Preparation and Application of Water-in-Oil Emulsions Stabilized by Modified Graphene Oxide
Abstract
:1. Introduction
2. Experimental Materials
2.1. Preparation of Graphene Oxide (GO)
2.2. Preparation of Alkyl Chain Modified Graphene Oxide (AmGO)
2.3. Preparation of Pickering Emulsions Stabilized by Amgo
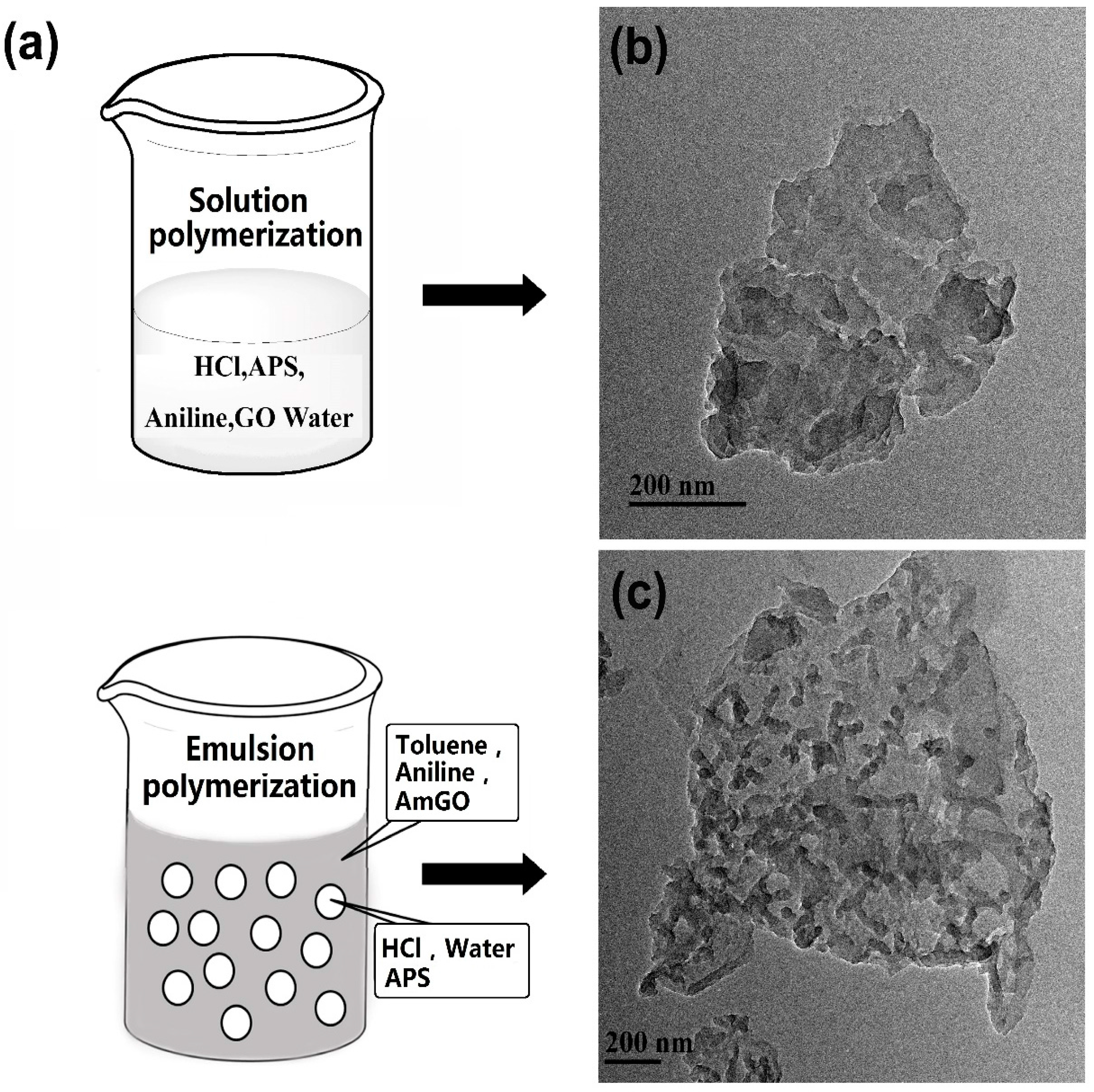
2.4. Preparation of Graphene/Polyaniline Nanocomposite
2.5. Characterization
3. Results and Discussion
3.1. Basic Characterization of AmGO
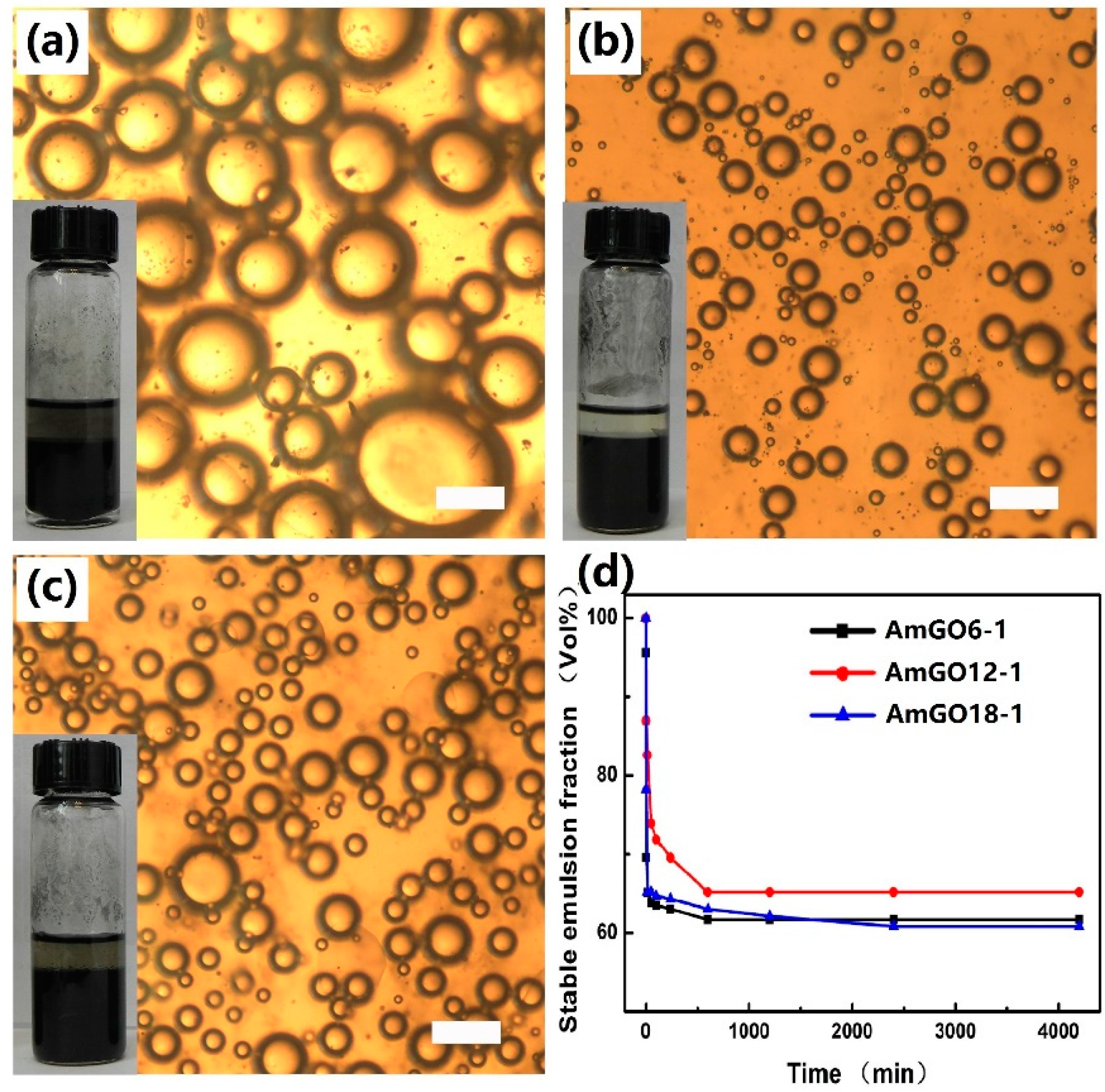
3.2. Effect of Alkyl Chain Length on the Emulsion Properties of AmGO
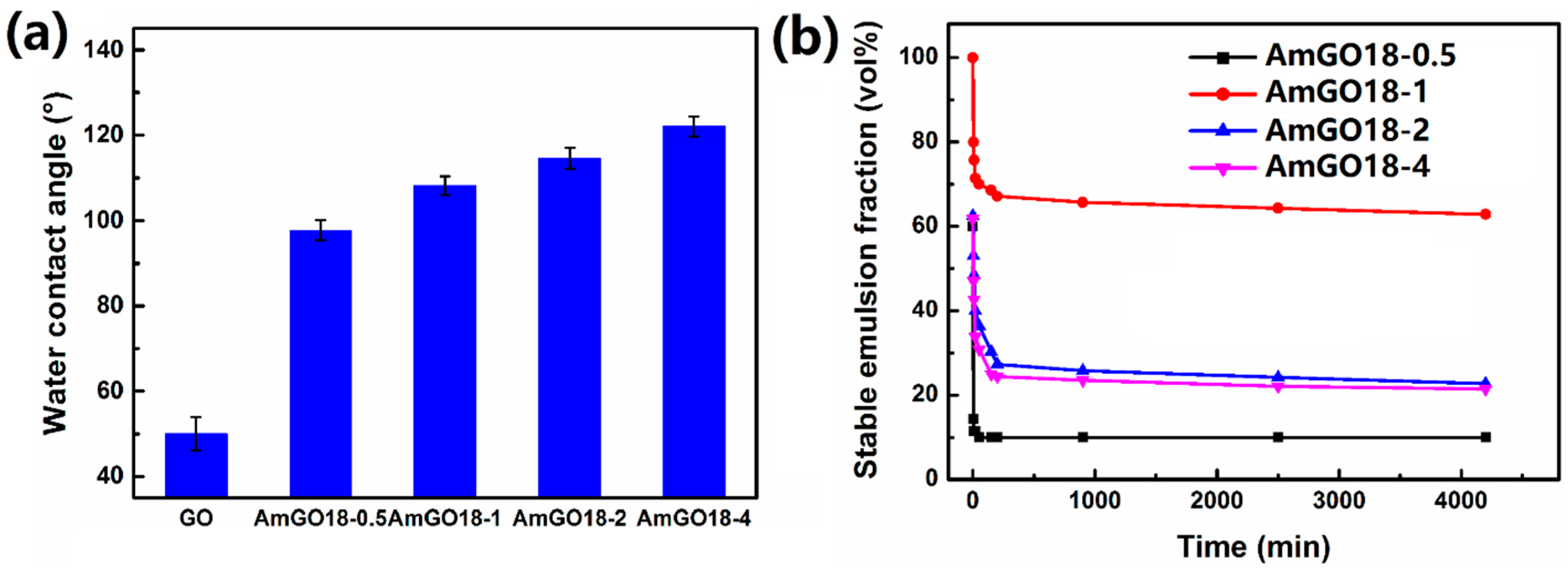
3.3. Effect of the Grafting Content of Alkyl Chain on the Emulsion Properties of AmGO
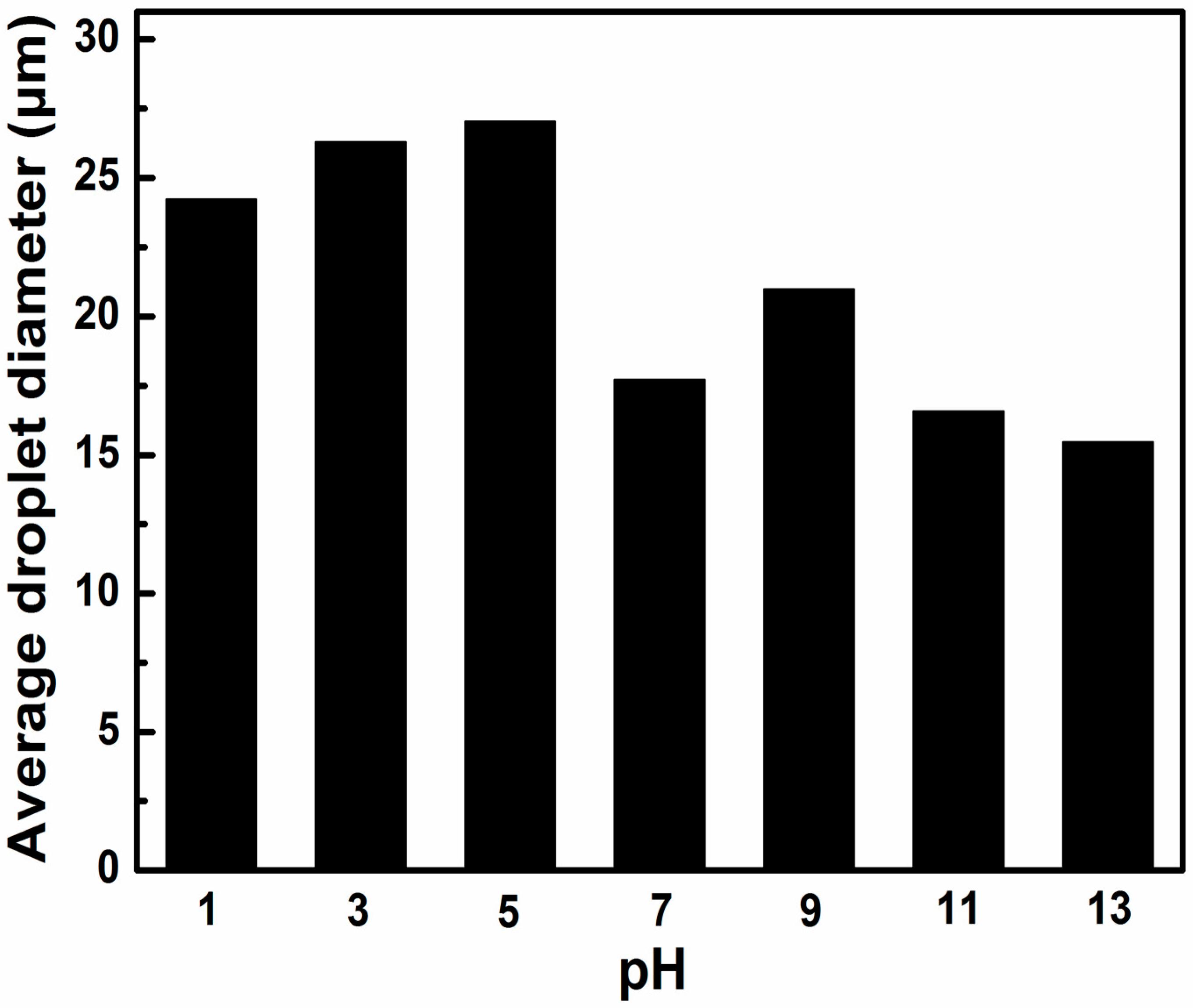
3.4. Effect of pH Value and NaCl Concentration on the Emulsion Properties of AmGO
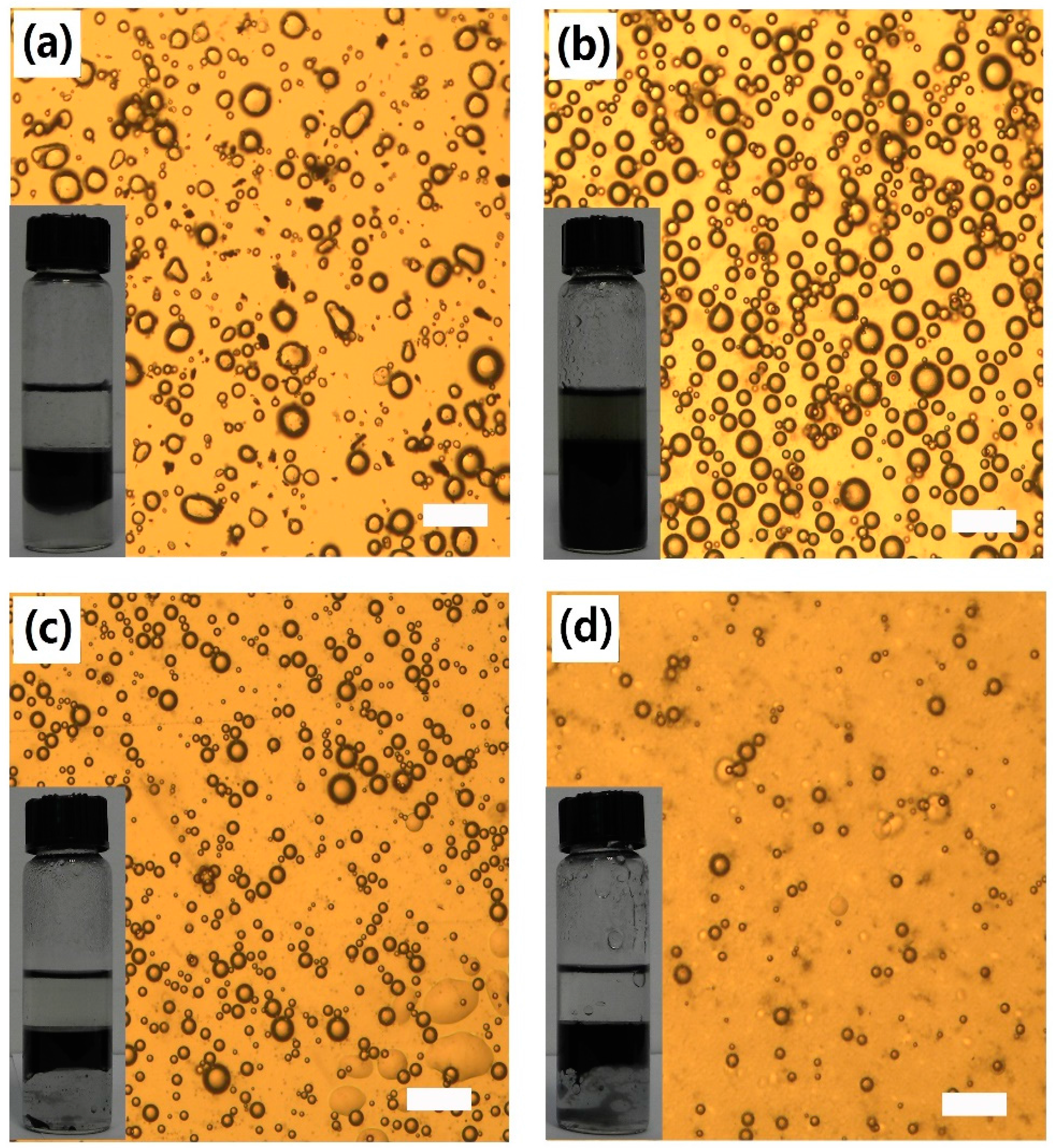
3.5. Effect of AmGO Concentration on the Emulsion Properties of AmGO
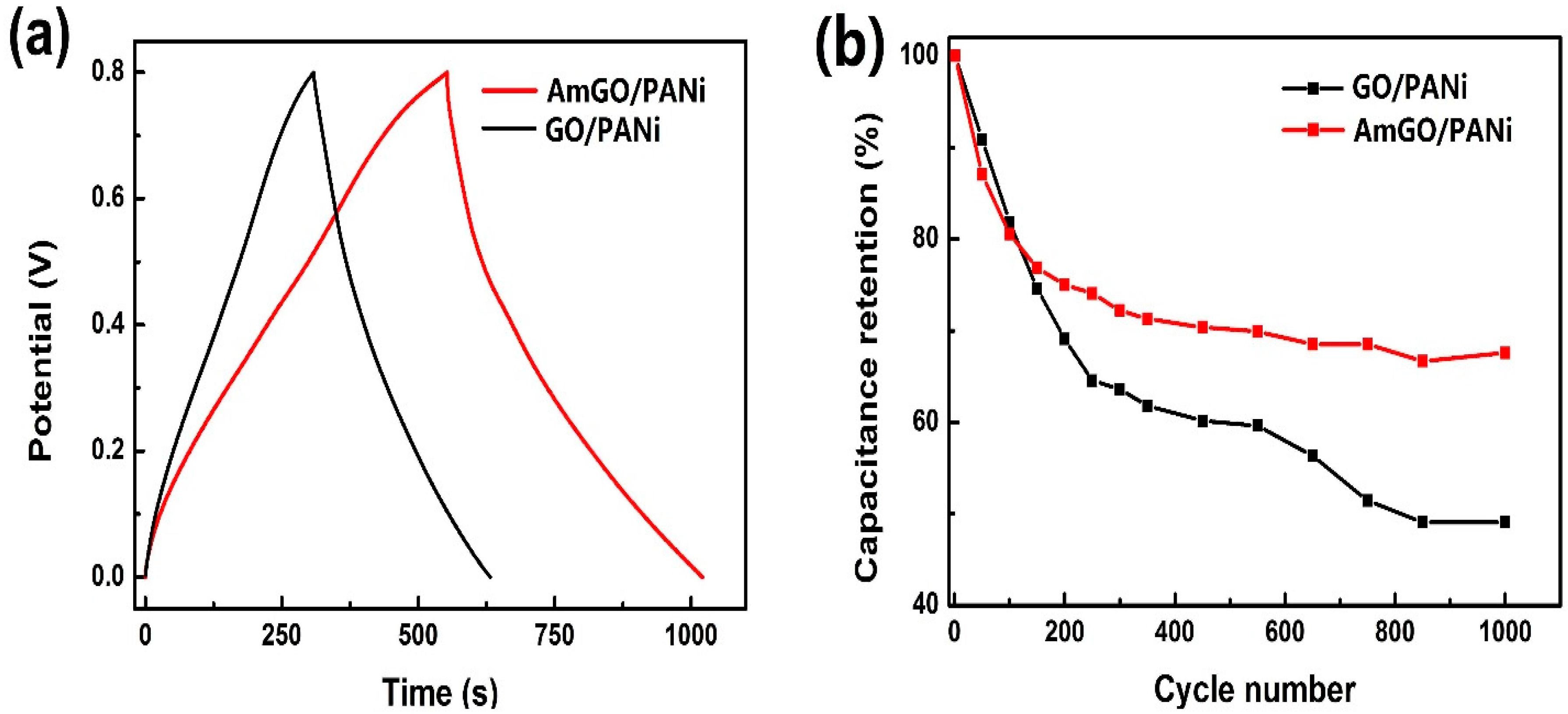
3.6. Preparation of AmGO/PANi and Its Supercapacitor Performance
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wassei, J.K.; Kaner, R.B. Graphene, a promising transparent conductor. Mater. Today 2010, 13, 52–59. [Google Scholar] [CrossRef]
