Stability and Hydrocarbon/Fluorocarbon Sorption of a Metal-Organic Framework with Fluorinated Channels
Abstract
:1. Introduction
2. Experimental Section
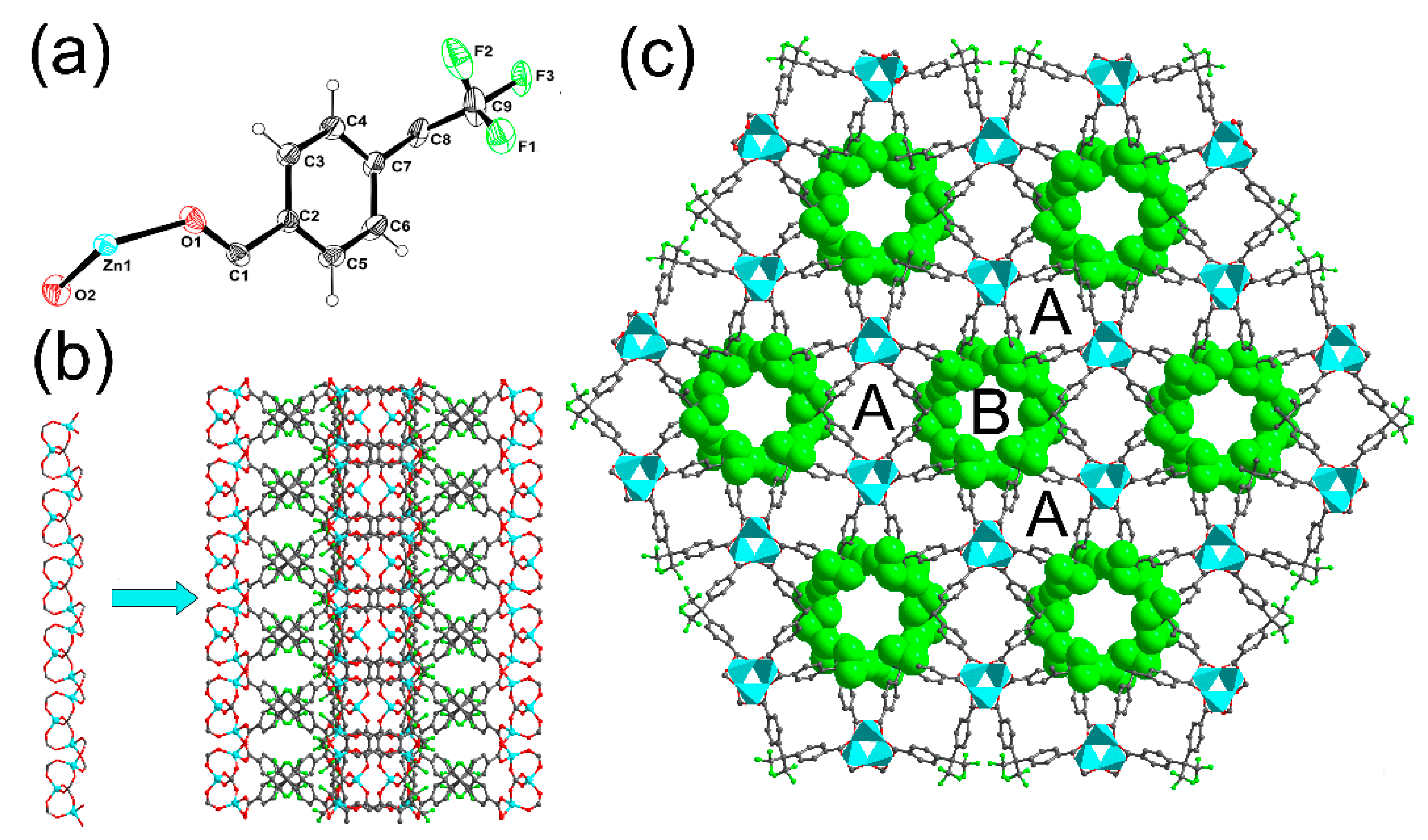
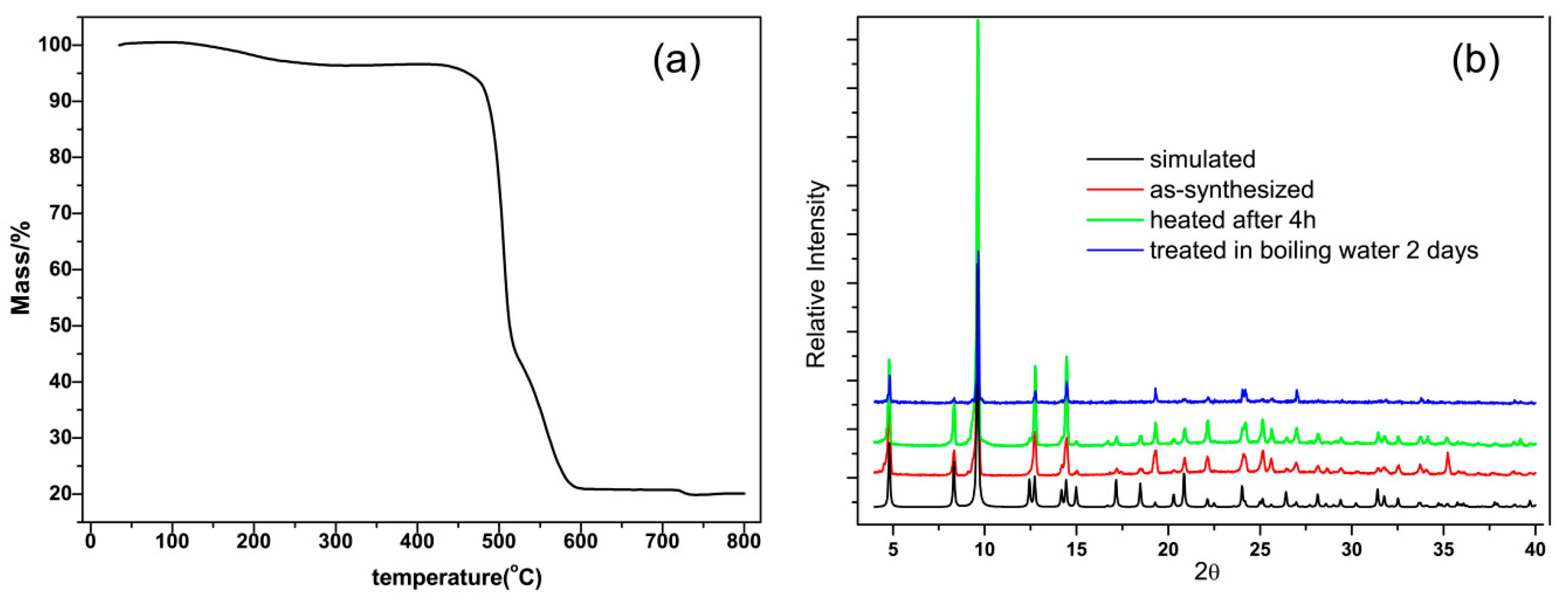
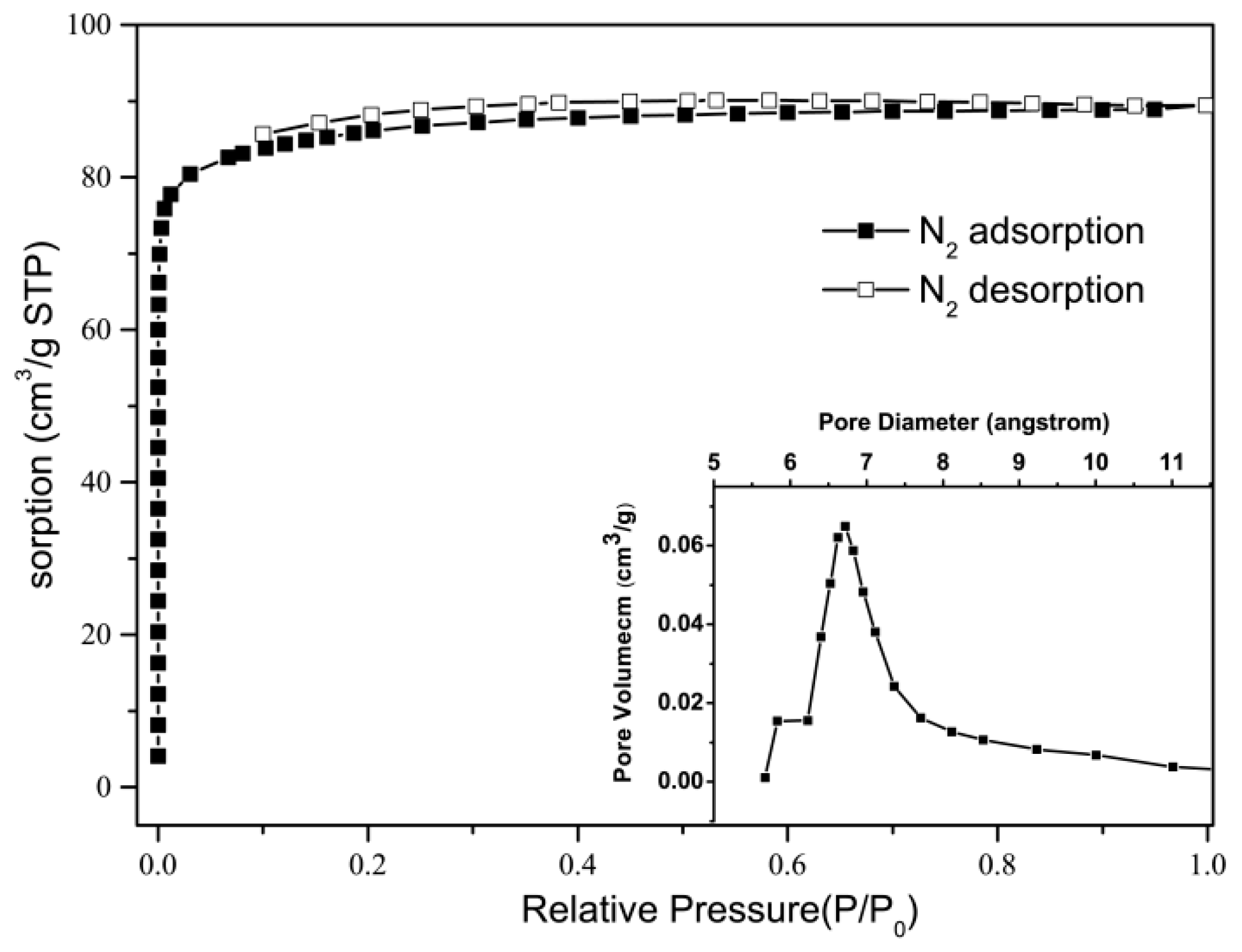
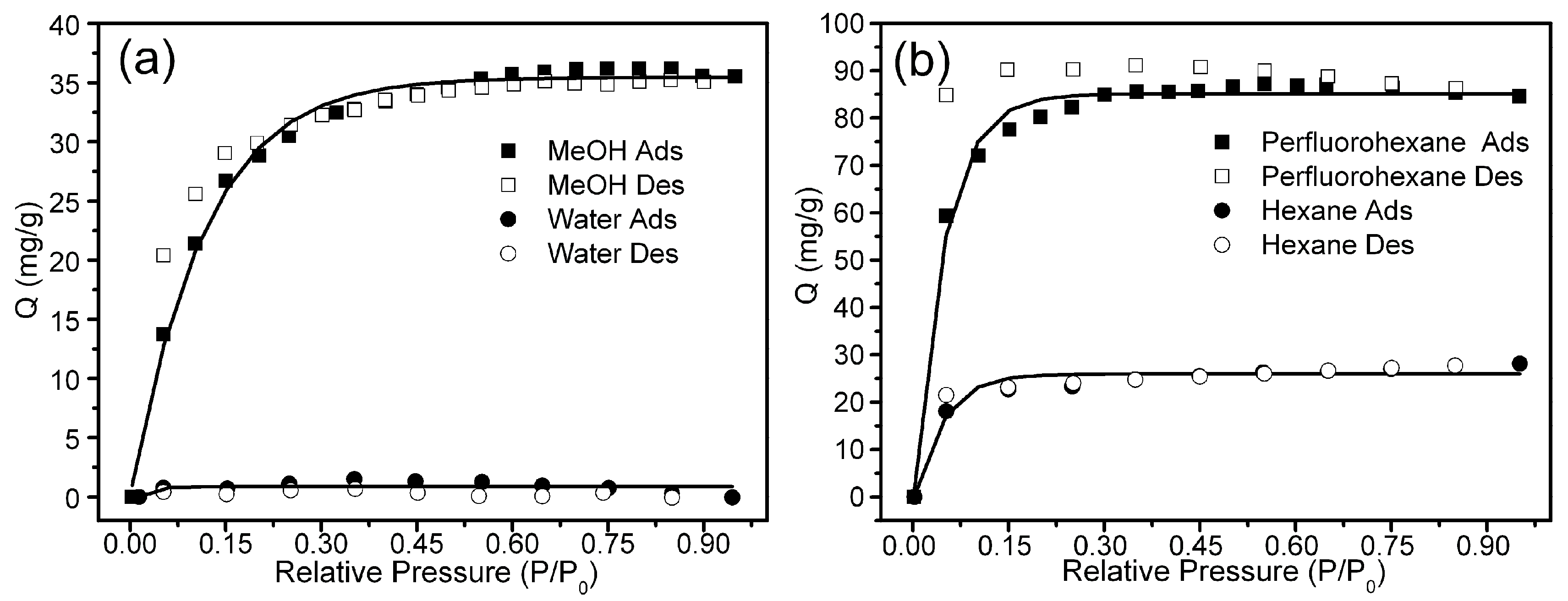
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.; Vilela, S.M.F.; Tome, J.P.C.; Paz, F.A.A. Multifunctional metal-organic frameworks: From academia to industrial applications. Chem. Soc. Rev. 2015, 44, 6774–6803. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, A. Adsorption—From theory to practice. Adv. Colloid Interface 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Alhamami, M.; Doan, H.; Cheng, C.H. A Review on Breathing Behaviors of Metal-Organic-Frameworks (MOFs) for Gas Adsorption. Materials 2014, 7, 3198–3250. [Google Scholar] [CrossRef]
- Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Burtch, N.C.; Jasuja, H.; Walton, K.S. Water Stability and Adsorption in Metal–Organic Frameworks. Chem. Rev. 2014, 114, 10575–10612. [Google Scholar] [CrossRef] [PubMed]
