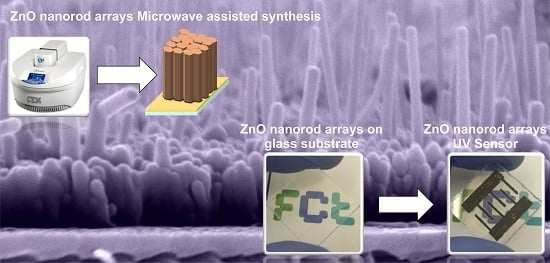
Microwave Synthesized ZnO Nanorod Arrays for UV Sensors: A Seed Layer Annealing Temperature Study
Abstract
:1. Introduction
2. Results and Discussion
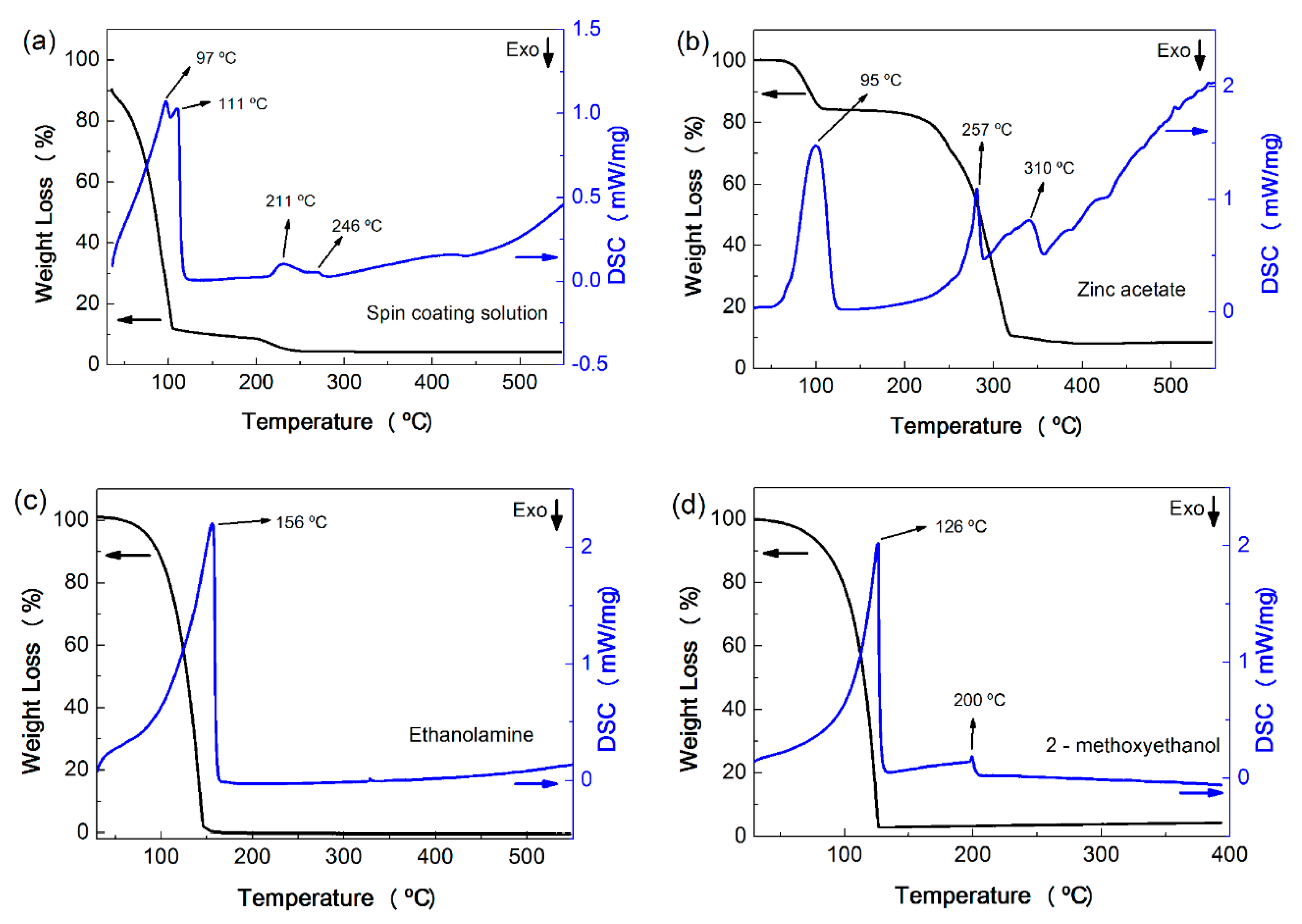
2.1. Thermal Analysis
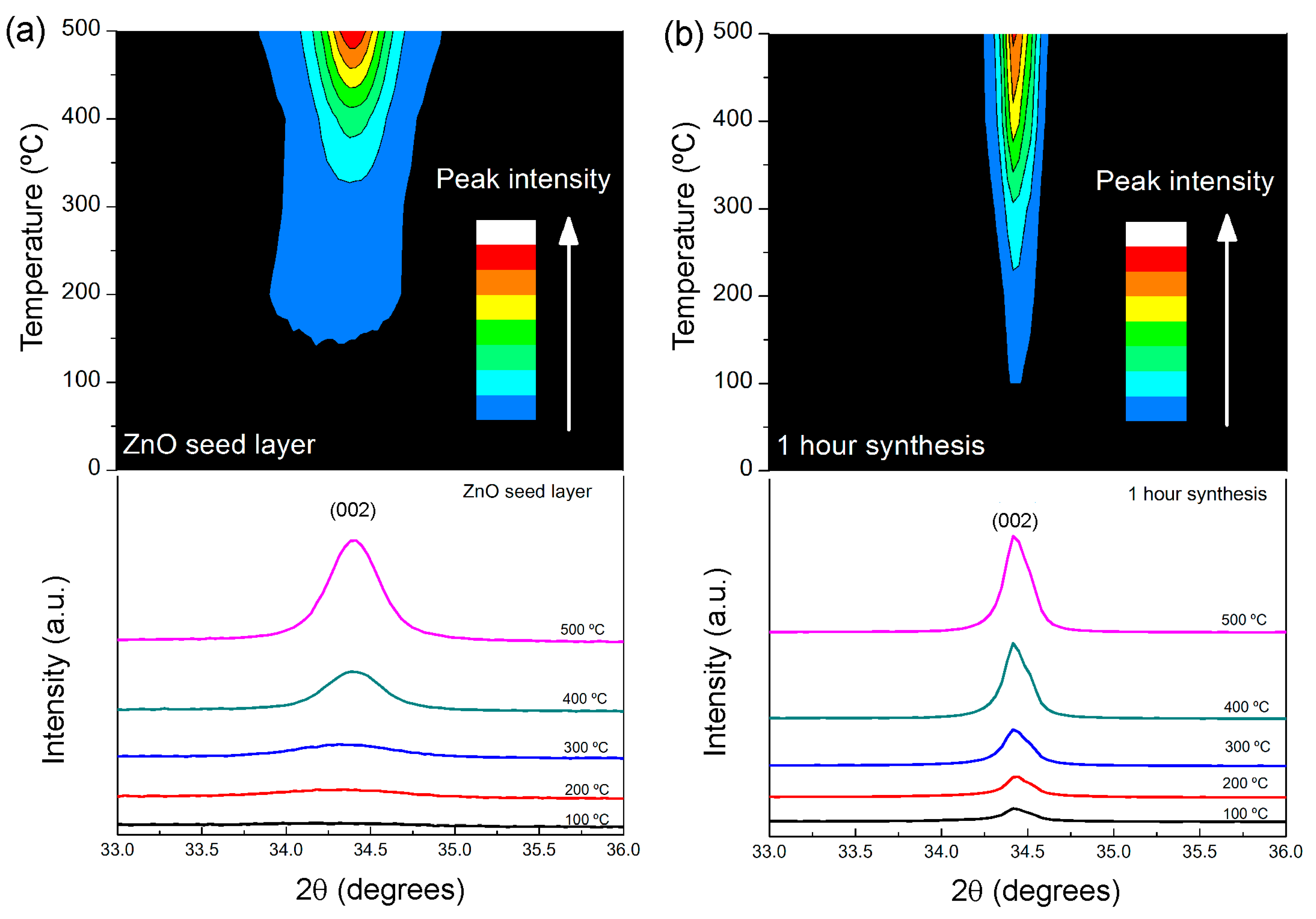
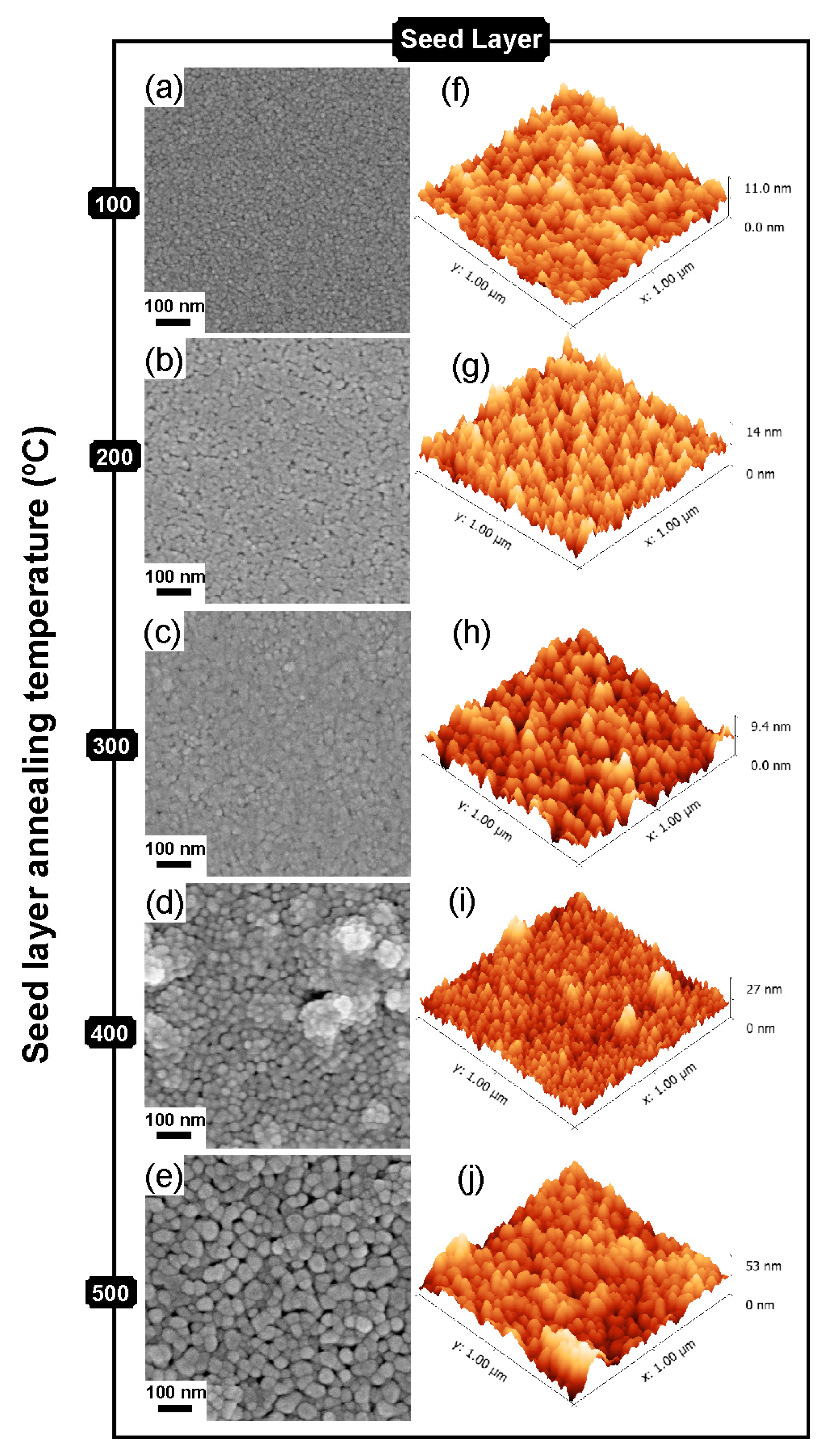
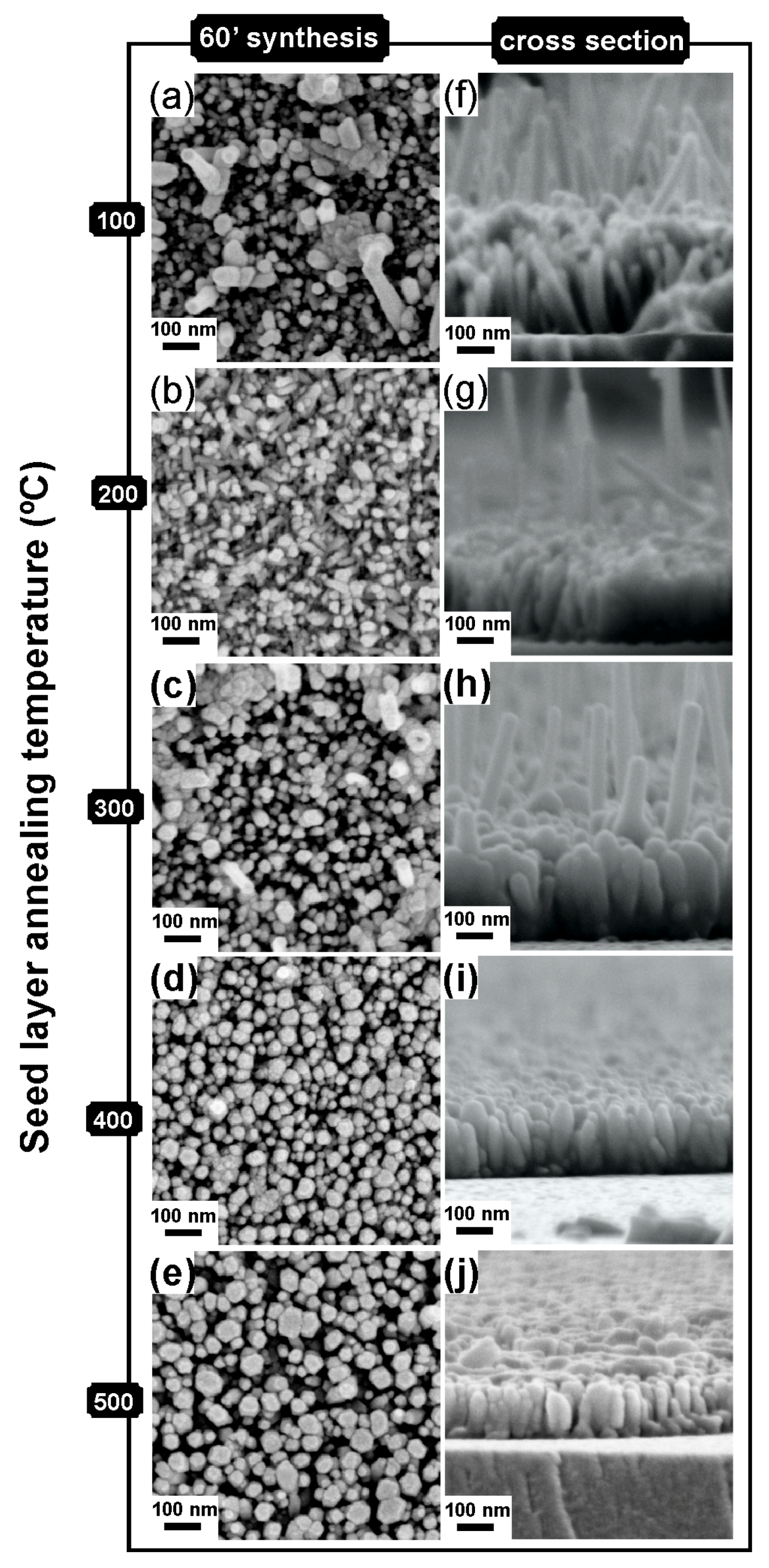
2.2. Structure and Morphology
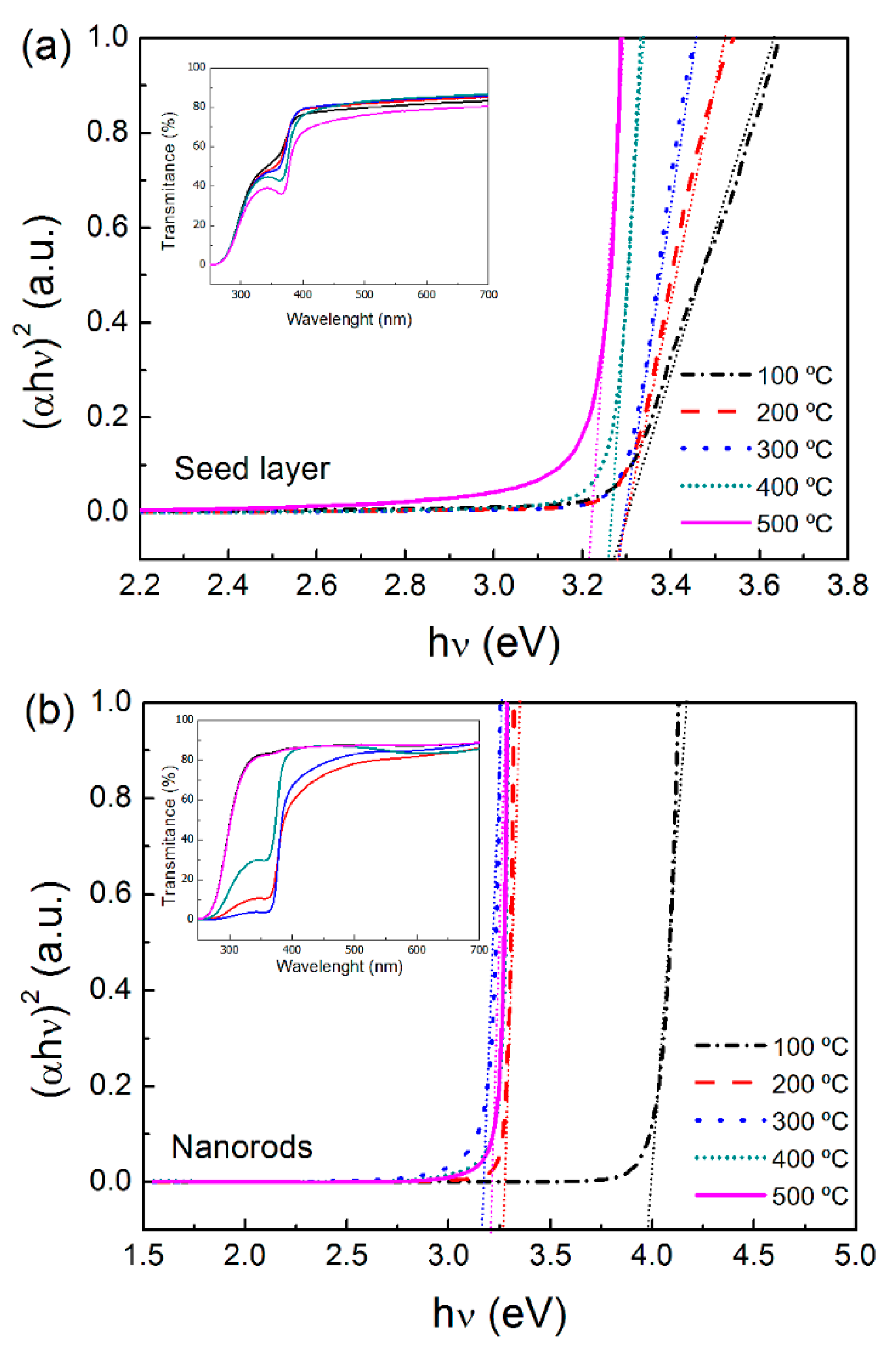
2.3. Optical Properties
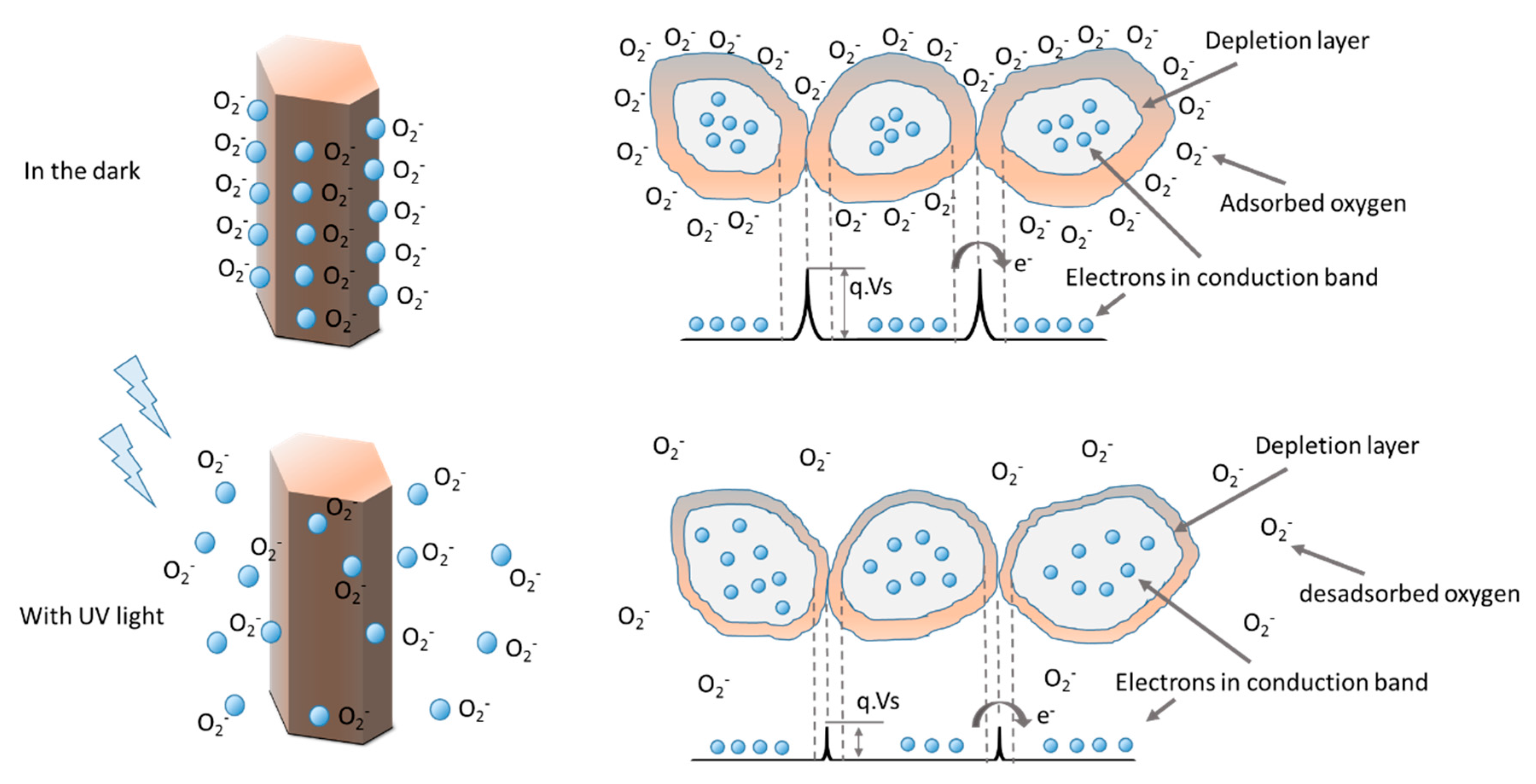
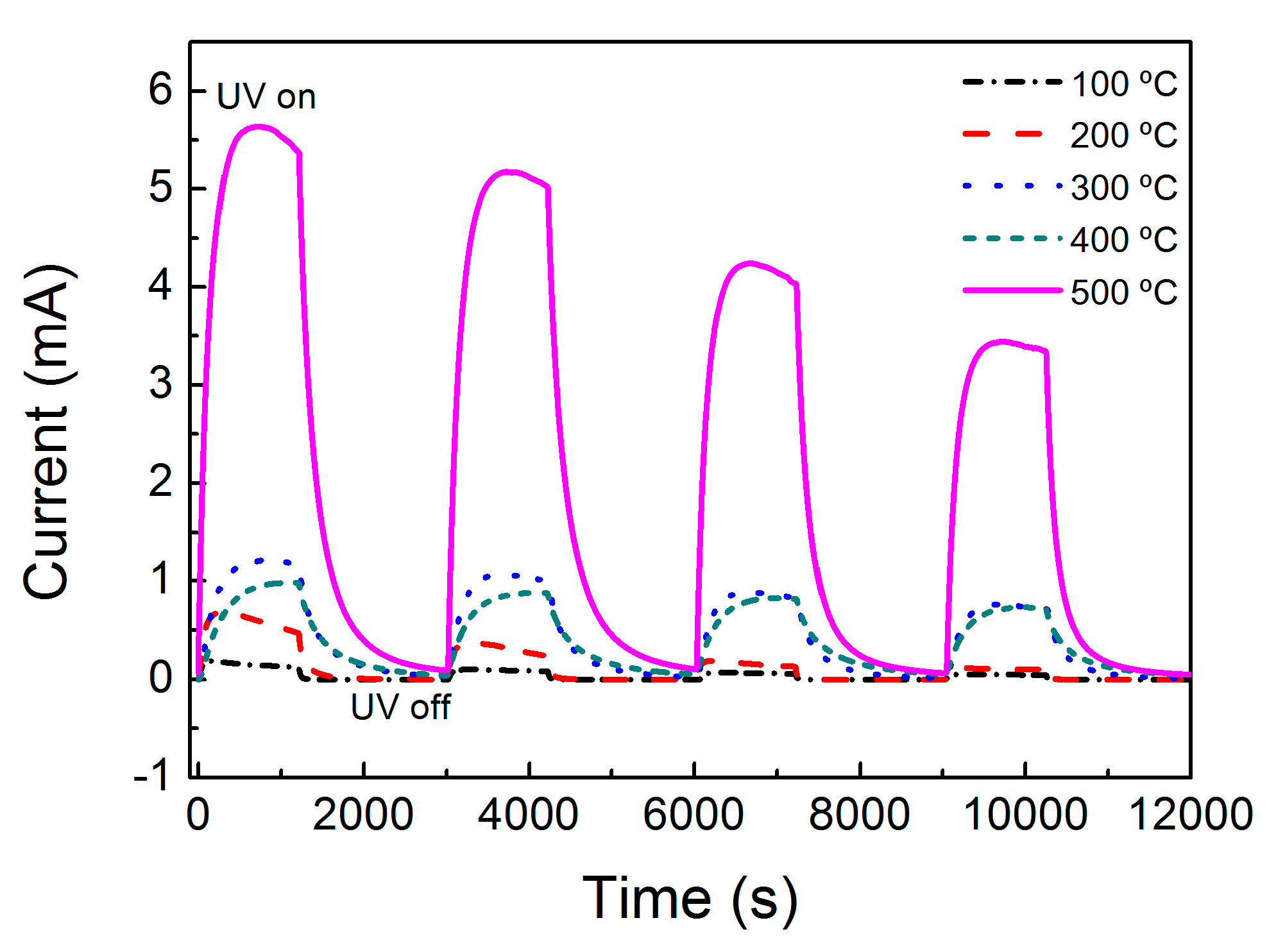
2.4. ZnO Nanorods Array UV Sensor
3. Experimental Details
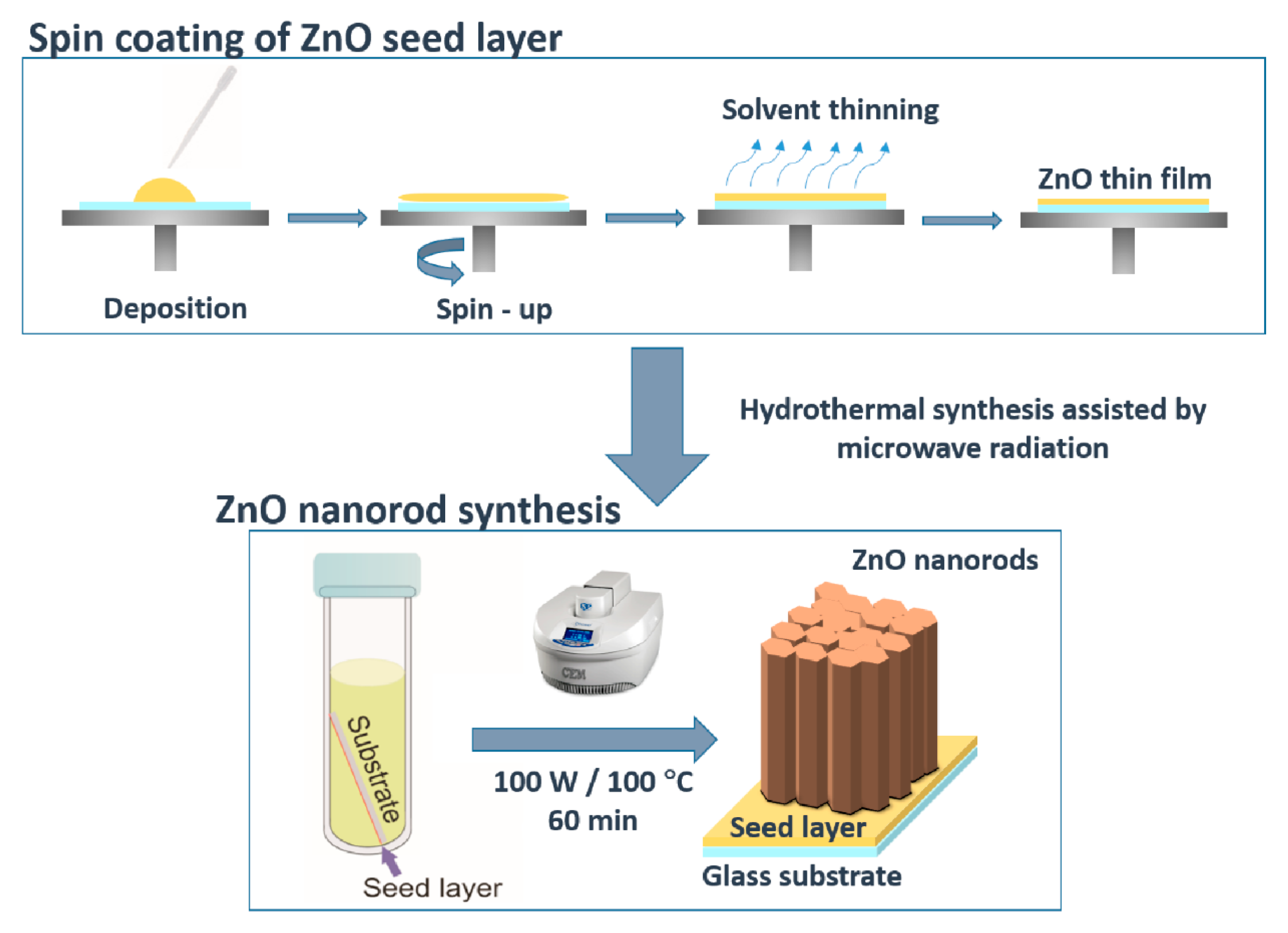
3.1. Synthesis of ZnO Nanostructures
3.2. Characterization Techniques
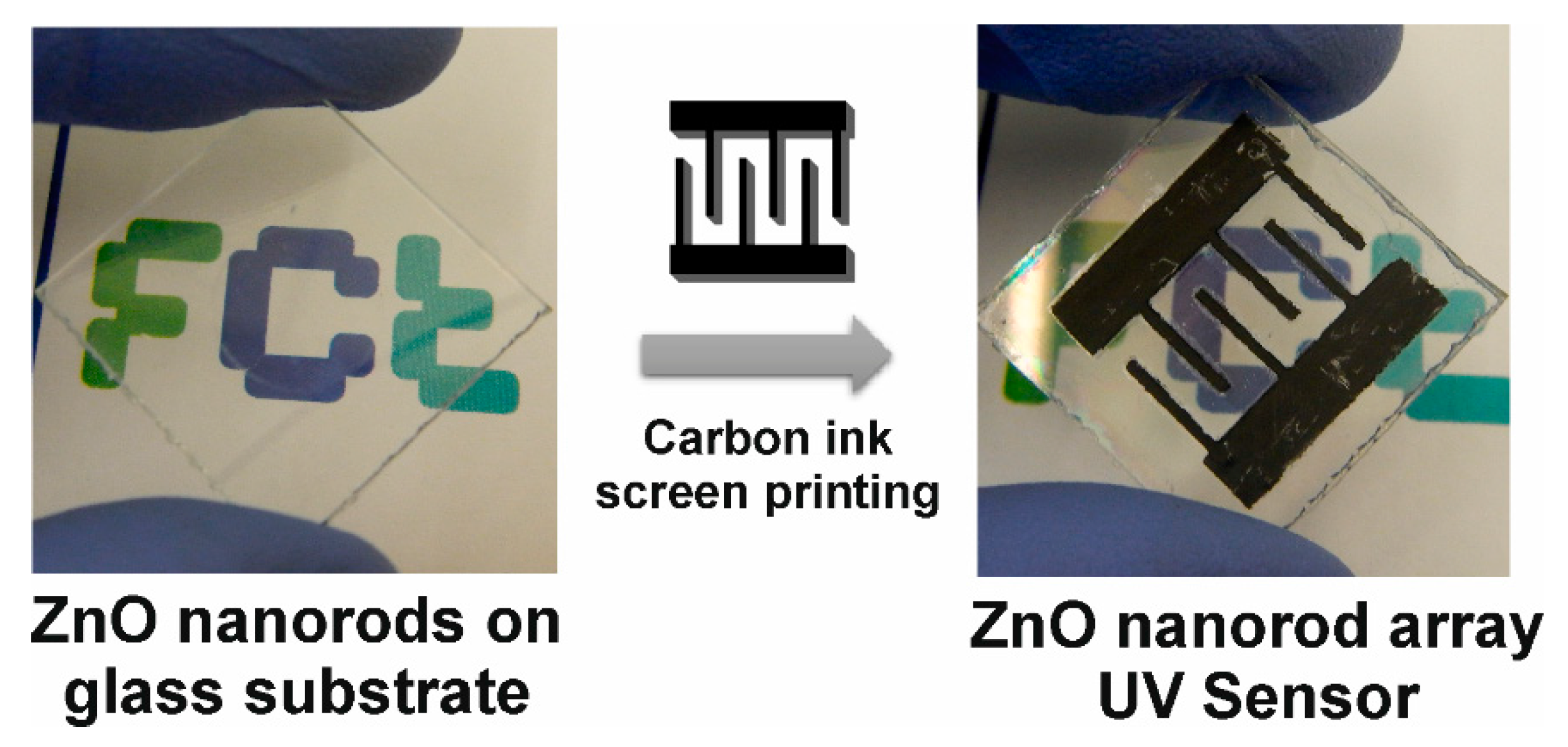
3.3. Characterization of ZnO Nanorod Arrays as a UV Sensor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Coleman, V.A.; Jagadish, C. Basic Properties and Applications of ZnO. In Zinc Oxide Bulk, Thin Films and Nanostructures; Jagadish, C., Pearton, S., Eds.; Elsevier Science Ltd.: Oxford, UK, 2006; Chapter 1; pp. 1–20. [Google Scholar]
- Barquinha, P.; Fortunato, E.; Gonçalves, A.; Pimentel, A.; Marques, A.; Pereira, L.; Martins, R. A Study on the Electrical Properties of ZnO Based Transparent TFTs. Mater. Sci. Forum 2006, 514–516, 68–72. [Google Scholar] [CrossRef]
- Fortunato, E.; Gonçalves, A.; Marques, A.; Pimentel, A.; Barquinha, P.; Águas, H.; Pereira, L.; Raniero, L.; Gonçalves, G.; Ferreira, I.; et al. Multifunctional Thin Film Zinc Oxide Semiconductors: Application to Electronic Devices. Mater. Sci. Forum 2006, 514–516, 3–7. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Ng, A.M.C.; Chen, X.Y. ZnO nanostructures for optoelectronics: Material properties and device applications. Prog. Quantum Electron. 2010, 34, 191–259. [Google Scholar] [CrossRef]
