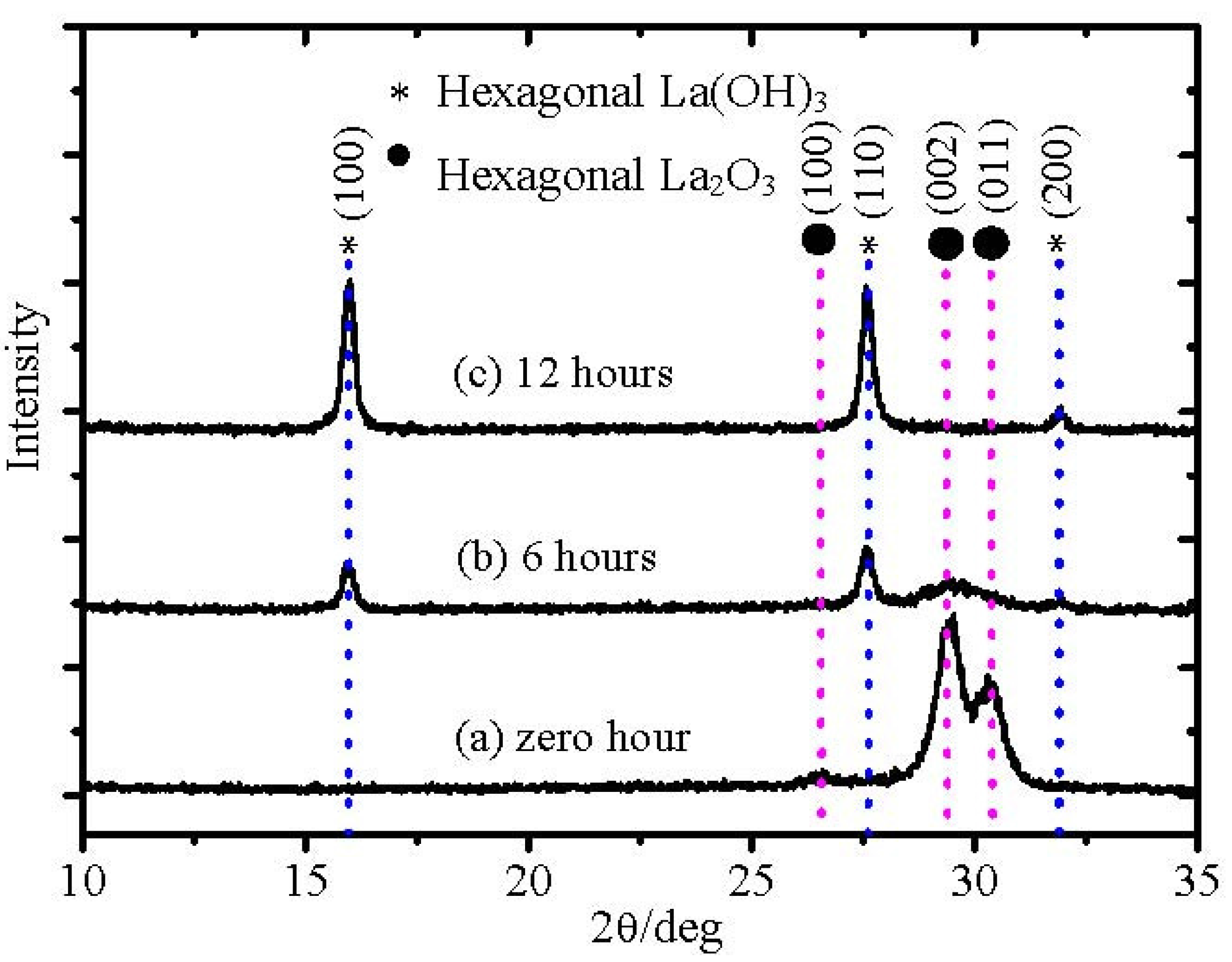
As discussed earlier, it has been concluded that the amount of hexagonal La(OH)
3 in the La
2O
3 film increases with time exposed to air. Although there is no report about the permittivity of hexagonal La(OH)
3, we can estimate the permittivity of hexagonal La(OH)
3 on the basis of an additivity rule of the polarizability from Shannon’s consideration [
7]. From the
Clausius-Mossotti relationship, the dielectric constant is described by:
where V
m and α
T denote molar volume and total polarizability respectively. For hexagonal La(OH)
3, α
T is 12.81 Å
3 from Shannon’s additivity rule [
7], (α
T(La(OH)
3) = α(La
3+) + 3α(OH
−)) and V
m is 71 Å
3 from Reference [
8]. With the above values, we can estimate the permittivity of hexagonal La(OH)
3 which is about 10. This result indicates that hexagonal La(OH)
3 has a much lower permittivity compared to La
2O
3. Therefore, the effective permittivity of La
2O
3 film exposed to air could be degraded. In fact, with time exposed to air,
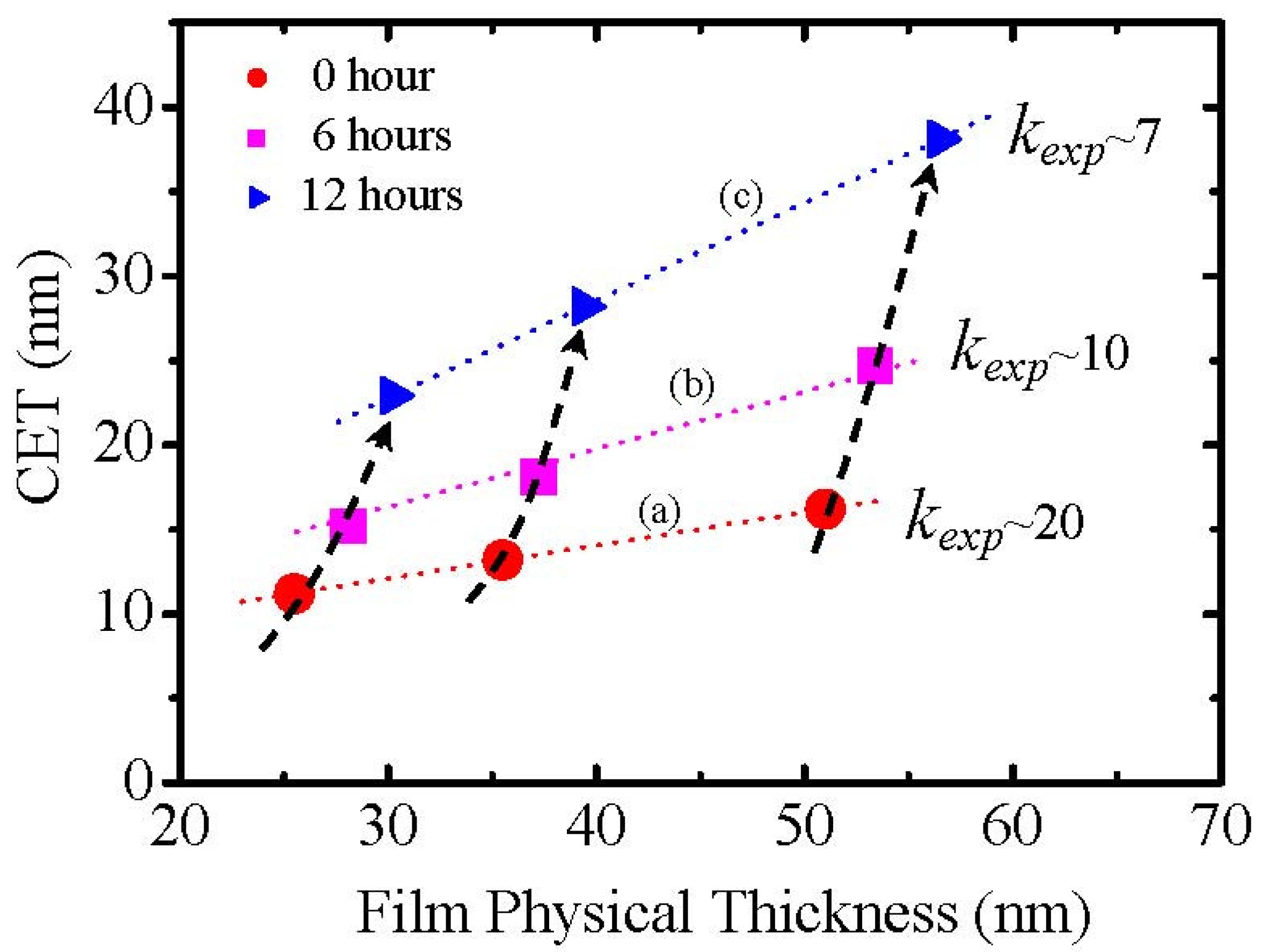
Figure 3 shows the degradation of
kexp (
k-value obtained experimentally from the slope), though it is necessary to take account of an inhomogeneity of the film due to the partial reaction of the La
2O
3 with moisture. Therefore, the moisture absorption which causes the formation of low permittivity lanthanum hydroxide should be a very possible reason for scattering of the permittivity value of La
2O
3 films in previous literatures [
4,
5,
6], although details of the process are not mentioned in the literature.
