MedicalSeg: A Medical GUI Application for Image Segmentation Management
Abstract
:1. Introduction
2. Materials and Digital Repository
2.1. Medical Digital Databases
2.1.1. Full-Digital Mammography Database
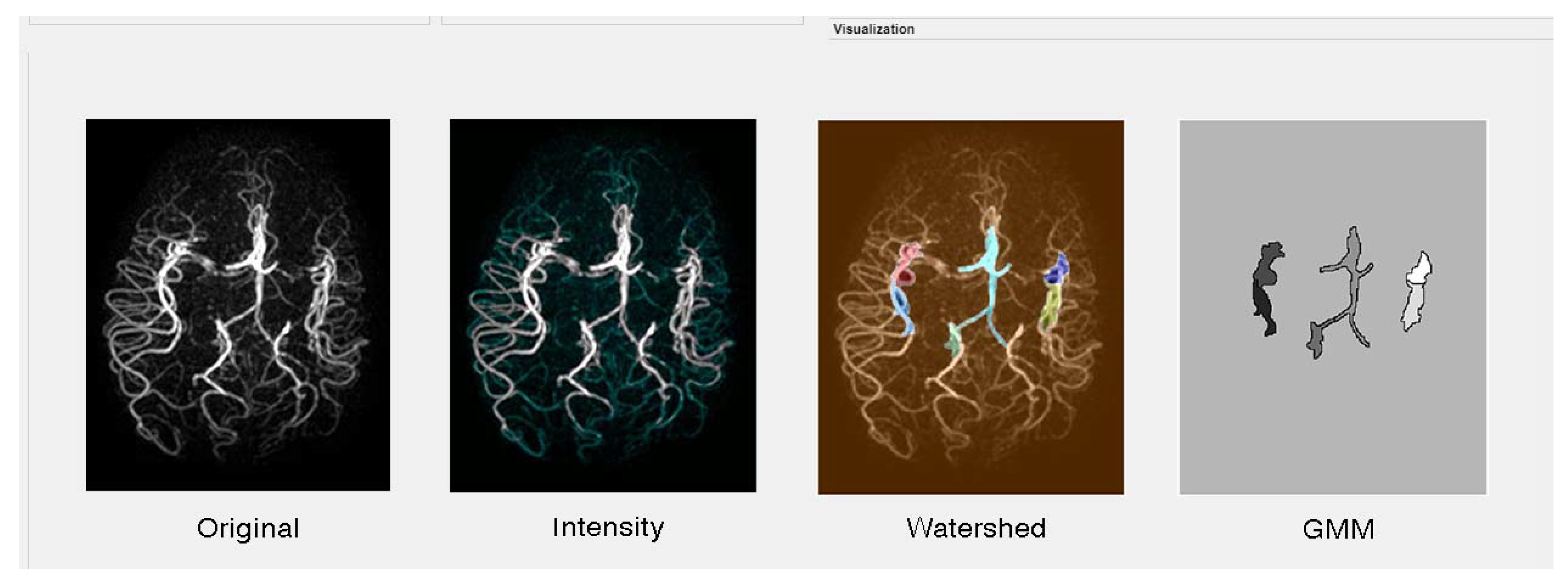
2.1.2. Magnetic Resonance Angiography (MRA) Vascular Brain Database
2.1.3. MRI Prostate Database
2.1.4. Digital Retinal Database
2.1.5. Ultrasound Database
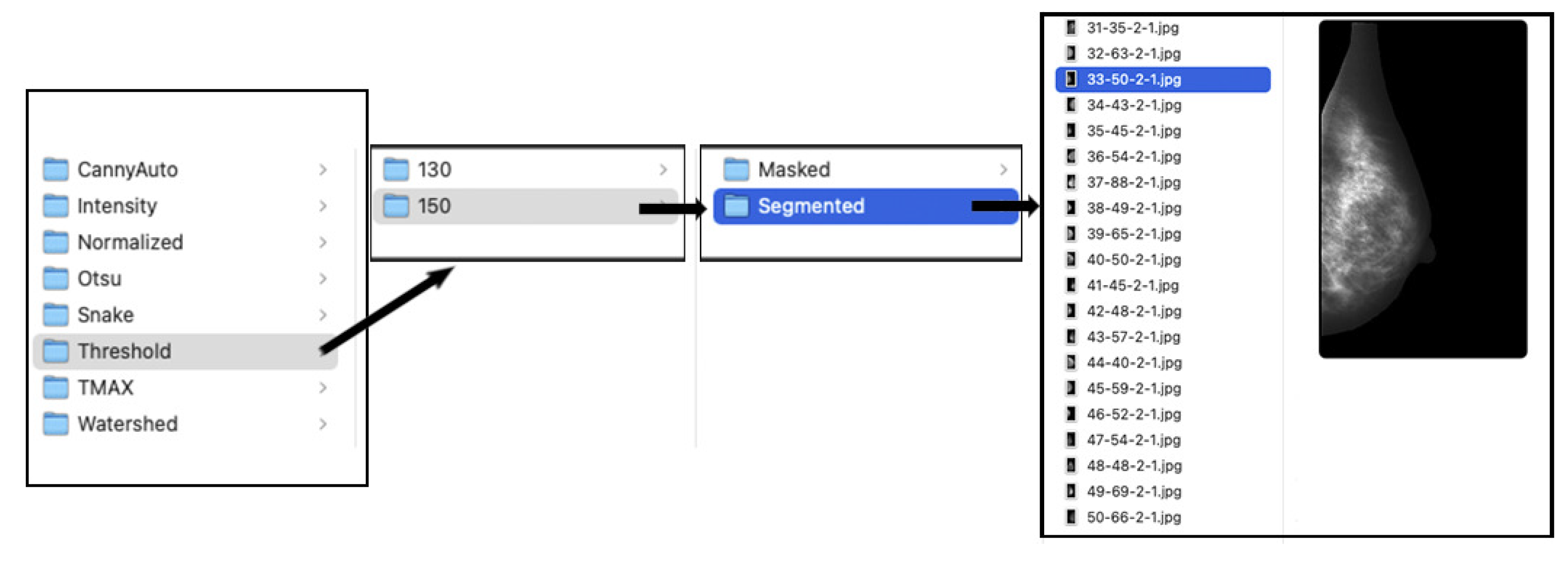
2.2. Digital Repository and Distribution
- 1.