- Jiang, H.J. Chemical preparation of graphene-based nanomaterials and their applications in chemical and biological sensors. Small 2011, 7, 2413–2427. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.Y.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-based ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.D.; Allen, M.J.; Tung, V.C.; Yang, Y.; Kaner, R.B.; Weiller, B.H. Practical chemical sensors from chemically derived graphene. ACS Nano 2009, 3, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yue, S.F.; Sui, Z.Y.; Zhang, X.T.; Hou, S.S.; Cao, G.P.; Yang, Y.S. What is the choice for supercapacitors: Graphene or graphene oxide? Energy Environ. Sci. 2011, 4, 2826–2830. [Google Scholar] [CrossRef]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Kaner, R.B. Graphene-Based Materials. Science 2008, 320, 1170–1171. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.; Erni, R.; Lee, Z.; Alem, N.; Gannett, W.; Zettl, A. Determination of the local chemical structure of graphene oxide and reduced graphene oxide. Adv. Mater. 2010, 40, 4467–4472. [Google Scholar] [CrossRef] [PubMed]
- Texter, J. Graphene oxide and graphene flakes as stabilizers and dispersing aids. Curr. Opin. Colloid Interface Sci. 2015, 20, 454–464. [Google Scholar] [CrossRef]
- Luo, J.Y.; Cote, L.J.; Tung, V.C.; Tan, A.T.L.; Goins, P.E.; Wu, J.S.; Huang, J.X. Graphene oxide nanocolloids. J. Am. Chem. Soc. 2010, 132, 17667–17669. [Google Scholar] [CrossRef] [PubMed]
- Cote, L.J.; Kim, J.Y.; Tung, V.C.; Luo, J.Y.; Kim, F.; Huang, J.X. Graphene oxide as surfactant sheets. Pure Appl. Chem. 2011, 83, 95–110. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J.X. Graphene oxide sheets at interfaces. J. Am. Chem. Soc. 2010, 132, 8180–8186. [Google Scholar] [CrossRef] [PubMed]
- He, Y.Q.; Wu, F.; Sun, X.Y.; Li, R.Q.; Guo, Y.Q.; Li, C.B.; Zhang, L.; Xing, F.B.; Wang, W.; Gao, J.P. Factors that affect pickering emulsions stabilized by graphene oxide. ACS Appl. Mater. Interfaces 2013, 5, 4843–4855. [Google Scholar] [CrossRef] [PubMed]
- Creighton, M.A.; Ohata, Y.; Miyawaki, J.; Bose, A.; Hurt, R.H. Two-dimensional materials as emulsion stabilizers: Interfacial thermodynamics and molecular barrier properties. Langmuir 2014, 30, 3687–3696. [Google Scholar] [CrossRef] [PubMed]
