Organic-Inorganic Hydrophobic Nanocomposite Film with a Core-Shell Structure
Abstract
:1. Introduction
2. Experiments
2.1. Raw Materials
2.2. Main Instruments and Characterization
2.3. Experimental Procedures
2.3.1. Preparation Process of Ternary SiO2–Al2O3–TiO2 Nanomaterials
2.3.2. Adsorption Processes of the Emulsifiers on the Nanomaterials
2.3.3. Preparation of Organic-Inorganic Hydrophobic Nanocomposite Emulsion
3. Results and Discussion
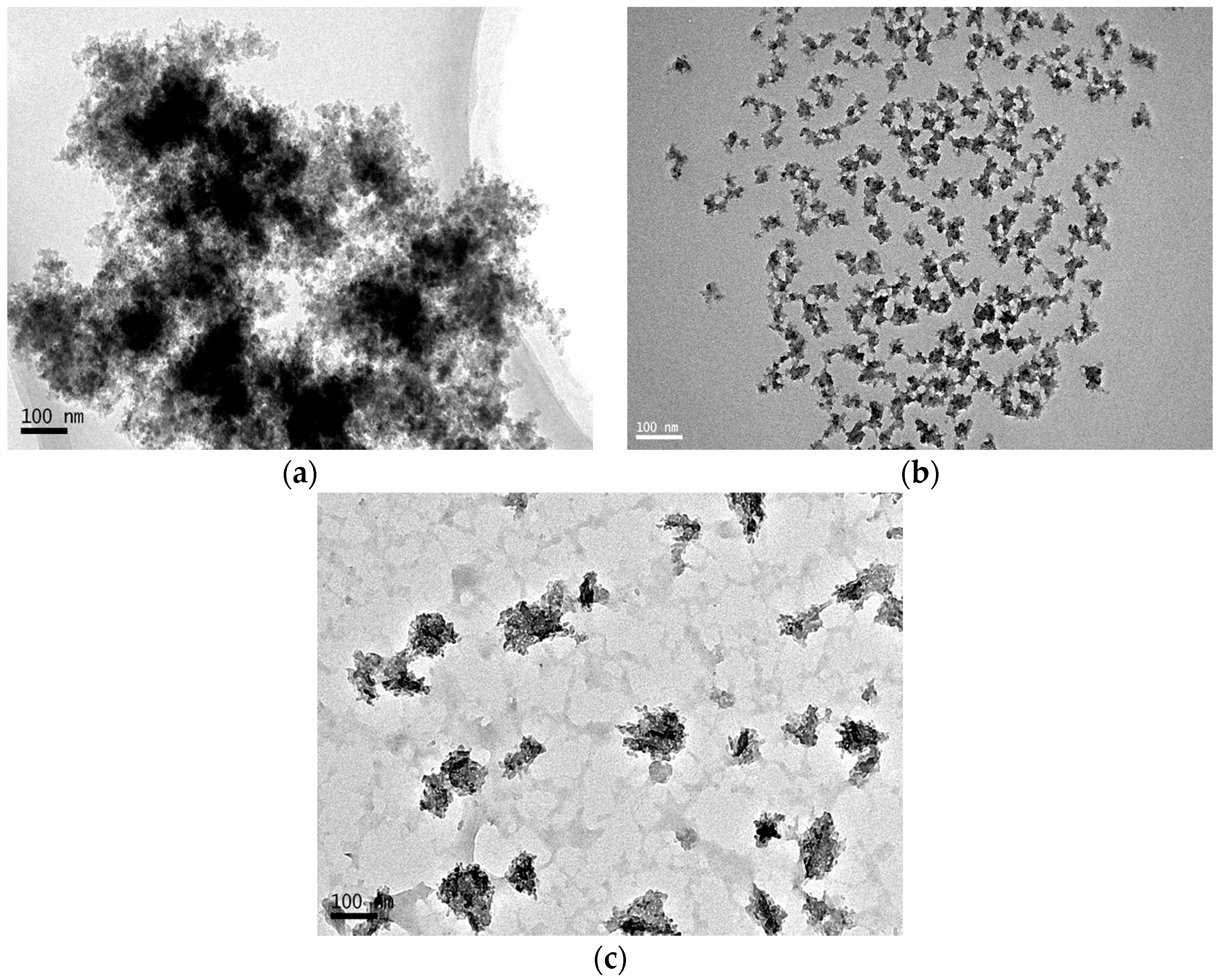
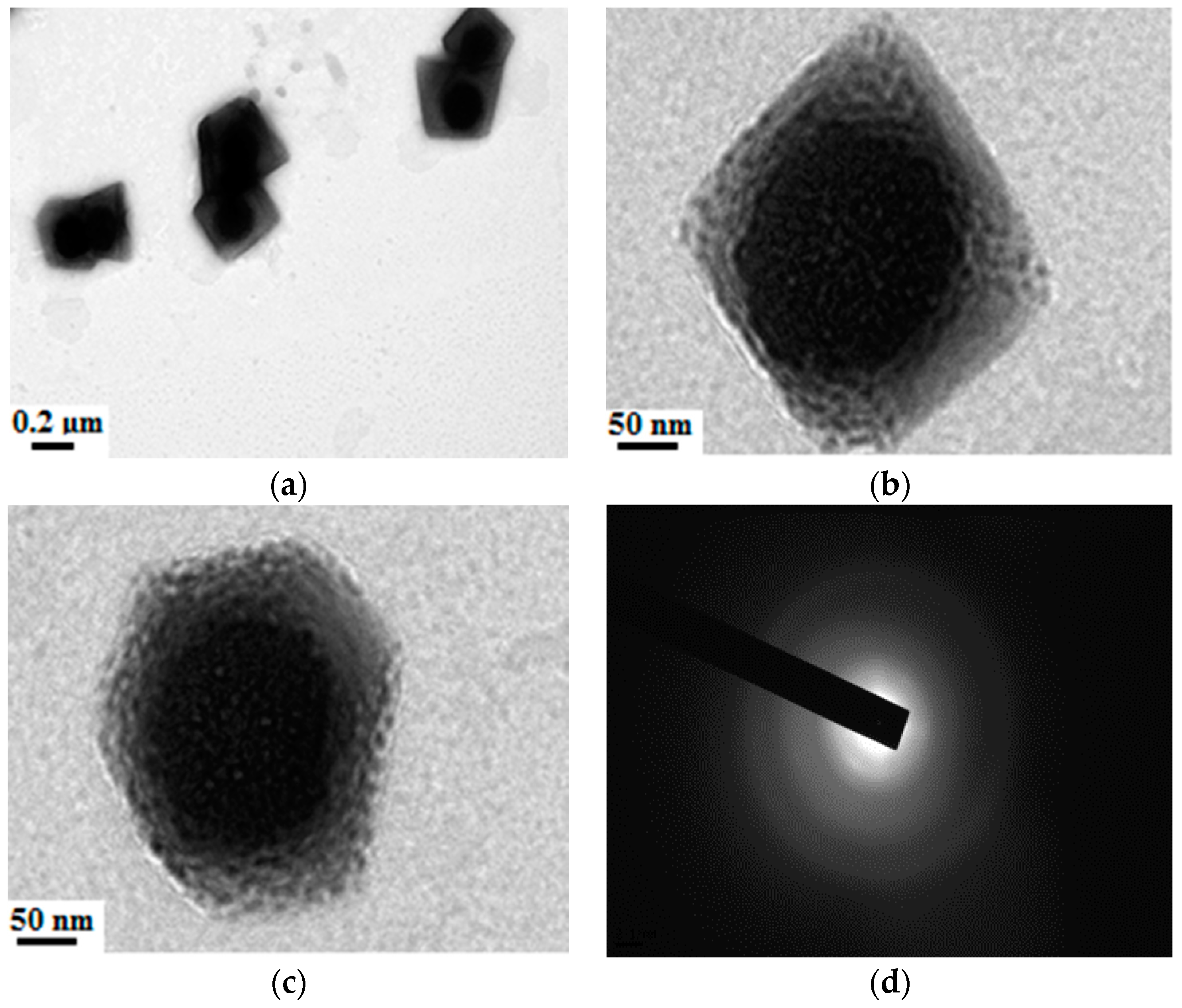
3.1. Synthesis of Ternary SiO2–Al2O3–TiO2 Nanomaterials
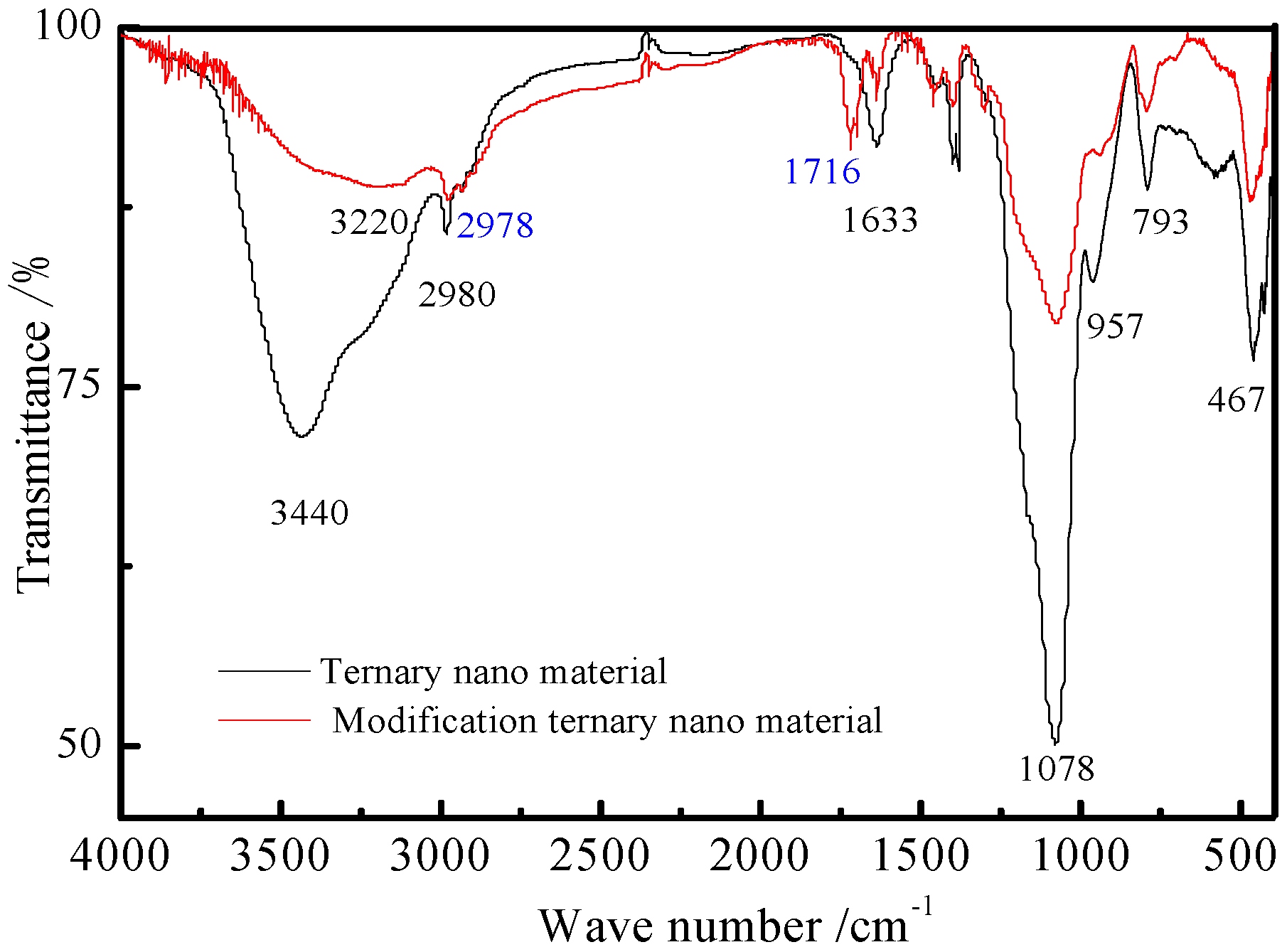
3.2. Modification of the Ternary Nanomaterials
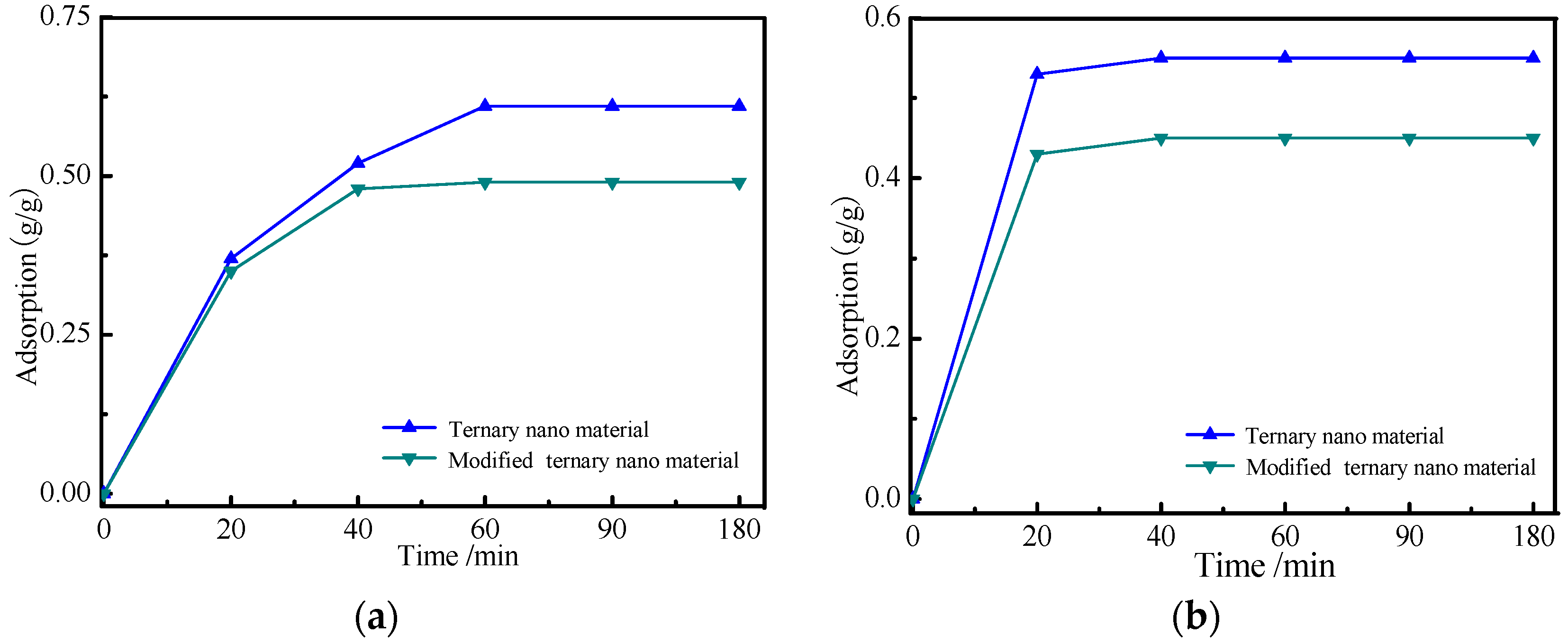
3.3. Adsorption of the Nanomaterials
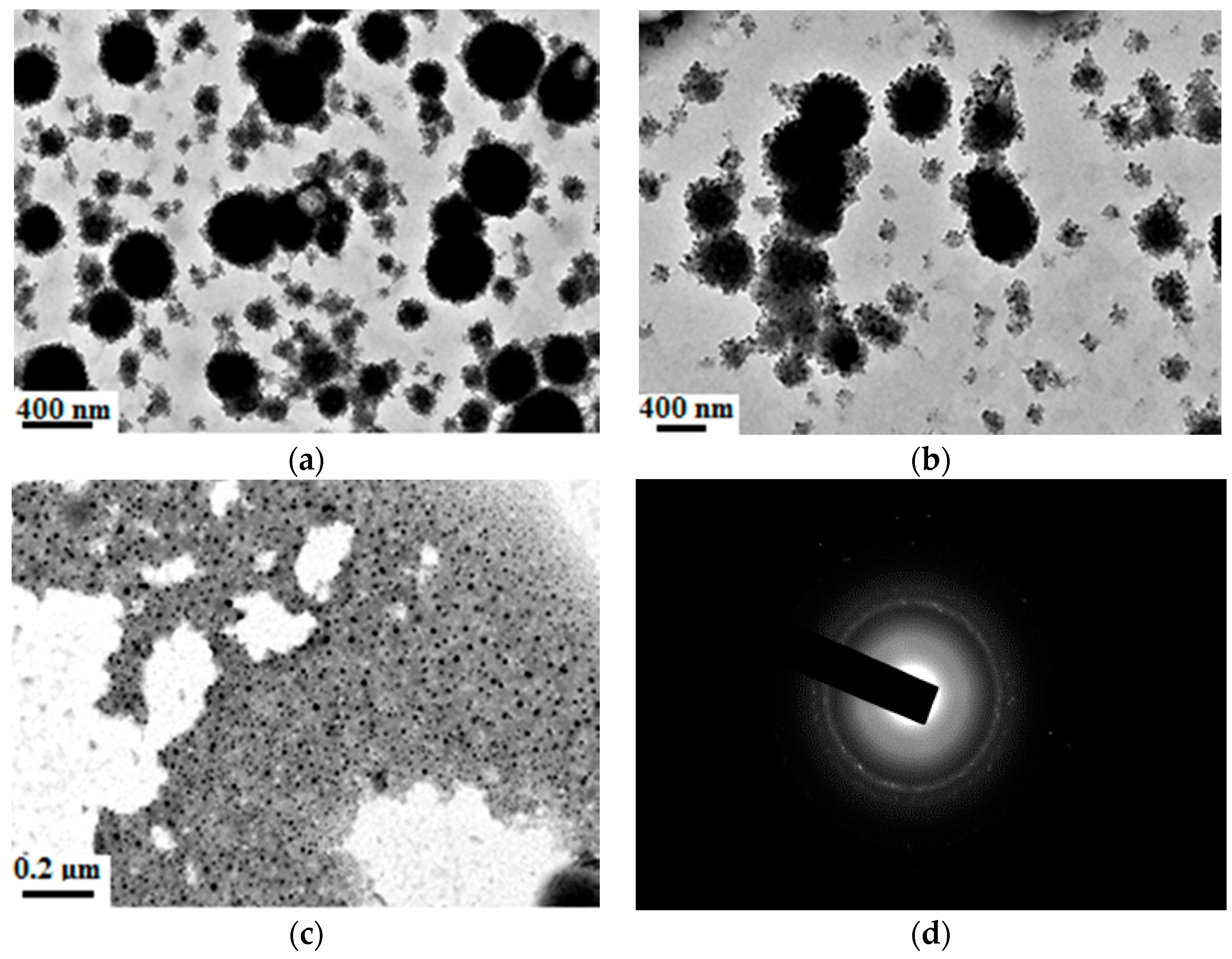
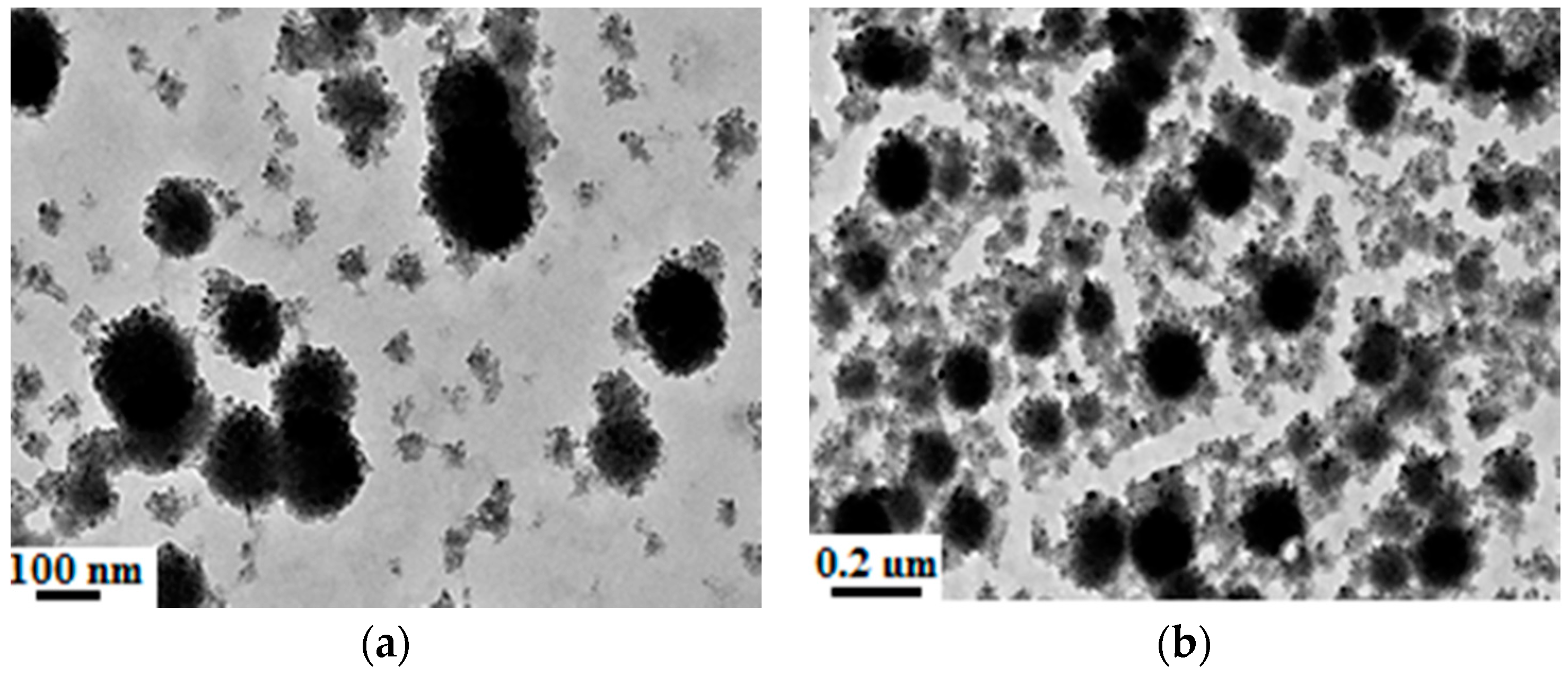
3.4. Synthesis of Organic-Inorganic Hydrophobic Nanocomposite Emulsion
3.5. Effect of Temperature on the Nanocomposite Particle
3.6. Effect of MA and HFMA on the Nanocomposite Particle
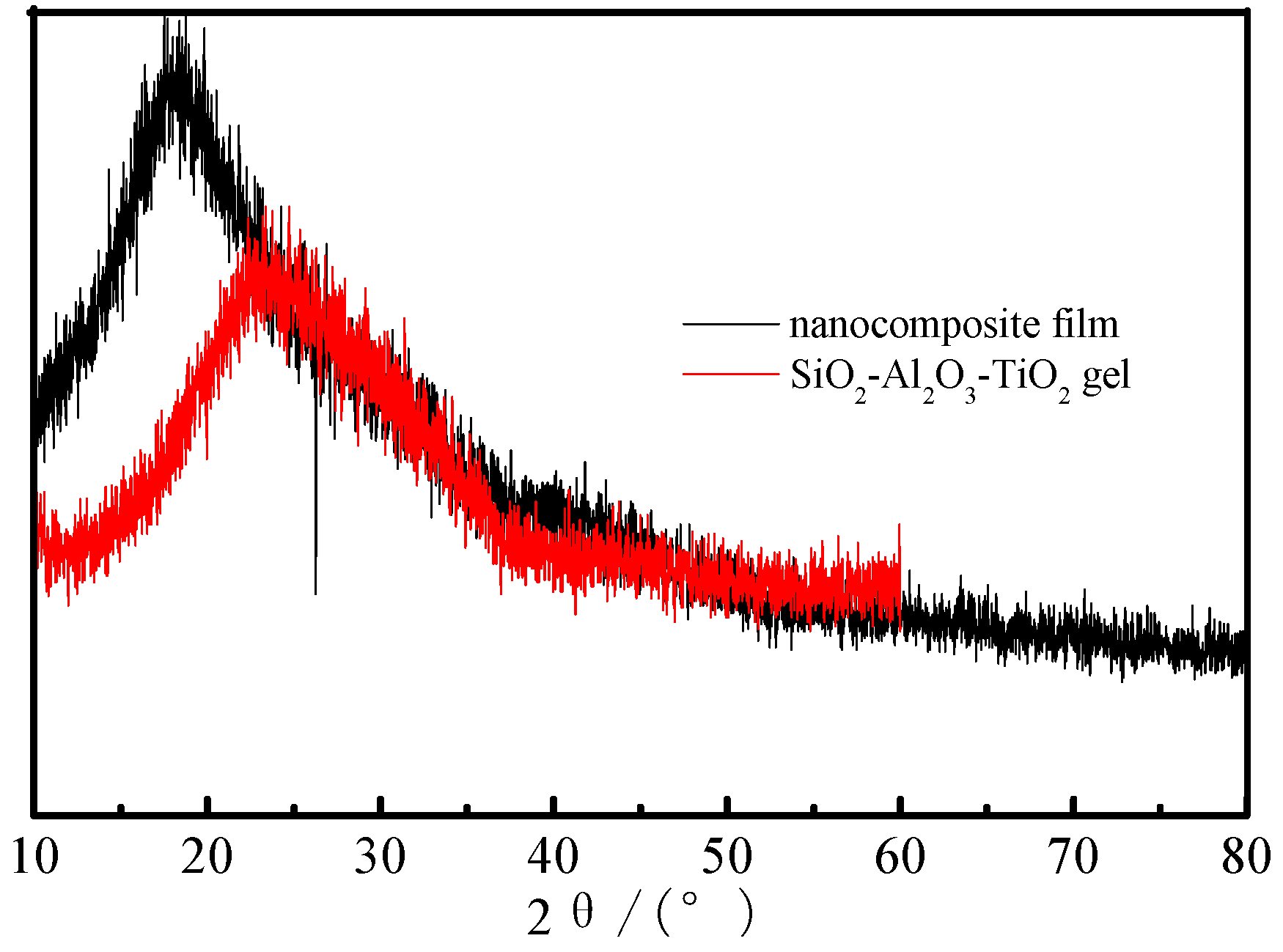
3.7. XRD Spectra of the Nanocomposite Film
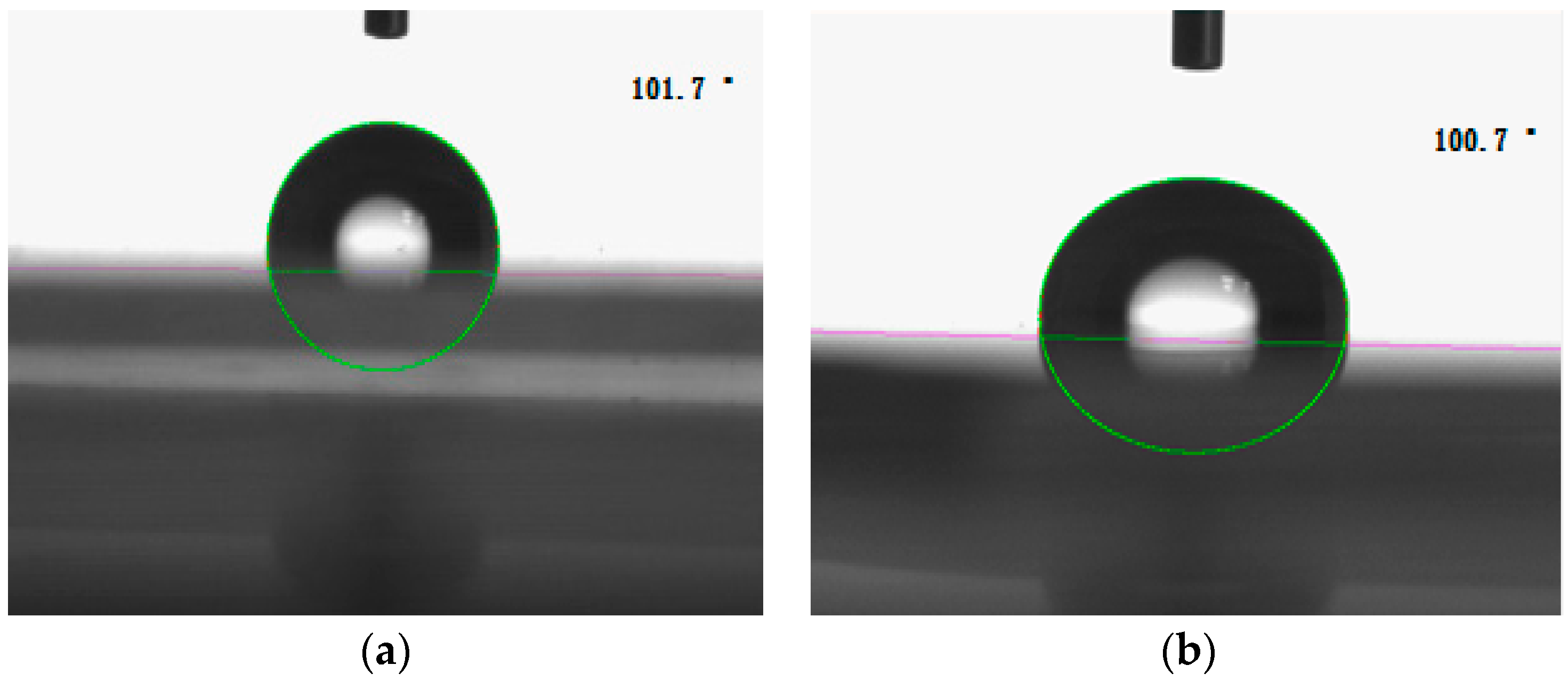
3.8. Hydrophobicity of the Nanocomposite Film
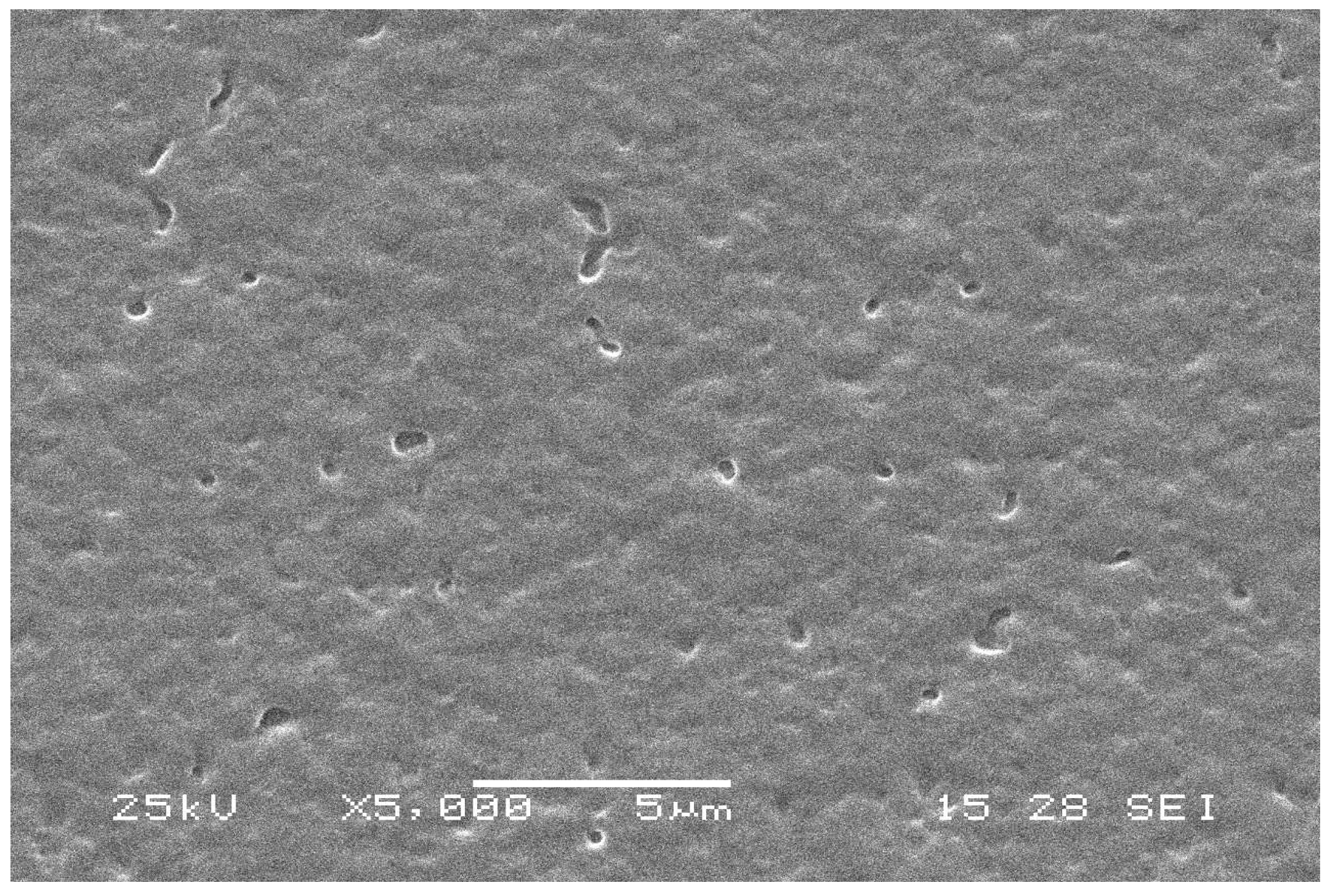
3.9. SEM Analysis of the Nanocomposite Film
