Improved Charge Separation in WO3/CuWO4 Composite Photoanodes for Photoelectrochemical Water Oxidation
Abstract
:1. Introduction
2. Results and Discussion
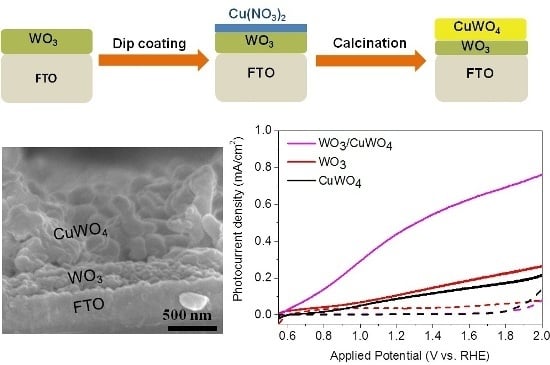
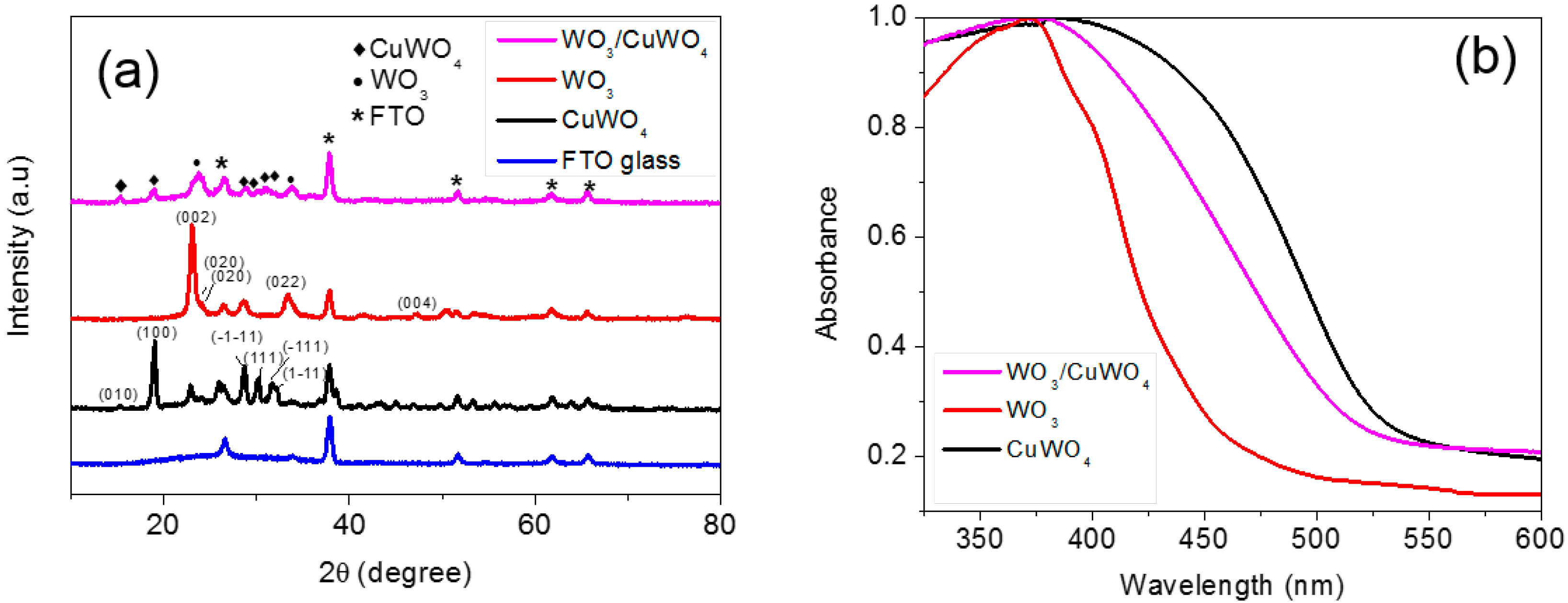
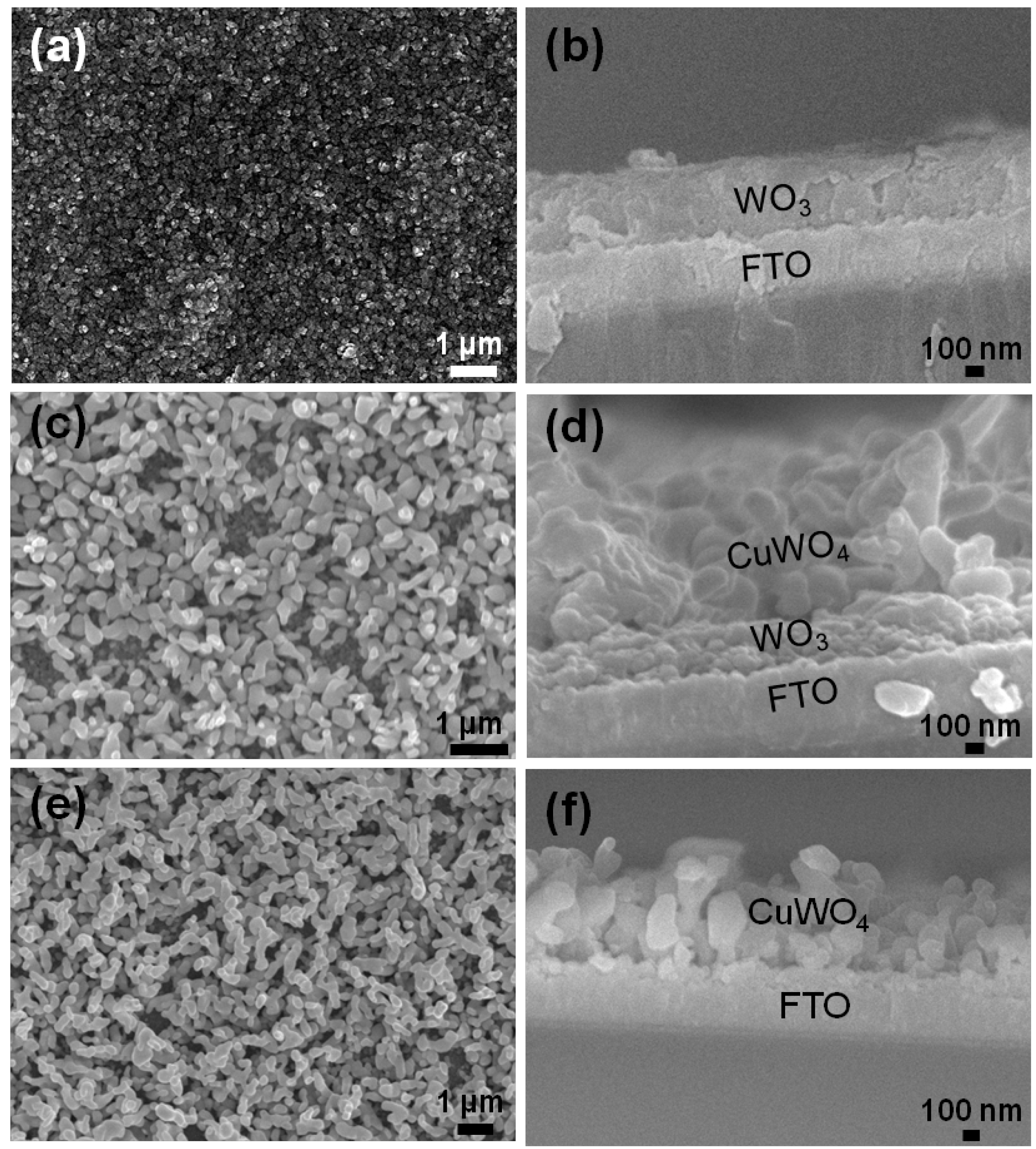
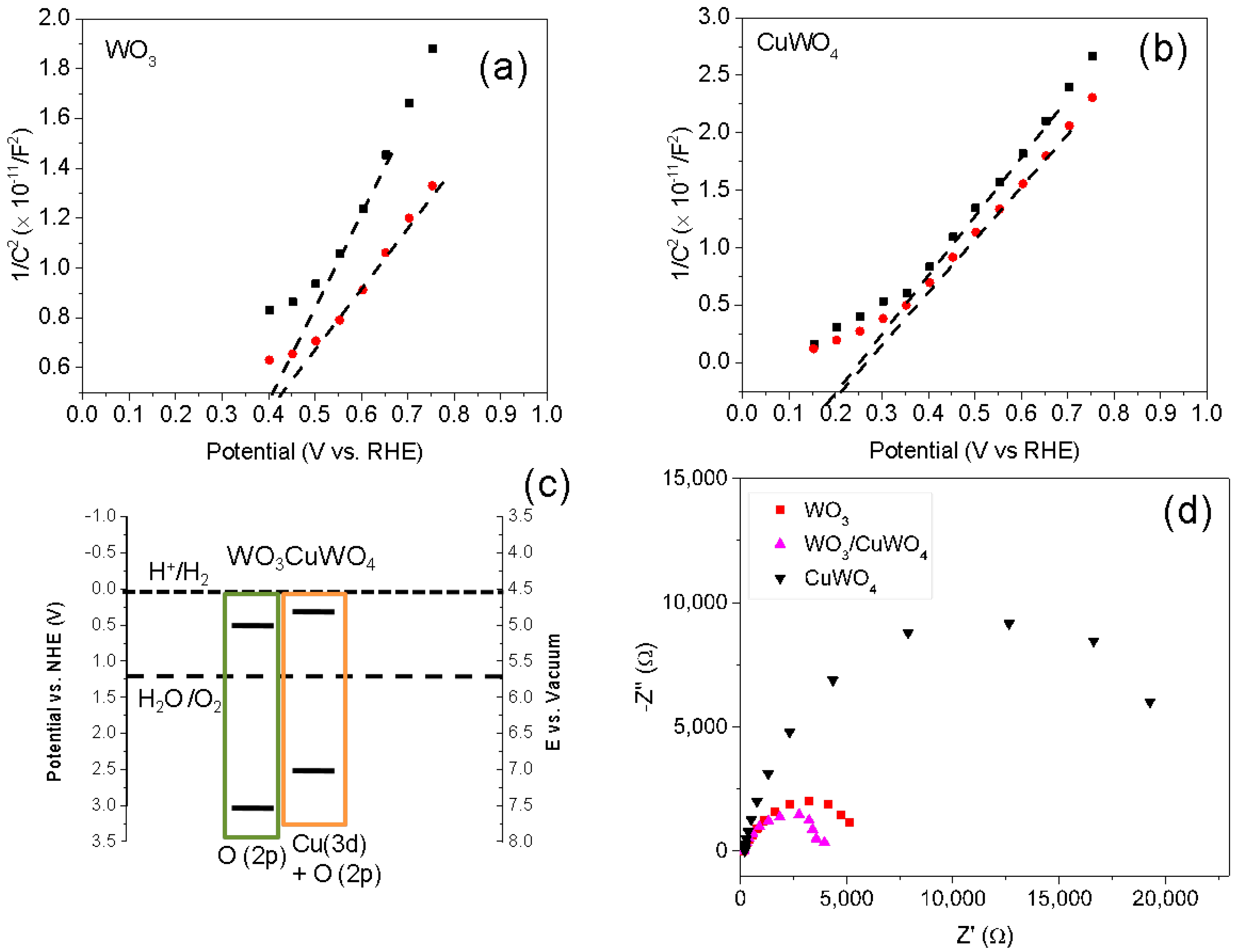
2.1. Synthesis and Characterization of Pristine WO3 and WO3/CuWO4 Composite Thin Films
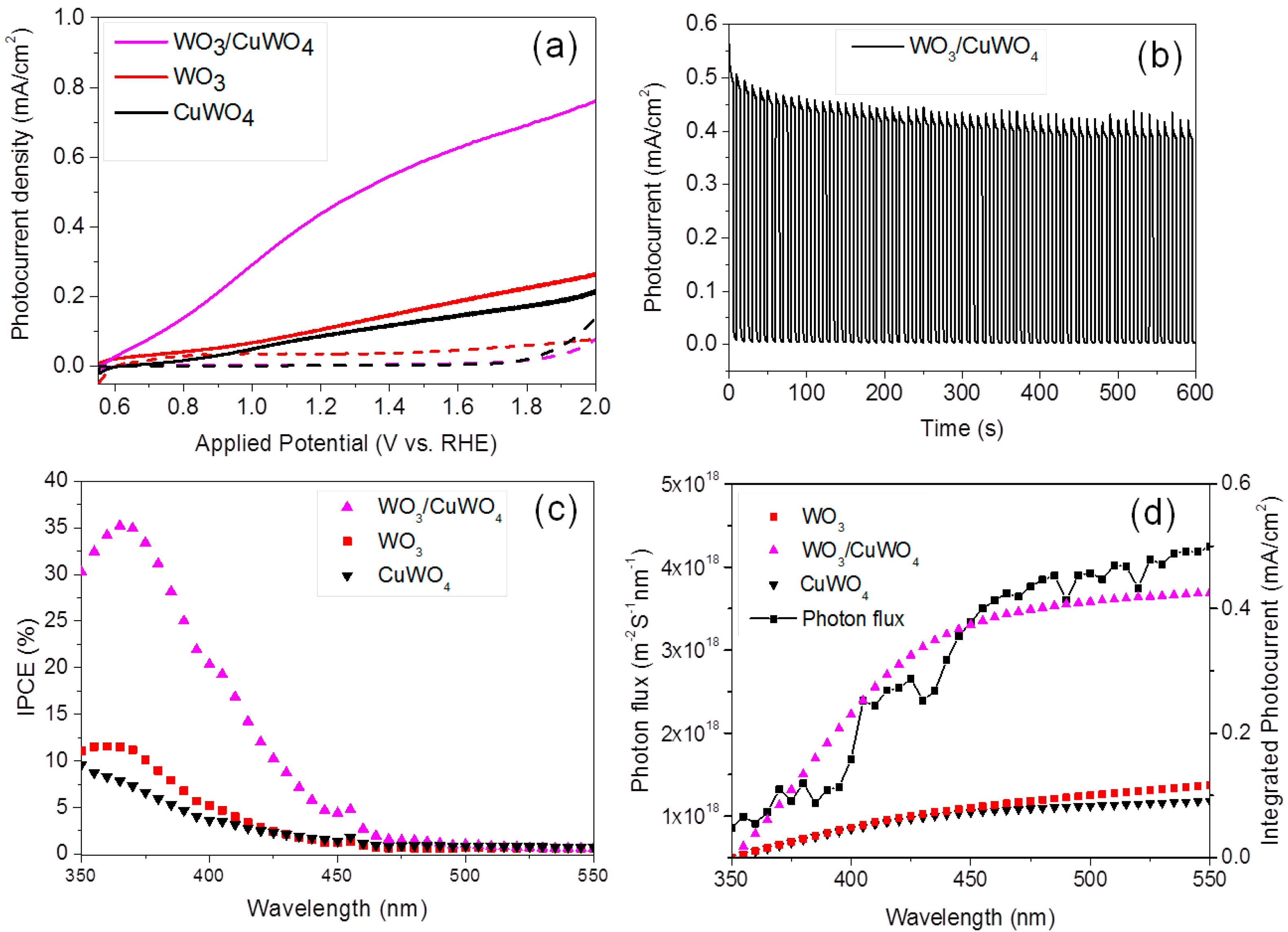
2.2. Photoelectrochemical Performance of Thin Films
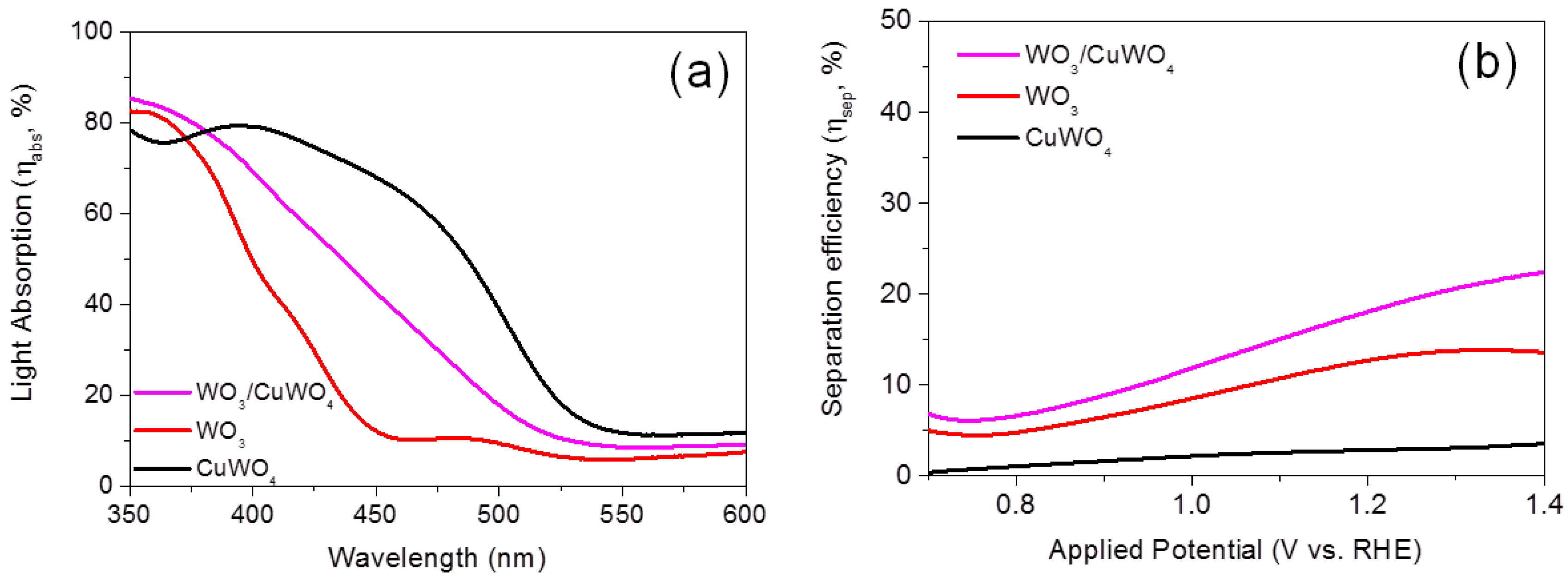
2.3. Comparison of Absorption Efficiency (ηabs), and Charge Separation Efficiency (ηsep) of WO3, CuWO4 and WO3/CuWO4 Thin Films
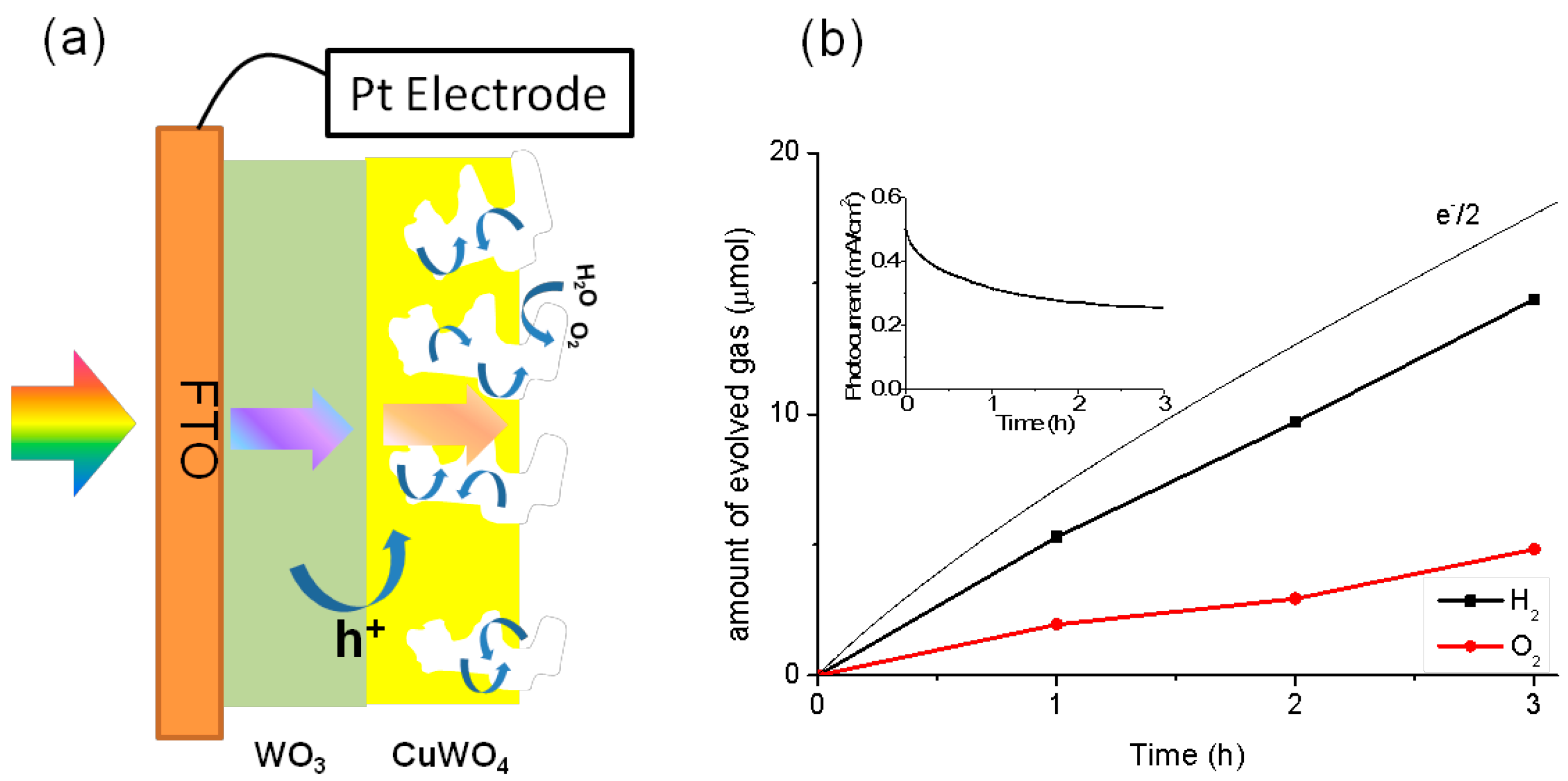
2.4. Photoelectrochemical Water Splitting
3. Materials and Methods
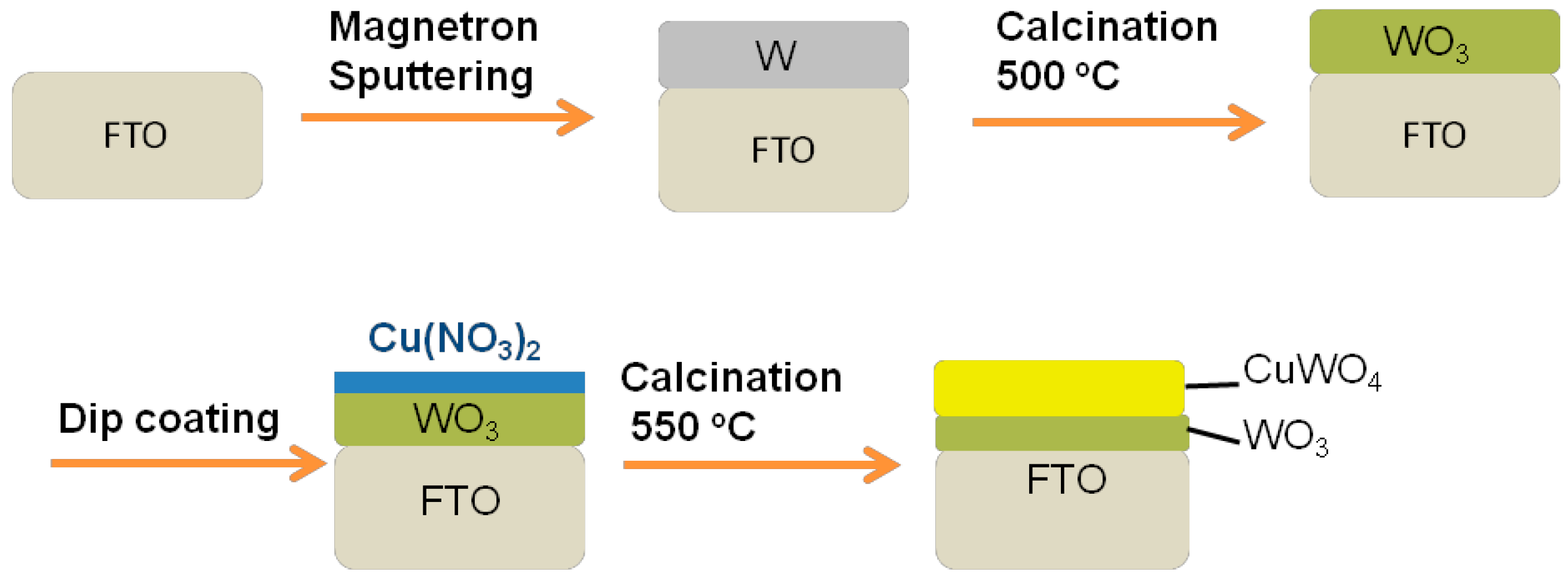
3.1. Preparation of W Thin Film from Magnetron Sputtering
3.2. Fabrication of WO3 Thin Film
3.3. Fabrication of WO3/CuWO4 Thin Films
3.4. Material Characterization
3.5. Photoelectrochemical (PEC) Measurement
3.6. Photoelectrochemical Water Splitting
