The Enhancement of Mg Corrosion Resistance by Alloying Mn and Laser-Melting
Abstract
:1. Introduction
2. Results and Discussion
2.1. Microstructure Characteristics
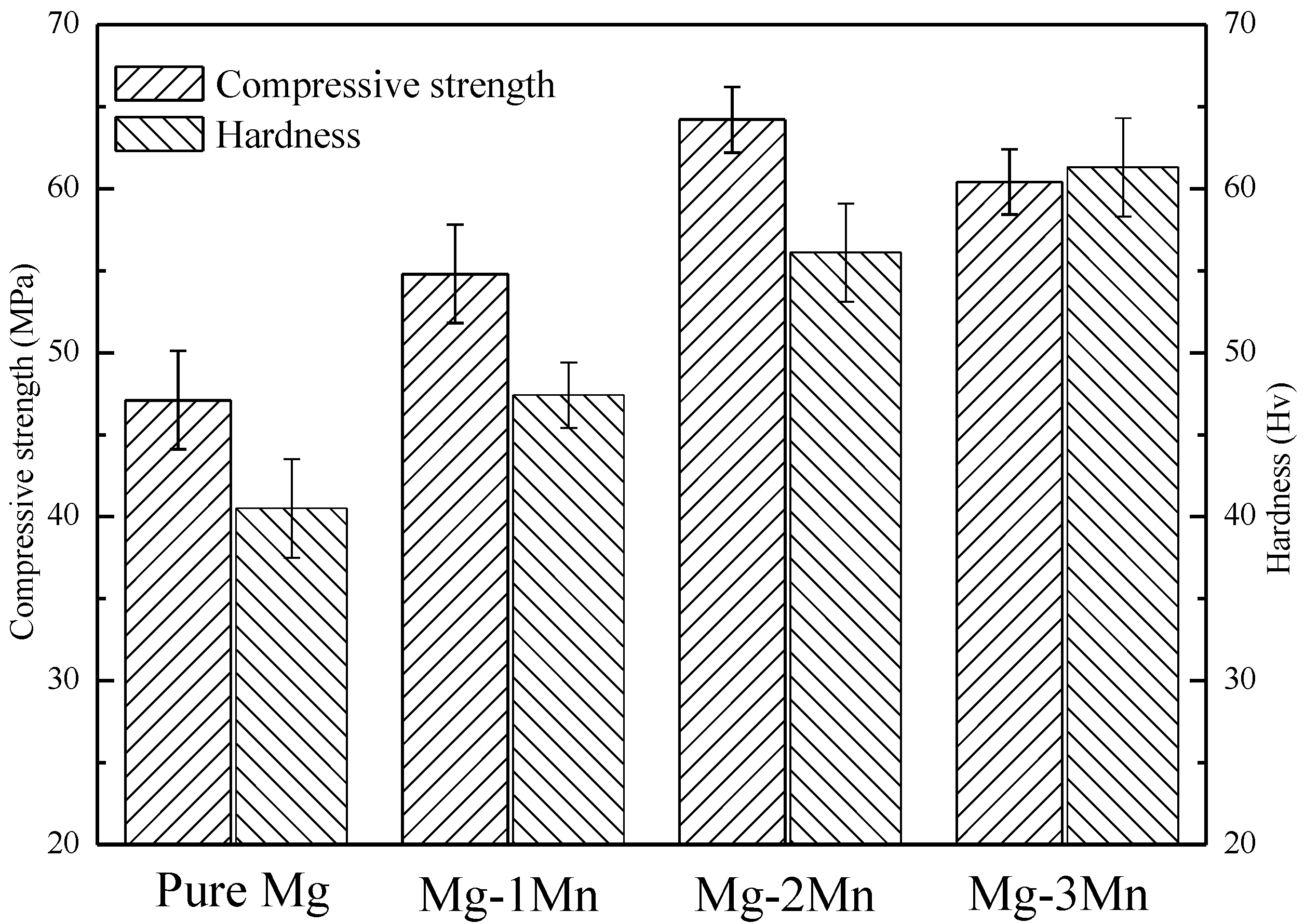
2.2. Mechanical Properties
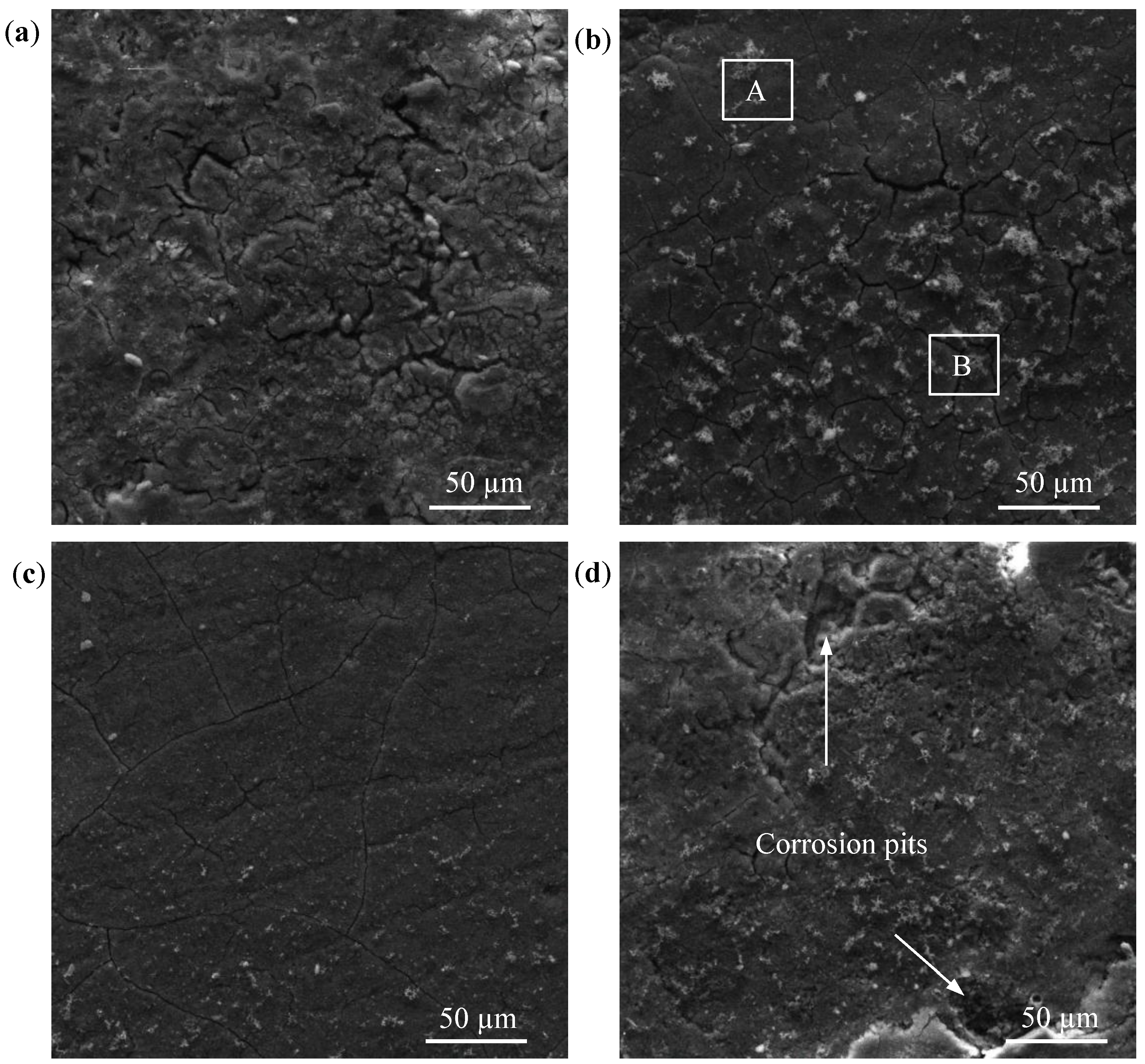
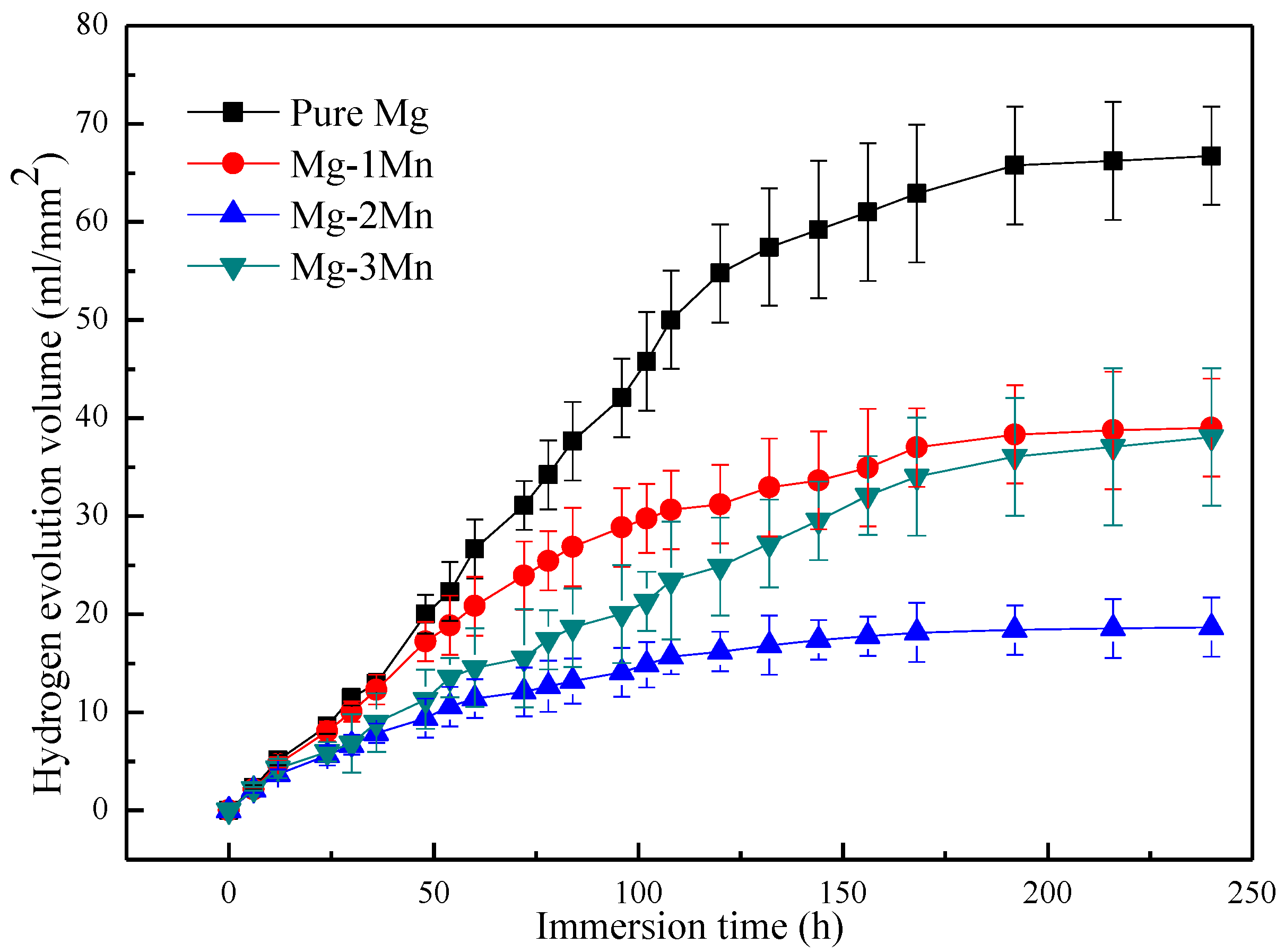
2.3. Corrosion Resistance
3. Materials and Methods
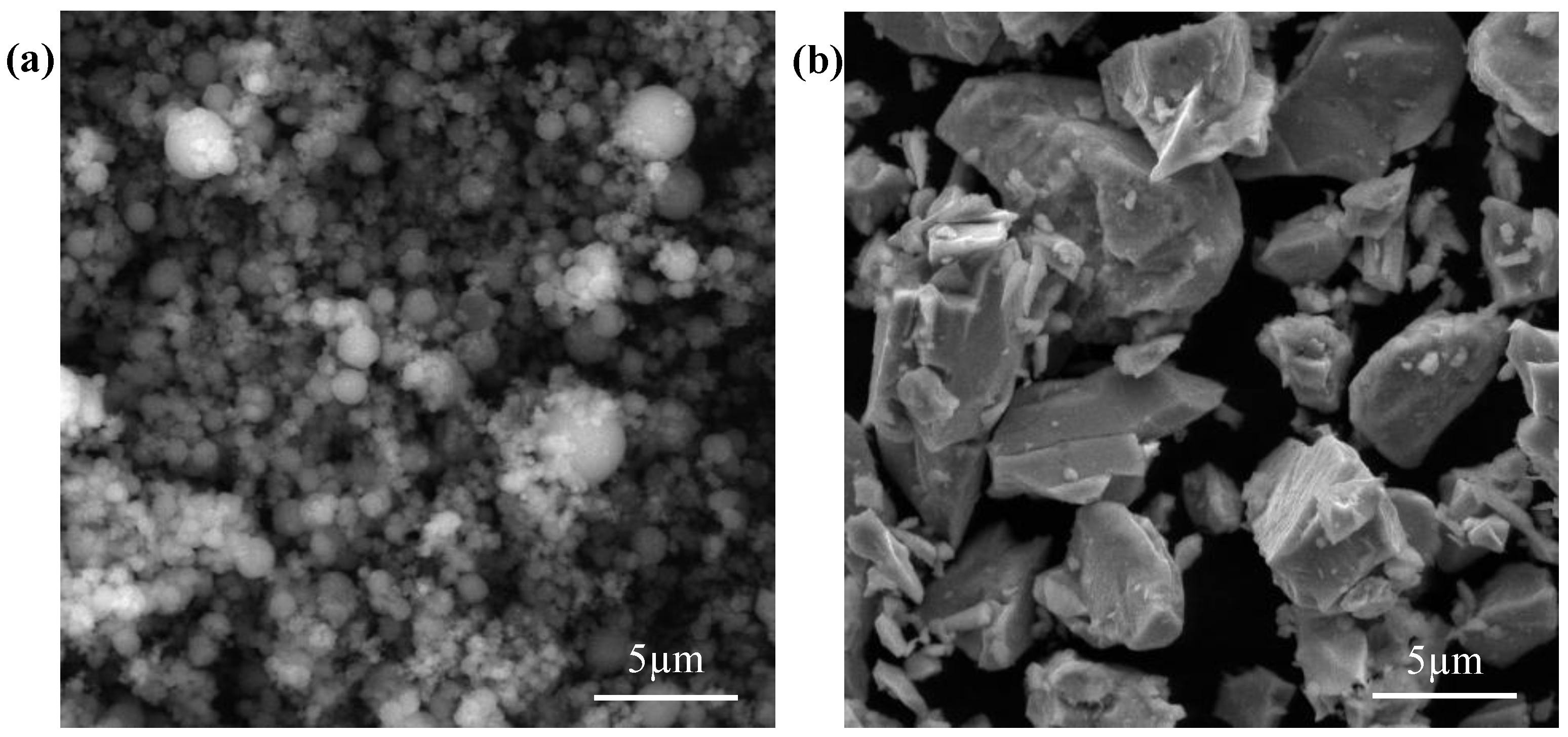
3.1. Materials
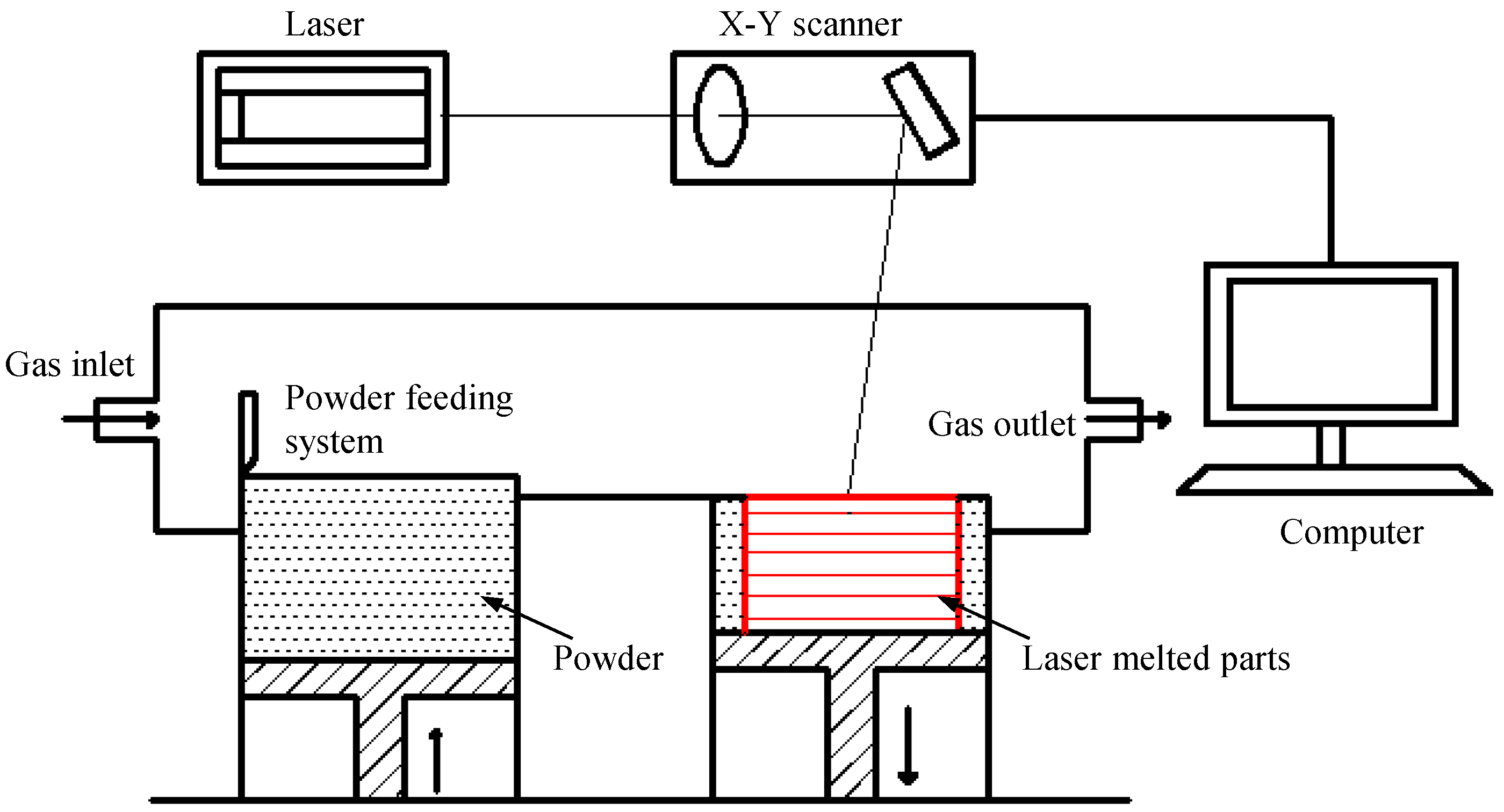
3.2. Laser-Melting Process
3.3. Microstructural Characterizations
3.4. Mechanical Tests
3.5. Immersion tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Myers, N.; Costello, B.J.; Chou, D.T.; Sfeir, C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 2015, 18, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Yin, D.; Xu, L.; Yang, L.; Yang, K. Microstructure, mechanical and corrosion properties and biocompatibility of Mg–Zn–Mn alloys for biomedical application. Mater. Sci. Eng. C 2009, 29, 987–993. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.F.; Hänzi, A.C.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. Magnesium alloys for temporary implants in osteosynthesis: In vivo studies of their degradation and interaction with bone. Acta Biomater. 2012, 8, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Chen, L.; Zhao, J.; Li, S.; Dai, Y.; Huang, Q.; Yu, Z. In vitro corrosion behavior and In vivo biodegradation of biomedical β-Ca3 (PO4)2/Mg–Zn composites. Acta Biomater. 2012, 8, 2845–2855. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, B.; Wang, Y.; Geng, L.; Jiao, X. Preparation and characterization of a new biomedical Mg–Zn–Ca alloy. Mater. Des. 2012, 34, 58–64. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gu, X.; Lou, S.; Zheng, Y. The development of binary Mg-Ca alloys for use as biodegradable materials within bone. Biomaterials 2008, 29, 1329–1344. [Google Scholar] [CrossRef] [PubMed]
- Salahshoor, M.; Guo, Y. Biodegradable orthopedic magnesium-calcium (MgCa) alloys, processing, and corrosion performance. Materials 2012, 5, 135–155. [Google Scholar] [CrossRef]
- Gu, X.; Xie, X.; Li, N.; Zheng, Y.; Qin, L. In vitro and In vivo studies on a Mg–Sr binary alloy system developed as a new kind of biodegradable metal. Acta Biomater. 2012, 8, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Bian, Y. Research on an Mg–Zn alloyas a degradable biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Zhao, J.; Qi, Y.; Li, Y.; Shen, L.; Xiao, M. Decreasing pores in a laser cladding layer with pulsed current. Chin. Opt. Lett. 2013, 11, 111401. [Google Scholar]