- McCoy, T.M.; Pottage, M.J.; Tabor, R.F. Graphene oxide-stabilized oil-in-water emulsions: pH-controlled dispersion and flocculation. J. Phys. Chem. C 2014, 118, 4529–4535. [Google Scholar] [CrossRef]
- Sun, Z.W.; Feng, T.; Russell, T.P. Assembly of graphene oxide at water/oil interfaces: Tessellated nanotiles. Langmuir 2013, 29, 13407–13413. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.Y.; Wang, X.R.; Wu, F.; Liu, Y.; Zhang, S.; Pang, X.B.; Li, X.X.; Qiu, H.X. Au nanoparticle/graphene oxide hybrids as stabilizers for Pickering emulsions and Au nanoparticle/graphene oxide@ polystyrene microspheres. Carbon 2014, 71, 238–248. [Google Scholar] [CrossRef]
- Gudarzi, M.M.; Sharif, F. Self assembly of graphene oxide at the liquid–liquid interface: A new route to the fabrication of graphene based composites. Soft Matter 2011, 7, 3432–3440. [Google Scholar] [CrossRef]
- Moghaddam, S.Z.; Saburya, S.; Sharif, F. Dispersion of rGO in polymeric matrices by thermodynamically favorable self-assembly of GO at oil–water interfaces. RSC Adv. 2014, 4, 8711–8719. [Google Scholar] [CrossRef]
- Thickett, S.C.; Zetterlund, P.B. Preparation of composite materials by using graphene oxide as a surfactant in Ab initio emulsion polymerization systems. ACS Macro Lett. 2013, 2, 630–634. [Google Scholar] [CrossRef]