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gust, D.; Moore, T.A.; Moore, A.L. Solar fuels via artificial photosynthesis. Acc. Chem. Res. 2009, 42, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Sivula, K.; Le Formal, F.; Gratzel, M. Solar water splitting: Progress using hematite (alpha-Fe2O3) photoelectrodes. Chemsuschem 2011, 4, 432–449. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.X.; Santori, E.A.; Lewis, N.S. Solar water splitting cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef] [PubMed]
- Youngblood, W.J.; Lee, S.-H.A.; Kobayashi, Y.; Hernandez-Pagan, E.A.; Hoertz, P.G.; Moore, T.A.; Moore, A.L.; Gust, D.; Mallouk, T.E. Photoassisted overall water splitting in a visible light-absorbing dye-sensitized photoelectrochemical cell. J. Am. Chem. Soc. 2009, 131, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Dareedwards, M.P.; Goodenough, J.B.; Hamnett, A.; Trevellick, P.R. Electrochemistry and photoelectrochemistry of iron(III) oxide. J. Chem. Soc. Faraday Trans. I 1983, 79, 2027–2041. [Google Scholar] [CrossRef]
- Kennedy, J.H.; Frese, K.W. Photo-oxidation of water at alpha-Fe2O3 electrodes. J. Electrochem. Soc. 1978, 125, 709–714. [Google Scholar] [CrossRef]
- Gardner, R.F.G.; Tanner, D.W.; Sweett, F. Electrical properties of alpha ferric oxide. 2. Ferric oxide of high purity. J. Phys. Chem. Solids 1963, 24, 1183–1196. [Google Scholar] [CrossRef]