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bai, R.-Q.; Qiu, T.; Han, F.; He, L.-F.; Li, X.-Y. Preparation and characterization of inorganic-organic trilayer core-shell polysilsesquioxane/polyacrylate/polydimethylsiloxane hybrid latex particles. Appl. Surf. Sci. 2012, 258, 7683–7688. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic-inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Pavlidou, S.; Papaspyrides, C.-D. A review on polymer-layered silicate nanocomposites. Prog. Polym. Sci. 2008, 33, 1119–1198. [Google Scholar] [CrossRef]
- Ma, J.-Z.; Liu, Y.-H.; Bao, Y.; Liu, J.-L.; Zhang, J. Research advances in polymer emulsion based on core-shell structure particle design. Adv. Colloid Interface Sci. 2013, 198, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Galo, J.-D. Design of functional nano-structured materials through the use of controlled hybrid organic-inorganic interfaces. C. R. Chim. 2003, 6, 1131–1151. [Google Scholar] [CrossRef]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Qin, Y.-C.; Ren, H.-B.; Zhu, F.H.; Zhang, L.; Shang, C.-W.; Wei, Z.-J.; Luo, M.-M. Preparation of POSS-based organic–inorganic hybrid mesoporous materials networks through Schiff base chemistry. Eur. Polym. J. 2011, 47, 853–860. [Google Scholar] [CrossRef]
- Hashemi-Nasab, R.; Mirabedini, S.-M. Effect of silica nanoparticles surface treatment on in situ polymerization of styrene-butyl acrylate latex. Prog. Org. Coat. 2013, 76, 1016–1023. [Google Scholar] [CrossRef]
- Chen, J.-H.; Cheng, C.-Y.; Chiu, W.-Y.; Lee, C.-F.; Liang, N.-Y. Synthesis of ZnO/polystyrene composites particles by pickering emulsion polymerization. Eur. Polym. J. 2008, 44, 3271–3279. [Google Scholar] [CrossRef]