- Sun, J.; Bi, H. Pickering emulsion fabrication and enhanced supercapacity of graphene oxide-covered polyaniline nanoparticles. Mater. Lett. 2012, 81, 48–51. [Google Scholar] [CrossRef]
- Zheng, Z.; Zheng, X.H.; Wang, H.T.; Du, Q.G. Macroporous graphene oxide–polymer composite prepared through Pickering high internal phase emulsions. ACS Appl. Mater. Interfaces 2013, 5, 7974–7982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.F.; Zhang, X.B.; Dong, Y.H.; Wang, L.M. Enhancing electrical conductivity of rubber composites by constructing interconnected network of self-assembled graphene with latex mixing. J. Mater. Chem. 2012, 22, 10464–10468. [Google Scholar] [CrossRef]
- Xie, P.F.; Ge, X.P.; Fang, B.; Li, Z.; Liang, Y.; Yang, C.Z. Pickering emulsion polymerization of graphene oxide-stabilized styrene. Colloid Polym. Sci. 2013, 7, 1631–1639. [Google Scholar] [CrossRef]
- Kattimuttathu, S.I.; Krishnappan, C.; Vellorathekkaepadil, V.; Nutenki, R.; Mandapati, V.R.; Cernik, M. Synthesis, characterization and optical properties of graphene oxide–polystyrene nanocomposites. Polym. Adv. Technol. 2015, 26, 214–222. [Google Scholar]
