Voltammetric Studies on Gold Electrodes Coated with Chitosan-Containing Layer-by-Layer Films
Abstract
:1. Introduction
2. Experimental Section
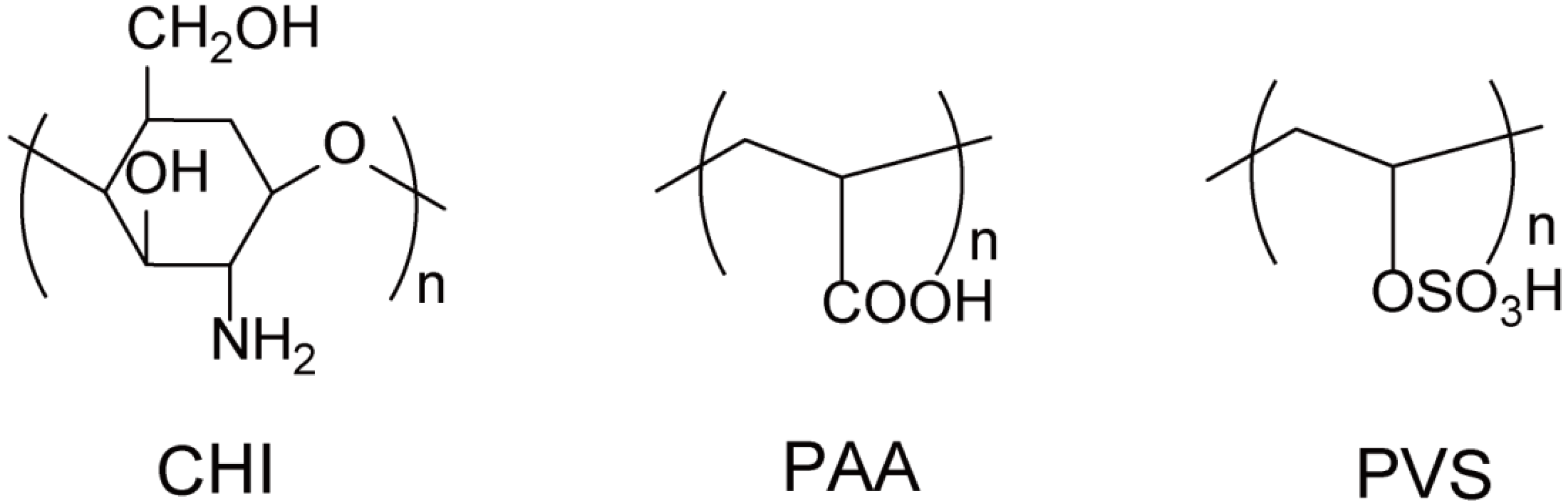
2.1. Materials
2.2. Apparatus
2.3. Preparation of LbL Film-Coated Electrodes
2.4. Gravimetric Analysis of LbL Films
2.5. AFM Imaging
2.6. Electrochemical Measurements
3. Results and Discussion
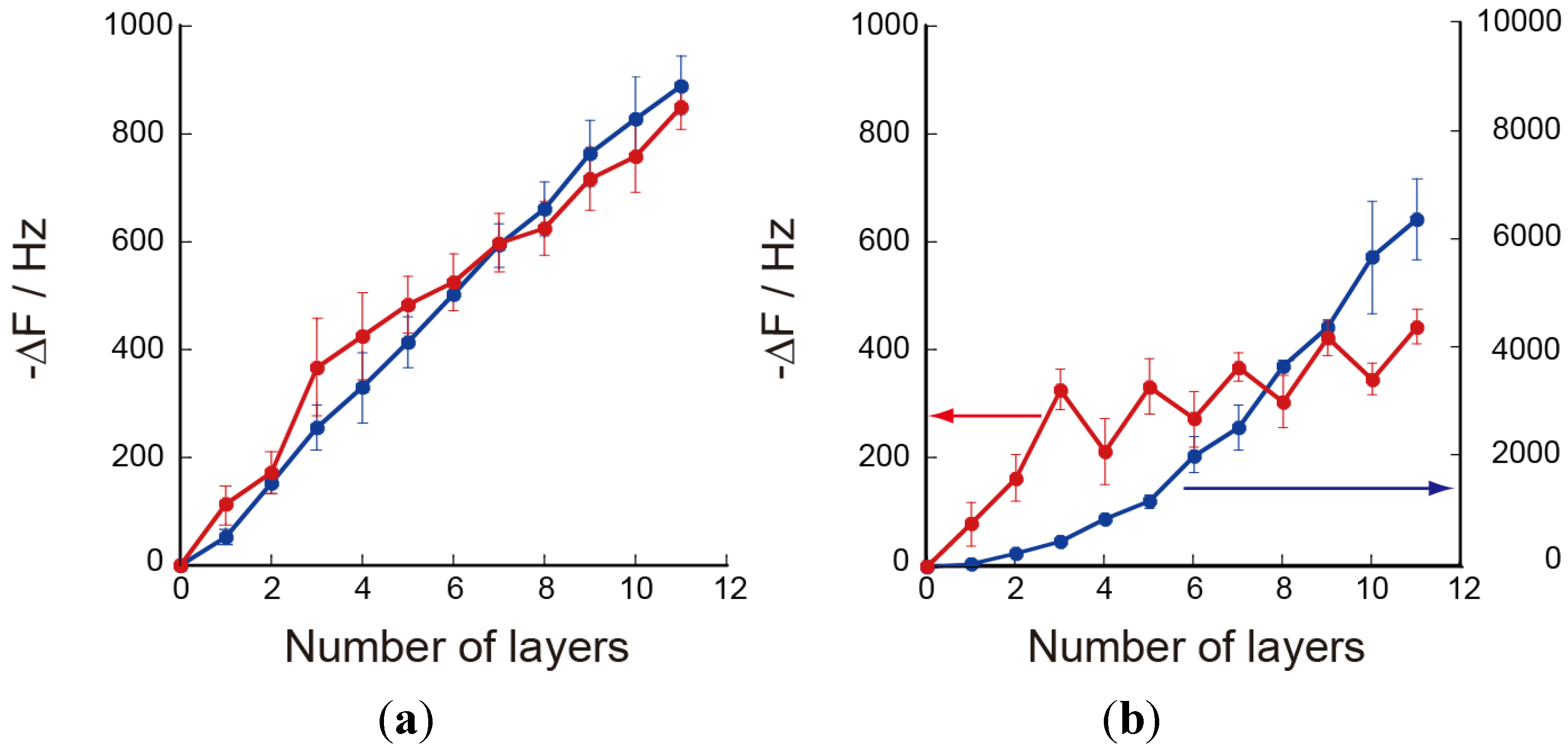
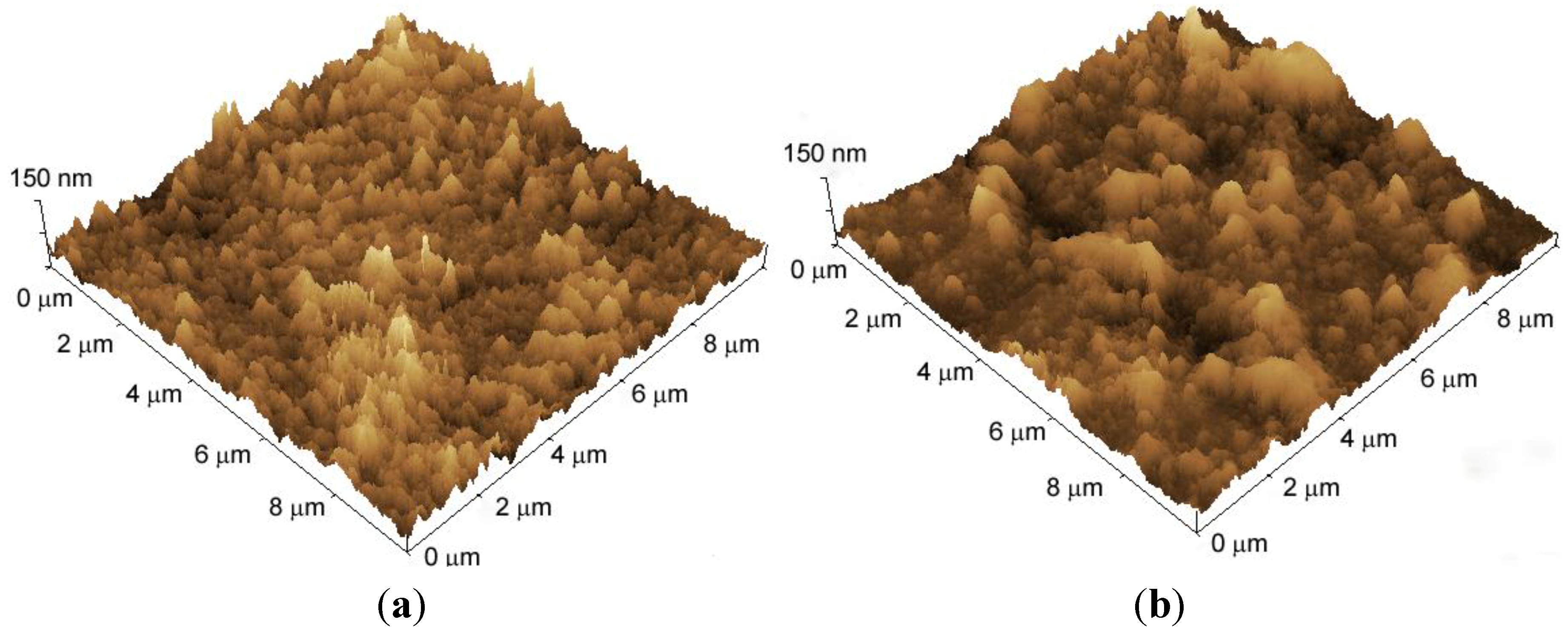
3.1. Preparation of Chitosan-Containing LbL Films
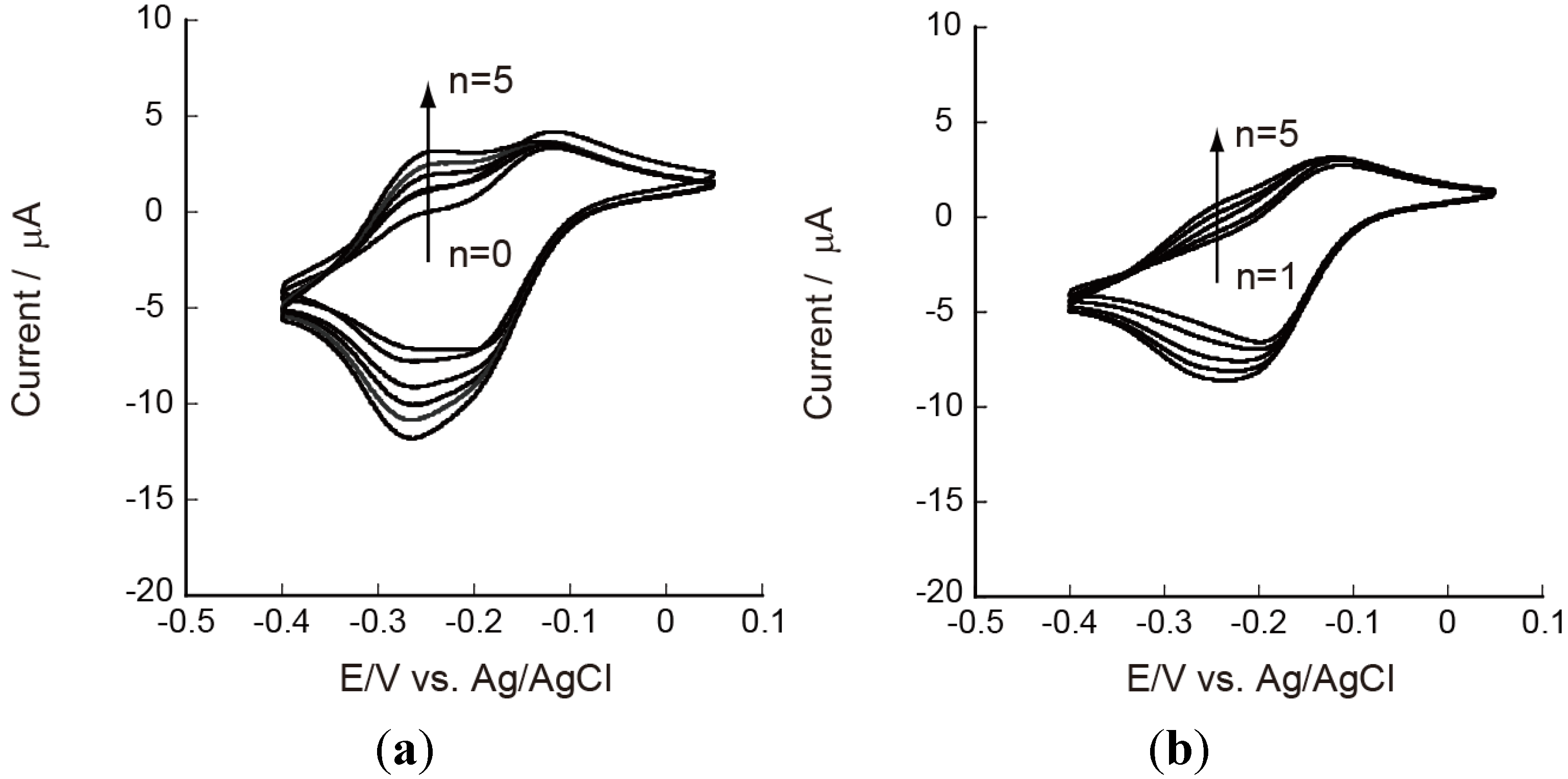
3.2. Redox Reactions of Ru(NH3)63+ and Fe(CN)63− on CHI/PVS and CHI/PAA Film-Modified Electrodes

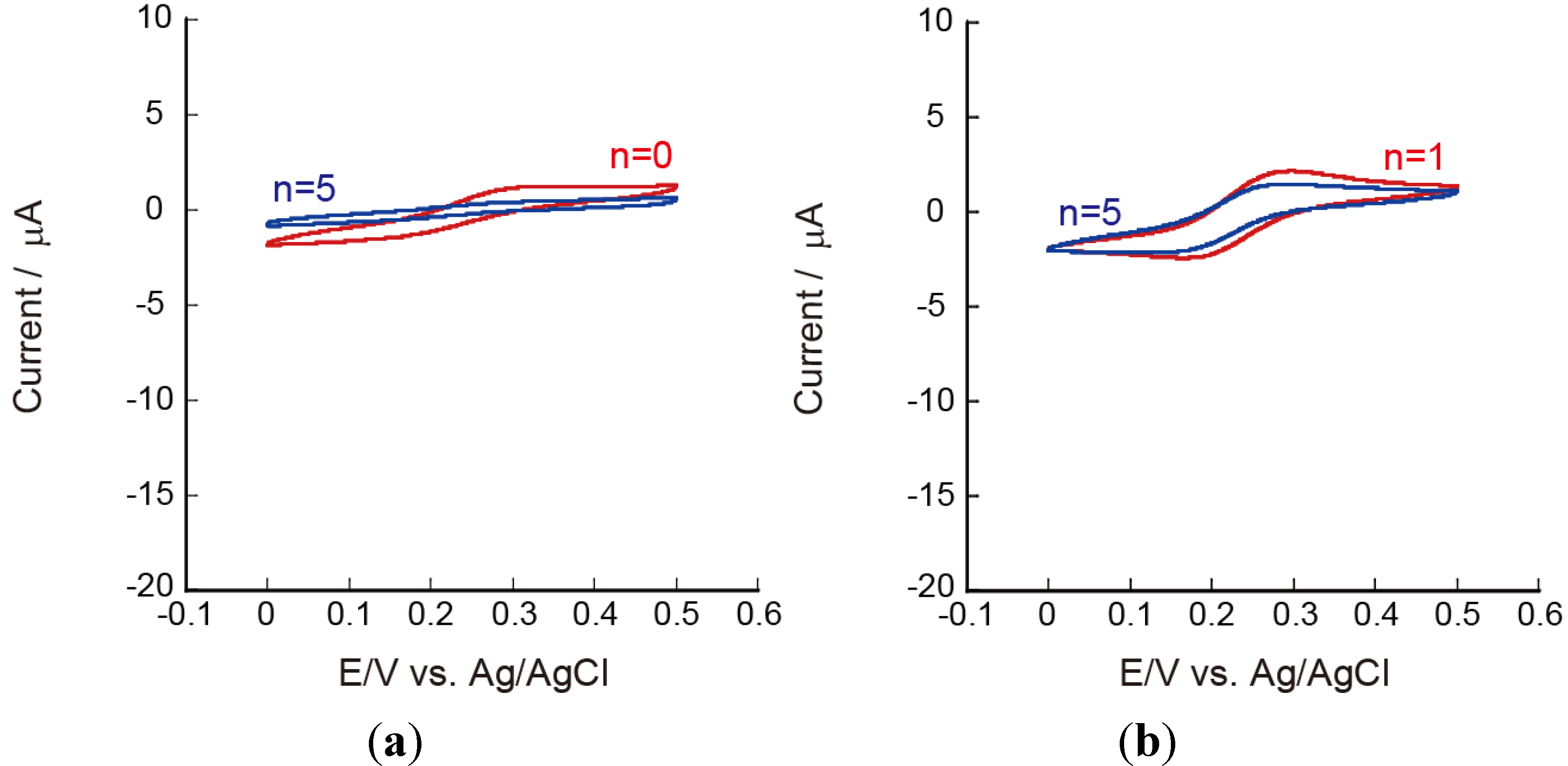

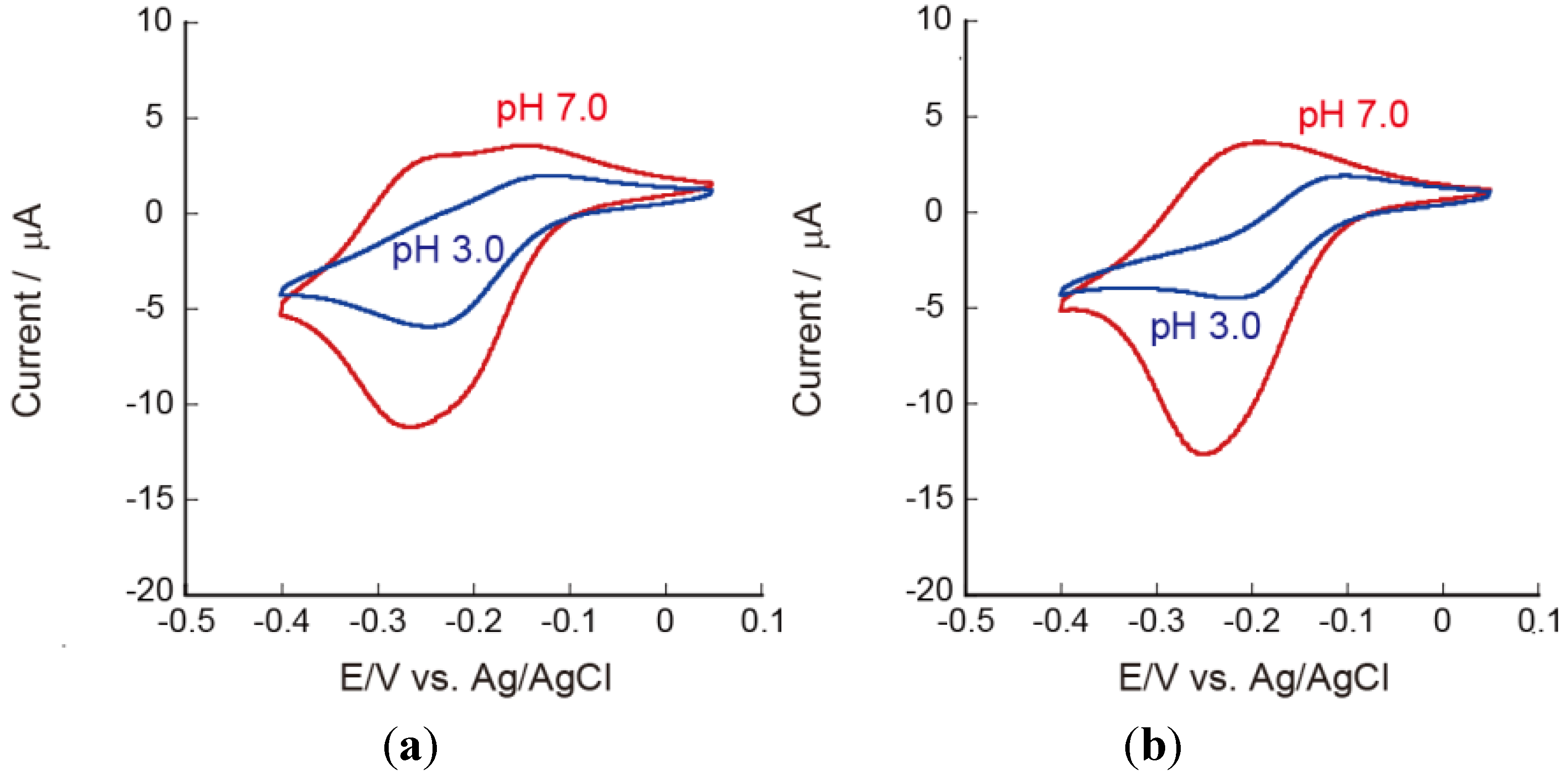
3.3. Effects of pH on the Redox Reactions of Ru(NH3)63+
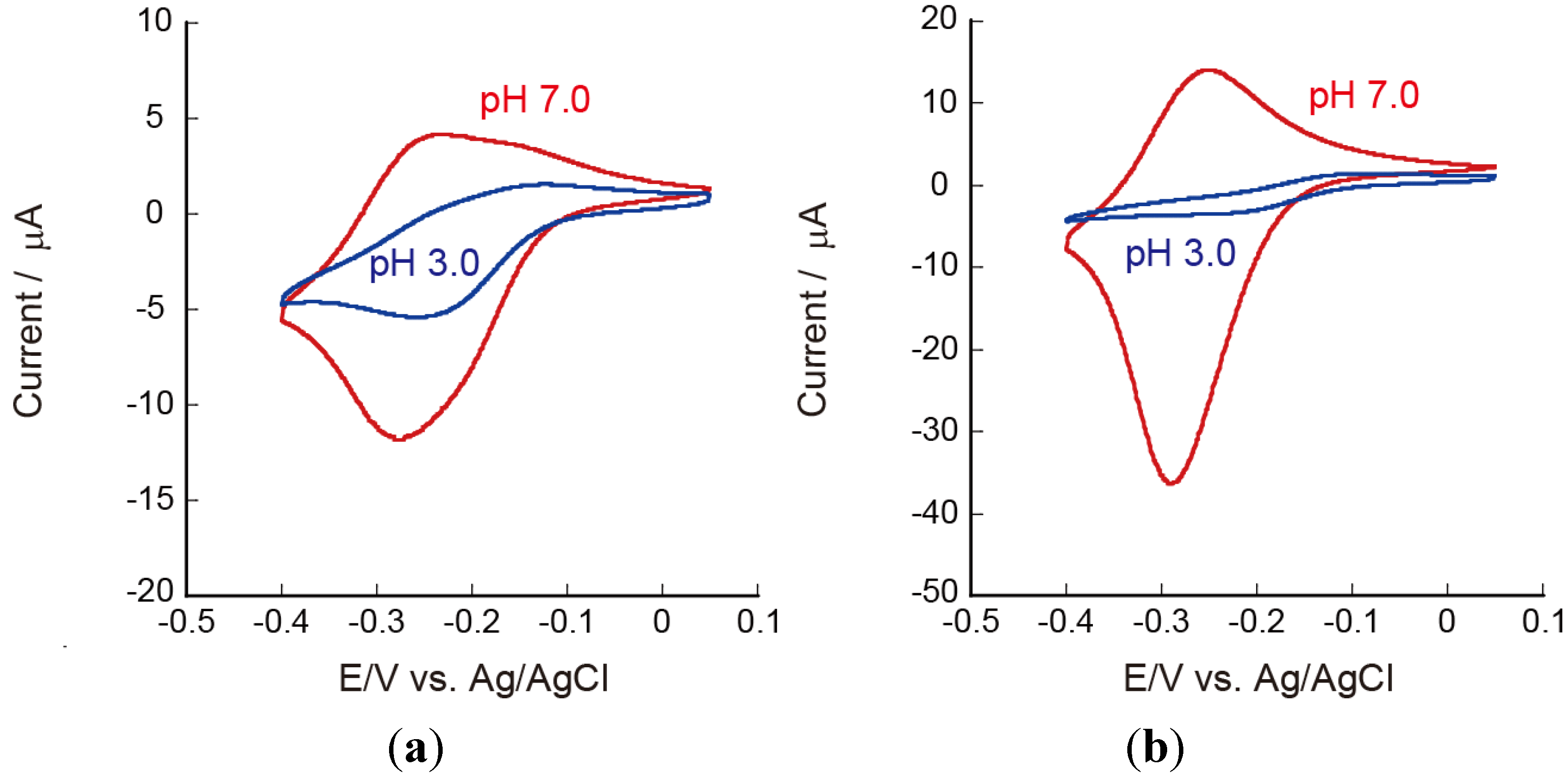
3.4. Stability of Ru(NH3)63+ Confined in LbL Films
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zheng, D.; Vashist, S.K.; Dykas, M.M.; Saha, S.; Al-Rubeaan, K.; Lam, E.; Luong, J.H.T.; Sheu, F.S. Graphene versus multi-walled carbon nanotubes for electrochemical glucose biosensing. Materials 2013, 6, 1011–1027. [Google Scholar] [CrossRef]