- Tan, J.; Yu, M.; Rao, X.; Zeng, Z. Fast and facile one-step synthesis of monodisperse thermo-responsive core–shell microspheres and applications. Polym. Chem. 2015, 6, 6698–6708. [Google Scholar] [CrossRef]
- Fielding, L.A.; Tonnar, J.; Armes, S.-P. All-acrylic film-forming colloidal polymer/silica nanocomposite particles prepared by aqueous emulsion polymerization. Langmuir 2011, 27, 11129–11144. [Google Scholar] [CrossRef] [PubMed]
- Seulki, C.; Nahae, K.; Soonjae, L.; Hoseok, L.; Lee, S.-H.; Kim, J.; Choi, J.-W. Use of hybrid composite particles prepared using alkoxysilane-functionalized amphiphilic polymer precursors for simultaneous removal of various pollutants from water. Chemosphere 2016, 156, 302–311. [Google Scholar]
- Yu, Q.-J.; Xu, J.-M.; Liu, J.; Li, B.-X.; Liu, Y.-J. Synthesis and properties of PANI/SiO2 organic-inorganic hybrid films. Appl. Surf. Sci. 2012, 263, 532–535. [Google Scholar] [CrossRef]
- Chang, G.; He, L.; Zheng, W.; Pan, A.-Z.; Liu, J.; Li, Y.-J.; Cao, R.-J. Well-defined inorganic/organic nanocomposite by nano silica core-poly (methyl methacrylate/butylacrylate/trifluoroethyl methacrylate) shell. J. Colloid Interface Sci. 2013, 396, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-H.; Zhang, L.; Ma, J.-Z. Fluorinated polyacrylate emulsifier-free emulsion mediated by poly(acrylic acid)-b-poly(hexafluorobutyl acrylate) trithiocarbonate via ab initio RAFT emulsion polymerization. Chem. Eng. J. 2013, 223, 8–17. [Google Scholar] [CrossRef]