- Nugent, P.; Belmabkhout, Y.; Burd, S.D.; Cairns, A.J.; Luebke, R.; Forrest, K.; Pham, T.; Ma, S.; Space, B.; Wojtas, L.; et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 2013, 495, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Nijem, N.; Canepa, P.; Kaipa, U.; Tan, K.; Roodenko, K.; Tekarli, S.; Halbert, J.; Oswald, I.W.H.; Arvapally, R.K.; Yang, C.; et al. Water cluster confinement and methane adsorption in the hydrophobic cavities of a fluorinated metal-organic framework. J. Am. Chem. Soc. 2013, 135, 12615–12626. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Kaipa, U.; Mather, Q.Z.; Wang, X.; Nesterov, V.; Venero, A.F.; Omary, M.A. Fluorous metal-organic frameworks with superior adsorption and hydrophobic properties toward oil spill cleanup and hydrocarbon storage. J. Am. Chem. Soc. 2011, 133, 18094–18097. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.G.; Li, B.; Wang, H.L.; Bao, Z.B.; Yildirim, T.; Yao, Z.Z.; Xiang, S.C.; Zhou, W.; Chen, B.L. A microporous metal-organic framework with polarized trifluoromethyl groups for high methane storage. Chem. Commun. 2015, 51, 14789–14792. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-Y.A.; Chen, S.-Y.; Jochems, A.P. Zirconium-based metal organic frameworks: Highly selective adsorbents for removal of phosphate from water and urine. Mater. Chem. Phys. 2015, 160, 168–176. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Omary, M.A. Fluorous metal-organic frameworks for high-density gas adsorption. J. Am. Chem. Soc. 2007, 129, 15454–15455. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Popov, I.; Kaveevivitchai, W.; Chuang, Y.C.; Chen, Y.S.; Jacobson, A.J.; Miljanic, O.S. Mesoporous Fluorinated Metal-Organic Frameworks with Exceptional Adsorption of Fluorocarbons and CFCs. Angew. Chem. 2015, 54, 13902–13906. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Popov, I.; Zenasni, O.; Daugulis, O.; Miljanic, O.S. Superhydrophobic perfluorinated metal-organic frameworks. Chem. Commun. 2013, 49, 6846–6848. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Kaveevivitchai, W.; Jacobson, A.J.; Miljanic, O.S. Adsorption of fluorinated anesthetics within the pores of a molecular crystal. Chem. Commun. 2015, 51, 14096–14098. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Popov, I.; Kaveevivitchai, W.; Chuang, Y.C.; Chen, Y.S.; Daugulis, O.; Jacobson, A.J.; Miljanic, O.S. Thermally robust and porous noncovalent organic framework with high affinity for fluorocarbons and CFCs. Nat. Commun. 2014, 5, 5131. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Olson, D.H.; Ciemnolonski, L.R.; Heddy, R.; Li, J. Separation of hydrocarbons with a microporous metal-organic framework. Angew. Chem. 2006, 45, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Lor, B.; Gutierrez-Puebla, E.; Iglesias, M.; Monge, M.A.; Ruiz-Valero, C.; Snejko, N. Novel 2D and 3D indium metal-organic frameworks: Topology and catalytic properties. Chem. Mater. 2005, 17, 2568–2573. [Google Scholar] [CrossRef]
- Gandara, F.; Gornez-Lor, B.; Gutierrez-Puebla, E.; Iglesias, M.; Monge, M.A.; Proserpio, D.M.; Snejko, N. An indium layered MOF as recyclable lewis acid catalyst. Chem. Mater. 2008, 20, 72–76. [Google Scholar] [CrossRef]
- Monge, A.; Snejko, N.; Gutierrez-Puebla, E.; Medina, M.; Cascales, C.; Ruiz-Valero, C.; Iglesias, M.; Gomez-Lor, B. One teflon-like channelled nanoporous polymer with a chiral and new uninodal 4-connected net: Sorption and catalytic properties. Chem. Commun. 2005, 10, 1291–1293. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Suh, M.P.; Park, H.J.; Prasad, T.K.; Lim, D.W. Hydrogen Storage in Metal-Organic Frameworks. Chem. Rev. 2012, 112, 782–835. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.A.; Veenstra, M.; Long, J.R. Evaluating metal-organic frameworks for natural gas storage. Chem. Sci. 2014, 5, 32–51. [Google Scholar] [CrossRef]