- Redistributions of source code must retain the above copyright notice [26], this list of conditions and the following disclaimer;
- 2.
- Redistributions in binary form must reproduce the above copyright notice, this list of conditions and the following disclaimer in the documentation and/or other materials provided with the distribution;
- 3.
- Neither the name of the copyright holder nor the names of its contributors may be used to endorse or promote products derived from this software without specific prior written permission;
- 4.
- Respect and to be responsible by giving credit and acknowledging of this work. As a code of ethics, users must be appreciate our effort and reference this work on their future scientific purposes.
3. Methods
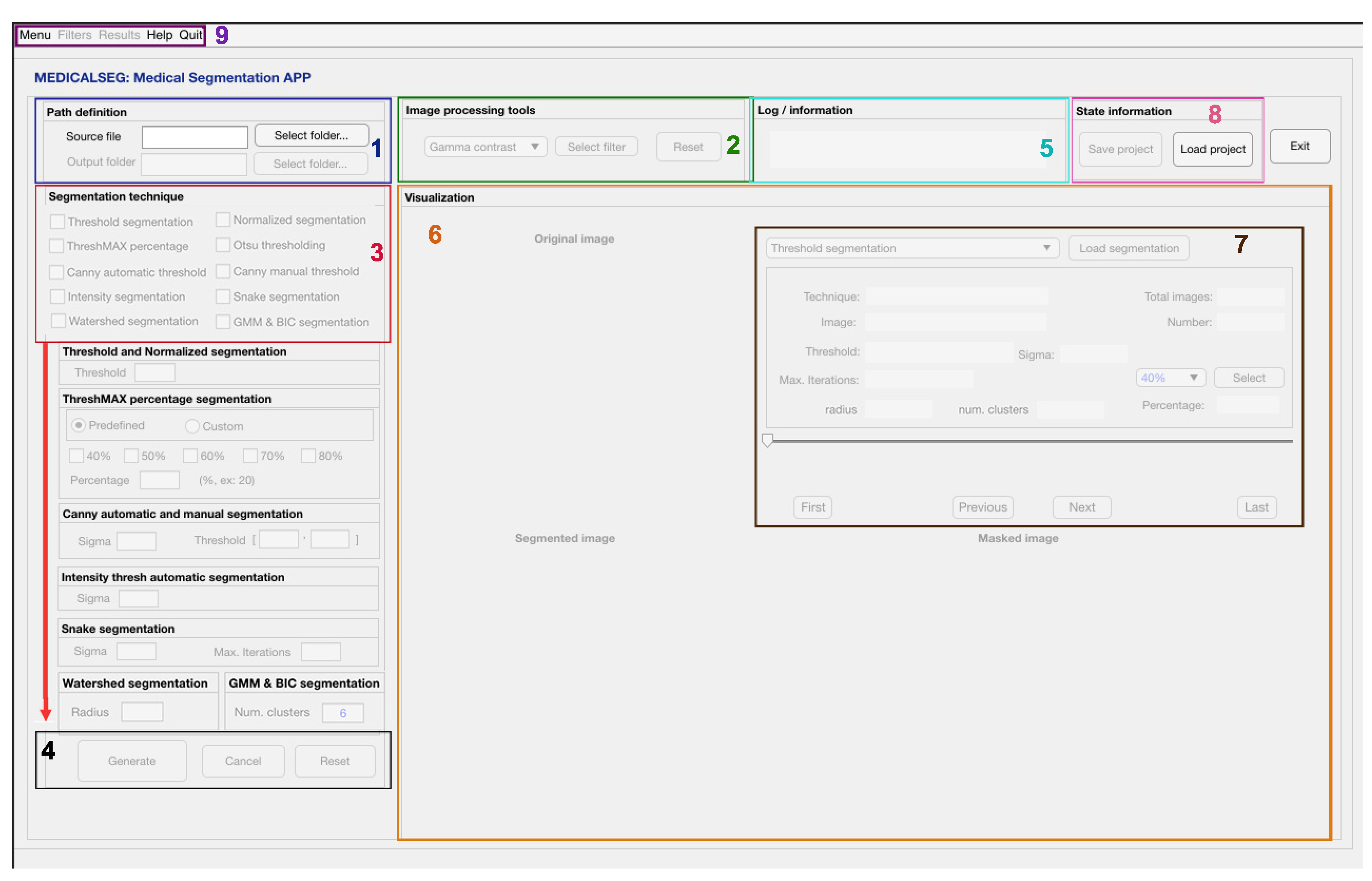
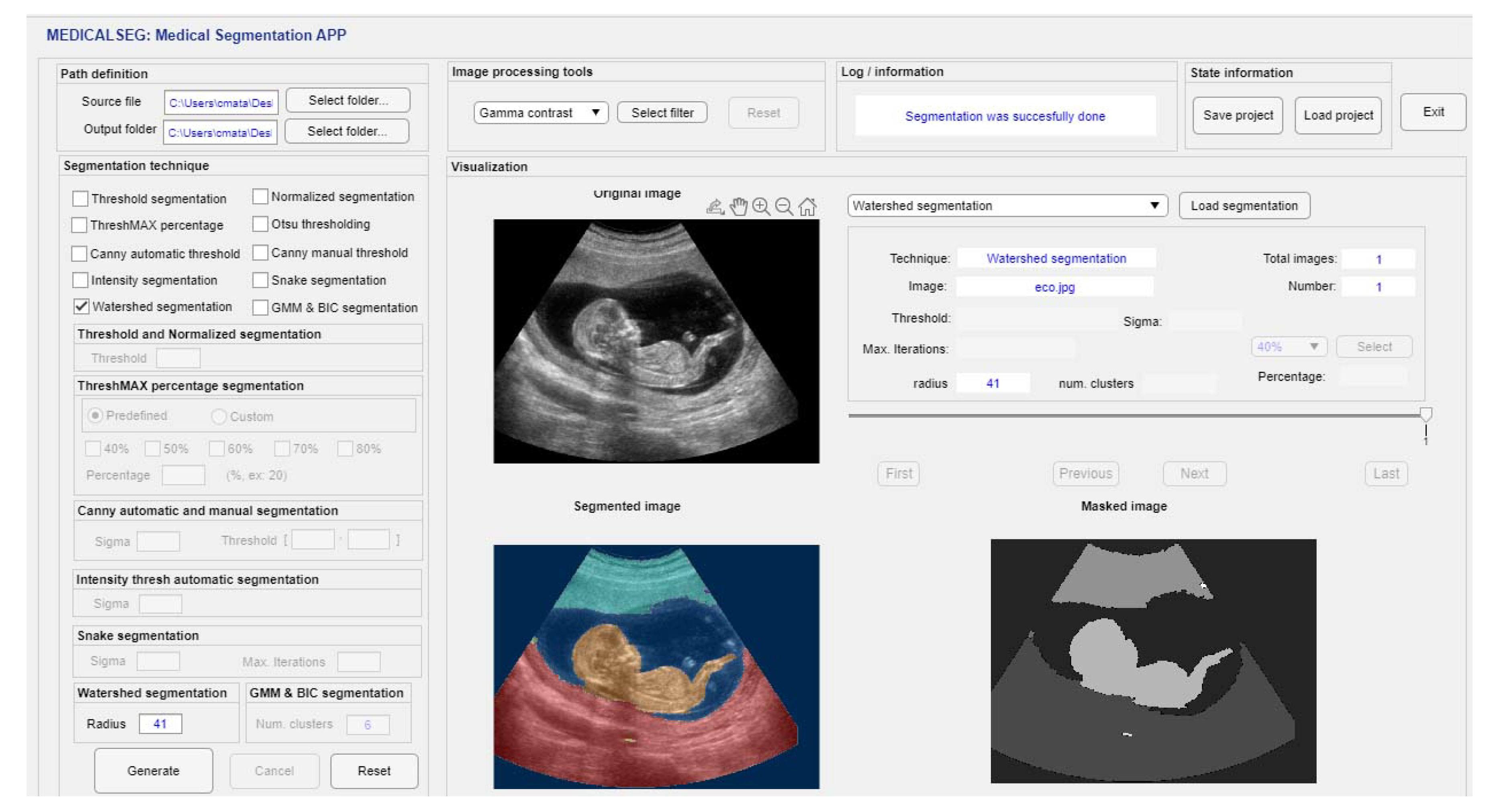
3.1. GUI Interface Design
3.2. Segmentation Algorithms
3.2.1. Threshold Segmentation
3.2.2. Otsu Segmentation
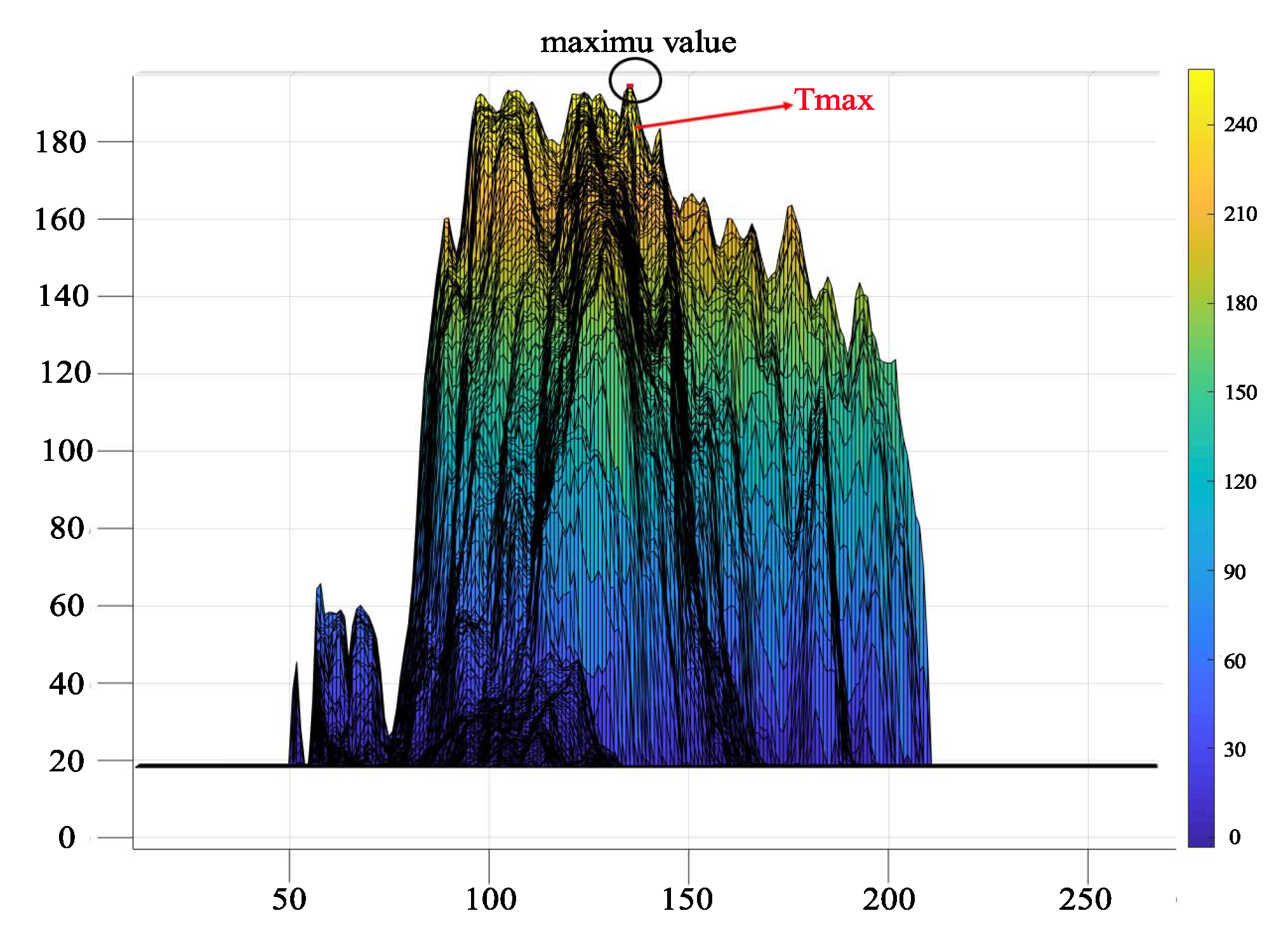
3.2.3. ThreshMAX Percentage Segmentation
3.2.4. Canny Edge Segmentation
3.2.5. Intensity Automatic Threshold
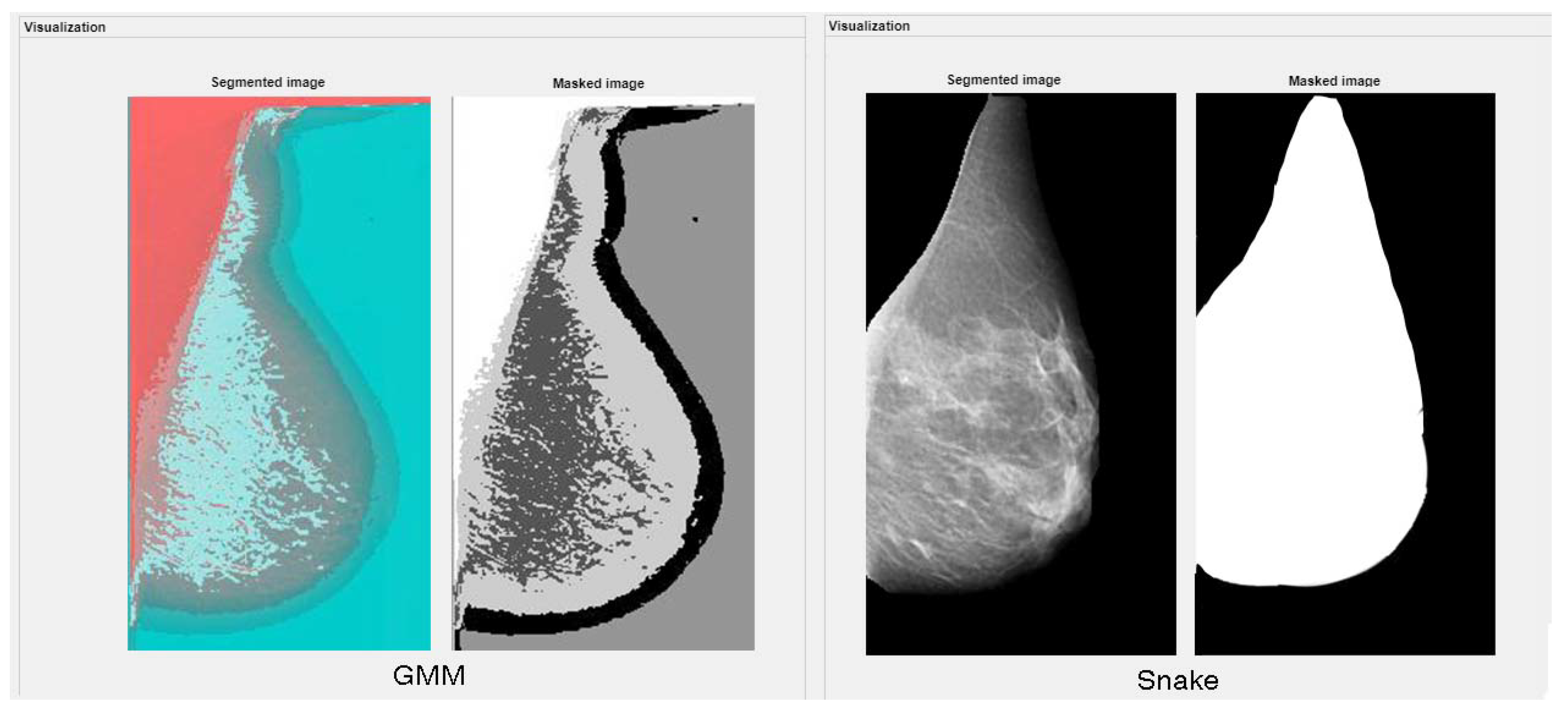
3.2.6. Active Contour Models: Snakes
3.2.7. Watershed Segmentation
3.2.8. Gaussian Mixture Model
3.3. Image Processing Tools
3.3.1. Gamma Contrast Filter
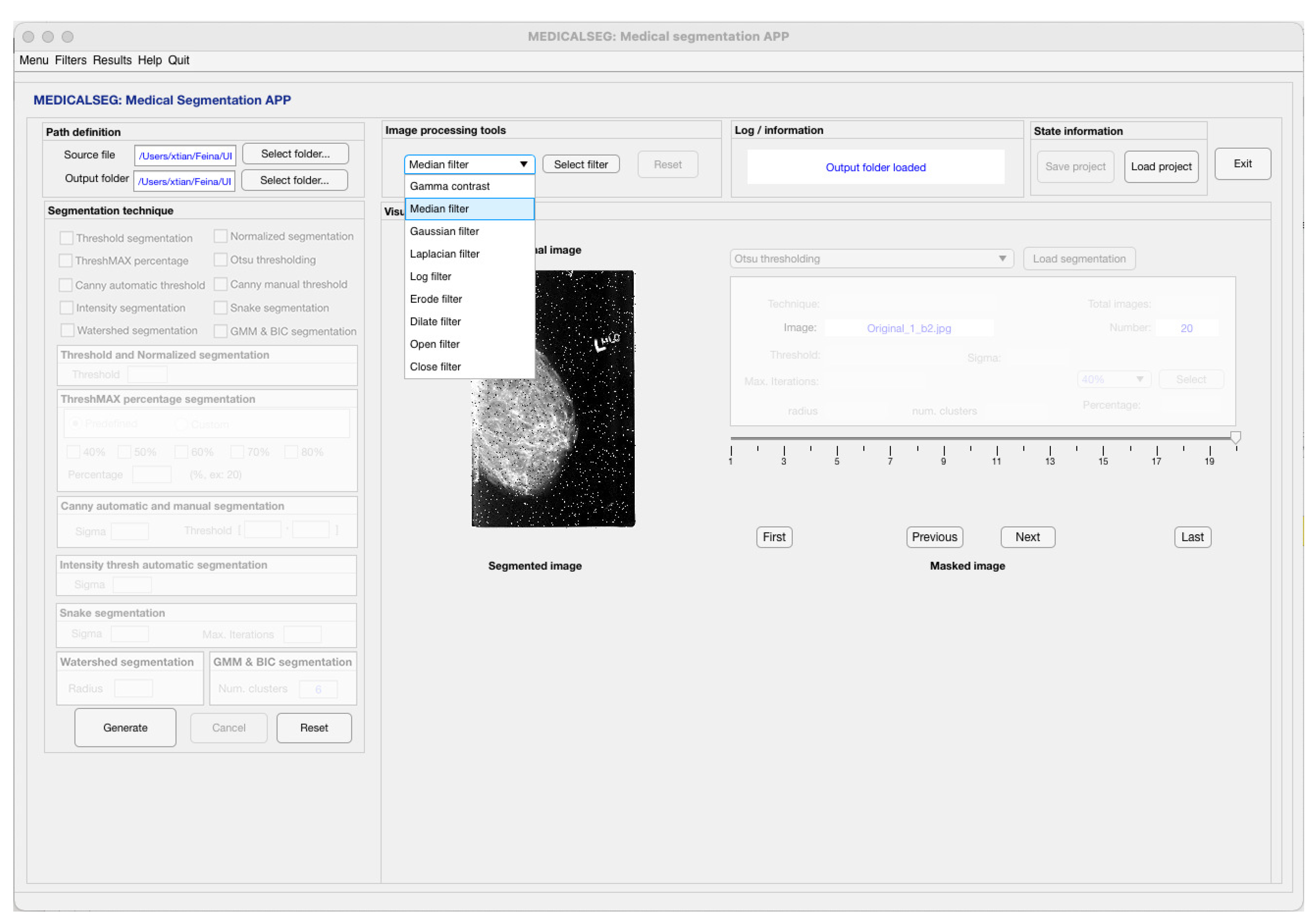
3.3.2. Gaussian and Median Filters
3.3.3. Laplacian and Log Filters
3.3.4. Morphological Filters
4. Results and Discussion
4.1. Pre-Processing Test
4.2. Experimental Test