- Vilaro, T.; Colin, C.; Bartout, J.D.; Nazé, L.; Sennour, M. Microstructural and mechanical approaches of the selective laser melting process applied to a nickel-base superalloy. Mater. Sci. Eng. A 2012, 534, 446–451. [Google Scholar] [CrossRef]
- Nayak, S.; Bhushan, B.; Jayaganthan, R.; Gopinath, P.; Agarwal, R.D.; Lahiri, D. Strengthening of Mg based alloy through grain refinement for orthopaedic application. J. Mech. Behav. Biomed. Mater. 2016, 59, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Orlov, D.; Ralston, K.D.; Birbilis, N.; Estrin, Y. Enhanced corrosion resistance of Mg alloy ZK60 after processing by integrated extrusion and equal channel angular pressing. Acta Mater. 2011, 59, 6176–6186. [Google Scholar] [CrossRef]
- Makar, G.L.; Kruger, J. Corrosion of magnesium. Int. Mater. Rev. 1993, 38, 138–153. [Google Scholar] [CrossRef]
- Leach, R.M.; Muenster, A.M.; Wien, E.M. Studies on the role of manganese in bone formation: II. Effect upon chondroitin sulfate synthesis in chick epiphyseal cartilage. Arch. Biochem. Biophys. 1969, 133, 22–28. [Google Scholar] [CrossRef]
- Liu, Y.; Koltick, D.; Byrne, P.; Wang, H.; Zheng, W.; Nie, L.H. Development of a transportable neutron activation analysis system to quantify manganese in bone in vivo: Feasibility and methodology. Physiol. Meas. 2013, 34, 1593. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zheng, Y.; Cheng, Y.; Zhong, S.; Xi, T. In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 2009, 30, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.; Okamoto, H. ASM Handbook, Volume 3, Alloy Phase Diagrams; ASM International the Materials Information Company: Materials Park, OH, USA, 1992. [Google Scholar]
