Active Nanofibrous Membrane Effects on Gingival Cell Inflammatory Response
Abstract
:1. Introduction
2. Results α-MSH
2.1. PGA-α-MSH Localization on EC and FB
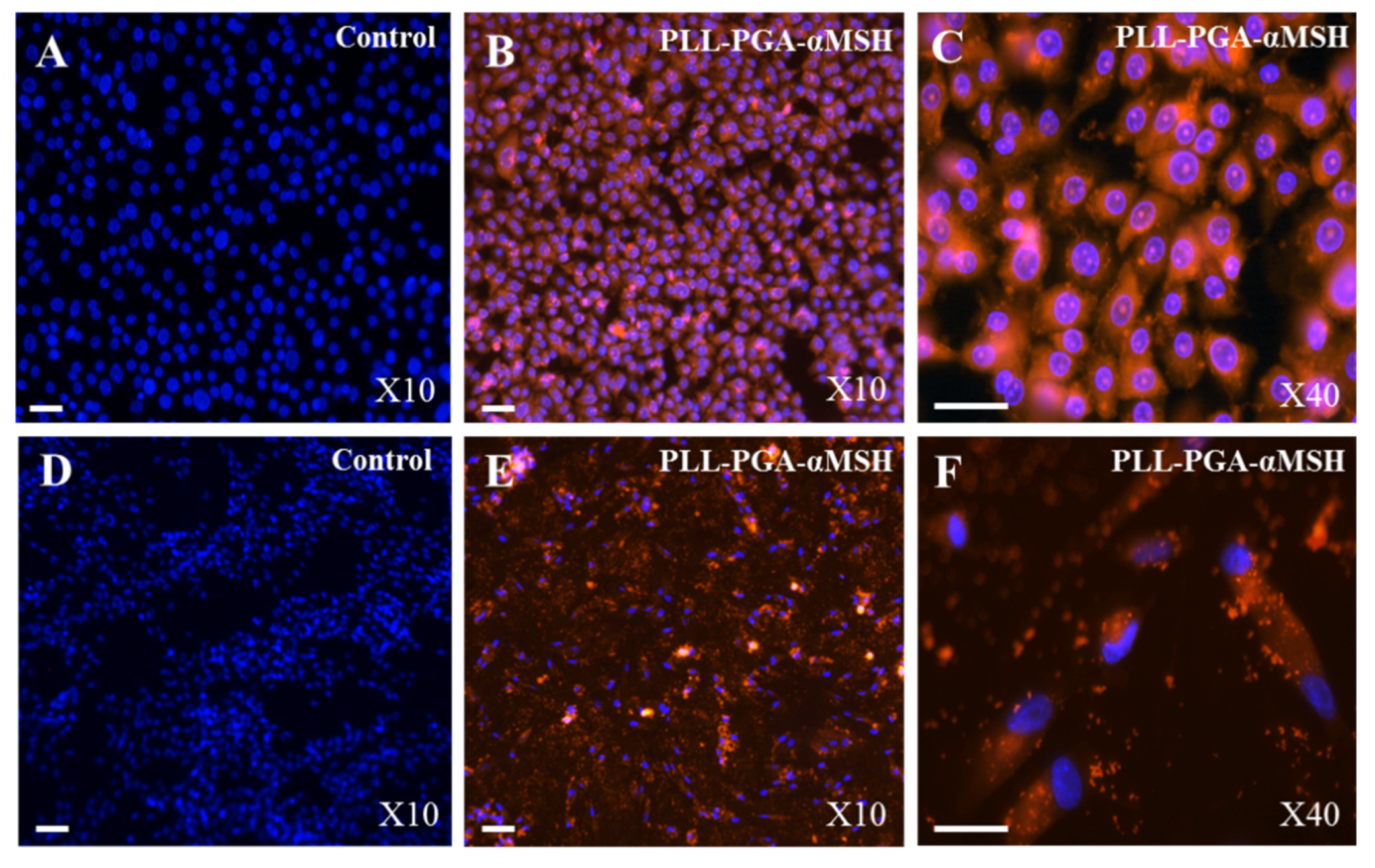
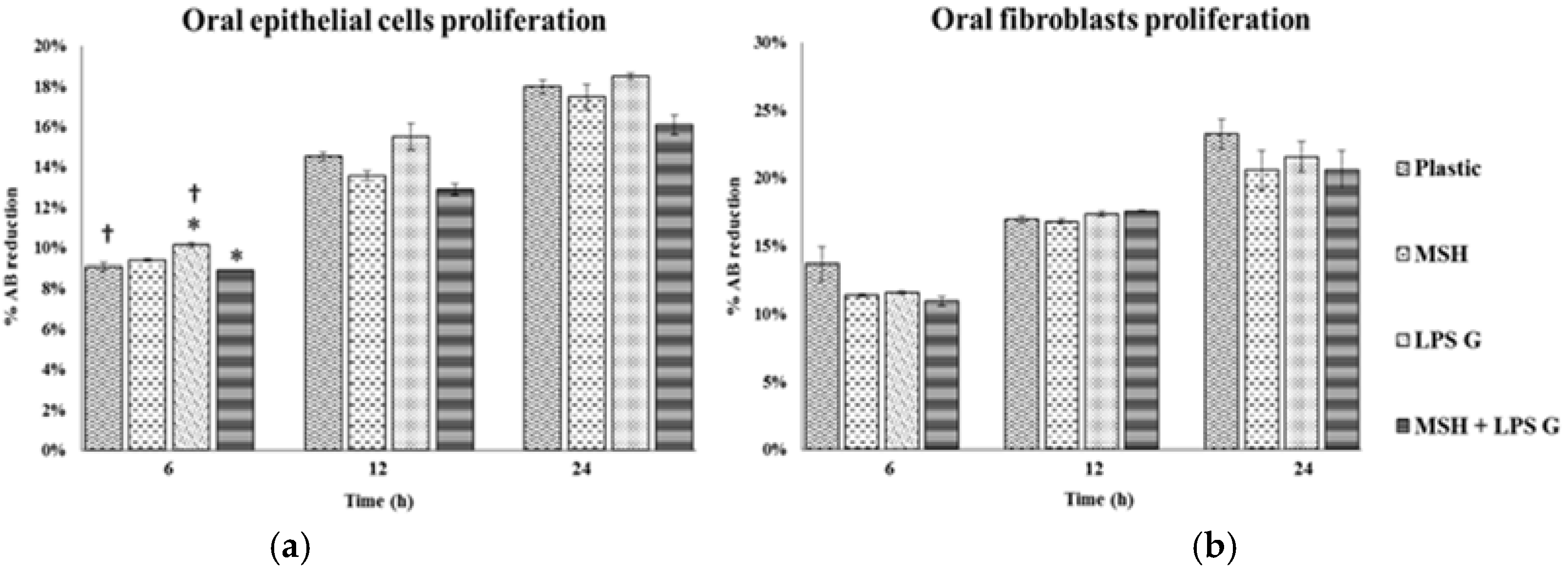
2.2. Effect of PGA-α-MSH on Cell Proliferation/Viability and Migration

2.3. Effect of PGA-α-MSH on Expression Profiles of IL-6, TGF-β and TNF-α in EC and FB
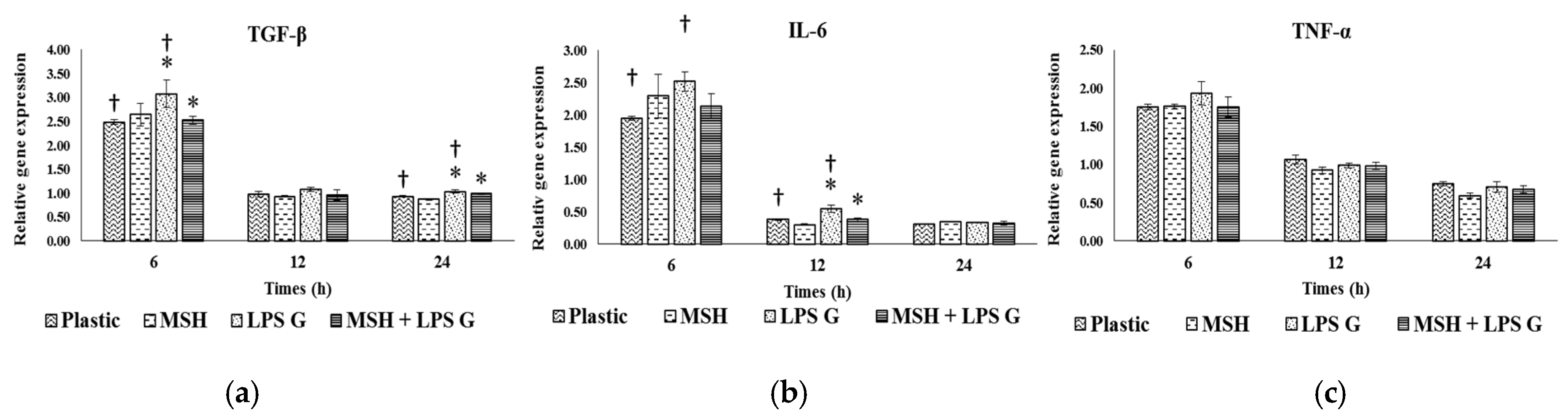
2.3.1. Effects of Pg-LPS Stimulation
2.3.2. Effects of PGA-α-MSH
2.4. PGA-α-MSH Localization and Morphology Analysis of EC, FB on PCL Membranes
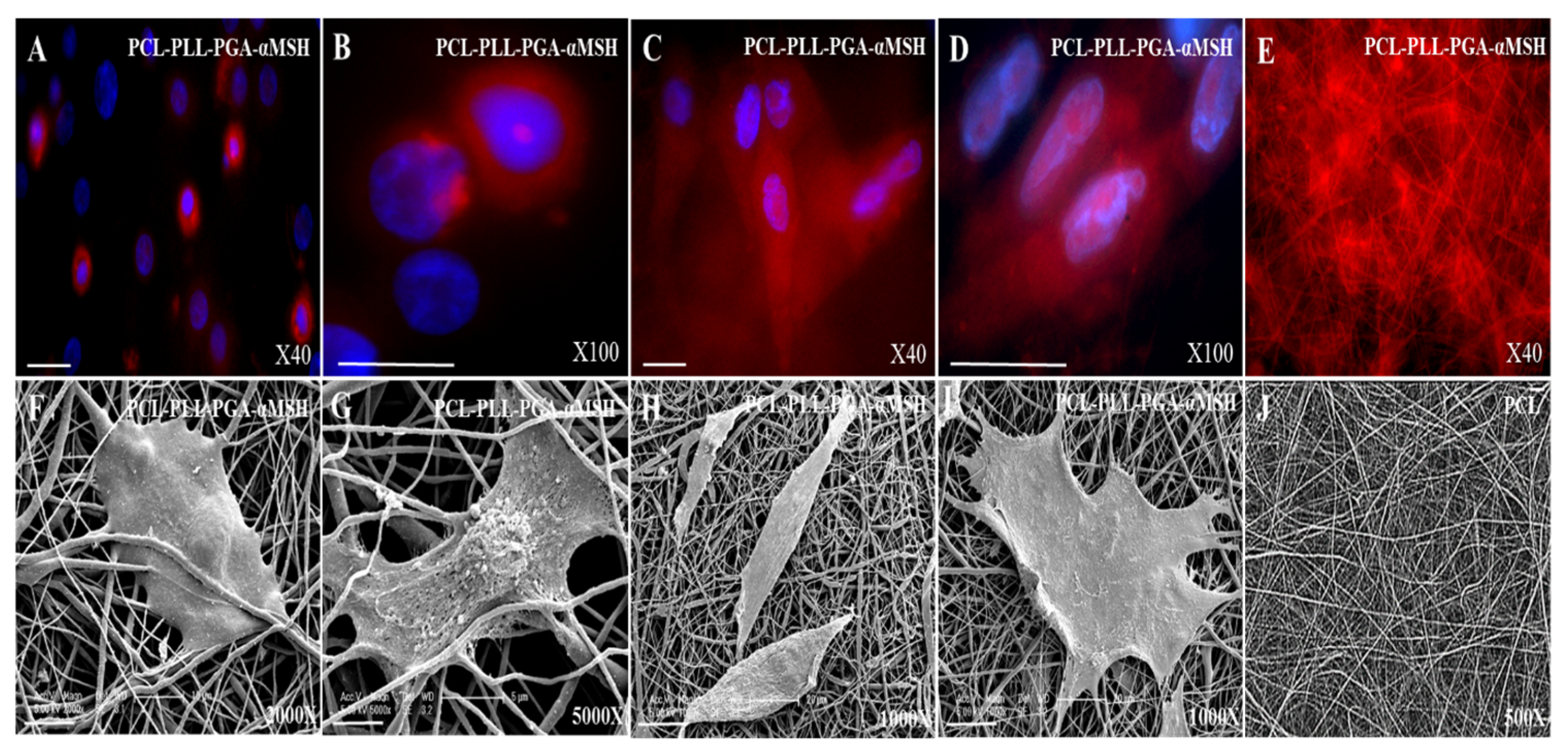
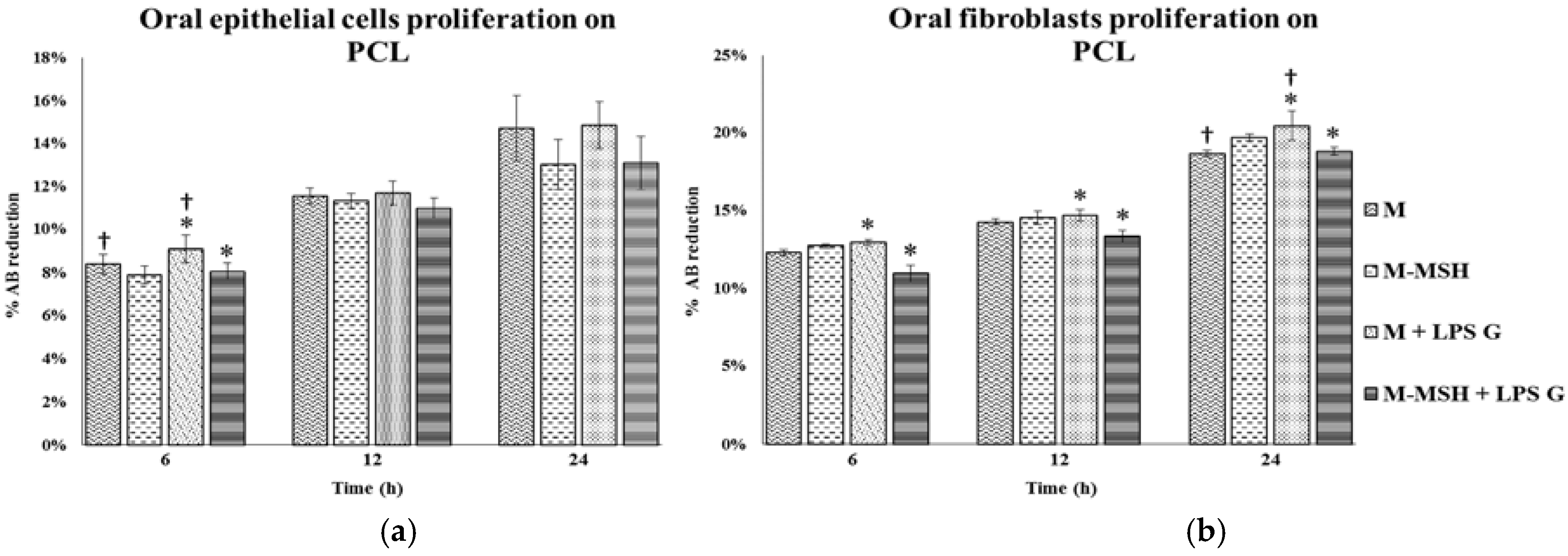
2.5. Effect of PCL Membranes Functionalized with PGA-α-MSH on Cell Proliferation
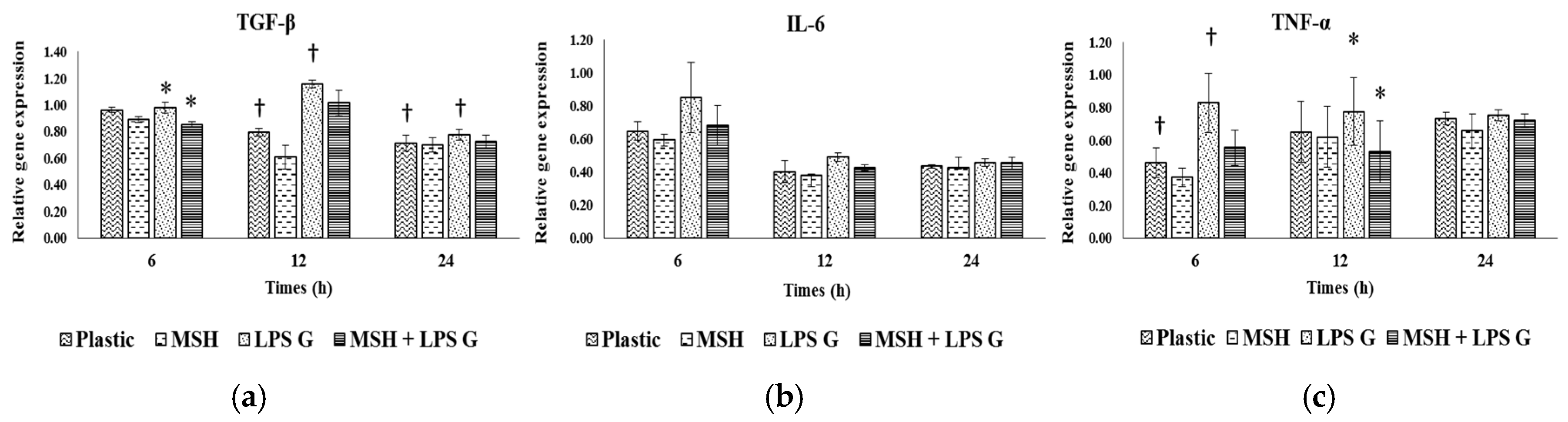
2.6. Effect of PCL Membranes Functionalized with PGA-α-MSH on Expression profile of IL-6, TGF-β and TNF-α in EC and in FB
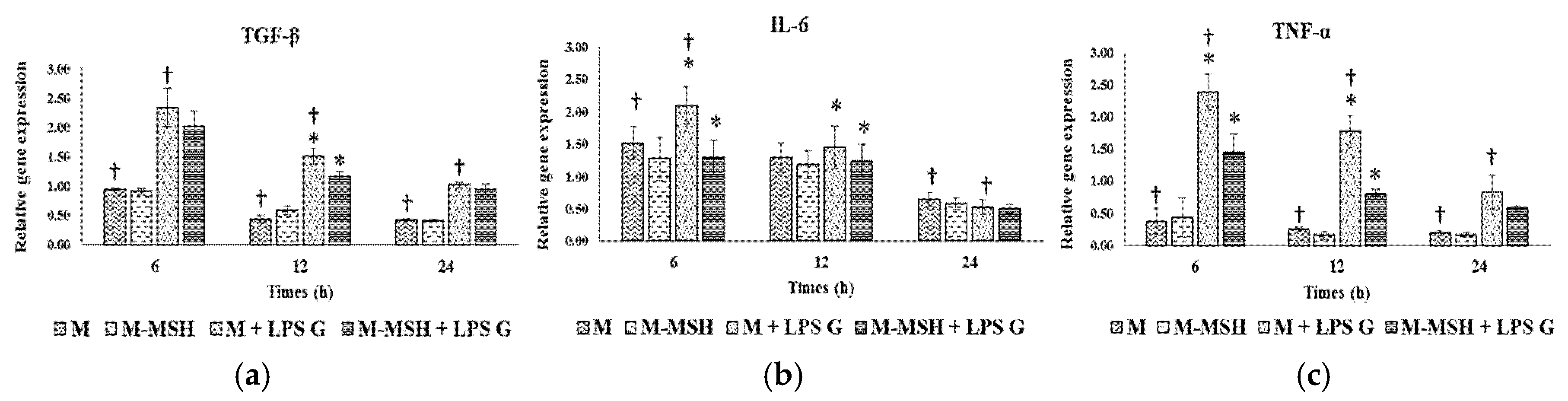
2.6.1. Effects of Pg-LPS Stimulation
2.6.2. Effects of PGA-α-MSH
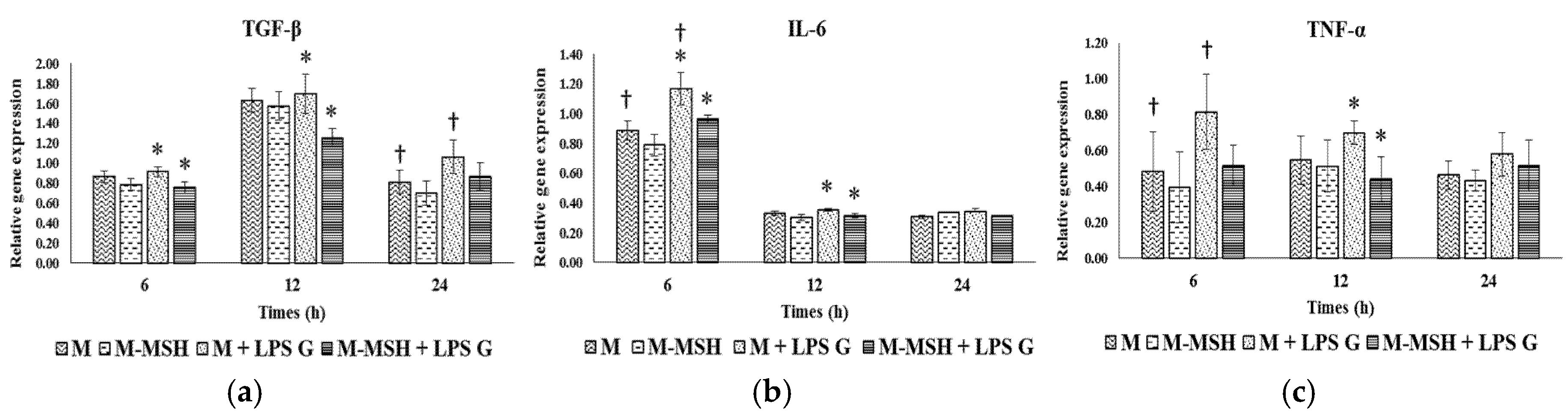
2.7. Specific Effect of PCL Membranes on EC and FB Responses to PGA-α-MSH
3. Discussion
4. Material and Methods
4.1. Electrospinning
4.2. PCL Membranes Functionalization with PGA-α-MSH
4.3. Cell Cultures
4.4. Cell Proliferation
4.5. Wound Closure Assay
4.6. Immunofluorescence
4.7. Scanning Electron Microscopy Observation
4.8. RNA Isolation and Reverse Transcription
4.9. Real-Time Quantitative RT-PCR Analysis (RT-qPCR)
| Gene Product | Primer Name | Primer Sequence |