- Pimentel, A.; Gonçalves, A.; Marques, A.; Martins, R.; Fortunato, E. Role of the thickness on the electrical and optical performances of undoped polycrystalline zinc oxide films used as UV detectors. J. Non Cryst. Solids 2006, 352, 1448–1452. [Google Scholar] [CrossRef]
- Pimentel, A.C.; Gonçalves, A.; Marques, A.; Martins, R.; Fortunato, E. Zinc Oxide Thin Films used as an Ozone Sensor at Room Temperature. MRS Proc. 2006, 915. [Google Scholar] [CrossRef]
- Bai, S.; Wu, W.; Qin, Y.; Cui, N.; Bayerl, D.J.; Wang, X. High-Performance Integrated ZnO Nanowire UV Sensors on Rigid and Flexible Substrates. Adv. Funct. Mater. 2011, 21, 4464–4469. [Google Scholar] [CrossRef]
- Arya, S.K.; Saha, S.; Ramirez-Vick, J.E.; Gupta, V.; Bhansali, S.; Singh, S.P. Recent advances in ZnO nanostructures and thin films for biosensor applications: Review. Anal. Chim. Acta 2012, 737, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kang, Z.; Yan, X.; Liao, Q. ZnO nanostructures in enzyme biosensors. Sci. China Mater. 2015, 58, 60–76. [Google Scholar] [CrossRef]
- Kumar, B.; Kim, S.-W. Energy harvesting based on semiconducting piezoelectric ZnO nanostructures. Nano Energy 2012, 1, 342–355. [Google Scholar] [CrossRef]
- Wang, Z.L.; Song, J. Piezoelectric nanogenerators based on zinc oxide nanowire arrays. Science 2006, 312, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.L. Zinc oxide nanostructures: Growth, properties and applications. J. Phys. Condens. Matter 2004, 16, R829–R858. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; MacManus-Driscoll, J.L. ZnO—Nanostructures, defects, and devices. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Baruah, S.; Dutta, J. Hydrothermal growth of ZnO nanostructures. Sci. Technol. Adv. Mater. 2009, 10. [Google Scholar] [CrossRef]
- Bai, S.; Chen, L.; Li, D.; Yang, W.; Yang, P.; Liu, Z.; Chen, A.; Liu, C.C. Different morphologies of ZnO nanorods and their sensing property. Sens. Actuators B Chem. 2010, 146, 129–137. [Google Scholar]
- Spencer, M.J.S. Gas sensing applications of 1D-nanostructured zinc oxide: Insights from density functional theory calculations. Prog. Mater. Sci. 2012, 57, 437–486. [Google Scholar] [CrossRef]
- Wang, L.; Kang, Y.; Liu, X.; Zhang, S.; Huang, W.; Wang, S. ZnO nanorod gas sensor for ethanol detection. Sens. Actuators B Chem. 2012, 162, 237–243. [Google Scholar] [CrossRef]
- Lupan, O.; Emelchenko, G.A.; Ursaki, V.V.; Chai, G.; Redkin, A.N.; Gruzintsev, A.N.; Tiginyanu, I.M.; Chow, L.; Ono, L.K.; Roldan Cuenya, B.; et al. Synthesis and characterization of ZnO nanowires for nanosensor applications. Mater. Res. Bull. 2010, 45, 1026–1032. [Google Scholar] [CrossRef]
- Pimentel, A.; Nunes, D.; Duarte, P.; Rodrigues, J.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Synthesis of Long ZnO Nanorods under Microwave Irradiation or Conventional Heating. J. Phys. Chem. C 2014, 118, 14629–14639. [Google Scholar] [CrossRef]
- Wu, L.; Song, F.; Fang, X.; Guo, Z.-X.; Liang, S. A practical vacuum sensor based on a ZnO nanowire array. Nanotechnology 2010, 21. [Google Scholar] [CrossRef] [PubMed]