From
Figure 1, it could be concluded that with time exposed to air, the amount of hexagonal La(OH)
3 in La
2O
3 film increases and then the density of the film is degraded. Therefore, the effect of moisture absorption on the surface roughness should be another concern of hygroscopic La
2O
3 film application. According to the above discussion, it seems that the reported low permittivity of the La
2O
3 can be attributed to moisture absorption phenomena. However, we also have to note that the permittivity of La
2O
3 film in air for 0 hour was still a little low, about 20. This value is much lower than the reported highest one, 27 [
9], although the possibility of moisture absorption still cannot be excluded, because the sample was exposed to the air. To exclude the effect on the permittivity of La
2O
3 films and obtain the permittivity of La
2O
3 films without moisture absorption, we used the
in-situ heating method in a high vacuum chamber. The La
2O
3 film was annealed at 400 °C in the high vacuum (HV) chamber (10
−6 Pa) to make lanthanum hydroxide decompose into La
2O
3 and H
2O and then followed by 6 nm SiO
2 layer deposition to prevent moisture absorption after removal from the sputtering chamber for the electrode deposition.
3.1. Hygroscopic Tolerance Enhancement of La2O3 Films
As discussed earlier, the moisture absorption process of La
2O
3 films is related with the formation of the OH ion. In the XRD pattern, peaks of hexagonal La(OH)
3 appeared after exposure to air for 6 hours (
Figure 1). Based on the consideration of possible reactions of the moisture absorption of La
2O
3 films, one very possible mechanism is the intrinsic reaction of La
2O
3 and H
2O.
Due to the high ionicity of La
2O
3, it can react with H
2O directly as per the following Equations:
This moisture absorption progress is mainly due to the small lattice energy of La
2O
3 that promotes the reaction [
10]. Lattice Energy (U) is the energy required to completely separate one mole of a solid ionic compound into gaseous ions which indicates the strength of the ionic bonds in an ionic lattice as shown below:
It has been reported that the lattice energy of ionic oxides is inversely proportional to the sum of the metal ion and oxygen ion radius [
11]. In other words, the oxide with a larger metal ion radius shows a smaller lattice energy. In the case for rare earth oxides, because lanthanum ions have the largest radius, La
2O
3 shows the smallest lattice energy within rare earth oxides [
12].
Thus, to enhance the hygroscopic tolerance of La
2O
3 films, it is necessary to enhance the lattice energy of La
2O
3. Furthermore, poorly crystallized film is looser than well crystallized. This makes water easier to diffuse into the film and react with La
2O
3. Therefore, one method to enhance the hygroscopic tolerance is to enhance the crystallinity of La
2O
3 film. As the poor crystallinity is intrinsic to La
2O
3, to enhance the crystallinity of La
2O
3, doping with other elements or oxides is necessary. When we select oxides for doping, we have to consider the lattice energy, and larger lattice energy oxides are preferred. From the phase diagram of the La
2O
3-Y
2O
3 system [
13], a high melting point of La
2−xY
xO
3 can be observed, which indicates a low crystallization temperature of La
2−xY
xO
3. On the other hand, Y
2O
3 shows a much lower crystallization temperature than La
2O
3. It is very possible that La
2−xY
xO
3 films could also exhibit a low crystallization temperature or be very easy to be crystallized [
14]. Furthermore, Y is in the same element group in the elements table as La and is the nearest element to La. It can be expected that La
2−xY
xO
3 might show similar properties as La
2O
3: for example permittivity, large band gap, and so on.
La2−xYxO3 films with different Y atomic concentrations (Y/La + Y = 0%, 10%, 40%, 70%, 90% and 100%) were deposited on the HF-last Si (100) substrates or thick Pt films deposited on SiO2/Si substrates by RF co-sputtering of La2O3 and Y2O3 targets (provided by Kojundo Chemical, Saitama, Japan) in Ar ambient at room temperature and then annealed at 600 °C in pure N2 or 0.1%-O2+N2 ambient for 30 seconds in a rapid thermal annealing (RTA) furnace. The Y concentrations were determined by x-ray photoelectron spectroscopy (XPS) measurement. Moisture absorption experiments were performed in room air. The temperature and relative humidity of the air was 25 °C and 25% respectively. The XRD patterns of films before and after the moisture absorption were investigated. The MIM (metal-insulator-metal) capacitors on thick Pt films deposited on SiO2/Si substrates were prepared by depositing the Au film on the La2−xYxO3 films to evaluate the permittivities. Au was also deposited on some La2O3 and La2−xYxO3 films on silicon to form Au/La2O3 or La2−xYxO3/Si metal insulator semiconductor (MIS) capacitors. The capacitance-voltage (C–V) with a frequency of 100 kHz and the gate current density-gate voltage (J–V) measurements were performed for MIS capacitors. The physical thicknesses of films were determined with grazing incident x-ray reflectivity (GIXR) and spectroscopic ellipsometry (SE) measurements.