|---|---|---|
| TNF-α | TNF-α-FW | CCTGCCCCAATCCCTTTATT |
| TNF-α-RW | CCCTAAGCCCCCAATTCTCT | |
| IL-6 | IL-6-FW | GCCTCAGATCTCCAGTCC |
| IL-6-RW | GCCTCAGATCTCCAGTCC | |
| TGF-β | TGF-β-FW | CCCAGCATCTGCAAAGCTC |
| TGF-β-RW | GTCAATGTACAGCTGCCGCA | |
| β-actin | β-actin-FW | GATGAGATTGGCATGGCTTT |
| β-actin-RW | CACCTTCACCGTTCCAGTTT |
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, J.H.; Park, C.H.; Perez, R.A.; Lee, H.Y.; Jang, J.H.; Lee, H.H.; Wall, I.B.; Shi, S.; Kim, H.W. Advanced Biomatrix Designs for Regenerative Therapy of Periodontal Tissues. J. Dent. Res. 2014, 93, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Polimeni, G.; Xiropaidis, A.V.; Wikesjö, U.M.E. Biology and principles of periodontal wound healing/regeneration. Periodontol. 2000 2006, 41, 30–47. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, C.A.; Rasperini, G.; Batia, S.; Giannobile, W.V. Advanced reconstructive technologies for periodontal tissue repair. Periodontol. 2000 2012, 59, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Giannobile, W.V. Host-response therapeutics for periodontal diseases. J. Periodontol. 2008, 79, 1592–1600. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.V.; Puleo, D.A. Infection, inflammation, and bone regeneration: a paradoxical relationship. J. Dent. Res. 2011, 90, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Yucel-Lindberg, T.; Båge, T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev. Mol. Med. 2013, 15, e7. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-M.; Zhang, J.; Zhang, M.; An, Y.; Chen, F.; Wu, Z.