- Ajimsha, R.S.; Manoj, R.; Aneesh, P.M.; Jayaraj, M.K. Violet luminescence from ZnO nanorods grown by room temperature pulsed laser deposition. Curr. Appl. Phys. 2010, 10, 693–697. [Google Scholar] [CrossRef]
- Shinde, S.D.; Patil, G.E.; Kajale, D.D.; Gaikwad, V.B.; Jain, G.H. Synthesis of ZnO nanorods by spray pyrolysis for H2S gas sensor. J. Alloys Compd. 2012, 528, 109–114. [Google Scholar] [CrossRef]
- Gurav, K.V.; Gang, M.G.; Shin, S.W.; Patil, U.M.; Deshmukh, P.R.; Agawane, G.L.; Suryawanshi, M.P.; Pawar, S.M.; Patil, P.S.; Lokhande, C.D.; et al. Gas sensing properties of hydrothermally grown ZnO nanorods with different aspect ratios. Sens. Actuators B Chem. 2014, 190, 439–445. [Google Scholar] [CrossRef]
- Pimentel, A.; Rodrigues, J.; Duarte, P.; Nunes, D.; Costa, F.M.; Monteiro, T.; Martins, R.; Fortunato, E. Effect of solvents on ZnO nanostructures synthesized by solvothermal method assisted by microwave radiation: A photocatalytic study. J. Mater. Sci. 2015, 50, 5777–5787. [Google Scholar] [CrossRef]
- Hassan, J.J.; Mahdi, M.A.; Chin, C.W.; Abu-Hassan, H.; Hassan, Z. Room temperature hydrogen gas sensor based on ZnO nanorod arrays grown on a SiO2/Si substrate via a microwave-assisted chemical solution method. J. Alloys Compd. 2013, 546, 107–111. [Google Scholar] [CrossRef]
- Rai, P.; Song, H.-M.; Kim, Y.-S.; Song, M.-K.; Oh, P.-R.; Yoon, J.-M.; Yu, Y.-T. Microwave assisted hydrothermal synthesis of single crystalline ZnO nanorods for gas sensor application. Mater. Lett. 2012, 68, 90–93. [Google Scholar] [CrossRef]
- Huang, J.-S.; Lin, C.-F. Influences of ZnO sol-gel thin film characteristics on ZnO nanowire arrays prepared at low temperature using all solution-based processing. J. Appl. Phys. 2008, 103. [Google Scholar] [CrossRef]