- Dao, T.D.; Efdenedelger, G.; Jeong, H.M. Water-dispersible graphene designed as a Pickering stabilizer for the suspension polymerization of poly(methyl methacrylate)/graphene core–shell microsphere exhibiting ultra-low percolation threshold of electrical conductivity. Polymer 2014, 55, 4709–4719. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339. [Google Scholar] [CrossRef]
- Li, W.; Tang, X.Z.; Zhang, H.B.; Jiang, Z.G.; Yu, Z.Z.; Du, X.S.; Mai, Y.W. Simultaneous surface functionalization and reduction of graphene oxide with octadecylamine for electrically conductive polystyrene composites. Carbon 2011, 49, 4724–4730. [Google Scholar] [CrossRef]
- Woltornist, S.J.; Carrillo, J.M.Y.; Xu, T.O.; Dobrynin, A.V.; Adamson, D.H. Polymer/pristine graphene based composites: From emulsions to strong, electrically conducting foams. Macromolecules 2015, 48, 687–693. [Google Scholar] [CrossRef]
- Mohaghedgh, N.; Tasviri, M.; Rahimi, E.; Gholami, M.R. A novel p–n junction Ag3PO4/BiPO4-based stabilized Pickering emulsion for highly efficient photocatalysis. RSC Adv. 2015, 5, 12944–12955. [Google Scholar] [CrossRef]