- Asgharzadeh, H.; Yoon, E.Y.; Chae, H.J.; Kim, T.S.; Lee, J.W.; Kim, H.S. Microstructure and mechanical properties of a Mg–Zn–Y alloy produced by a powder metallurgy route. J. Alloys Compd. 2014, 586, S95–S100. [Google Scholar] [CrossRef]
- Mora, E.; Garcés, G.; Onorbe, E.; Pérez, P.; Adeva, P. High-strength Mg–Zn–Y alloys produced by powder metallurgy. Scr. Mater. 2009, 60, 776–779. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, G. Analysis Methods in Materials Science—X-ray Diffraction and Electron Microscopy in Materials Science, 2nd ed.; Harbin Institute of Technology Press: Harbin, China, 2007. [Google Scholar]
- Ng, C.C.; Savalani, M.M.; Lau, M.L.; Man, H.C. Microstructure and mechanical properties of selective laser melted magnesium. Appl. Surf. Sci. 2011, 257, 7447–7454. [Google Scholar] [CrossRef]
- Baril, G.; Pebere, N. The corrosion of pure magnesium in aerated and deaerated sodium sulphate solutions. Corros. Sci. 2001, 43, 471–484. [Google Scholar] [CrossRef]
- Shuai, C.; Gao, C.; Nie, Y.; Hu, H.; Qu, H.; Peng, S. Structural design and experimental analysis of a selective laser sintering system with nano-hydroxyapatite powder. J. Biomed. Nanotechnol. 2010, 6, 370–374. [Google Scholar] [CrossRef] [PubMed]
- American Society for Testing Materials. Standard Test Methods for Tension Testing of Metallic Materials; ASTM E8-04; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
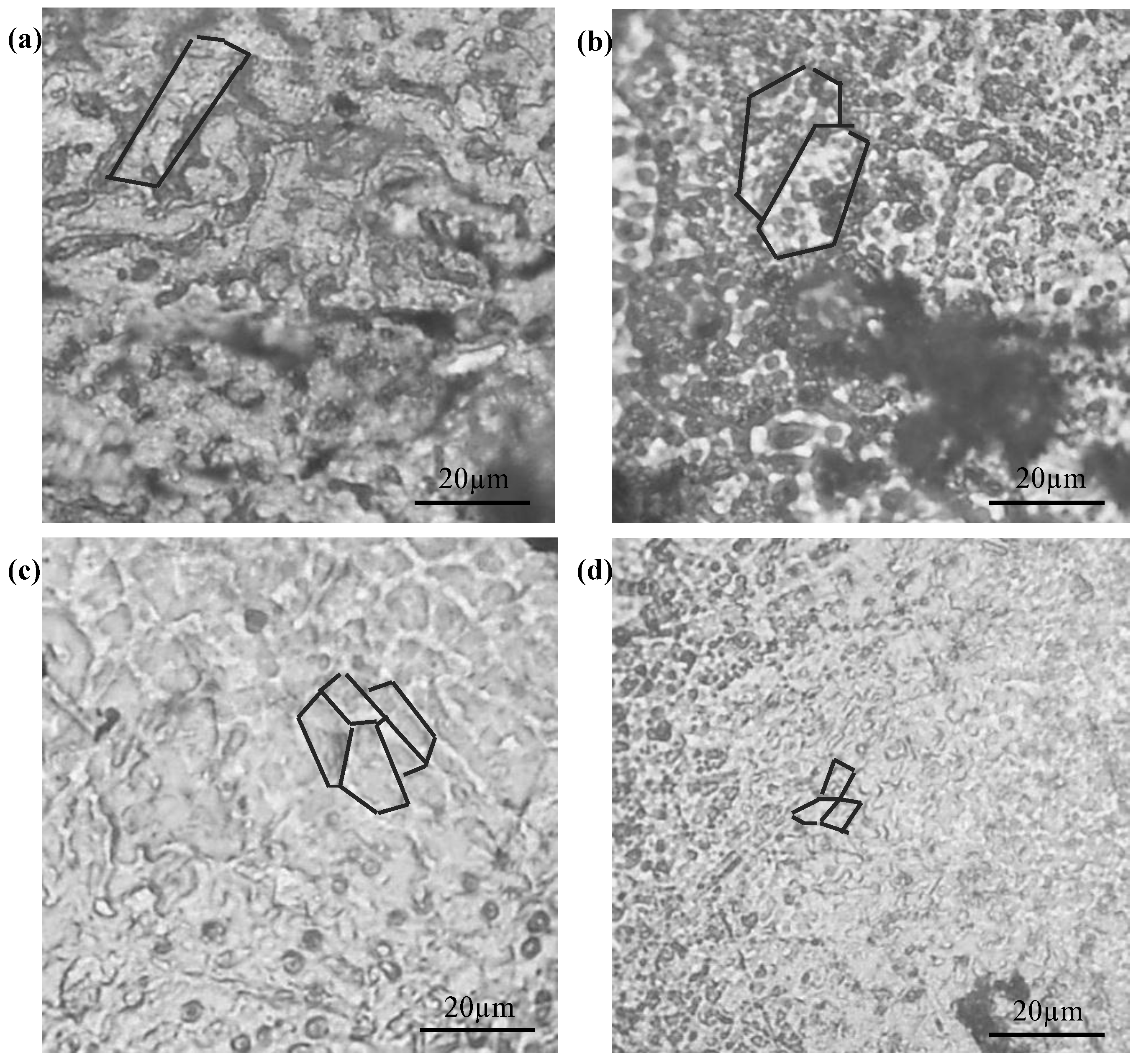
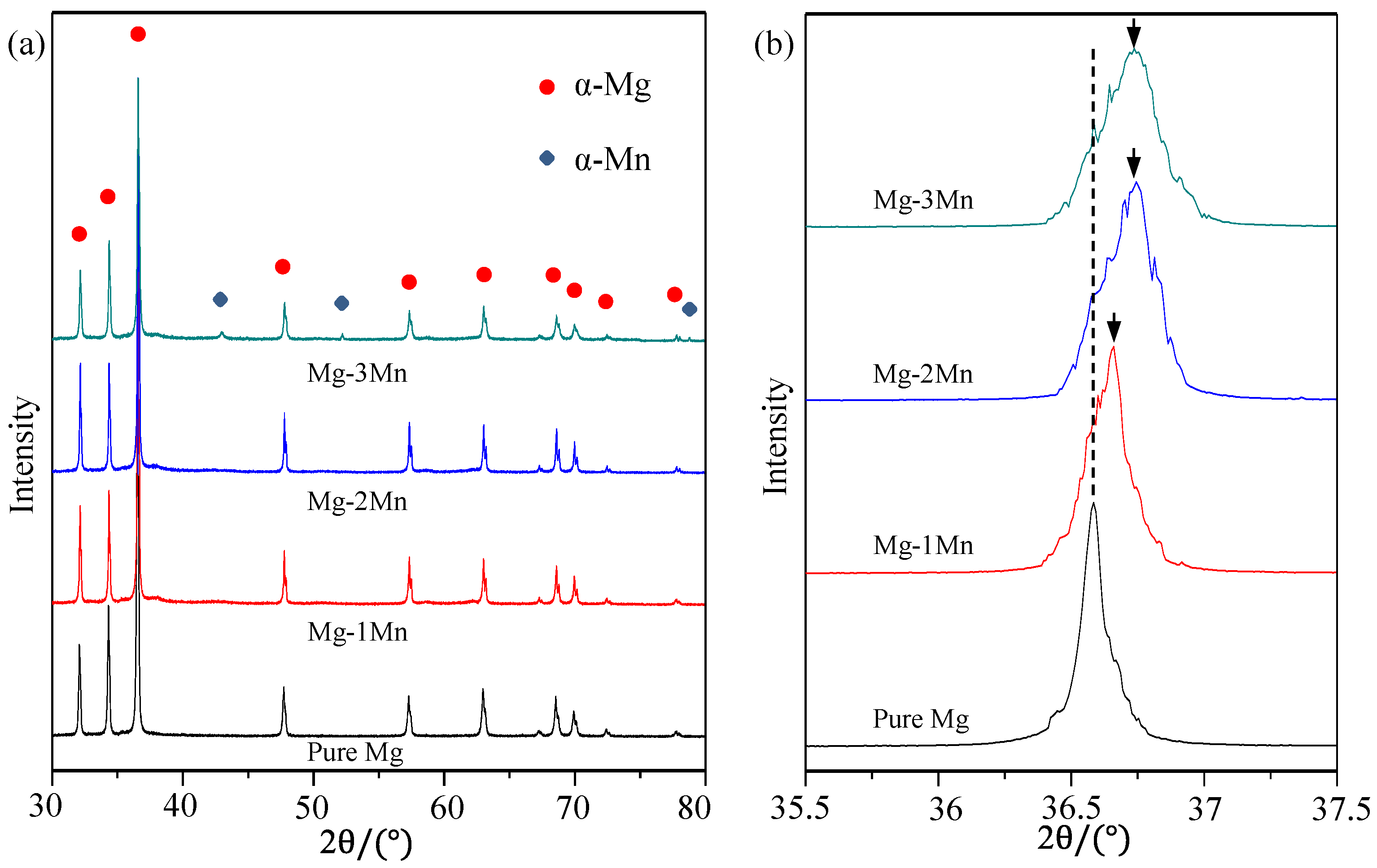

| Sample | Standard | Pure Mg | Mg-1Mn | Mg-2Mn | Mg-3Mn |
|---|---|---|---|---|---|
| 2θ location (°) | 36.53 | 36.53 | 36.59 | 36.71 | 36.72 |
| Lattice spacing (Å) | 2.45772 | 2.45772 | 2.45382 | 2.44608 | 2.44543 |