4.3. Performance Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, K. Medical Image Segmentation: Methods and Applications in Functional Imaging. In Handbook of Biomedical Image Analysis; Topics in Biomedical Engineering International Book, Series; Suri, J.S., Wilson, D.L., Laxminarayan, S., Eds.; Springer: Boston, MA, USA, 2005. [Google Scholar] [CrossRef]
- Norouzi, A.; Shafry, M.; Rahim, M.; Altameem, A.; Saba, T.; Rad, A.; Rehman, A.; Uddin, M. Medical Image Segmentation Methods, Algorithms, and Applications. IETE Tech. Rev. 2014, 31, 199–213. [Google Scholar] [CrossRef]
- Rogowska, J. Overview and Fundamentals of Medical Image Segmentation. In Handbook of Medical Imaging, Processing and Analysis; Elsevier: Amsterdam, The Netherlands, 2000; pp. 69–85. [Google Scholar] [CrossRef]
- Ma, J. Cutting-edge 3D Medical Image Segmentation Methods in 2020: Are Happy Families All Alike? arXiv 2021, arXiv:2101.00232. [Google Scholar]
- Zgallai, W. Biomedical Signal Processing and Artificial Intelligence in Healthcare; Developments in Biomedical Engineering and Bioelectronics; Elsevier: Amsterdam, The Netherlands, 2020; pp. 241–247. [Google Scholar] [CrossRef]
- O’Mahony, N.; Campbell, S.; Carvalho, A.; Harapanahalli, S.; Velasco-Hernandez, G.; Krpalkova, L.; Riordan, D.; Walsh, J. Advances in Computer Vision. In Advances in Intelligent Systems and Computing; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef] [Green Version]
- Mr Gloom GitHub. Awesome Semantic Segmentation. Available online: https://resume.github.io/?mrgloom (accessed on 24 February 2022).