- Butler, M.A. Photoelectrolysis and physical-properties of semiconducting electrode WO3. J. Appl. Phys. 1977, 48, 1914–1920. [Google Scholar] [CrossRef]
- Yourey, J.E.; Bartlett, B.M. Electrochemical deposition and photoelectrochemistry of CuWO4, a promising photoanode for water oxidation. J. Mater. Chem. 2011, 21, 7651–7660. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Hwang, Y.J.; Chae, S.Y.; Joo, O.S. Facile growth of aligned WO3 nanorods on FTO substrate for enhanced photoanodic water oxidation activity. J. Mater. Chem. A 2013, 1, 3479–3488. [Google Scholar] [CrossRef]
- Waller, M.R.; Townsend, T.K.; Zhao, J.; Sabio, E.M.; Chamousis, R.L.; Browning, N.D.; Osterloh, F.E. Single-crystal tungsten oxide nanosheets: Photochemical water oxidation in the quantum confinement regime. Chem. Mater. 2012, 24, 698–704. [Google Scholar] [CrossRef]
- Su, J.Z.; Feng, X.J.; Sloppy, J.D.; Guo, L.J.; Grimes, C.A. Vertically aligned WO3 nanowire arrays grown directly on transparent conducting oxide coated glass: Synthesis and photoelectrochemical properties. Nano Lett. 2011, 11, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.Y.; Song, G.; Kim, C.W.; Kang, Y.S. Facile