Research on Glass Frit Deposition Based on the Electrospray Process
Abstract
:1. Introduction
2. Materials and Methods
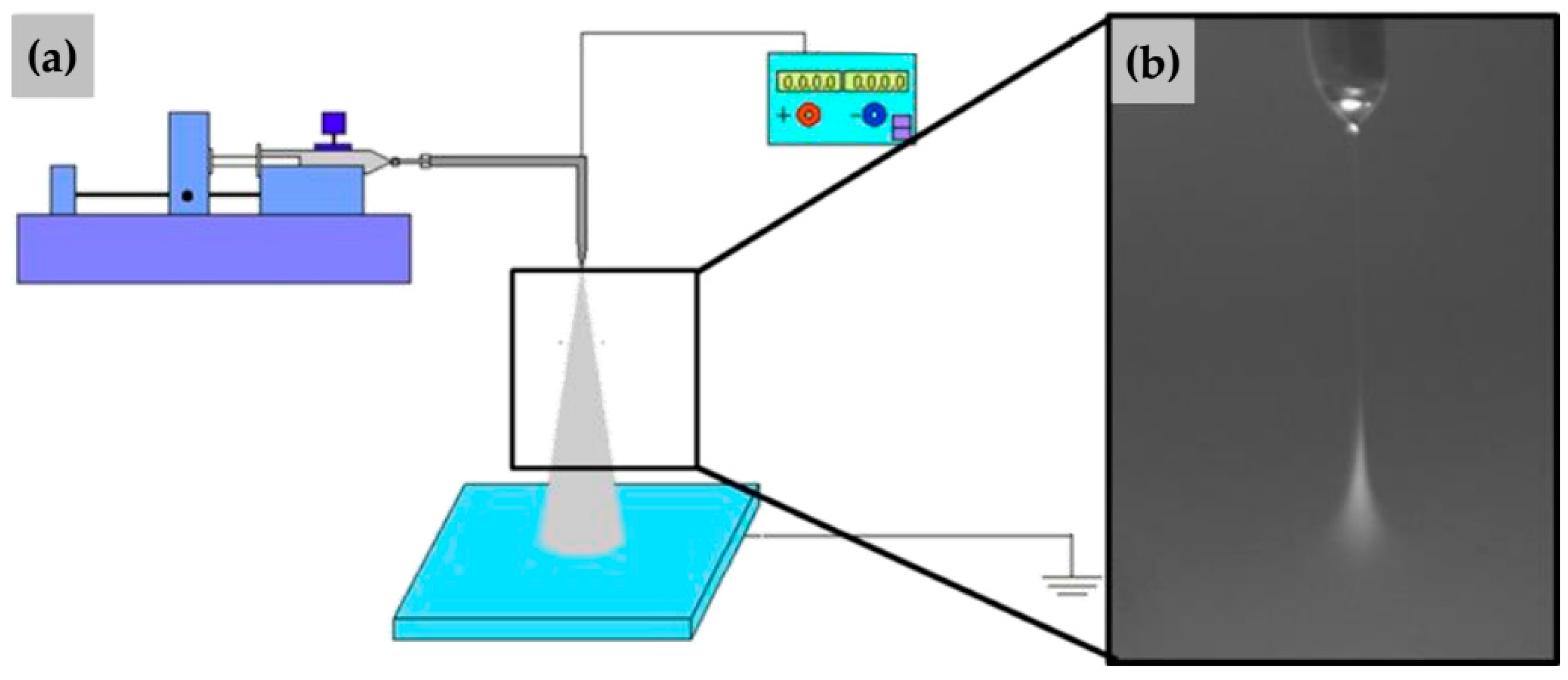
2.1. Experimental Facilities
2.2. Materials
2.3. Details of Far-Field Electrospray Experiments
2.4. Details of Near-Field Electrospray Experiments
3. Results and Discussion
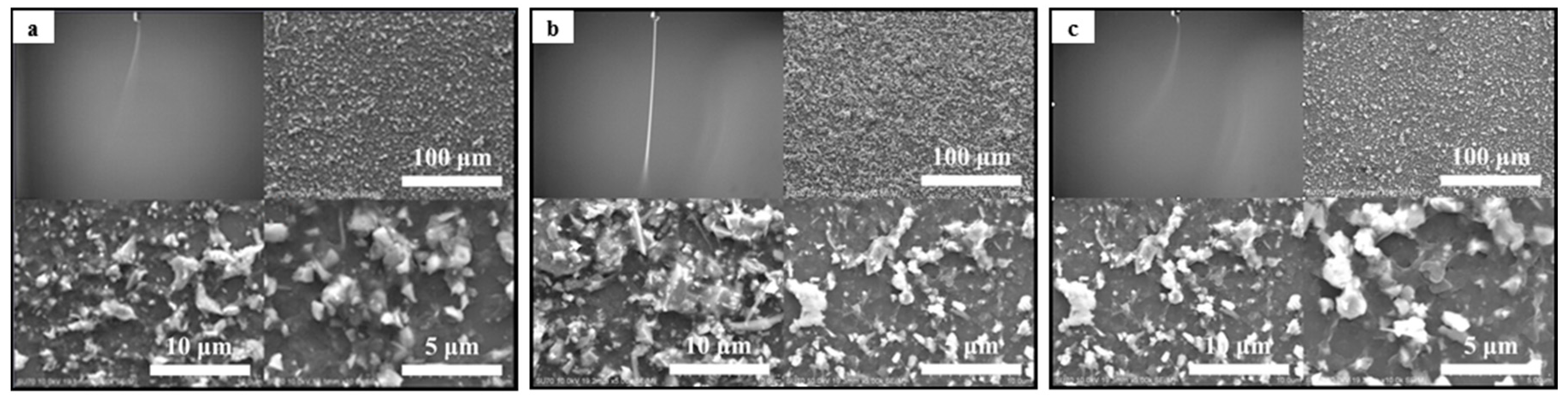
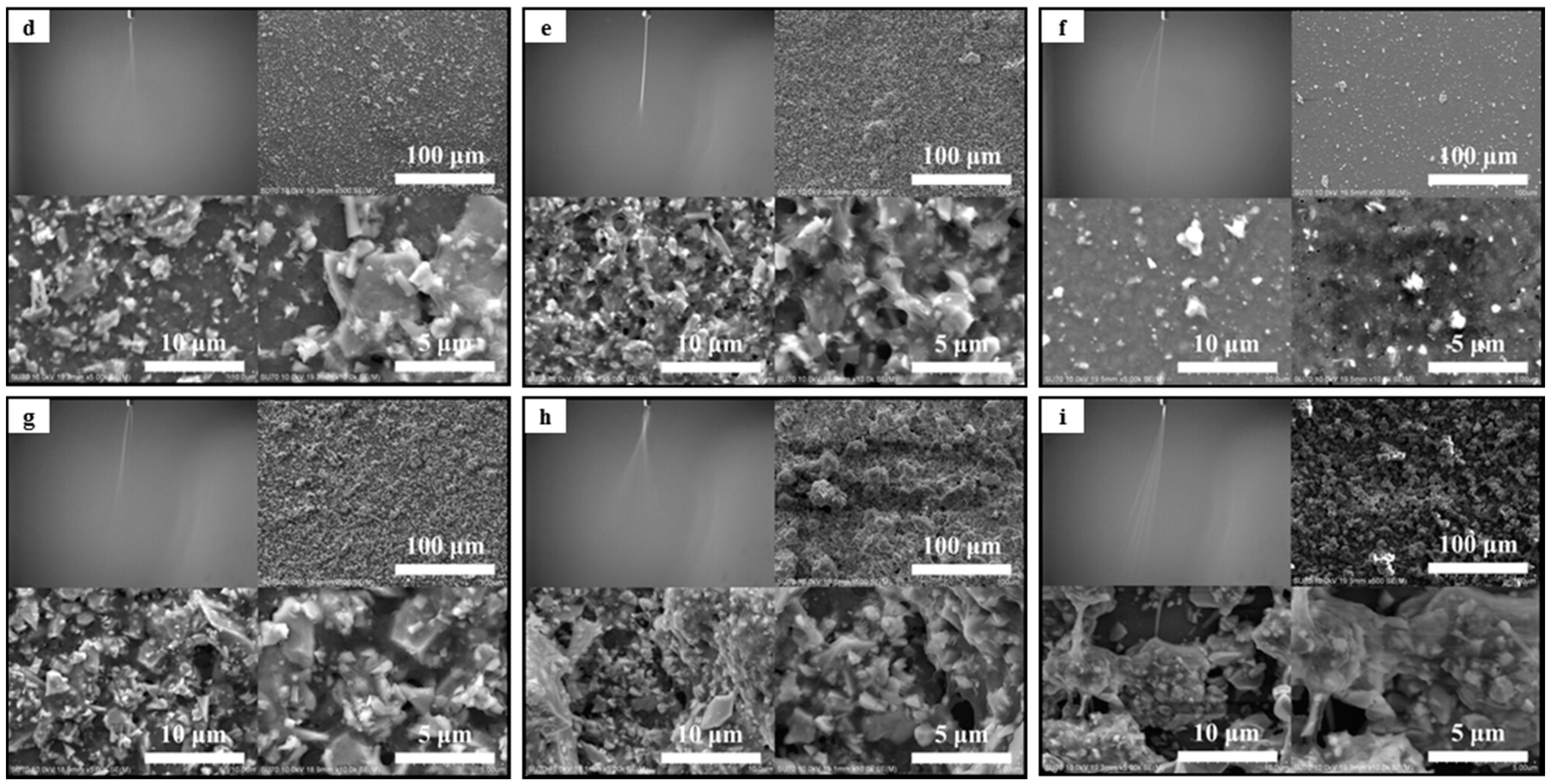
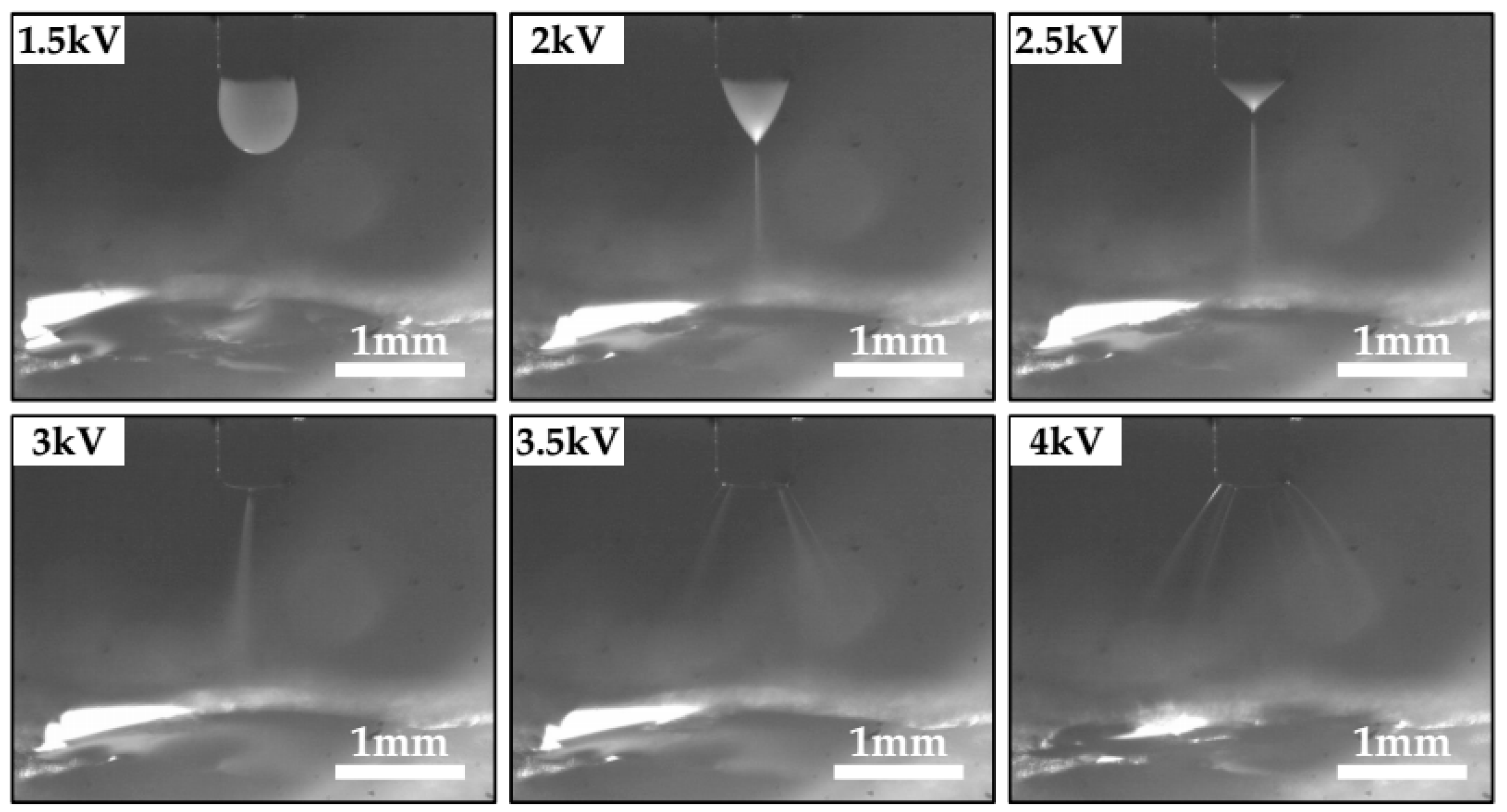
3.1. The Stability of Spray Plume and the Deposition Profile of Aerosol
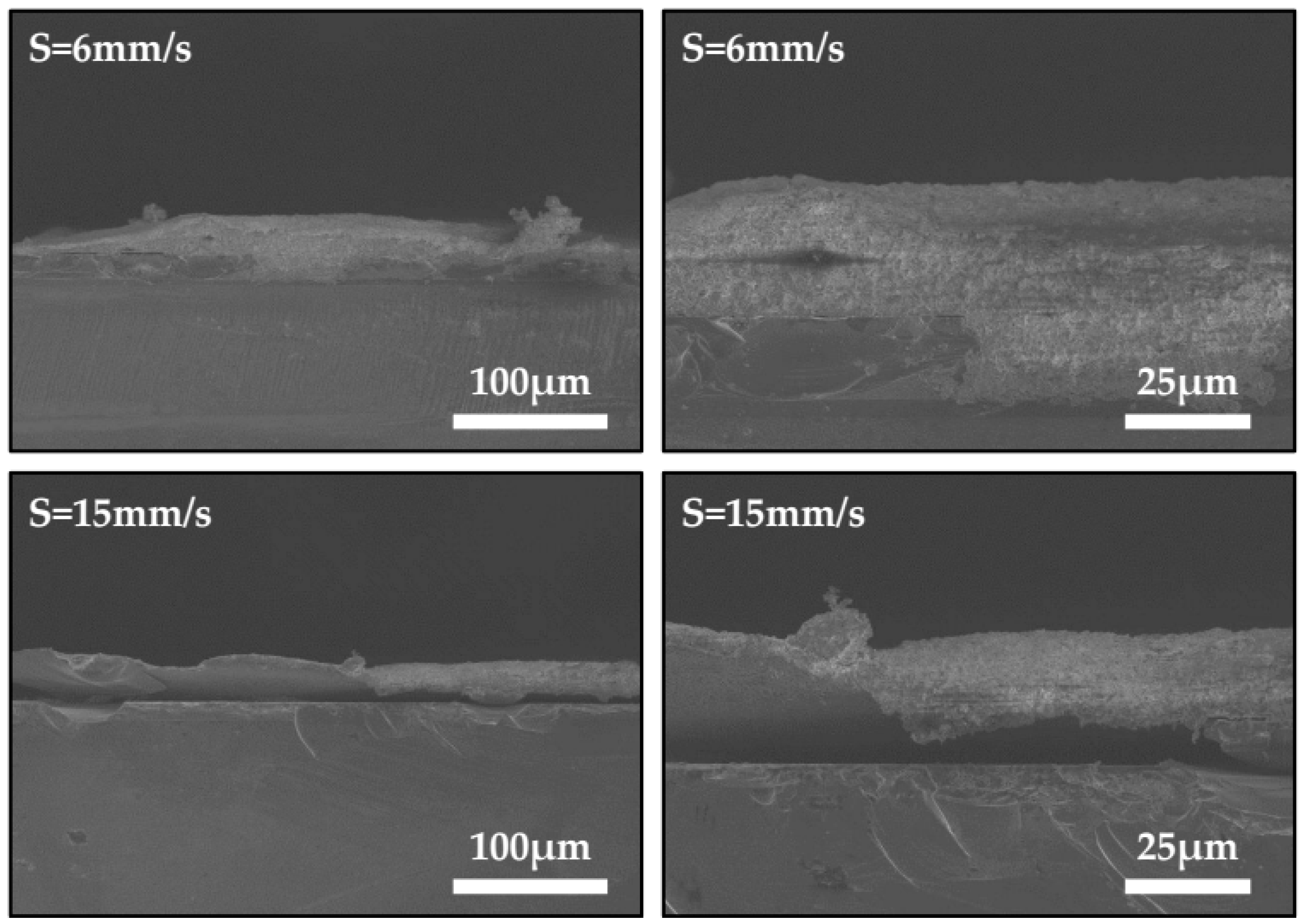
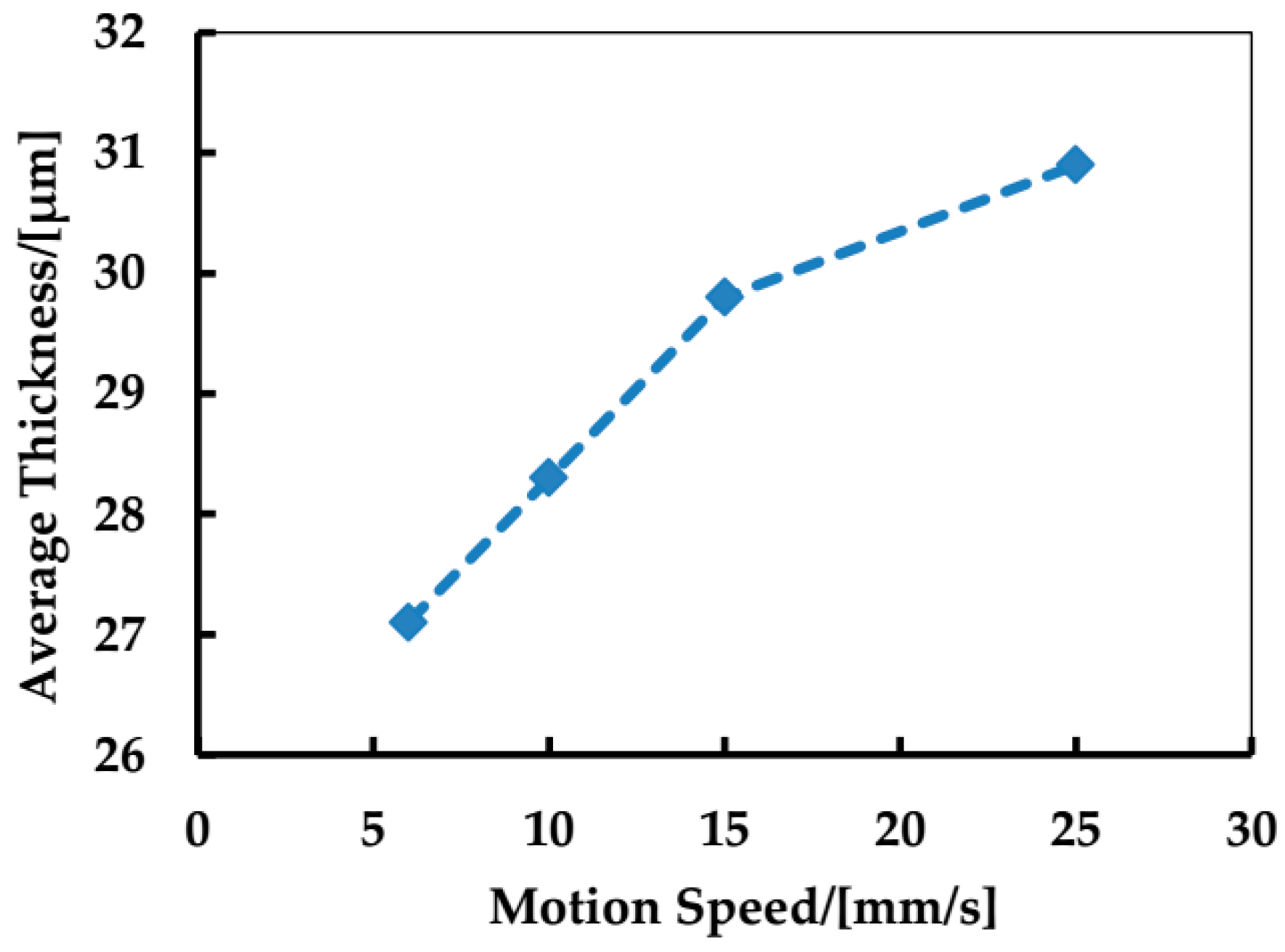
3.2. Preparation of Micro-Scale Linear Film
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MEMS | Micro-electromechanical Systems |
| CCD | Charge Coupled Device |
| SEM | Scanning Electron Microscope |
References
- Knechtel, R.; Wiemer, M.; Frömel, J. Wafer level encapsulation of microsystems using glass frit bonding. Microsyst. Technol. 2006, 12, 468–472. [Google Scholar] [CrossRef]