- Radau, P.; Lu, Y.; Connelly, K.; Paul, G.; Dick, A.; Wright, G. Evaluation Framework for Algorithms Segmenting Short Axis Cardiac MRI. MIDAS J. 2009. Available online: https://doi.org/10.54294/g80ruo (accessed on 15 March 2022). [CrossRef]
- Lequan, Y.; Hao, C.; Qi, D.; Jing, Q.; Pheng-Ann, H. Automated Melanoma Recognition in Dermoscopy Images via Very Deep Residual Networks. IEEE Trans. Med. Imaging 2017, 36, 994–1004. [Google Scholar] [CrossRef]
- Chang, C.-S.; Lin, J.-F.; Lee, M.-C.; Palm, C. Semantic Lung Segmentation Using Convolutional Neural Networks. In Bildverarbeitung für die Medizin 2020; Springer: Wiesbaden, Germany, 2020; pp. 75–80. [Google Scholar] [CrossRef]
- GitHub imlab-uiip Repository. Mammography Segmentation. 2021. Available online: https://github.com/imlab-uiip (accessed on 10 March 2022).
- Scotty Kwok, GitHub Repository. Cervix. 2017. Available online: https://github.com/scottykwok/cervix-roi-segmentation-by-unet (accessed on 18 February 2022).
- Li, W.; Qin, S.; Li, F.; Wang, L. MAD-UNet: A deep U-shaped network combined with an attention mechanism for pancreas segmentation in CT images. Med. Phys. 2021, 48, 329–341. [Google Scholar] [CrossRef]
- Christ, P.F.; Ettlinger, F.; Grün, F.; Elshaera, M.E.A.; Lipkova, J.; Schlecht, S.; Ahmaddy, F.; Tatavarty, S.; Bickel, M.; Bilic, P.; et al. Automatic Liver and Lesion Segmentation in CT Using Cascaded Fully Convolutional Neural Networks and 3D Conditional Random Fields; Springer International Publishing: Berlin, Germany, 2016; pp. 415–423. [Google Scholar] [CrossRef] [Green Version]
- Marko Jocic GitHub Repository. Ultrasound Nerve Segmentation. 2018. Available online: https://github.com/jocicmarko/ultrasound-nerve-segmentation (accessed on 20 February 2022).
- Anthimopoulos, M.; Christodoulidis, S.; Ebner, L.; Christe, A.; Mougiakakou, S. Lung Pattern Classification for Interstitial Lung Diseases Using a Deep Convolutional Neural Network. IEEE Trans. Med. Imaging 2016, 35, 1207–1216. [Google Scholar] [CrossRef]
- Mendrik, A.M.; Vincken, K.L.; Kuijf, H.J.; Breeuwer, M.; Bouvy, W.; de Bresser, J.; Alansary, A.; de Bruijne, M.; Carass, A.; El-Baz, A.; et al. MRBrainS Challenge: Online Evaluation Framework for Brain Image Segmentation in 3T MRI Scans. Comput. Intell. Neurosci. 2015, 2015, 813696. [Google Scholar] [CrossRef] [Green Version]
- Ronneberger, O.; Fischer, P.; Brox, T. U-Net: Convolutional Networks for Biomedical Image Segmentation. arXiv 2015, arXiv:1505.04597. [Google Scholar]
- Varma, D. Managing DICOM images: Tips and tricks for the radiologist. Indian J. Radiol. Imaging 2012, 22, 4–13. [Google Scholar] [CrossRef]
- Mata, C.; Freixenet, J.; Lladó, X.; Oliver, A. Texture descriptors applied to digital mammography. In Erasmus-Mundus Master in Computer Vision and Robotics (VIBOT); UPC Commons: Barcelona, Spain, 2008; pp. 105–110. [Google Scholar]
- Hospital Sant Joan de Déu (HSJD), Esplugues de Llobregat, Barcelona (Spain). Available online: https://www.sjdhospitalbarcelona.org/en (accessed on 11 January 2022).
- Rodríguez, J.; Ochoa-Ruiz, G.; Mata, C. A Prostate MRI Segmentation Tool Based on Active Contour Models Using a Gradient Vector Flow. Appl. Sci. 2020, 10, 6163. [Google Scholar] [CrossRef]
- Mata, C.; Walker, P.; Oliver, A.; Brunotte, F.; Martí, J.; Lalande, A. Prostateanalyzer: Web-based medical application for the management of prostate cancer using multiparametric mr images. Inform. Health Soc. Care 2015, 87, 1–21. [Google Scholar] [CrossRef]
- Kaggle Grandmaster. DRIVE: Digital Retinal Images for Vessel Extraction. 2021. Available online: https://www.kaggle.com/datasets/andrewmvd/drive-digital-retinal-images-for-vessel-extraction (accessed on 18 April 2022).