-F. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials 2010, 31, 7892–7927. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kohli, M.; Zhou, Q.; Graves, D.T.; Amar, S. Short- and Long-Term Effects of IL-1 and TNF Antagonists on Periodontal Wound Healing. J. Immunol. 2004, 173, 3514–3523. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte-fibroblast interactions in wound healing. J. Investig. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- De Souza, K.S.; Cantaruti, T.A.; Azevedo, G.M.; Galdino, D.A.; Rodrigues, C.M.; Costa, R.A.; Vaz, N.M.; Carvalho, C.R. Improved cutaneous wound healing after intraperitoneal injection of alpha-melanocyte-stimulating hormone. Exp. Dermatol. 2015, 24, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, F.; Mendoza-Palomares, C.; Helms, M.; Alam, D.A.; Richert, L.; Arntz, Y.; Rinckenbach, S.; Garnier, F.; Haïkel, Y.; Gangloff, S.C.; et al. Nanostructured assemblies for dental application. ACS Nano 2010, 4, 3277–3287. [Google Scholar] [CrossRef] [PubMed]
- Muffley, L.A.; Zhu, K.Q.; Engrav, L.H.; Gibran, N.S.; Hocking, A.M. Spatial and temporal localization of the melanocortin 1 receptor and its ligand α-melanocyte-stimulating hormone during cutaneous wound repair. J. Histochem. Cytochem. 2011, 59, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Böhm, M.; Luger, T.A.; Tobin, D.J.; García-Borrón, J.C. Melanocortin Receptor Ligands: New Horizons for Skin Biology and Clinical Dermatology. J. Investig. Dermatol. 