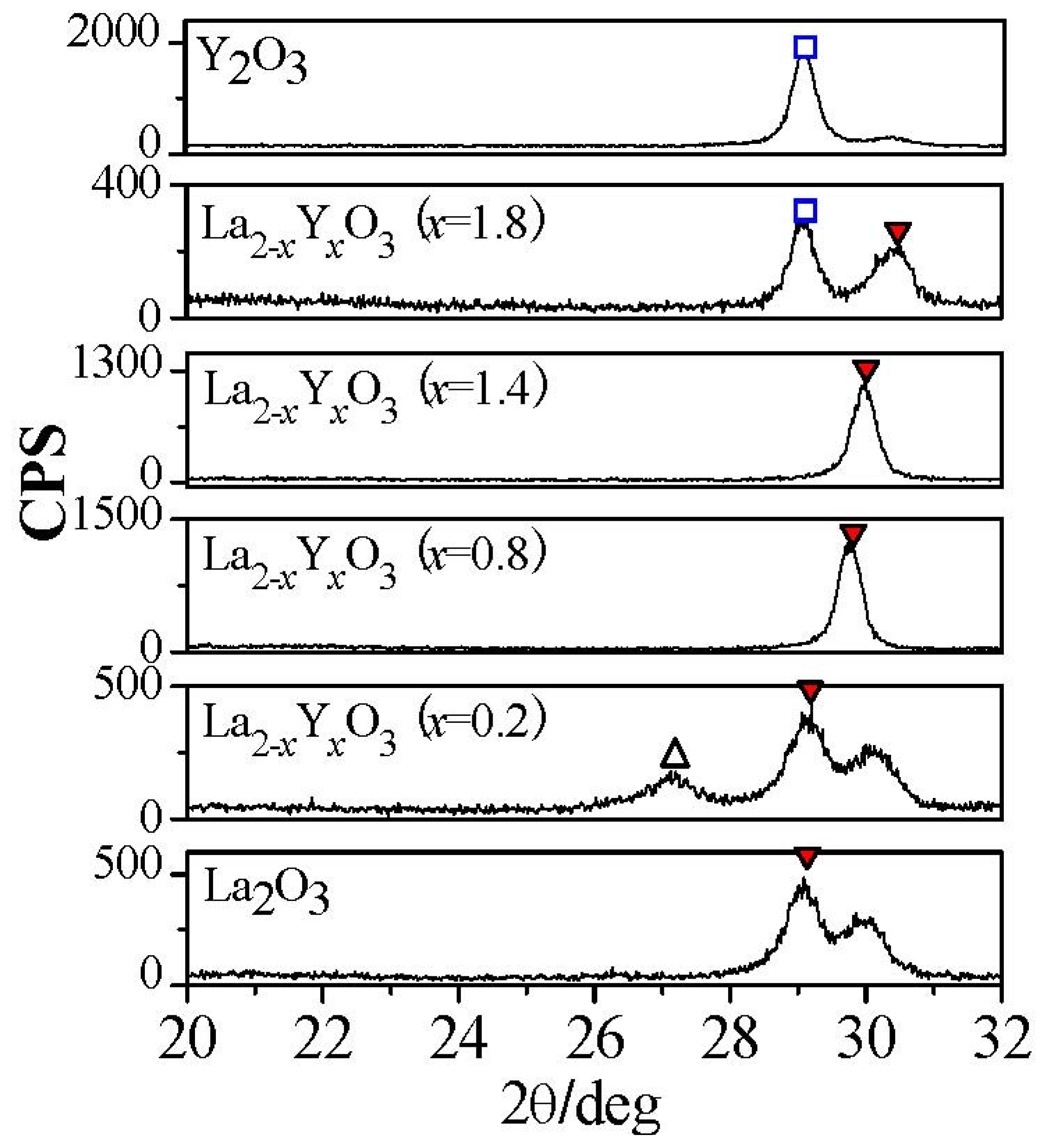
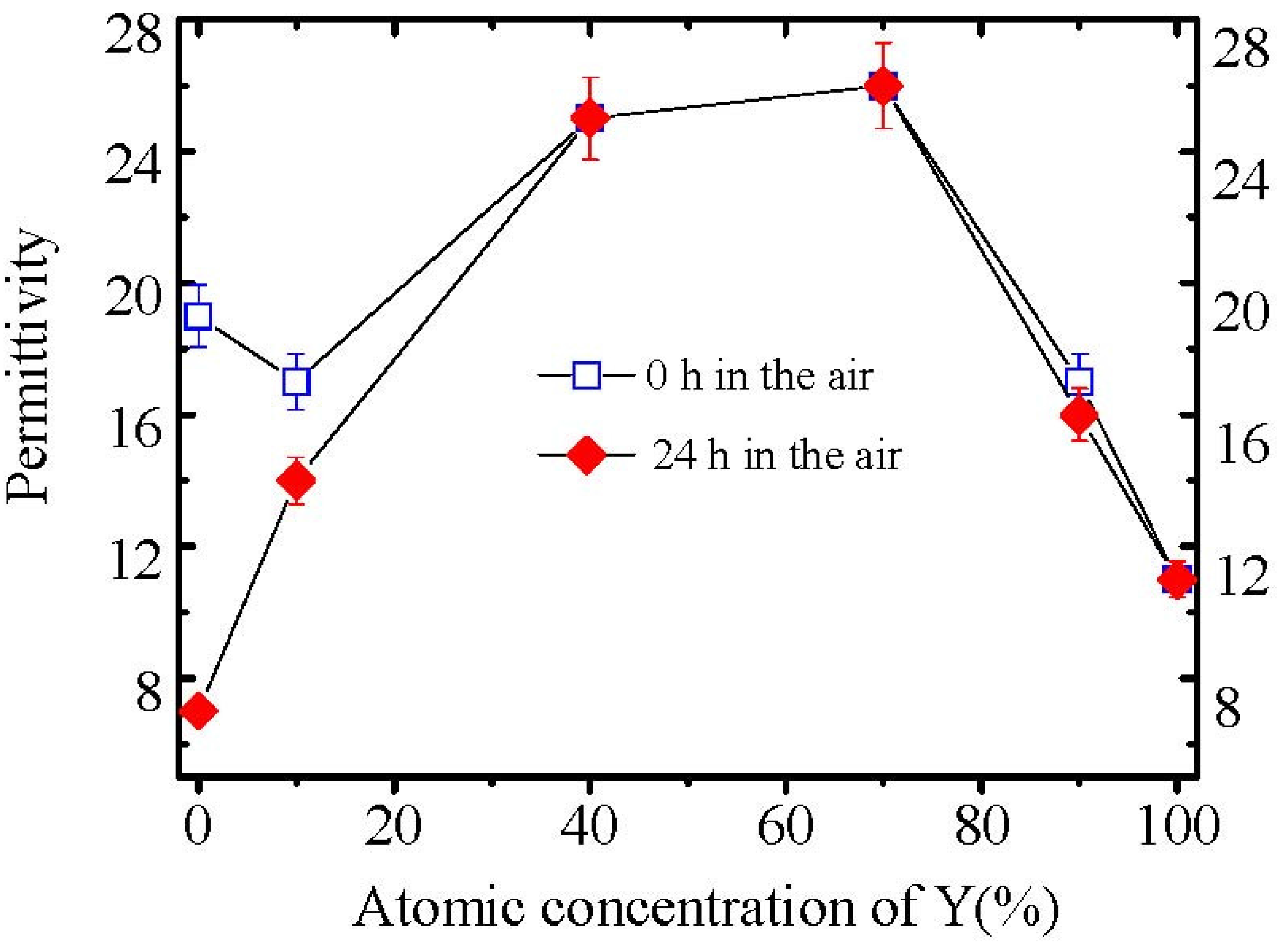
Figure 5 shows the permittivities of all La
2−xY
xO
3 films after exposed to air for 0 and 24 hours. No permittivity degradation of La
2−xY
xO
3 (
x = 0.8), La
2−xY
xO
3 (
x = 1.4), La
2−xY
xO
3 (
x = 1.8) and Y
2O
3 films was observed after films were exposed to air for 24 hours. However, the permittivities of La
2−xY
xO
3 (
x = 0.2) film and La
2O
3 film decrease dramatically after exposure to air for 24 hours, due to the formation of low permittivity hydroxide (
Figure 6). The XRD patterns of all La
2−xY
xO
3 films exposed to air for 24 hours are shown in
Figure 6. The characteristic peaks attributed to hexagonal hydroxide due to the moisture absorption appear in XRD patterns of La
2−xY
xO
3 (
x = 0.2) film and La
2O
3 film, while those are not found in XRD patterns of La
2−xY
xO
3 (
x = 0.8), La
2−xY
xO
3 (
x = 1.4), La
2−xY
xO
3 (
x = 1.8), and Y
2O
3 films. This means that when the Y concentration is higher than, or equal to, 40% (
x = 0.8), the La
2−xY
xO
3 film will exhibit good moisture resistance. From the electrical properties measurements, we can also know the strong moisture-resistance of La
2−xY
xO
3 films. No degradation of C–V characteristics of La
2−xY
xO
3 (
x = 1.4) film is observed after exposed to air for 24 hours. At the same time, the gate leakage current of Au/La
2−xY
xO
3 (
x = 1.4)/Si MIS capacitor shows no apparent increase after exposed to the air for 24 hours. On the contrary, for the La
2O
3 film after exposure to air for 24 hours, the maximum capacitance decrease in the accumulation side of the C–V curve and the flat band shift are observed. The gate leakage current of the Au/La
2O
3/Si MIS capacitor also increased by about two orders of magnitude when the La
2O
3 film was exposed to air for 24 hours before Au deposition.