preparation of p-CuO and p-CuO/n-CuWO4 junction thin films and their photoelectrochemical properties. Electrochim. Acta 2012, 69, 340–344. [Google Scholar] [CrossRef]
- Yourey, J.E.; Pyper, K.J.; Kurtz, J.B.; Bartlett, B.M. Chemical stability of CuWO4 for photoelectrochemical water oxidation. J. Phys. Chem. C 2013, 117, 8708–8718. [Google Scholar] [CrossRef]
- Yourey, J.E.; Kurtz, J.B.; Bartlett, B.M. Water oxidation on a CuWO4-WO3 composite electrode in the presence of [Fe(CN)6]3−: Toward solar z-scheme water splitting at zero bias. J. Phys. Chem. C 2012, 116, 3200–3205. [Google Scholar] [CrossRef]
- Pilli, S.K.; Deutsch, T.G.; Furtak, T.E.; Brown, L.D.; Turner, J.A.; Herring, A.M. BiVO4/CuWO4 heterojunction photoanodes for efficient solar driven water oxidation. Phys. Chem. Chem. Phys. 2013, 15, 3273–3278. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.K.; Bhave, N.S.; Kharat, R.B. Spray deposition process of polycrystalline thin films of CuWO4 and study on its photovoltaic electrochemical properties. Mater. Lett. 2005, 59, 3149–3155. [Google Scholar] [CrossRef]