- Qu, A.-L.; Wen, X.-F.; Pi, P.-H.; Cheng, J.; Yang, Z.-R. Synthesis of composite particles through emulsion polymerization based on silica/fluoroacrylate siloxane using anionic reactive and nonionic surfactants. J. Colloid Interface Sci. 2008, 317, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Guido, K. Concepts for the incorporation of inorganic building blocks into organic polymers on a nanoscale. Prog. Polym. Sci. 2003, 28, 83–114. [Google Scholar]
- Matthew, A.-H.; Margherita, M.; Rafael, M.-E. Synthetic Strategies in the Preparation of Polymer/Inorganic Hybrid Nanoparticles. Materials 2014, 7, 4057–4087. [Google Scholar]
- Ma, H.-Y.; Wang, T.-L.; Chang, P.-Y.; Yang, C.-H. High refractive Organic–inorganic hybrid films prepared by low water sol-gel and UV-Irradiation processes. Nanomaterials 2016, 6, 44–53. [Google Scholar] [CrossRef]
- Hedayati, M.-K.; Abdelaziz, M.; Etrich, C.; Homaeigohar, S.; Rockstuhl, C.; Elbahri, M. Broadband anti-reflective coating based on plasmonic nanocomposite. Materials 2016, 9, 636–645. [Google Scholar] [CrossRef]
- Pan, Y.; Pan, H.-F.; Yuan, B.-H.; Hong, N.-N.; Zhan, J.; Wang, B.-B.; Song, L.; Hu, Y. Construction of organic inorganic hybrid nano-coatings containing zirconium phosphate with high efficiency for reducing re hazards of exible polyurethane foam. Mater. Chem. Phys. 2015, 163, 107–115. [Google Scholar] [CrossRef]