Figure 5.
Variation of the permittivities of La2−xYxO3 films with Y concentration. The permittivities were determined by MIM capacitors.
Figure 5.
Variation of the permittivities of La2−xYxO3 films with Y concentration. The permittivities were determined by MIM capacitors.
Figure 6.
XRD patterns of La
2−xY
xO
3 films with different Y concentrations after exposure to air for 24 hours. Temperature and relative humidity of the air is 25 °C and 50% respectively. The films were annealed at 600 °C. (
![Materials 05 01413 i001]()
: hydroxide).
Figure 6.
XRD patterns of La
2−xY
xO
3 films with different Y concentrations after exposure to air for 24 hours. Temperature and relative humidity of the air is 25 °C and 50% respectively. The films were annealed at 600 °C. (
![Materials 05 01413 i001]()
: hydroxide).
As discussed earlier, the moisture absorption reaction is intrinsically due to the small lattice energy of La2O3. The larger lattice energy could induce stronger moisture resistance due to the suppression of a reaction between La2O3 and H2O. It is possible that the well crystallized film should exhibit a relatively larger lattice energy than the amorphous or poorly crystallized film. In our experiments, La2O3 had poorer crystallinity (full-width at half-maximum (FWHM) ≈ 1.4 degree) than 40%Y (x = 0.8) and 70%Y (x = 1.4) La2−xYxO3 films (FWHM ≈ 0.4 degree) from the XRD patterns. This indicates that the lattice energy of 40%Y (x = 0.8) and 70%Y (x = 1.4) La2−xYxO3 films might be larger than that of La2O3, thanks to better crystallinity. Furthermore, Y2O3 exhibits a much larger lattice energy of 158.47 eV/mol than that of La2O3 (146.83 eV/mol). Therefore, Y2O3 doping could effectively enhance the lattice energy of La2O3. Furthermore, the lattice energy is related to the crystal forms of the film. One thing we should note is that 40%Y (x = 0.8) and 70%Y (x = 1.4) La2−xYxO3 films also show a much higher permittivity (~26) than La2O3 film in our study. The high permittivities are due to the formation of high permittivity hexagonal phase of La2−xYxO3 films with very good crystallinity after the annealing. The permittivity of lanthanum based oxides will be discussed in more details in the later paragraphs. These results indicate that La2−xYxO3 films not only show strong moisture resistance, also show a high permittivity when the Y concentration is between 40% (x = 0.8) and 70% (x = 1.4).
Therefore, due to the introduction of Y2O3, 40%Y (x = 0.8) and 70%Y (x = 1.4) La2−xYxO3 films after annealed at 600 °C exhibit much larger lattice energy than La2O3 film which induces stronger hygroscopic tolerance of La2−xYxO3 films. The results also indicate that phase control is an effective method to enhance the moisture-robustness of La2O3 films.
To further understand the mechanism for enhancing moisture resistance via second oxide doping, thermodynamic analysis of moisture absorption phenomena in high-
k gate dielectrics has been performed [
15]. Intrinsically, the moisture absorption phenomenon in high-
k oxides is the reaction between the solid oxide (
MmOn) film and gaseous state water (
H2O) in air, which can be expressed by Equation (5) as discussed above.
It is well known that the rate of a chemical reaction can be indexed by the Gibbs free energy change, Δ
G, of the reaction, which is given by Equation (6) [
16].
where, Δ
H is the enthalpy change of the reaction, Δ
S is the entropy change of the reaction, and
T is the ambient temperature. Both of Δ
S and Δ
H are calculated by subtracting the sum (entropy or enthalpy) of the left side of the reaction equation to that of the right side of the reaction equation. The entropy and enthalpy data of
H2O,
M(
OH) and
MmOn were obtained from the database of HSC Chemistry software [
17] and Reference [
18] (only for Hf(OH)
4). The negative Δ
G, meaning the decrease in system energy after the reaction, indicates a possibility for the occurrence of the reaction. Furthermore, when Δ
G is negative, a larger absolute value of Δ
G means a larger reaction rate. However, note that in the real case of high-
k oxide films, the reaction rate is influenced by many other factors. In our study, we focused on the thermodynamic process of the moisture absorption reaction, which could be the main factor for determining the reaction rate.