- Wu, G.; Xu, D.; Sun, X.; Xiong, B.; Wang, Y. Wafer-level vacuum packaging for microsystems using glass frit bonding. Compon. Packag. Manuf. Technol. IEEE Trans. 2013, 3, 1640–1646. [Google Scholar] [CrossRef]
- Wu, G.; Xu, D.; Xiong, B.; Wang, Y.; Wang, Y.; Ma, Y. Wafer-level vacuum packaging for MEMS resonators using glass frit bonding. Microelectromech. Syst. 2012, 21, 1484–1491. [Google Scholar] [CrossRef]
- Cui, Y.; Zhang, X.; Lei, W.; Wang, Q.; Di, Y.; Li, C.; Chen, J. Study on large current field-emission carbon nanotube cathode. High Power Laser Part. Beams 2013, 6, 1509–1512. (In Chinese) [Google Scholar]
- Zhang, L.; Zhong, J.; Chen, Y. High performance organic phototransistor fabricated by spray technology. High Power Laser Part. Beams 2013, 3, 787–790. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, L.; Zhong, J.; Chen, Y. Electrostatic spray assisted vapour deposition of fluorine doped tin oxide. J. Cryst. Growth 2001, 231, 215–221. [Google Scholar]
- Li, X.; Huang, J.; Edirisinghe, M.J. Novel patterning of nano-bioceramics: Template-assisted electrohydrodynamic atomization spraying. J. R. Soc. Interface 2008, 5, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Li, C.; Yang, W.; Lojewski, B.; An, L.; Deng, W. Near-field electrospray microprinting of polymer-derived ceramics. Microelectromech. Syst. 2013, 22, 1–3. [Google Scholar] [CrossRef]
- Khan, S.; Doh, Y.H.; Khan, A.; Rahman, A.; Choi, K.H.; Kim, D.S. Direct patterning and electrospray deposition through EHD for fabrication of printed thin film transistors. Curr. Appl. Phys. 2011, 11, S271–S279. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A.T. Electrospraying route to nanotechnology: An overview. J. Electrost. 2008, 66, 197–219. [Google Scholar] [CrossRef]
- Jaworek, A.; Sobczyk, A.; Czech, T.; Krupa, A. Corona discharge in electrospraying. J. Electrost. 2014, 72, 166–178. [Google Scholar] [CrossRef]
- Hartman, R.P.A.; Brunner, D.J.; Camelot, D.M.A.; Marijnissen, J.C.M.; Scarlett, B. Jet break-up in electrohydrodynamic atomization in the cone-jet mode. J. Aerosol Sci. 2000, 31, 65–95. [Google Scholar] [CrossRef]