2006, 126, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Haycock, J.W.; Rowe, S.J.; Cartledge, S.; Wyatt, A.; Ghanem, G.; Morandini, R.; Rennie, I.G.; MacNeil, S. α-Melanocyte-stimulating Hormone Reduces Impact of Proinflammatory Cytokine and Peroxide-generated Oxidative Stress on Keratinocyte and Melanoma Cell Lines. J. Biol. Chem. 2000, 275, 15629–15636. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.P.; MacNeil, S.; Haycock, J.W. Melanocyte stimulating hormone peptides inhibit TNF-α signaling in human dermal fibroblast cells. Peptides 2006, 27, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Ferrand, A.; Eap, S.; Richert, L.; Lemoine, S.; Kalaskar, D.; Demoustier-Champagne, S.; Atmani, H.; Mély, Y.; Fioretti, F.; Schlatter, G.; et al. Osteogenetic Properties of Electrospun Nanofibrous PCL Scaffolds Equipped With Chitosan-Based Nanoreservoirs of Growth Factors. Macromol. Biosci. 2014, 14, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Vaquette, C.; Fan, W.; Xiao, Y.; Hamlet, S.; Hutmacher, D.W.; Ivanovski, S. A biphasic scaffold design combined with cell sheet technology for simultaneous regeneration of alveolar bone/periodontal ligament complex. Biomaterials 2012, 33, 5560–5573. [Google Scholar] [CrossRef] [PubMed]
- Bashutski, J.D.; Wang, H.-L. Periodontal and endodontic regeneration. J. Endod. 