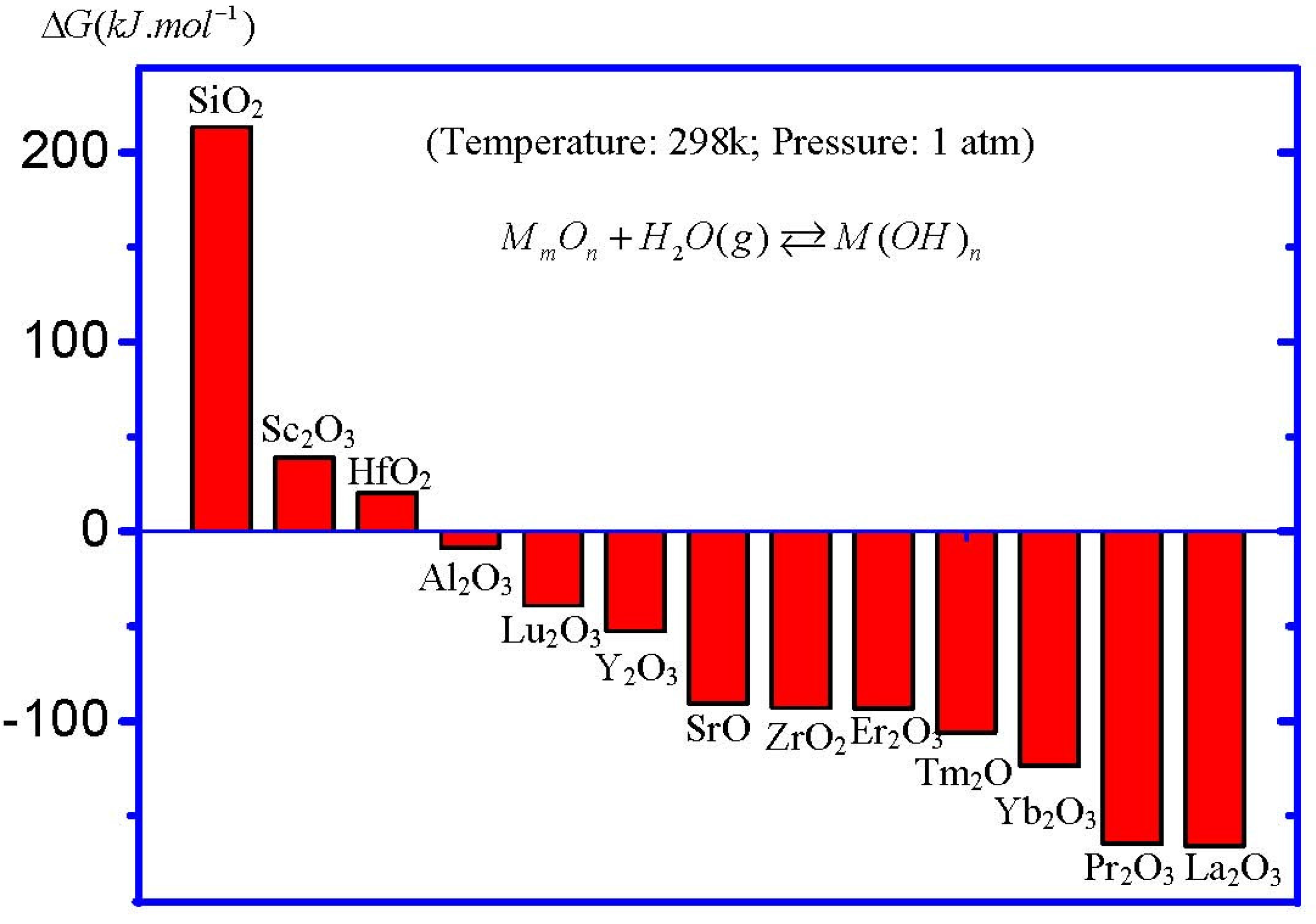
Figure 7 shows the calculated Δ
G of the moisture absorption reactions of main high-
k oxide candidates. For the purpose of comparison, the data of the reaction between SiO
2 and H
2O is also included in the figure. It can be obviously observed that, under standard conditions (temperature = 298.15 K, pressure = 1 atm), the moisture absorption reaction in SiO
2 could not occur, since the Δ
G of the reaction is positive. This fact is the chemical reason for the stable SiO
2 film in the air as a gate oxide. On the other hand, a large range of Δ
G values in high-
k oxides, indicating different moisture-absorption-reaction rates, could be observed. Hafnium oxide (HfO
2), the most studied high-
k gate dielectric so far, shows a positive Δ
G, meaning a small moisture-absorption-reaction rate. This result is coincident with the experimental results, since there have been few reports about the moisture absorption phenomenon in HfO
2. On the contrary, note that zirconium oxide (ZrO
2), which is also thought to be a promising high
k oxide, shows a large negative Δ
G. However, the moisture absorption phenomenon in ZrO
2 film as a high
k gate dielectric has not been emphasized in the literature yet. As a matter of fact, the formation of zirconium hydroxide at the surface of ZrO
2 film has been reported [
19]. Furthermore, La
2O
3 shows the most negative Δ
G among all main high-
k oxide candidates. This is the reason for the serious moisture absorption phenomenon in La
2O
3 films. This fact also suggests that the moisture absorption phenomenon in La
2O
3 films is the intrinsic property of La
2O
3, rather than caused by some external factors. On the other hand, it can be found from
Figure 7 that all rare earth oxides show a large moisture-absorption-reaction rate, except for scandium oxide (Sc
2O
3), meaning that most pure rare earth oxides might not be suitable as high-
k gate oxides, although they usually show high permittivities.
Figure 7.
Δ
G of the moisture absorption reactions in high-
k oxides under standard conditions. All entropy and enthalpy data of oxides, H
2O and hydroxides were obtained from the database of HSC chemistry software, except for Hf(OH)
4, which is cited from Reference [
18].
Figure 7.
Δ
G of the moisture absorption reactions in high-
k oxides under standard conditions. All entropy and enthalpy data of oxides, H
2O and hydroxides were obtained from the database of HSC chemistry software, except for Hf(OH)
4, which is cited from Reference [
18].
Next, we discuss how to enhance the moisture resistance of rare earth oxides, especially that of La
2O
3. Through considering the thermodynamic process of the moisture absorption reaction as shown in Equation (5), the most direct method of enhancing the moisture resistance or decreasing the moisture-absorption-reaction rate of an oxide film is doping with a second oxide that exhibits a stronger resistance to moisture absorption. We have observed that Y
2O
3 doped La
2O
3 films show much stronger strong moisture resistance than La
2O
3 [
20,
21], which is a demonstration of this method (
Figure 6).
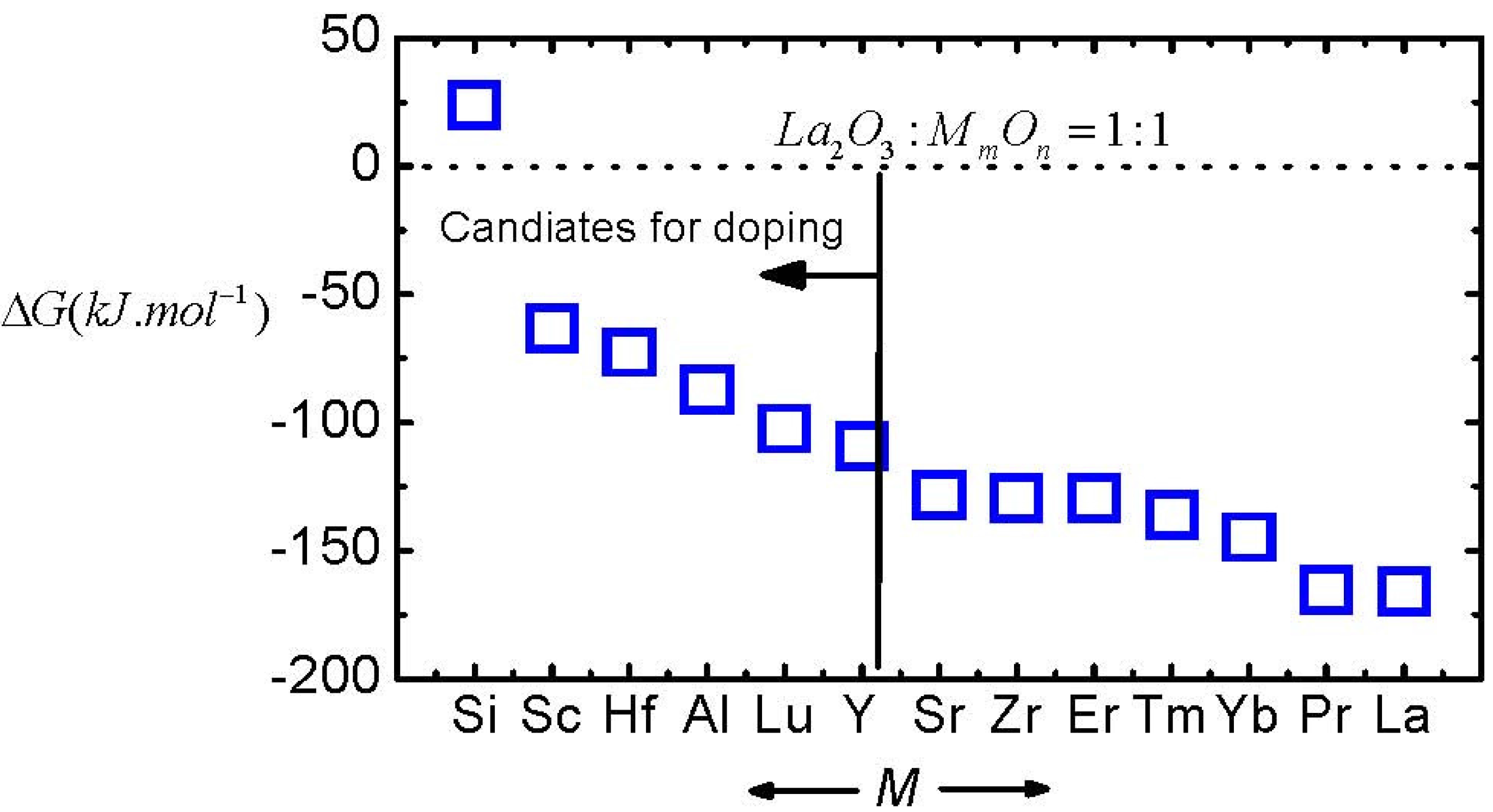
Figure 8 shows the Δ
G of several La-based ternary oxides with a molecule ratio of 1:1 between La
2O
3 and the second oxide, which are simply calculated by averaging the Δ
G of the moisture absorption reaction of La
2O
3 and the second oxides.