- Ali, G.; Abbas, D.-T. New organic-inorganic hybrid material based on functional cellulose nano whisker, polypseudorotaxane and Au nanorods. Carbohydr. Polym. 2016, 152, 196–206. [Google Scholar]
- Xu, L.-H.; Shen, Y.; Wang, L.-M.; Ding, Y.; Cai, Z.-S. Preparation of vinyl silica-based organic/inorganic nanocomposites and superhydrophobic polyester surfaces from it. Colloid Polym. Sci. 2015, 293, 2359–2371. [Google Scholar] [CrossRef]
- Serkis, M.; SpIrkova, M.; Kredatusova, J.; Hodan, J.; Bures, R. Organic–inorganic nanocomposite films made from polyurethane dispersions and colloidal silica particles. Compos. Interface 2016, 23, 1–17. [Google Scholar] [CrossRef]
- Mallakpour, S.; Khadem, E. Recent development in the synthesis of polymer nanocomposites based on nano-alumina. Prog. Polym. Sci. 2015, 51, 74–93. [Google Scholar] [CrossRef]
- Wang, L.; Song, L.-Y.; Chao, Z.-Y.; Chen, P.-P.; Nie, W.-Y.; Zhou, Y.-F. Role of surface functionality on the formation of raspberry-like polymer/silica composite particles: Weak acid–base interaction and steric effect. Appl. Surf. Sci. 2015, 342, 92–100. [Google Scholar] [CrossRef]
- Yan, W.; Han, Z.-J.; Phung, B.-T.; Faupel, F.; Ostrikov, K. High-Voltage insulation organic-inorganic nanocomposites by plasma polymerization. Materials 2014, 7, 563–575. [Google Scholar] [CrossRef]