- Fukuda, T.; Suzuki, A.; Liao, Y.; Suzuki, K. Influence of spray conditions on droplet charge per unit volume for electrospray deposition. J. Aerosol Sci. 2014, 77, 38–49. [Google Scholar] [CrossRef]
- Ku, B.K.; Kim, S.S. Electrospray characteristics of highly viscous liquids. J. Aerosol Sci. 2002, 33, 1361–1378. [Google Scholar] [CrossRef]
- Gañán-Calvo, A.M.; Montanero, J.M. Revision of capillary cone-jet physics: Electrospray and flow focusing. Phys. Rev. E 2009, 79. [Google Scholar] [CrossRef] [PubMed]
- Jaworek, A.; Sobczyk, A.; Krupa, A.; Lackowski, M.; Czech, T. Electrostatic deposition of nanothin films on metal substrate. Bull. Pol. Acad. Sci. Tech. Sci. 2009, 57, 63–70. [Google Scholar] [CrossRef]
- Rha, S.K.; Chou, T.P.; Cao, G.; Lee, Y.-S.; Lee, W.-J. Characteristics of silicon oxide thin films prepared by sol electrophoretic deposition method using tetraethylorthosilicate as the precursor. Curr. Appl. Phys. 2009, 9, 551–555. [Google Scholar] [CrossRef]