Figure 8.
The ΔG of the moisture absorption reactions of La-based ternary oxides, calculated by averaging the ΔG of La2O3 and the second oxide. The molecule ratio between La2O3 and the second oxide in ternary oxides is 1:1.
Figure 8.
The ΔG of the moisture absorption reactions of La-based ternary oxides, calculated by averaging the ΔG of La2O3 and the second oxide. The molecule ratio between La2O3 and the second oxide in ternary oxides is 1:1.
As shown in
Figure 8, doping a second oxide is an effective method for decreasing moisture-absorption-reaction speed. Furthermore, SiO
2, Sc
2O
3, HfO
2, Al
2O
3, Lu
2O
3, and Y
2O
3 are better candidates than other oxides for doping to enhance the moisture resistance of La
2O
3. On the other hand, note that the permittivity of the doped La
2O
3 has to be considered when we select a second oxide. This issue will be discussed further later. Furthermore, the moisture resistance of an oxide film is also affected by several external factors, like the crystallinity [
20] of the film and oxygen vacancies in the film [
22], which will be discussed in more detail later. We can understand these behaviors by a more detailed analysis of the moisture absorption reaction. The reaction in Equation (5) could be divided into three steps as shown in Equations (7), (8) and (9).
The Equations (7) and (8) are the key reactions for determining the rate of the whole moisture absorption reaction. Physically, the reaction rate of Equation (7) is determined by the lattice energy of the oxide, which is mainly determined by the ionicity (or electronegativity) of
M ion and could also be affected by the crystallinity of the oxide in the case of a thin film. The larger electronegativity means a larger ionicity, resulting in a smaller lattice energy and a larger reaction rate of Equation (7). In fact, the Δ
G results in
Figure 7 coincide well with the reported electronegativity data [
23]. On the other hand, the reaction Equation (4) is responsible for the formation of OH
−, resulting in the formation of hydroxide after being combined with
Mn+ (Equation (9)). The oxygen vacancies, however, can also induce the formation of OH
−, which is the reason for the more serious moisture absorption phenomenon in oxygen-deficient La
2O
3 films. However, as shown in
Figure 7 a thermodynamic process could be the main and intrinsic factor for determining the rate of the moisture absorption reaction.
In summary, the moisture absorption phenomena in main high-k gate oxides have been theoretically discussed by comparing the Gibbs free energy change of the moisture absorption reactions of these oxides. The results show that moisture absorption could occur in most high-k oxides, especially in rare earth oxides. On the other hand, La2O3 shows the largest moisture-absorption-reaction speed among main high-k oxide candidates. To enhance the moisture resistance of La2O3, doping a second oxide, which has a stronger moisture resistance than La2O3, could be an applicable solution.
3.2. Hygroscopic Tolerance Enhancement of La2O3 Films by Ultraviolet Ozone Treatment
In our experiments, we found that the oxygen-ambient-annealing La
2O
3 film shows stronger moisture resistance than nitrogen-ambient-annealing La
2O
3 film, although the moisture absorption phenomenon was still observed after being in air for several days. So, it seems that moisture absorption is partly related to oxygen vacancies in the films. In other words, if the oxygen vacancies in La
2O
3 film could be eliminated, moisture resistance could be enhanced to some degree. The most direct method is to eliminate or heal the oxygen vacancy. It has been reported that ultraviolet (UV) ozone treatment at room temperature can eliminate oxygen vacancies in oxide films [
24]. Thus, moisture absorption suppression is expected with UV ozone post treatment, thanks to the healing of oxygen vacancies. The low temperature of UV ozone treatment merits the CMOS process which could prevent the formation of a thick interface layer. The interface layer could enhance the total EOT (Equivalent Oxide Thickness) of the gate dielectric. La
2O
3 films were deposited on HF-last Si by sputtering the La
2O
3 target in argon at ambient room temperature and then annealed at 600 °C in pure N
2 or 0.1%-O
2+N
2 ambient for 30 seconds in a rapid thermal annealing (RTA) furnace. Some samples were treated with UV ozone for 9 minutes at room temperature.
The moisture absorption experiments were performed in room air. The temperature and relative humidity of the air were 25 °C and 25%, respectively. The root-mean-square (rms) surface roughnesses and XRD patterns of films before and after the moisture absorption were investigated. Au was also deposited on some La2O3 films on silicon to form Au/La2O3/Si metal insulator semiconductor (MIS) capacitors. The capacitance-voltage (C–V) with a frequency of 100 kHz and the gate current density–gate voltage (Jg–Vg) measurements were performed for MIS capacitors. The physical thickness films were determined with grazing incident x-ray reflectivity (GIXR) and spectroscopic ellipsometry (SE) measurements.
Since, as reported, that the UV ozone treatment can eliminate oxygen vacancies in the oxide films, moisture absorption suppression is expected with the UV ozone post treatment, thanks to the healing of oxygen vacancies.