- Patolea, A.S.; Patoleb, S.P.; Kanga, H.; Yoob, J.B.; Kima, T.H.; Ahn, J.H. A facile approach to the fabrication of graphene/polystyrene nanocomposite by in situ microemulsion polymerization. J. Colloid Interface Sci. 2010, 350, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Song, X.H.; Yang, Y.F.; Liu, J.C.; Zhao, A.H.Y. PS colloidal particles stabilized by graphene oxide. Langmuir 2011, 27, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wei, S.; Haldolaarachchige, N.; Colorado, H.; Luo, Z.; Young, D.P. Magnetoresistive conductive polyaniline–barium titanate nanocomposites with negative permittivity. J. Phys. Chem. C 2012, 116, 15731–15740. [Google Scholar] [CrossRef]
- Gu, H.; Huang, Y.; Zhang, X.; Wang, Q.; Zhu, J.; Shao, L. Magnetoresistive polyaniline-magnetite nanocomposites with negative dielectrical properties. Polymer 2012, 46, 801–809. [Google Scholar] [CrossRef]
- Zhu, J.; Gu, H.; Luo, Z.; Haldolaarachige, N.; Young, D.P.; Wei, S. Carbon nanostructure-derived polyaniline metacomposites: Electrical, dielectric, and giant magnetoresistive properties. Langmuir 2012, 28, 10246–10255. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.X.