- Motkuri, R.K.; Annapureddy, H.V.R.; Vijaykumar, M.; Schaef, H.T.; Martin, P.F.; McGrail, B.P.; Dang, L.X.; Krishna, R.; Thallapally, P.K. Fluorocarbon adsorption in hierarchical porous frameworks. Nat. Commun. 2014, 5, 4368. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, J.; Sun, F.; Wang, C.; Pan, Q. Stability and Hydrocarbon/Fluorocarbon Sorption of a Metal-Organic Framework with Fluorinated Channels. Materials 2016, 9, 327. https://doi.org/10.3390/ma9050327
Xie J, Sun F, Wang C, Pan Q. Stability and Hydrocarbon/Fluorocarbon Sorption of a Metal-Organic Framework with Fluorinated Channels. Materials. 2016; 9(5):327. https://doi.org/10.3390/ma9050327
Chicago/Turabian StyleXie, Jijiang, Fuxing Sun, Chunrui Wang, and Qikun Pan. 2016. "Stability and Hydrocarbon/Fluorocarbon Sorption of a Metal-Organic Framework with Fluorinated Channels" Materials 9, no. 5: 327. https://doi.org/10.3390/ma9050327
APA StyleXie, J., Sun, F., Wang, C., & Pan, Q. (2016). Stability and Hydrocarbon/Fluorocarbon Sorption of a Metal-Organic Framework with Fluorinated Channels. Materials, 9(5), 327. https://doi.org/10.3390/ma9050327