- Molina, M.-J.; Mariscal, R.; Granados, M.-L. Synthesis of silica xerogelpoly(styrene sulphonic acid) nanocomposites as acid catalysts: Effects of temperature and polymer concentration on their textural and chemical properties. J. Sol-Gel Sci. Technol. 2015, 75, 164–179. [Google Scholar] [CrossRef]
- Kalan, R.-E.; Yaparatne, S.; Amirbahman, A.; Tripp, C.-P. P25 titanium dioxide coated magnetic particles: Preparation, characterization and photocatalytic activity. Appl. Catal. B Environ. 2016, 187, 249–258. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Yi, S.-S.; Lv, Z.-S.; Huang, C. Facile fabrication of superhydrophobic coatings based on two silica sols. Colloid Polym. Sci. 2016, 294, 1503–1509. [Google Scholar] [CrossRef]
- Ma, X.-F.; Zhang, B.; Cong, Q.; He, X.-C.; Gao, M.-J.; Li, G. Organic/inorganic nanocomposites of ZnO/CuO/chitosan with improved properties. Mater. Chem. Phys. 2016, 178, 88–97. [Google Scholar] [CrossRef]
- Zhang, J.-F.; Ma, C.; Liu, J.-T.; Chen, L.-B.; Pan, A.-Q.; Wei, W.-F. Solid polymer electrolyte membranes based on organic/inorganic nanocomposites with star-shaped structure for high performance lithium ion battery. J. Membr. Sci. 2016, 509, 138–148. [Google Scholar] [CrossRef]
- Kormanyos, A.; Endrodi, B.; Ondok, R.; Sapi, A.; Janaky, C. Controlled photocatalytic synthesis of core–shell SiC/Polyaniline hybrid nanostructures. Materials 2016, 9, 201. [Google Scholar] [CrossRef]