2009, 35, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Palomares, C.; Ferrand, A.; Facca, S.; Fioretti, F.; Ladam, G.; Kuchler-Bopp, S.; Regnier, T.; Mainard, D.; Benkirane-Jessel, N. Smart hybrid materials equipped by nanoreservoirs of therapeutics. ACS Nano 2012, 6, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Schultz, P.; Vautier, D.; Richert, L.; Jessel, N.; Haikel, Y.; Schaaf, P.; Voegel, J.-C.; Ogier, J.; Debry, C. Polyelectrolyte multilayers functionalized by a synthetic analogue of an anti-inflammatory peptide, alpha-MSH, for coating a tracheal prosthesis. Biomaterials 2005, 26, 2621–2630. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.; Szabo, M.; Ghanem, G.E.; Morandini, R.; Kemp, E.H.; MacNeil, S.; Haycock, J.W. Inhibition of Tumor Necrosis Factor-α Stimulated NFκB/p65 in Human Keratinocytes by α-Melanocyte Stimulating Hormone and Adrenocorticotropic Hormone Peptides. J. Investig. Dermatol. 2002, 119, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Eves, P.; Haycock, J.; Layton, C.; Wagner, M.; Kemp, H.; Szabo, M.; Morandini, R.; Ghanem, G.; García-Borrón, J.C.; Jiménez-Cervantes, C.; Mac Neil, S. Anti-inflammatory and anti-invasive effects of α-melanocyte-stimulating hormone in human melanoma cells. Br. J. Cancer 2003, 89, 2004–2015. [Google Scholar] [CrossRef] [PubMed]
- Eves, P.C.; MacNeil, S.; Haycock, J.W. α-Melanocyte stimulating hormone, inflammation and human melanoma. Peptides 2006, 27, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Kocgozlu, L.; Elkaim, R.; Tenenbaum, H.; Werner, S. Variable cell responses to P. gingivalis lipopolysaccharide. J. Dent. Res. 2009, 88, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Ara, T.; Kurata, K.; Hirai, K.; Uchihashi, T.; Uematsu, T.; Imamura, Y.; Furusawa, K.; Kurihara, S.; Wang, P.