- Hayes, B.L. Microwave Synthesis: Chemistry at the Speed of Light; CEM Publishing: Matthews, NC, USA, 2002. [Google Scholar]
- Nunes, D.; Santos, L.; Duarte, P.; Pimentel, A.; Pinto, J.V.; Barquinha, P.; Carvalho, P.A.; Fortunato, E.; Martins, R. Room temperature synthesis of Cu2O nanospheres: Optical properties and thermal behavior. Microsc. Microanal. 2015, 21, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Resende, J.; Marques, A.C.; Pinto, J.V.; Nunes, D.; Marie, A.; Goncalves, R.; Pereira, L.; Martins, R.; Fortunato, E. Smart optically active VO2 nanostructured layers applied in roof-type ceramic tiles for energy efficiency. Sol. Energy Mater. Sol. Cells 2016, 150, 1–9. [Google Scholar] [CrossRef]
- Ohyama, M.; Kouzuka, H.; Yoko, T. Sol-gel preparation of ZnO films with extremely preferred orientation along (002) plane from zinc acetate solution. Thin Solid Films 1997, 306, 78–85. [Google Scholar] [CrossRef]
- Ohyama, M.; Kozuka, H.; Yoko, T.; Sakka, S. Preparation of ZnO films with preferential orientation by sol-gel method. J. Ceram. Soc. Jpn. 1996, 104, 296–300. [Google Scholar] [CrossRef]
- Znaidi, L. Sol-gel-deposited ZnO thin films: A review. Mater. Sci. Eng. B 2010, 174, 18–30. [Google Scholar] [CrossRef]
- Lin, C.-C.; Li, Y.-Y. Synthesis of ZnO nanowires by thermal decomposition of zinc acetate dihydrate. Mater. Chem. Phys. 2009, 113, 334–337. [Google Scholar] [CrossRef]
- Khomyak, V.V.; Slyotov, M.M.; Shtepliuk, I.I.; Lashkarev, G.V.; Slyotov, O.M.; Marianchuk, P.D.; Kosolovskiy, V.V. Annealing effect on the near-band edge emission of ZnO. J. Phys. Chem. Solids 2013, 74, 291–297. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X ray Diffraction; Addison-Wesley Publisher Company, Inc.: Boston, MA, USA, 1956. [Google Scholar]