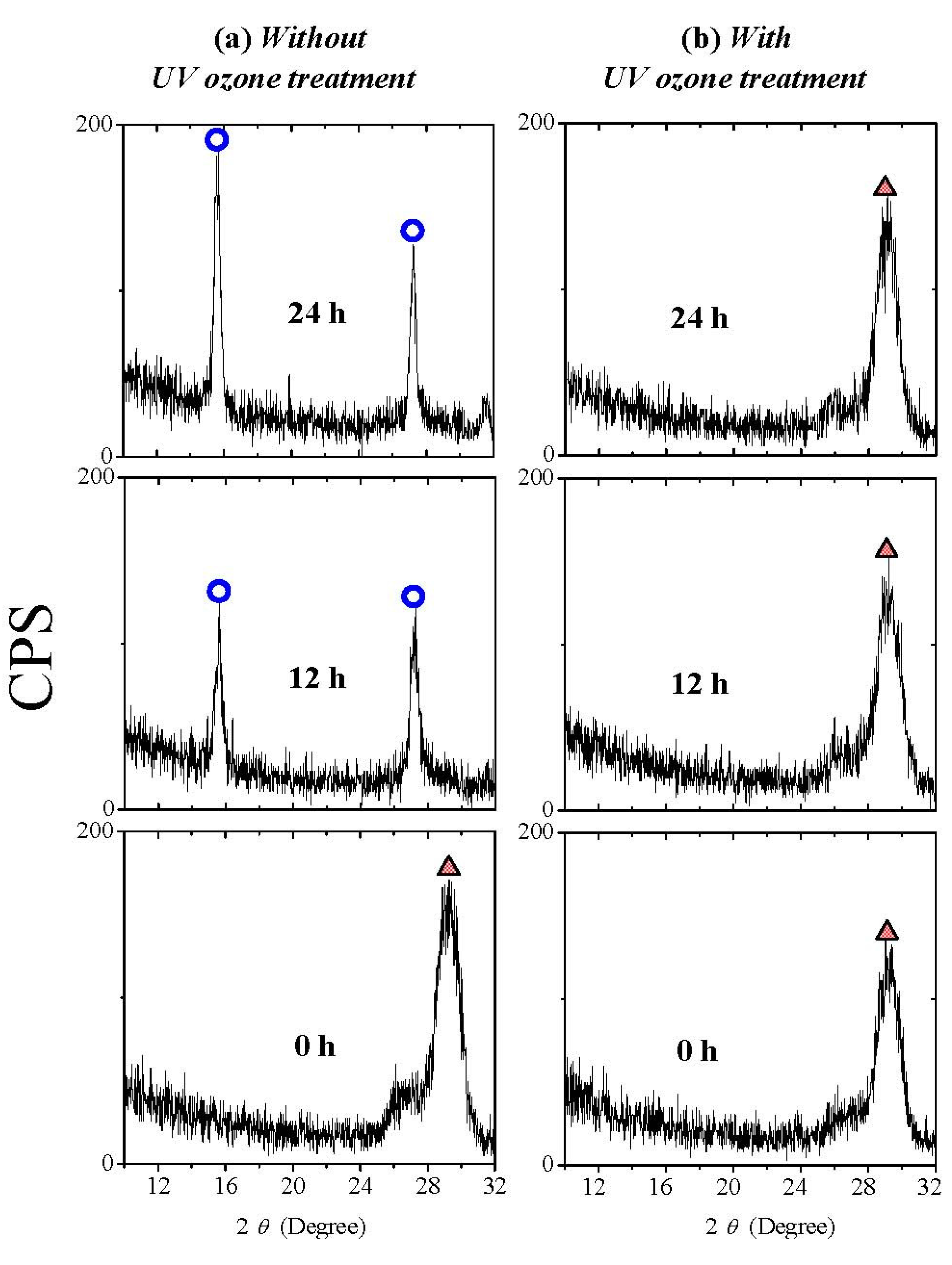
Figure 9 shows the XRD patterns of La
2O
3 films with and without UV ozone post treatment after N
2 annealing. 0 hour in air means that the sample was measured as soon as possible after annealing or UV ozone post treatment. It is found that both are poly-crystallized in the hexagonal phase when they are exposed to air for 0 hour. After exposure to air for 24 hours, in the XRD pattern of the La
2O
3 film without UV ozone post treatment after N
2 annealing, the characteristic peaks attributed to hexagonal La(OH)
3 due to moisture absorption appear, while these peaks are not found in the XRD pattern of the La
2O
3 film with UV ozone post treatment.
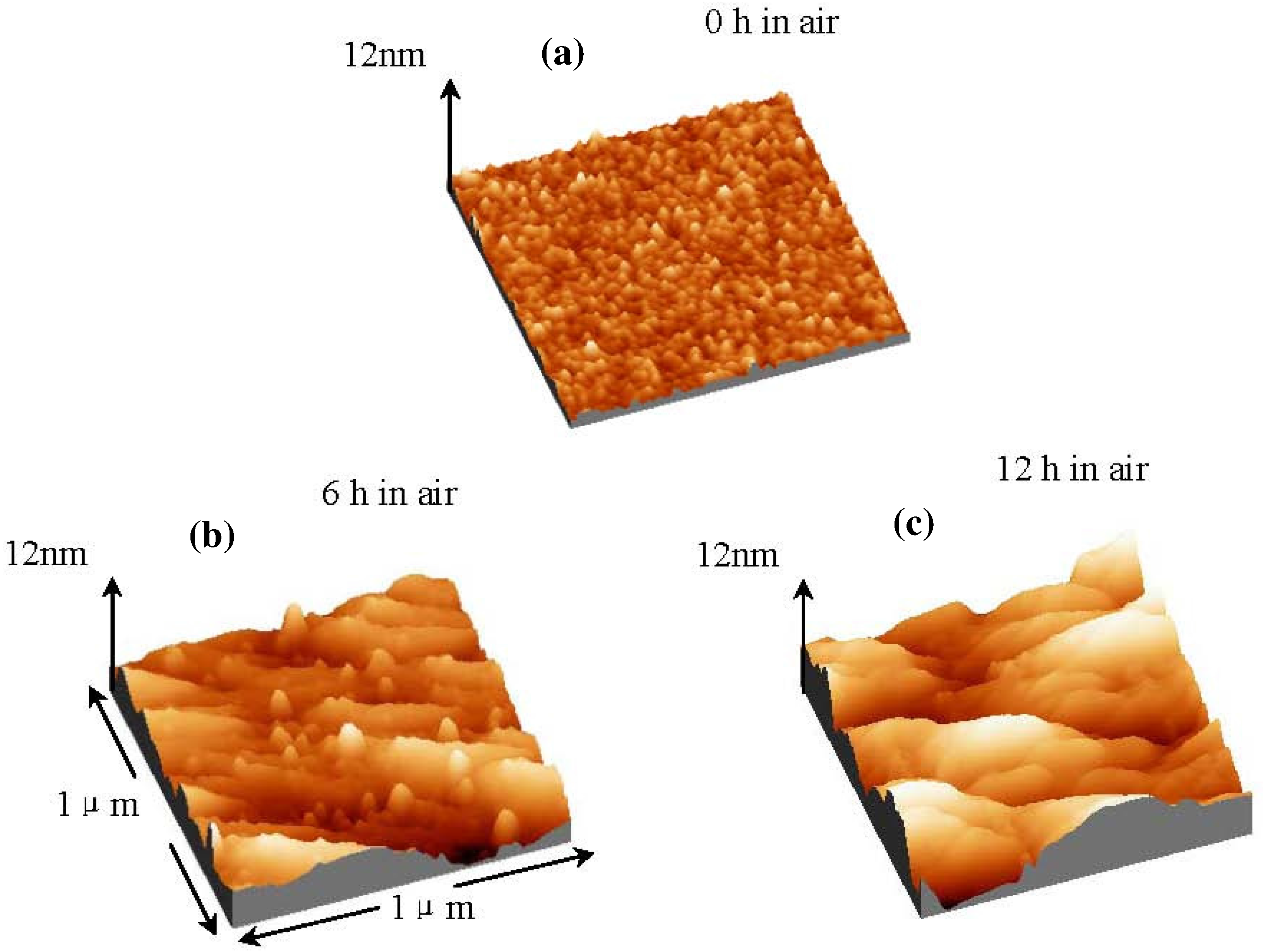
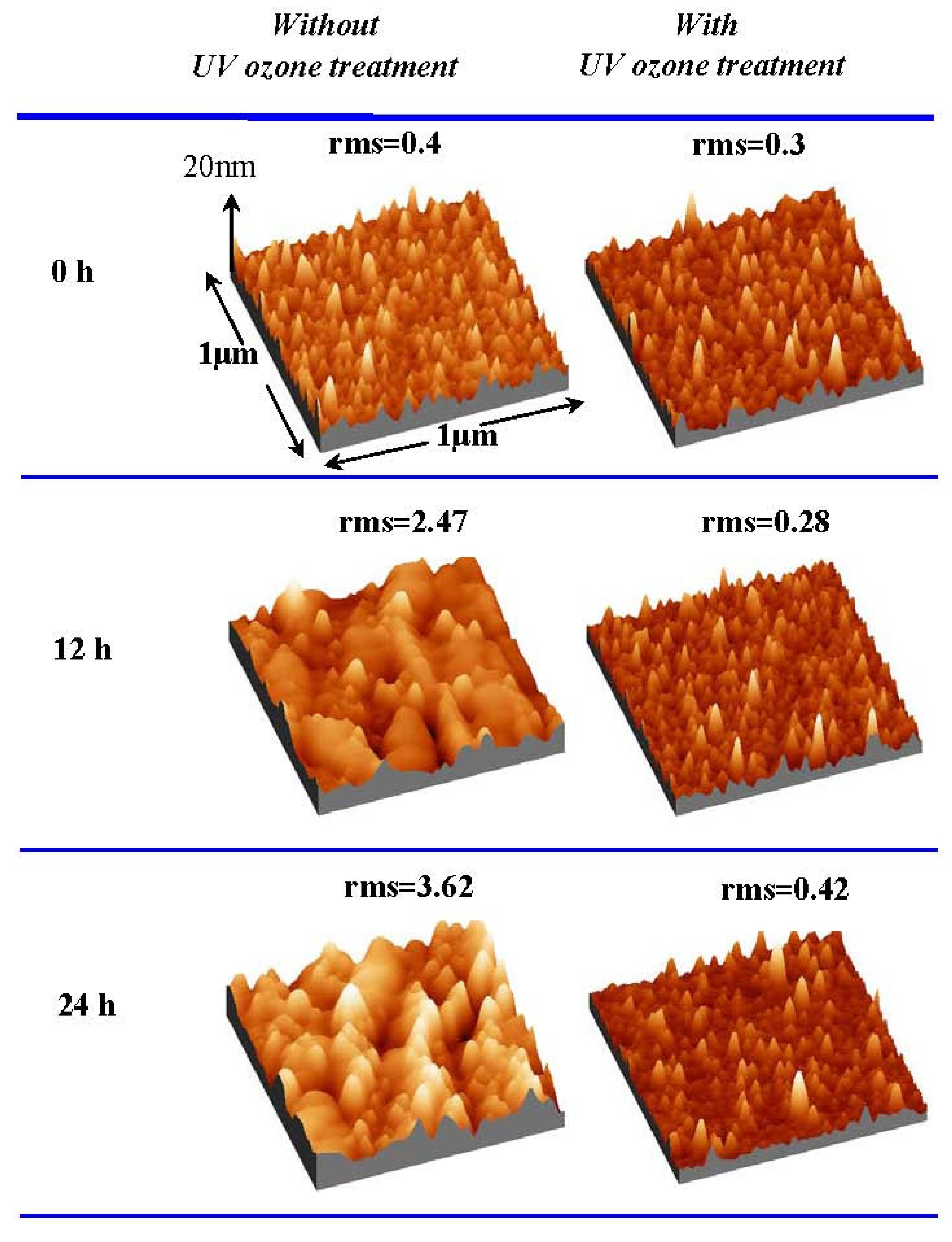
Figure 10 shows AFM images of La
2O
3 films with and without UV ozone post treatment after the films were exposed to air for different times. The root-mean-square (rms) surface roughness of the La
2O
3 film without UV ozone post treatment after N
2 annealing increases with time exposed to air, due to the formation of low density hexagonal La(OH)
3. In contrast, the surface roughness of the La
2O
3 film with UV ozone post treatment after N
2 annealing increases very little even after the film was exposed to the air for 24 hours. The above results suggest that UV ozone treatment can suppress the moisture absorption of La
2O
3 films.
To investigate the origin of the suppression effect with UV ozone treatment, moisture resistances of La2O3 films with ambient oxygen (0.1%-O2 + N2) annealing and as-deposited La2O3 film (without annealing or post treatment) were also investigated. It was clearly observed (data is not shown here) that the rms surface roughness of the UV ozone post treatment film and ambient oxygen annealing film show almost no increase with the time exposed to air. On the contrary, as-deposited and N2 annealing films’ rms surface roughnesses rapidly increase with time exposure to air. Since UV ozone post treatment and ambient oxygen annealing cause the same effect of healing the oxygen vacancies, it is reasonable to think that the origin of the moisture absorption suppression with the UV ozone post treatment might be the healing of the oxygen vacancies in La2O3