- Van den Heuvel, T.; De Bruijn, D.; De Korte, C. Automated measurement of fetal head circumference using 2D ultrasound images. PLoS ONE 2018, 13, e0200412. [Google Scholar] [CrossRef]
- Mata, C. MEDICALSEG Application. 2022. Available online: https://github.com/xtianu/medicalsegapp (accessed on 5 June 2022).
- Gonzalez, R.; Woods, R. Digital Image Processing, 3rd ed.; Prentice-Hall, Inc.: Hoboken, NJ, USA, 2007; p. 976. [Google Scholar]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef] [Green Version]
- Bajaj, P.; Otsu Thresholding. MATLAB Central File Exchange. Available online: https://www.mathworks.com/matlabcentral/fileexchange/74380-otsu-thresholding) (accessed on 7 June 2022).
- Kothwala, A.; Image Segmentation Using Otsu Method. MATLAB Central File Exchange. Available online: https://es.mathworks.com/matlabcentral/fileexchange/51297-image-segmentation-using-otsu-method (accessed on 7 June 2022).
- Canny, J. A Computational Approach to Edge Detection. IEEE Trans. Pattern Anal. Mach. Intell. 1986, PAMI-8, 679–698. [Google Scholar] [CrossRef]
- Farras, A.; Auto-Thresholding Canny Edge Detection. Auto-Thresholding Canny Edge Detection. Available online: https://www.mathworks.com/matlabcentral/fileexchange/61237-auto-thresholding-canny-edge-detection) (accessed on 8 September 2021).
- Trung-Thien, T.; Chan-Su, B.; Young-Nam, K.; Hyo-Moon, C.; Sang-Bock, C. An Adaptive Method for Lane Marking Detection Based on HSI Color Model. Commun. Comput. Inf. Sci. 2010, 93, 304–311. [Google Scholar] [CrossRef]
- Kass, M.; Witkin, A.; Terzopoulos, D. Snakes: Active contour models. Int. J. Comput. Vis. 1988, 1, 321–331. [Google Scholar] [CrossRef]
- Meyer, F. Topographic distance and watershed lines. Signal Process. 1994, 38, 113–125. [Google Scholar] [CrossRef]
- Preim, B.; Botha, C. Chapter 4—Image Analysis for Medical Visualization. In Visual Computing for Medicine, 2nd ed; Theory, Algorithms, and Applications; O’Reilly Media, Inc.: Sebastopol, CA, USA, 2014; p. 976. [Google Scholar] [CrossRef]
- Mohamed, O.; Jaïdane-Saïdane, M. Generalized Gaussian mixture model. In Proceedings of the 17th European Signal Processing Conference (EUSIPCO), Glasgow, UK, 24–28 August 2009; pp. 2273–2277. [Google Scholar] [CrossRef]
- Moitra, A. Algorithmic Aspects of Machine Learning; Cambridge University Press: Cambridge, UK, 2018; pp. 107–131. [Google Scholar] [CrossRef]
- Saleh, S. Robust Variable Selection Based on Schwarz Information Criterion for Linear Regression Models. Math. Stat. Eng. Appl. 2022, 71, 54–68. [Google Scholar] [CrossRef]
- McLachlan, G.; Peel, D. Finite Mixture Models; John Wiley & Sons: Hoboken, NJ, USA, 2000; Volume 6, pp. 355–378. [Google Scholar] [CrossRef]