- Hoon, J.-W.; Chan, K.-Y.; Krishnasamy, J.; Tou, T.-Y.; Knipp, D. Direct current magnetron sputter-deposited ZnO thin films. Appl. Surf. Sci. 2011, 257, 2508–2515. [Google Scholar] [CrossRef]
- Ridhuan, N.S.; Razak, K.A.; Lockman, Z.; Abdul Aziz, A. Structural and morphology of ZnO nanorods synthesized using ZnO seeded growth hydrothermal method and its properties as UV sensing. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Baruah, S.; Dutta, J. Effect of seeded substrates on hydrothermally grown ZnO nanorods. J. Sol Gel Sci. Technol. 2009, 50, 456–464. [Google Scholar] [CrossRef]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B 2013, 120, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Huang, J.; He, H.; Fan, Z.; Shao, J. Influence of different post-treatments on the structure and optical properties of zinc oxide thin films. Appl. Surf. Sci. 2005, 242, 346–352. [Google Scholar] [CrossRef]
- Panda, S.K.; Jacob, C. Preparation of transparent ZnO thin films and their application in UV sensor devices. Solid State Electron. 2012, 73, 44–50. [Google Scholar] [CrossRef]
- Chou, C.-S.; Wu, Y.-C.; Lin, C.-H. Oxygen sensor utilizing ultraviolet irradiation assisted ZnO nanorods under low operation temperature. RSC Adv. 2014, 4, 52903–52910. [Google Scholar] [CrossRef]
- Zhai, T.; Fang, X.; Liao, M.; Xu, X.; Zeng, H.; Yoshio, B.; Golberg, D. A Comprehensive Review of One-Dimensional Metal-Oxide Nanostructure Photodetectors. Sensors 2009, 9, 6504–6529. [Google Scholar] [CrossRef] [PubMed]