| Intensity (CPS) | - | 5709 | 5309 | 5016 | 4220 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wu, P.; Wang, Q.; Wu, H.; Liu, Y.; Deng, Y.; Zhou, Y.; Shuai, C. The Enhancement of Mg Corrosion Resistance by Alloying Mn and Laser-Melting. Materials 2016, 9, 216. https://doi.org/10.3390/ma9040216
Yang Y, Wu P, Wang Q, Wu H, Liu Y, Deng Y, Zhou Y, Shuai C. The Enhancement of Mg Corrosion Resistance by Alloying Mn and Laser-Melting. Materials. 2016; 9(4):216. https://doi.org/10.3390/ma9040216
Chicago/Turabian StyleYang, Youwen, Ping Wu, Qiyuan Wang, Hong Wu, Yong Liu, Youwen Deng, Yuanzhuo Zhou, and Cijun Shuai. 2016. "The Enhancement of Mg Corrosion Resistance by Alloying Mn and Laser-Melting" Materials 9, no. 4: 216. https://doi.org/10.3390/ma9040216
APA StyleYang, Y., Wu, P., Wang, Q., Wu, H., Liu, Y., Deng, Y., Zhou, Y., & Shuai, C. (2016). The Enhancement of Mg Corrosion Resistance by Alloying Mn and Laser-Melting. Materials, 9(4), 216. https://doi.org/10.3390/ma9040216