-L. Human gingival fibroblasts are critical in sustaining inflammation in periodontal disease. J. Periodontal Res. 2009, 44, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Herath, T.D.K.; Wang, Y.; Seneviratne, C.J.; Lu, Q.; Darveau, R.P.; Wang, C.-Y.; Jin, L. Porphyromonas gingivalis lipopolysaccharide lipid A heterogeneity differentially modulates the expression of IL-6 and IL-8 in human gingival fibroblasts. J. Clin. Periodontol. 2011, 38, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, A.; Uehara, A.; Iki, K.; Matsushita, K.; Nakamura, R.; Ogawa, T.; Sugawara, S.; Takada, H. Activation of human gingival epithelial cells by cell-surface components of black-pigmented bacteria: augmentation of production of interleukin-8, granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor and expression of intercellular adhesion molecule 1. J. Med. Microbiol. 2002, 51, 27–33. [Google Scholar] [PubMed]
- Spiekstra, S.W.; Breetveld, M.; Rustemeyer, T.; Scheper, R.J.; Gibbs, S. Wound-healing factors secreted by epidermal keratinocytes and dermal fibroblasts in skin substitutes. Wound Repair Regen. 2007, 15, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hori, K.; Ding, J.; Huang, Y.; Kwan, P.; Ladak, A.; Tredget, E.E. Toll-like receptors expressed by dermal fibroblasts contribute to hypertrophic scarring. J. Cell. Physiol. 2011, 226, 1265–1273. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, G.; Paoletti, I.; Buommino, E.; Antonietta Tufano, M.; Baroni, A. α-MSH reduces the internalization of Staphylococcus aureus and down-regulates HSP 70, integrins and cytokine expression in human keratinocyte cell lines. Exp. Dermatol. 2004, 13, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Kasaj, A.; Reichert, C.; Götz, H.; Röhrig, B.; Smeets, R.; Willershausen, B. In vitro evaluation of various bioabsorbable and nonresorbable barrier membranes for guided tissue regeneration. Head Face Med. 2008, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-I.; Wang, Y. Cell Responses to Surface and Architecture of Tissue Engineering Scaffolds. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; Eberli, D., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Gümüşderelioğlu, M.; Betül Kaya, F.; Beşkardeş, I.G. Comparison of epithelial and fibroblastic cell behavior on nano/micro-topographic PCL membranes produced by crystallinity control. J. Colloid Interface Sci. 2011, 358, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Daubiné, F.; Cortial, D.; Ladam, G.; Atmani, H.; Haïkel, Y.; Voegel, J.-C.; Clézardin, P.; Benkirane-Jessel, N. Nanostructured polyelectrolyte multilayer drug delivery systems for bone metastasis prevention. Biomaterials 2009, 30, 6367–6373. [Google Scholar] [CrossRef] [PubMed]
- Klapperich, C.M.; Bertozzi, C.R. Global gene expression of cells attached to a tissue engineering scaffold. Biomaterials 2004, 25, 5631–5641. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Sun, L.; Cairns, D.M.; Rainbow, R.S.; Preda, R.C.; Kaplan, D.L.; Zeng, L. The influence of scaffold material on chondrocytes in inflammatory conditions. Acta Biomater. 2013, 9, 6563–6575. [Google Scholar] [CrossRef] [PubMed]