- Madou, M.J.; Morrison, S.R. Chemical Sensing with Solid State Devices; Academic Press, Inc.: New York, NY, USA, 1989. [Google Scholar]
- Fryxell, G.E.; Cao, G. Environmental Applications of Nanomaterials: Synthesis, Sorbents and Sensors; Imperial College Press: London, UK, 2007. [Google Scholar]
- Mamat, M.H.; Khusaimi, Z.; Zahidi, M.; Mahmood, M.R. Nanorods; Yaln, O., Ed.; InTech: Vienna, Austria, 2012. [Google Scholar]
- Zhou, Q.; Chen, W.; Xu, L.; Peng, S. Hydrothermal synthesis of various hierarchical ZnO nanostructures and their methane sensing properties. Sensors 2013, 13, 6171–6182. [Google Scholar] [CrossRef] [PubMed]
- Mamat, M.H.; Khalin, M.I.C.; Mohammad, N.N.H.N.; Khusaimi, Z.; Sin, N.D.M.; Shariffudin, S.S.; Zahidi, M.M.; Mahmood, M.R. Effects of Annealing Environments on the Solution-Grown, Aligned Aluminium-Doped Zinc Oxide Nanorod-Array-Based Ultraviolet Photoconductive Sensor. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Chang, H.; Sun, Z.; Ho, K.Y.-F.; Tao, X.; Yan, F.; Kwok, W.-M.; Zheng, Z. A highly sensitive ultraviolet sensor based on a facile in situ solution-grown ZnO nanorod/graphene heterostructure. Nanoscale 2011, 3, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Nunes, D.; Pimentel, A.; Pinto, J.V.; Calmeiro, T.R.; Nandy, S.; Barquinha, P.; Pereira, L.; Carvalho, P.A.; Fortunato, E.; Martins, R. Photocatalytic behavior of TiO2 films synthesized by microwave irradiation. Catal. Today 2015. [Google Scholar] [CrossRef]
- Pankove, J.I. Optical Processes in Semiconductors; Dover Publications, Inc.: Mineola, NY, USA, 1971. [Google Scholar]