- Janssen, K.P.F.; Knez, K.; Spasic, D.; Lammertyn, J. Nucleic acid for ultra-sensitive protein detection. Sensors 2013, 13, 1353–1384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hasebe, Y. Uricase-adsorbed carbon-felt reactor coupled with a peroxidase-modified carbon-felt-based H2O2 detector for highly sensitive amperometric flow determination of uric acid. J. Pharmaceut. Biomed. Anal. 2012, 57, 125–132. [Google Scholar] [CrossRef]
- Lvov, Y.; Decher, G.; Möhwald, H. Assembly, structural characterization, and thermal behavior of layer-by-layer deposited ultrathin films of poly(vinyl sulfate) and poly(allylamine). Langmuir 1993, 9, 481–486. [Google Scholar] [CrossRef]
- Shiratori, S.S.; Rubner, M.F. pH-dependent thickness behavior of sequentially adsorbed layers of weak polyelectrolytes. Macromolecules 2000, 33, 4213–4219. [Google Scholar] [CrossRef]
- Suzuki, I.; Egawa, Y.; Mizukawa, Y.; Hoshi, T.; Anzai, J. Construction of positively-charged layered assemblies assisted by cyclodextrin complexation. Chem. Commun. 2002, 21, 164–165. [Google Scholar] [CrossRef]
- Hoshi, T.; Akase, S.; Anzai, J. Preparation of multilayer thin films containing avidin through sugar-lectin interactions and their binding properties. Langmuir 2002, 18, 7024–7028. [Google Scholar] [CrossRef]
- Vogt, C.; Mertz, D.; Benmlih, K.; Hemmerlé, J.; Voegel, J.-C.; Schaaf, P.; Lavalle, P. Layer-by-layer enzymatic platform for stretched-induced reactive release. ACS Macro Lett. 2012, 21, 797–801. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Q.; Fan, X.R.; Sun, X.J.; Huang, P.H. Layer-by-layer self-assembly immobilization of catalases on wool fabrics. Appl. Biochem. Biotechnol. 2013, 169, 2212–2222. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Lu, Z.; Lvov, Y.M.; Desamero, R.Z.B.; Frank, H.A.; Rusling, J.F. Direct electrochemistry of cofactor redox sites in a bacterial photosynthetic reaction center protein. J. Am. Chem. Soc. 1998, 120, 7371–7372. [Google Scholar] [CrossRef]
- Lvov, Y. Electrostatic layer-by-layer assembly of proteins and polyions. In Protein Architectures: Inter Facial Molecular Assemblies and Immobilization Biotechnology; Lvov, Y., Möhwald, H., Eds.; Marcel Dekker: New York, NY, USA, 1999; pp. 135–137. [Google Scholar]
- Sato, K.; Suzuki, I.; Anzai, J. Preparation of polyelectrolyte-layered assemblies containing cyclodextrin and their binding properties. Langmuir 2003, 19, 7406–7412. [Google Scholar] [CrossRef]
- Gribova, V.; Auzey-Velty, R.; Picart, C. Polyelectrolyte multilayer assemblies on materials surfaces: From cell adhesion to tissue engineering. Chem. Mater. 2011, 24, 854–869. [Google Scholar] [CrossRef]
- Zhou, D.; Xiao, H.; Meng, F.; Zhou, S.; Guo, J.; Li, X.; Jing, X.; Huang, Y. Layer-by-layer assembled polypeptide capsules for platinum-based pro-drug delivery. Bioconjugate Chem. 2012, 23, 2335–2343. [Google Scholar] [CrossRef]