- Lenggoro, I.W.; Lee, H.M.; Okuyama, K. Nanoparticle assembly on patterned “plus/minus” surfaces from electrospray of colloidal dispersion. J. Coll. Interface Sci. 2006, 303, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Z.; Jayasinghe, S.N.; Edirisinghe, M.J.; Luklinska, Z.B. Coaxial electrohydrodynamic direct writing of nano-suspensions. J. Nanopart. Res. 2007, 9, 825–831. [Google Scholar] [CrossRef]
- Chen, C.H.; Kelder, E.M.; Schoonman, J. Electrostatic sol-spray deposition (ESSD) and characterisation of nanostructured TiO2 thin films. Thin Solid Films 1999, 342, 35–41. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Schoonman, J.; Lumbreras, M.; Siadat, M. Electrostatic spray deposited zinc oxide films for gas sensor applications. Appl. Surf. Sci. 2007, 253, 7483–7489. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, G.; He, G.; Wei, J.; Liu, H.; Lin, Y.; Zheng, J.; Sun, D. Electrohydrodynamic direct-writing ZnO nanofibers for device applications. Mater. Lett. 2013, 109, 58–61. [Google Scholar] [CrossRef]
- Koo, B.R.; Ahn, H.J. Structural, electrical, and optical properties of Sb-doped SnO2 transparent conductive oxides fabricated using an electrospray technique. Ceram. Int. 2014, 40, 4375–4381. [Google Scholar] [CrossRef]
- Higashi, K.; Uchida, K.; Hotta, A.; Hishida, K.; Miki, N. Micropatterning of silica nanoparticles by electrospray deposition through a stencil mask. J. Lab. Autom. 2014, 19, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Yamagata, Y.; Higuchi, T. Thin-film fabrication method for organic light-emitting diodes using electrospray deposition. Adv. Mater. 2009, 21, 4343–4347. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Asaki, H.; Asano, T.; Takagi, K.; Honda, Z.; Kamata, N.; Ju, J.; Yamagata, Y. Surface morphology of fluorene thin film fabricated by electrospray deposition technique using two organic solvents: Application for organic light-emitting diodes. Thin Solid Films 2011, 520, 600–605. [Google Scholar] [CrossRef]
- Cloupeau, M.; Prunet-Foch, B. Electrostatic spraying of liquids: Main functioning modes. J. Electrost. 1990, 25, 165–184. [Google Scholar] [CrossRef]
- Kim, H.-H.; Kim, J.-H.; Ogata, A. Time-resolved high-speed camera observation of electrospray. J. Aerosol Sci. 2011, 42, 249–263. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, D.; Zhan, Z.; Li, C.; Zheng, J.; Sun, D. Research on Glass Frit Deposition Based on the Electrospray Process. Materials 2016, 9, 292. https://doi.org/10.3390/ma9040292
Liu Y, Chen D, Zhan Z, Li C, Zheng J, Sun D. Research on Glass Frit Deposition Based on the Electrospray Process. Materials. 2016; 9(4):292. https://doi.org/10.3390/ma9040292
Chicago/Turabian StyleLiu, Yifang, Daner Chen, Zhan Zhan, Chenlei Li, Jianyi Zheng, and Daoheng Sun. 2016. "Research on Glass Frit Deposition Based on the Electrospray Process" Materials 9, no. 4: 292. https://doi.org/10.3390/ma9040292
APA StyleLiu, Y., Chen, D., Zhan, Z., Li, C., Zheng, J., & Sun, D. (2016). Research on Glass Frit Deposition Based on the Electrospray Process. Materials, 9(4), 292. https://doi.org/10.3390/ma9040292