- Gaillard, N.; Chang, Y.C.; DeAngelis, A.; Higgins, S.; Braun, A. A nanocomposite photoelectrode made of 2.2 ev band gap copper tungstate (CuWO4) and multi-wall carbon nanotubes for solar-assisted water splitting. Int. J. Hydrog. Energy 2013, 38, 3166–3176. [Google Scholar] [CrossRef]
- Benko, F.A.; Maclaurin, C.L.; Koffyberg, F.P. CuWO4 and Cu3WO6 as anodes for the photoelectrolysis of water. Mater. Res. Bull. 1982, 17, 133–136. [Google Scholar] [CrossRef]
- Hill, J.C.; Choi, K.S. Synthesis and characterization of high surface area CuWO4 and Bi2WO6 electrodes for use as photoanodes for solar water oxidation. J. Mater. Chem. A 2013, 1, 5006–5014. [Google Scholar] [CrossRef]
- Koo, H.J.; Whangbo, M.H. Spin dimer analysis of the anisotropic spin exchange interactions in the distorted wolframite-type oxides CuWO4, CuMoO4-III, and Cu(Mo0.25W0.75)O4. Inorg. Chem. 2001, 40, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Montini, T.; Gombac, V.; Hameed, A.; Felisari, L.; Adami, G.; Fornasiero, P. Synthesis, characterization and photocatalytic performance of transition metal tungstates. Chem. Phys. Lett. 2010, 498, 113–119. [Google Scholar] [CrossRef]
- Lalic, M.V.; Popovic, Z.S.; Vukajlovic, F.R. Electronic structure and optical properties of CuWO4: An ab initio study. Comput. Mater. Sci. 2012, 63, 163–167. [Google Scholar] [CrossRef]