- Liu, A.; Anzai, J. Ferrocene-containing polyelectrolyte multilayer films: Effects of electrochemically inactive surface layers on the redox properties. Langmuir 2003, 19, 4043–4046. [Google Scholar] [CrossRef]
- Takahashi, S.; Sato, K.; Anzai, J. Layer-by-layer construction of protein architectures through avidin-biotin and lectin-sugar interactions for biosensor applications. Anal. Bioanal. Chem. 2012, 402, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhou, J.; Okezaki, Y.; Suye, S. Construction of L-lysine sensor by layer-by-layer adsorption of L-lysine 6-dehydrogenase and ferrocene-labeled high molecular weight coenzyme derivative on gold electrode. Electroanalysis 2008, 20, 2685–2691. [Google Scholar] [CrossRef]
- Sato, K.; Kodama, D.; Naka, Y.; Anzai, J. Electrochemically induced disintegration of layer-by-layer-assembled thin films composed of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Biomacromolecules 2006, 7, 3302–3305. [Google Scholar] [CrossRef] [PubMed]
- Sukhishvili, S.A. Responsive polymer films and capsules via layer-by-layer assembly. Curr. Opin. Colloid Interf. Sci. 2005, 10, 37–44. [Google Scholar] [CrossRef]
- Inoue, H.; Anzai, J. Stimuli-sensitive thin films prepared by a layer-by-layer deposition of 2-iminobiotin-labeled poly(ethyleneimine) and avidin. Langmuir 2005, 21, 8354–8359. [Google Scholar] [CrossRef]