- Le, M.-T.; Huang, S.-C. Thermal and mechanical behavior of hybrid polymer nanocomposite reinforced with graphene nanoplatelets. Materials 2015, 8, 5526–5536. [Google Scholar] [CrossRef]
- Tan, J.-B.; Fu, L.-L.; Zhang, X.-C.; Bai, Y.-H.; Zhang, L. Photosynthesis of poly(glycidyl methacrylate) microspheres: A component for making covalently cross-linked colloido somes and organic/inorganic nanocomposites. J. Mater. Sci. 2016, 51, 9455–9471. [Google Scholar] [CrossRef]
- Naffakh, M.; Ana, M.-D.; Marco, C.; Ellis, G.J.; Marian, A.-G. Opportunities and challenges in the use of inorganic fullerene-like nanoparticles to produce advanced polymer nanocomposites. Prog. Polym. Sci. 2013, 38, 1163–1231. [Google Scholar] [CrossRef]
- Bai, R.-Q.; Qiu, T.; Duan, M.; Ma, G.-L.; He, L.-F.; Li, X.-Y. Synthesis and characterization of core-shell polysilsesquioxane-poly (styrene-butyl acrylate-fluorinated acrylate) hybrid latex particles. Colloids Surf. A 2012, 396, 251–257. [Google Scholar] [CrossRef]
- Rabiee, A.; Baharvand, H. An organic inorganic polymeric alumina hybrid nanocomposite. Polym. Sci. 2015, 57, 264–273. [Google Scholar] [CrossRef]
- Ma, W.; Li, J.; Liu, Y.; Ren, X.-H.; Gu, Z.-G.; Xie, Z.-W.; Liang, J. Preparation and characterization of excellent antibacterial TiO2/N-halamines nanoparticles. Colloids Surf. A 2016, 506, 284–290. [Google Scholar] [CrossRef]