- Krisanaprakornkit, S.; Kimball, J.R.; Weinberg, A.; Darveau, R.P.; Bainbridge, B.W.; Dale, B.A. Inducible Expression of Human?-Defensin 2 by Fusobacterium nucleatum in Oral Epithelial Cells: Multiple Signaling Pathways and Role of Commensal Bacteria in Innate Immunity and the Epithelial Barrier. Infect. Immun. 2000, 68, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morand, D.-N.; Huck, O.; Keller, L.; Jessel, N.; Tenenbaum, H.; Davideau, J.-L. Active Nanofibrous Membrane Effects on Gingival Cell Inflammatory Response. Materials 2015, 8, 7217-7229. https://doi.org/10.3390/ma8105376
Morand D-N, Huck O, Keller L, Jessel N, Tenenbaum H, Davideau J-L. Active Nanofibrous Membrane Effects on Gingival Cell Inflammatory Response. Materials. 2015; 8(10):7217-7229. https://doi.org/10.3390/ma8105376
Chicago/Turabian StyleMorand, David-Nicolas, Olivier Huck, Laetitia Keller, Nadia Jessel, Henri Tenenbaum, and Jean-Luc Davideau. 2015. "Active Nanofibrous Membrane Effects on Gingival Cell Inflammatory Response" Materials 8, no. 10: 7217-7229. https://doi.org/10.3390/ma8105376
APA StyleMorand, D.-N., Huck, O., Keller, L., Jessel, N., Tenenbaum, H., & Davideau, J.-L. (2015). Active Nanofibrous Membrane Effects on Gingival Cell Inflammatory Response. Materials, 8(10), 7217-7229. https://doi.org/10.3390/ma8105376