- Wood, K.C.; Zacharie, N.S.; Schmidt, D.J.; Wrightman, S.N.; Andaya, B.J.; Hammond, P.T. Electroactive controlled release thin films. Proc. Natl. Acad. Sci. USA 2008, 105, 2280–2285. [Google Scholar] [CrossRef] [PubMed]
- Esser-Kahn, A.P.; Odom, S.A.; Scotts, N.R.; White, S.R.; Moore, J.S. Triggered release from polymer capsules. Macrommolecules 2011, 44, 5539–5553. [Google Scholar] [CrossRef]
- Manna, U.; Patil, S. Glucose-triggered drug delivery from boronate mediated layer-by-layer self-assembly. ACS Appl. Mater. Interf. 2010, 2, 1521–1527. [Google Scholar] [CrossRef]
- Sato, K.; Takahashi, S.; Anzai, J. Layer-by-layer thin films and microcapsules for biosensors and controlled release. Anal. Sci. 2012, 28, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Anzai, J. Redox properties of the ferricyanide ion on electrodes coated with layer-by-layer thin films composed of polysaccharide and poly(allylamine). Langmuir 2006, 22, 2870–2875. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Anzai, J. Redox reactions of ferricyanide ions in layer-by-layer deposited polysaccharide films: A significant effect of the type of polycation in the films. Langmuir 2007, 23, 7378–7384. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Noguchi, T.; Anzai, J. Layer-by-layer thin film-coated electrodes for electrocatalytic determination of ascorbic acid. Talanta 2007, 72, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E. Chitosan: A versatile biomaterial. Adv. Exp. Med. Biol. 2004, 553, 59–68. [Google Scholar] [PubMed]
- Lu, H.; Hu, N. Loading behavior of {chitosan/hyaluronic acid}n layer-by-layer assembly films toward myoglobin: and electrochemical study. J. Phys. Chem. B 2006, 110, 23710–23718. [Google Scholar] [CrossRef] [PubMed]
- Lundin, M.; Blomberg, E.; Tilton, R.D. Polymer dynamics in layer-by-layer assemblies of chitosan and heparin. Langmuir 2010, 26, 3242–3251. [Google Scholar] [CrossRef] [PubMed]
- Crouzier, T.; Boudou, T.; Picart, C. Polysaccharide-based polyelectrolyte multilayers. Curr. Opin. Colloid Interf. Sci. 2010, 15, 417–426. [Google Scholar] [CrossRef]
- Khopade, A.J.; Caruso, F. Investigation of the factors influencing the formation of dendrimer/polyanion multilayer films. Langmuir 2002, 18, 7669–7676. [Google Scholar] [CrossRef]
- Lvov, Y.; Ariga, K.; Ichinose, I.; Kunitake, T. Assembly of multicomponent protein films by means of electrostatic layer-by-layer adsorption. J. Am. Chem. Soc. 1995, 117, 6117–6123. [Google Scholar] [CrossRef]
- Lvov, Y.; Ariga, K.; Onda, M.; Ichinose, I.; Kunitake, T. A careful examination of the adsorption step in the alternate layer-by-layer assembly of linear polyanion and polycations. Colloid Surf. A 1999, 146, 337–346. [Google Scholar] [CrossRef]