- Zhou, J.-H.; Chen, X.; Duan, H.; Ma, J.-Z.; Ma, Y.-R. Synthesis and characterization of nano-SiO2 modified fluorine-containing polyacrylate emulsifier-free emulsion. Appl. Surf. Sci. 2015, 331, 504–511. [Google Scholar] [CrossRef]
- Lee, J.-W.; Othman, M.-R.; Eom, Y.; Lee, T.-G.; Kim, W.-S.; Kim, J. The effects of sonification and TiO2 deposition on the micro-characteristics of the thermally treated SiO2/TiO2 spherical core-shell particles for photo-catalysis of methyl orange. Microporous Mesoporous Mater. 2008, 116, 561–568. [Google Scholar] [CrossRef]
- Qin, Z.-G.; Zhang, J.; Chi, H.-H.; Cao, F. Organic-inorganic hybrid nanocomposites based on chitosan derivatives and layered double hydroxides with intercalated phacolysin as ocular delivery system. J. Nanopart. Res. 2015, 17, 1–15. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Chen, Y.; Yu, Z. Organic-Inorganic Hydrophobic Nanocomposite Film with a Core-Shell Structure. Materials 2016, 9, 1021. https://doi.org/10.3390/ma9121021
Liu P, Chen Y, Yu Z. Organic-Inorganic Hydrophobic Nanocomposite Film with a Core-Shell Structure. Materials. 2016; 9(12):1021. https://doi.org/10.3390/ma9121021
Chicago/Turabian StyleLiu, Peng, Ying Chen, and Zhiwu Yu. 2016. "Organic-Inorganic Hydrophobic Nanocomposite Film with a Core-Shell Structure" Materials 9, no. 12: 1021. https://doi.org/10.3390/ma9121021
APA StyleLiu, P., Chen, Y., & Yu, Z. (2016). Organic-Inorganic Hydrophobic Nanocomposite Film with a Core-Shell Structure. Materials, 9(12), 1021. https://doi.org/10.3390/ma9121021






