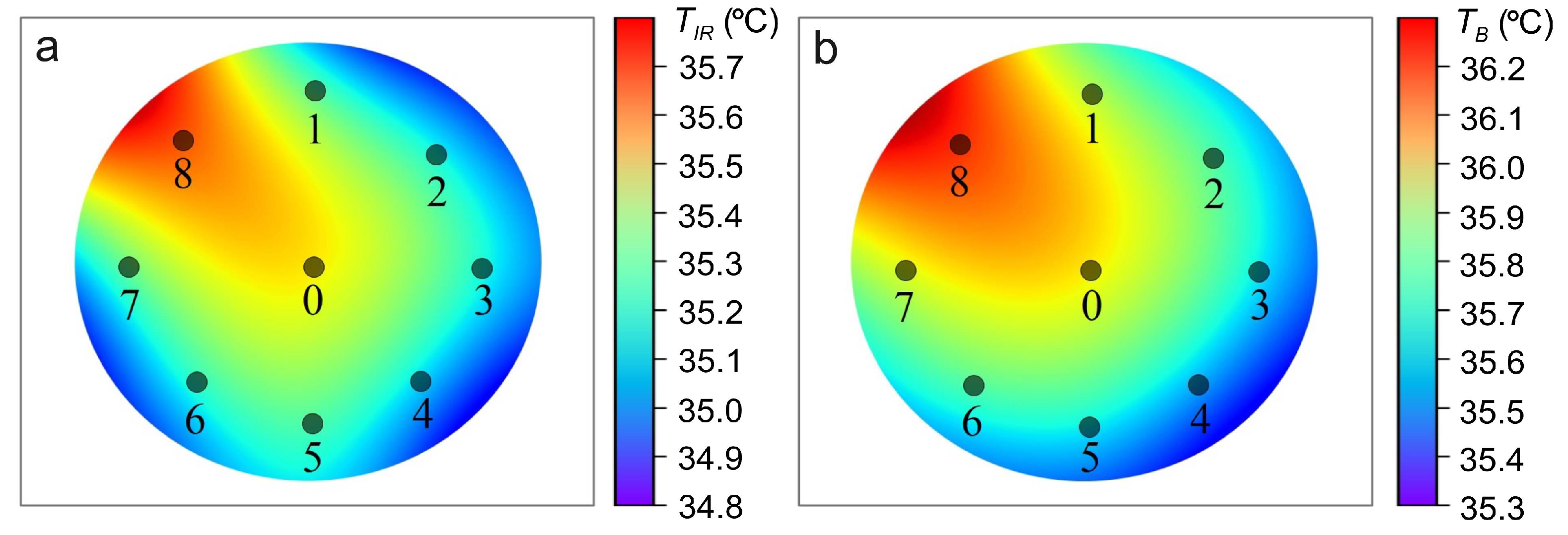
| , W/(m·K) | C, J/(kg·K) | , kg/m | , W/m | , S/m | ||
|---|---|---|---|---|---|---|
| Skin | 0.21–0.54 | 3391–3690 | 1180–1215 | 380–410 | 1.1–2.4 | 40–50 |
| Muscles | 0.4–0.56 | 3421–3790 | 1070–1100 | 675–690 | 0.44–0.7 | 54–56 |
| Fat | 0.18–0.34 | 2348–2690 | 900–915 | 356–370 | 0.03 | 4.4–6 |
| Blood | 0.45–0.6 | 3800–4200 | 1046–1058 | 0 | 0.9–1.2 | 64–85 |
| Glands | 0.4–0.5 | 3700–3790 | 1035–1041 | 450–610 | 0.56–0.61 | 10.6–12 |
| Connective tissue | 0.44–0.5 | 3340–3400 | 1006–1020 | 604–620 | 0.3–0.36 | 38–40 |
| Cancers | 0.45–0.58 | 3710–3800 | 1045–1054 | 3000–71,000 | 0.79–1.5 | 42–50 |
| 1 cm | 2 cm | 3 cm | 4 cm | 5 cm | |
|---|---|---|---|---|---|
| 1 cm | 0 | 0.016 | 0.027 | 0.041 | 0.059 |
| 2 cm | 0.016 | 0 | 0.011 | 0.026 | 0.043 |
| 3 cm | 0.027 | 0.011 | 0 | 0.015 | 0.033 |
| 4 cm | 0.041 | 0.026 | 0.015 | 0 | 0.019 |
| 5 cm | 0.059 | 0.043 | 0.033 | 0.019 | 0 |
| Topology 1 | Topology 2 | Topology 3 | Topology 4 | |
|---|---|---|---|---|
| Number of layers | 8 | 6 | 5 | 4 |
| Number of neurons on the 1st layer | 20 | 20 | 20 | 20 |
| Number of neurons on the 2nd layer | 20 | 20 | 20 | 10 |
| Number of neurons on the 3rd layer | 20 | 10 | 14 | 3 |
| Number of neurons on the 4th layer | 20 | 6 | 3 | 2 |
| Number of neurons on the 5th layer | 20 | 4 | 2 | – |
| Number of neurons on the 6th layer | 20 | 2 | – | – |
| Number of neurons on the 7th layer | 20 | – | – | – |
| Number of neurons on the 8th layer | 2 | – | – | – |
| 0.74 | 0.66 | 0.86 | 0.81 | |
| 0.67 | 0.61 | 0.82 | 0.71 | |
| 0.7 | 0.63 | 0.84 | 0.76 | |
| F1 | 0.72 | 0.61 | 0.83 | 0.75 |
| 0.46 | 0.27 | 0.63 | 0.54 |
| Predicted Condition | |||