- Handari, A.K.; Maurya, S.K. Gamma corrected reflectance for low contrast image enhancement using guided filter. Multimed. Tools Appl. 2022, 81, 6009–6030. [Google Scholar] [CrossRef]
- Bhairannawar, S. Chapter 4—Efficient Medical Image Enhancement Technique Using Transform HSV Space and Adaptive Histogram Equalization. In Visual Computing for Medicine, 2nd ed.; Theory, Algorithms, and Applications; ScienceDirect Academic Press: Cambridge, MA, USA, 2014; pp. 51–60. [Google Scholar] [CrossRef]
- Moore, C.; Bell, D. Variational Analysis. Radiopaedia Artif. Intell. 2020, 2020, 75056. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.E.K. IH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]


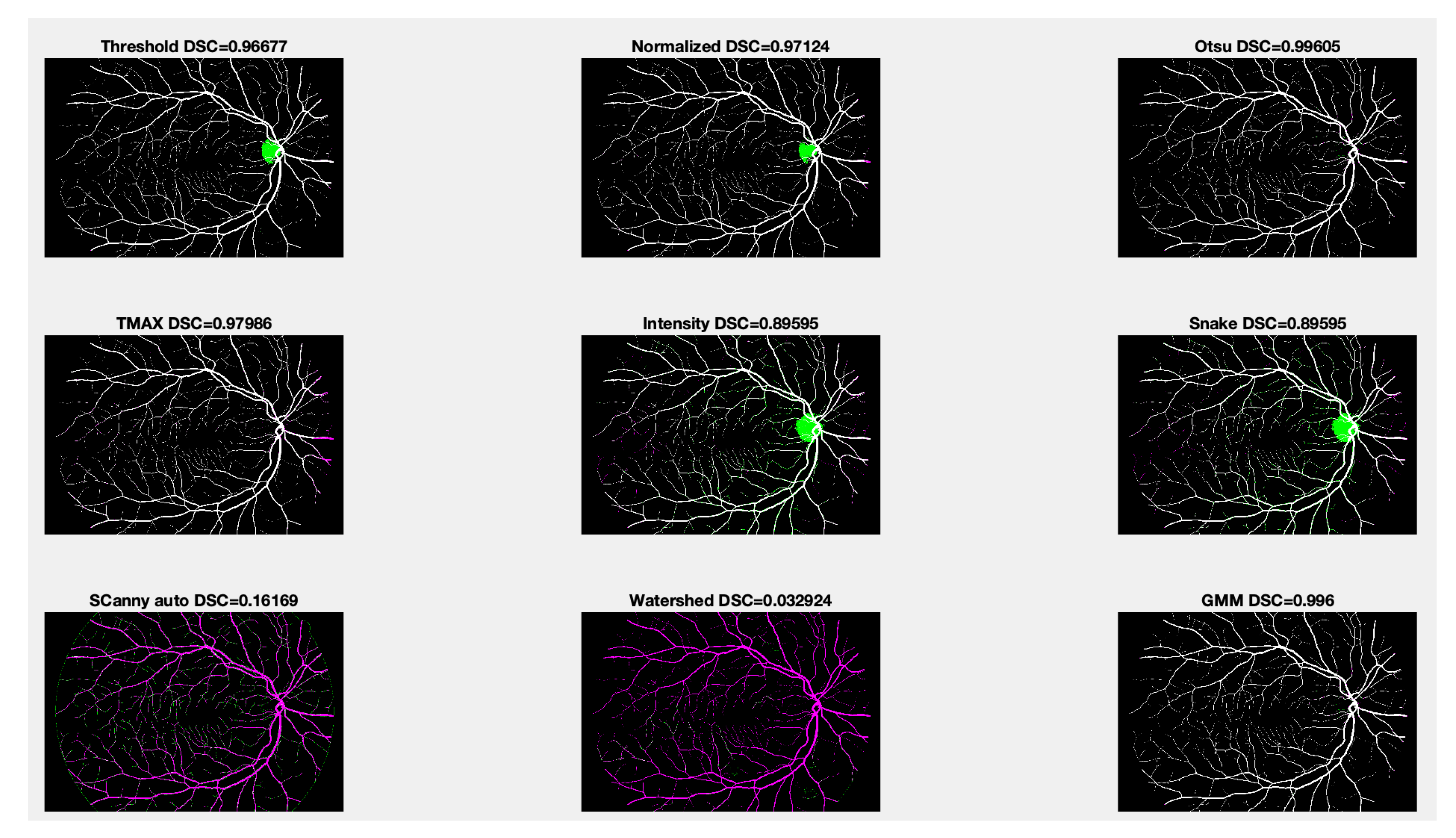
| Dice Similarity Index (DSC) | |||
|---|---|---|---|
| GT vs. MedicalSeg | GT vs. ImageJ | MedicalSeg vs. ImageJ | |
| Threshold | 0.96677 | 0.97550 | 0.99127 |
| Normalized | 0.97124 | 0.96998 | 0.99874 |
| Otsu | 0.99605 | 0.98256 | 0.98651 |
| Snake | 0.89595 | 0.91422 | 0.98713 |
| Canny auto | 0.16169 | 0.17881 | 0.98309 |
| Watershed | 0.032924 | 0.02749 | 0.99455 |
| GMM | 0.99600 | 0.92772 | 0.93172 |
| Images | Thres | ThMAX | Otsu | CannyAuto | Intensity | Snake | Watershed | GMM | All |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.73 | 1.15 | 0.76 | 1.89 | 1.27 | 1.03 | 2.35 | 3.61 | 3.61 |
| 10 | 1.04 | 2.09 | 3.84 | 12.79 | 2.46 | 1.81 | 4.39 | 18.19 | 18.19 |
| 20 | 2.78 | 3.92 | 10.93 | 23.69 | 4.50 | 2.14 | 7.39 | 34.50 | 34.50 |
| 50 | 4.96 | 5.43 | 13.21 | 60.04 | 6.28 | 5.12 | 10.31 | 85.45 | 85.45 |
| 100 | 10.18 | 10.04 | 33.81 | 117.25 | 11.81 | 10.12 | 22.48 | 177.28 | 177.28 |
| 200 | 17.14 | 20.62 | 65.25 | 220.29 | 25.45 | 22.21 | 46.43 | 336.57 | 336.57 |
| 500 | 58.32 | 64.54 | 189.60 | 533.18 | 82.10 | 56.71 | 114.65 | 1033.09 | 1033.09 |
| 1000 | 127.01 | 119.20 | 396.98 | 922.89 | 156.39 | 127.99 | 216.73 | 1670.10 | 1670.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mata, C.; Munuera, J.; Lalande, A.; Ochoa-Ruiz, G.; Benitez, R. MedicalSeg: A Medical GUI Application for Image Segmentation Management. Algorithms 2022, 15, 200. https://doi.org/10.3390/a15060200
Mata C, Munuera J, Lalande A, Ochoa-Ruiz G, Benitez R. MedicalSeg: A Medical GUI Application for Image Segmentation Management. Algorithms. 2022; 15(6):200. https://doi.org/10.3390/a15060200
Chicago/Turabian StyleMata, Christian, Josep Munuera, Alain Lalande, Gilberto Ochoa-Ruiz, and Raul Benitez. 2022. "MedicalSeg: A Medical GUI Application for Image Segmentation Management" Algorithms 15, no. 6: 200. https://doi.org/10.3390/a15060200
APA StyleMata, C., Munuera, J., Lalande, A., Ochoa-Ruiz, G., & Benitez, R. (2022). MedicalSeg: A Medical GUI Application for Image Segmentation Management. Algorithms, 15(6), 200. https://doi.org/10.3390/a15060200