- Lalic, M.V.; Popovic, Z.S.; Vukajlovic, F.R. Ab initio study of electronic, magnetic and optical properties of CuWO4 tungstate. Comput. Mater. Sci. 2011, 50, 1179–1186. [Google Scholar] [CrossRef]
- Chang, Y.C.; Braun, A.; Deangelis, A.; Kaneshiro, J.; Gaillard, N. Effect of thermal treatment on the crystallographic, surface energetics, and photoelectrochemical properties of reactively cosputtered copper tungstate for water splitting. J. Phys. Chem. C 2011, 115, 25490–25495. [Google Scholar] [CrossRef]
- Hill, J.C.; Ping, Y.; Galli, G.A.; Choi, K.S. Synthesis, photoelectrochemical properties, and first principles study of n-type CuW1-xMoxO4 electrodes showing enhanced visible light absorption. Energy Environ. Sci. 2013, 6, 2440–2446. [Google Scholar] [CrossRef]
- Valenti, M.; Dolat, D.; Biskos, G.; Schmidt-Ott, A.; Smith, W.A. Enhancement of the photoelectrochemical performance of CuWO4 thin films for solar water splitting by plasmonic nanoparticle functionalization. J. Phys. Chem. C 2015, 119, 2096–2104. [Google Scholar] [CrossRef]