|---|---|---|---|
| Total 118 | Positive 56 | Negative 62 | |
Actual condition | Positive 51 | 44 | 7 |
| Negative 67 | 12 | 55 | |
| F1 | |||||
|---|---|---|---|---|---|
| 9-point (CNN) | 0.74 | 0.62 | 0.68 | 0.7 | 0.36 |
| 17-point (CNN) | 0.79 | 0.64 | 0.71 | 0.71 | 0.44 |
| 9-point (SVM) | 0.76 | 0.69 | 0.72 | 0.71 | 0.45 |
| 17-point (SVM) | 0.8 | 0.71 | 0.75 | 0.74 | 0.51 |
| 9-point (KNN) | 0.71 | 0.6 | 0.65 | 0.64 | 0.31 |
| 17-point (KNN) | 0.75 | 0.63 | 0.69 | 0.67 | 0.38 |
| 9-point (NBC) | 0.72 | 0.62 | 0.67 | 0.65 | 0.33 |
| 17-point (NBC) | 0.73 | 0.63 | 0.68 | 0.67 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoperskov, A.V.; Polyakov, M.V. Improving the Efficiency of Oncological Diagnosis of the Breast Based on the Combined Use of Simulation Modeling and Artificial Intelligence Algorithms. Algorithms 2022, 15, 292. https://doi.org/10.3390/a15080292
Khoperskov AV, Polyakov MV. Improving the Efficiency of Oncological Diagnosis of the Breast Based on the Combined Use of Simulation Modeling and Artificial Intelligence Algorithms. Algorithms. 2022; 15(8):292. https://doi.org/10.3390/a15080292
Chicago/Turabian StyleKhoperskov, Alexander V., and Maxim V. Polyakov. 2022. "Improving the Efficiency of Oncological Diagnosis of the Breast Based on the Combined Use of Simulation Modeling and Artificial Intelligence Algorithms" Algorithms 15, no. 8: 292. https://doi.org/10.3390/a15080292
APA StyleKhoperskov, A. V., & Polyakov, M. V. (2022). Improving the Efficiency of Oncological Diagnosis of the Breast Based on the Combined Use of Simulation Modeling and Artificial Intelligence Algorithms. Algorithms, 15(8), 292. https://doi.org/10.3390/a15080292