- Rodahl, M.; Höök, F.; Fredriksson, C.; Keller, C.A.; Krozer, A.; Brzezinski, P.; Voinova, M.; Kasemo, B. Simultaneous frequency and dissipation factor QCM measurements of biomolecular adsorption and cell adhesion. Faraday Discuss. 1997, 107, 229–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, K.; Sun, D.; Xia, X.; Chen, H. Potentiodynamic deposition of Prussian blue from a solution containing single component of ferricyanide and its mechanism investigation. J. Solid State Electrochem. 2003, 7, 561–566. [Google Scholar] [CrossRef]
- Valota, A.T.; Kinloch, I.A.; Novoselov, K.S.; Casiraghi, C.; Eckmann, A.; Hill, E.W.; Dryfe, R.A.W. Electrochemical behavior of monolayer and bilayer grapheme. ACS Nano 2011, 5, 8809–8815. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Anzai, J. Phenylboronic acid monolayer-modified electrodes sensitive to sugars. Langmuir 2005, 21, 5102–5107. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guan, H.; Dai, H.; Tong, Y.; Zhao, X.; Qi, W.; Majeed, S.; Xu, G. An amperometric sensor for the determination of benzophenone in food packaging materials based on the electropolymerized molecular imprinted poly-o-phenylenediamine film. Talanta 2012, 99, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Khramov, A.N.; Munos, J.; Collinson, M.M. Preparation and characterization of macroporous silicate films. Langmuir 2001, 17, 8112–8117. [Google Scholar] [CrossRef]