- Zhang, H.; Yilmaz, P.; Ansari, J.O.; Khan, F.F.; Binions, R.; Krause, S.; Dunn, S. Incorporation of Ag nanowires in CuWO4 for improved visible light-induced photoanode performance. J. Mater. Chem. A 2015, 3, 9638–9644. [Google Scholar] [CrossRef]
- Nam, K.M.; Cheon, E.A.; Shin, W.J.; Bard, A.J. Improved photoelectrochemical water oxidation by the WO3/CuWO4 composite with a manganese phosphate electrocatalyst. Langmuir 2015, 31, 10897–10903. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zandi, O.; Hamann, T.W. Atomic layer stack deposition-annealing synthesis of CuWO4. J. Mater. Chem. A 2016, 4, 2826–2830. [Google Scholar] [CrossRef]
- Zhan, F.; Li, J.; Li, W.; Liu, Y.; Xie, R.; Yang, Y.; Li, Y.; Chen, Q. In situ formation of CuWO4/WO3 heterojunction plates array films with enhanced photoelectrochemical properties. Int. J. Hydrog. Energy 2015, 40, 6512–6520. [Google Scholar] [CrossRef]
- Jiang, Z.; Tang, Y.; Tay, Q.; Zhang, Y.; Malyi, O.I.; Wang, D.; Deng, J.; Lai, Y.; Zhou, H.; Chen, X.; et al. Understanding the role of nanostructures for efficient hydrogen generation on immobilized photocatalysts. Adv. Energy Mater. 2013, 3, 1368–1380. [Google Scholar] [CrossRef]
- Van de Krol, R.; Gratzel, M. (Eds.) Photoelectrochemical Hydrogen Production; Springer: New York, NY, USA, 2012; p. 54.