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.H.; Chen, M.J.; Qu, H.L.; Zhang, X.; Wei, H.G.; Luo, Z.P.; Colorado, H.A.; Wei, S.Y.; Guo, Z.H. Interfacial polymerized polyaniline/graphite oxide nanocomposites toward electrochemical energy storage. Polymer 2012, 53, 5953–5964. [Google Scholar] [CrossRef]



© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, X.; Xia, L.; Chen, M.; Wei, W.; Luo, J.; Liu, X. Preparation and Application of Water-in-Oil Emulsions Stabilized by Modified Graphene Oxide. Materials 2016, 9, 731. https://doi.org/10.3390/ma9090731
Fei X, Xia L, Chen M, Wei W, Luo J, Liu X. Preparation and Application of Water-in-Oil Emulsions Stabilized by Modified Graphene Oxide. Materials. 2016; 9(9):731. https://doi.org/10.3390/ma9090731
Chicago/Turabian StyleFei, Xiaoma, Lei Xia, Mingqing Chen, Wei Wei, Jing Luo, and Xiaoya Liu. 2016. "Preparation and Application of Water-in-Oil Emulsions Stabilized by Modified Graphene Oxide" Materials 9, no. 9: 731. https://doi.org/10.3390/ma9090731
APA StyleFei, X., Xia, L., Chen, M., Wei, W., Luo, J., & Liu, X. (2016). Preparation and Application of Water-in-Oil Emulsions Stabilized by Modified Graphene Oxide. Materials, 9(9), 731. https://doi.org/10.3390/ma9090731