- Trouillon, R.; Combs, Z.; Patel, B.A.; O’Hare, D. Comparative study of the effect of various electrode membranes on biofouling and electrochemical measurements. Electrochem. Commun. 2009, 11, 1409–1413. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Takahashi, S.; Watahiki, R.; Tomida, K.; Wang, B.; Anzai, J.-i. Voltammetric Studies on Gold Electrodes Coated with Chitosan-Containing Layer-by-Layer Films. Materials 2013, 6, 5427-5439. https://doi.org/10.3390/ma6115427
Takahashi S, Watahiki R, Tomida K, Wang B, Anzai J-i. Voltammetric Studies on Gold Electrodes Coated with Chitosan-Containing Layer-by-Layer Films. Materials. 2013; 6(11):5427-5439. https://doi.org/10.3390/ma6115427
Chicago/Turabian StyleTakahashi, Shigehiro, Ryota Watahiki, Kohji Tomida, Baozhen Wang, and Jun-ichi Anzai. 2013. "Voltammetric Studies on Gold Electrodes Coated with Chitosan-Containing Layer-by-Layer Films" Materials 6, no. 11: 5427-5439. https://doi.org/10.3390/ma6115427
APA StyleTakahashi, S., Watahiki, R., Tomida, K., Wang, B., & Anzai, J.-i. (2013). Voltammetric Studies on Gold Electrodes Coated with Chitosan-Containing Layer-by-Layer Films. Materials, 6(11), 5427-5439. https://doi.org/10.3390/ma6115427