- Li, Z.; Feng, J.; Yan, S.; Zou, Z. Solar fuel production: Strategies and new opportunities with nanostructures. Nano Today 2015, 10, 468–486. [Google Scholar] [CrossRef]
- Dotan, H.; Sivula, K.; Gratzel, M.; Rothschild, A.; Warren, S.C. Probing the photoelectrochemical properties of hematite (α-Fe2O3) electrodes using hydrogen peroxide as a hole scavenger. Energy Environ. Sci. 2011, 4, 958–964. [Google Scholar] [CrossRef]
- Kim, E.S.; Kang, H.J.; Magesh, G.; Kim, J.Y.; Jang, J.-W.; Lee, J.S. Improved photoelectrochemical activity of CaFe2O4/BiVO4 heterojunction photoanode by reduced surface recombination in solar water oxidation. ACS Appl. Mater. Interfaces 2014, 6, 17762–17769. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Luo, W.; Feng, J.; Li, M.; Li, Z.; Yu, T.; Zou, Z. Quantitative analysis and visualized evidence for high charge separation efficiency in a solid-liquid bulk heterojunction. Adv. Energy Mater. 2014, 4. [Google Scholar] [CrossRef]
- Lin, J.D.; Hu, P.; Zhang, Y.; Fan, M.T.; He, Z.M.; Ngaw, C.K.; Loo, J.S.C.; Liao, D.W.; Tan, T.T.Y. Understanding the photoelectrochemical properties of a reduced graphene oxide-WO3 heterojunction photoanode for efficient solar-light-driven overall water splitting. RSC Adv. 2013, 3, 9330–9336. [Google Scholar] [CrossRef]
| Sample | Thickness of WO3 (nm) | Thickness of CuWO4 (nm) |
|---|---|---|
| Pristine WO3 | 415 | 0 |
| WO3/CuWO4 | 150 | 600 |
| CuWO4 | 0 | 1000 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Bassi, P.S.; Qi, H.; Zhao, X.; Gurudayal; Wong, L.H.; Xu, R.; Sritharan, T.; Chen, Z. Improved Charge Separation in WO3/CuWO4 Composite Photoanodes for Photoelectrochemical Water Oxidation. Materials 2016, 9, 348. https://doi.org/10.3390/ma9050348
Wang D, Bassi PS, Qi H, Zhao X, Gurudayal, Wong LH, Xu R, Sritharan T, Chen Z. Improved Charge Separation in WO3/CuWO4 Composite Photoanodes for Photoelectrochemical Water Oxidation. Materials. 2016; 9(5):348. https://doi.org/10.3390/ma9050348
Chicago/Turabian StyleWang, Danping, Prince Saurabh Bassi, Huan Qi, Xin Zhao, Gurudayal, Lydia Helena Wong, Rong Xu, Thirumany Sritharan, and Zhong Chen. 2016. "Improved Charge Separation in WO3/CuWO4 Composite Photoanodes for Photoelectrochemical Water Oxidation" Materials 9, no. 5: 348. https://doi.org/10.3390/ma9050348
APA StyleWang, D., Bassi, P. S., Qi, H., Zhao, X., Gurudayal, Wong, L. H., Xu, R., Sritharan, T., & Chen, Z. (2016). Improved Charge Separation in WO3/CuWO4 Composite Photoanodes for Photoelectrochemical Water Oxidation. Materials, 9(5), 348. https://doi.org/10.3390/ma9050348