- Tauc, J. Optical properties and electronic structure of amorphous Ge and Si. Mater. Res. Bull. 1968, 3, 37–46. [Google Scholar] [CrossRef]
| Annealing Temperature | 100 °C | 200 °C | 300 °C | 400 °C | 500 °C |
|---|---|---|---|---|---|
| Seed Layer (nm) | 9.426 | 11.055 | 13.388 | 23.449 | 25.725 |
| MW 60 min (nm) | 38.621 | 40.779 | 47.235 | 54.972 | 56.274 |
| Annealing Temperature | 100 °C | 200 °C | 300 °C | 400 °C | 500 °C |
|---|---|---|---|---|---|
| Dark current (A) | 5.0 × 10−7 | 1.0 × 10−6 | 3.0 × 10−5 | 4.0 × 10−5 | 9.0 × 10−5 |
| Photocurrent (A) | 1.0 × 10−4 | 3.7 × 10−4 | 8.7 × 10−4 | 1.0 × 10−3 | 5.0 × 10−3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimentel, A.; Ferreira, S.H.; Nunes, D.; Calmeiro, T.; Martins, R.; Fortunato, E. Microwave Synthesized ZnO Nanorod Arrays for UV Sensors: A Seed Layer Annealing Temperature Study. Materials 2016, 9, 299. https://doi.org/10.3390/ma9040299
Pimentel A, Ferreira SH, Nunes D, Calmeiro T, Martins R, Fortunato E. Microwave Synthesized ZnO Nanorod Arrays for UV Sensors: A Seed Layer Annealing Temperature Study. Materials. 2016; 9(4):299. https://doi.org/10.3390/ma9040299
Chicago/Turabian StylePimentel, Ana, Sofia Henriques Ferreira, Daniela Nunes, Tomas Calmeiro, Rodrigo Martins, and Elvira Fortunato. 2016. "Microwave Synthesized ZnO Nanorod Arrays for UV Sensors: A Seed Layer Annealing Temperature Study" Materials 9, no. 4: 299. https://doi.org/10.3390/ma9040299
APA StylePimentel, A., Ferreira, S. H., Nunes, D., Calmeiro, T., Martins, R., & Fortunato, E. (2016). Microwave Synthesized ZnO Nanorod Arrays for UV Sensors: A Seed Layer Annealing Temperature Study. Materials, 9(4), 299. https://doi.org/10.3390/ma9040299