As discussed previously, the hygroscopic phenomena in La2O3 films are due to the low lattice energy of La2O3. Therefore, it is considered that the oxygen vacancy can decrease the lattice energy of La2O3. The oxygen vacancy could enlarge the charge transfer between La and O atoms and then make the La-O bond more ionic, resulting in a smaller lattice energy of La2O3 films.
Figure 9.
XRD patterns of La2O3 films (a) with and (b) without UV ozone post treatment after N2 annealing. Films were exposed to air (Temperature 25 °C and relative humidity about 25% respectively) for different times.
Figure 9.
XRD patterns of La2O3 films (a) with and (b) without UV ozone post treatment after N2 annealing. Films were exposed to air (Temperature 25 °C and relative humidity about 25% respectively) for different times.
On the other hand, it means that if we can heal oxygen vacancies in the La2O3 films, moisture absorption should be suppressed to some degree. Ozone (O3) can enhance the kinetics of oxidation (or oxygen vacancy healing) compared with conventional thermal oxidation (ambient oxygen annealing). For the La2O3 films containing oxygen vacancies (La2O3−x), the oxidation reaction can occur at low temperatures, and can heal the oxygen vacancies in the La2O3 films during UV ozone treatment.
Figure 10.
Surface AFM images (1 μm × 1 μm) of La2O3 films with and without UV ozone treatment after N2 annealing at 600 °C. Films were exposed to air for different times (Temperature and relative humidity of air: 25 °C and 25% respectively).
Figure 10.
Surface AFM images (1 μm × 1 μm) of La2O3 films with and without UV ozone treatment after N2 annealing at 600 °C. Films were exposed to air for different times (Temperature and relative humidity of air: 25 °C and 25% respectively).
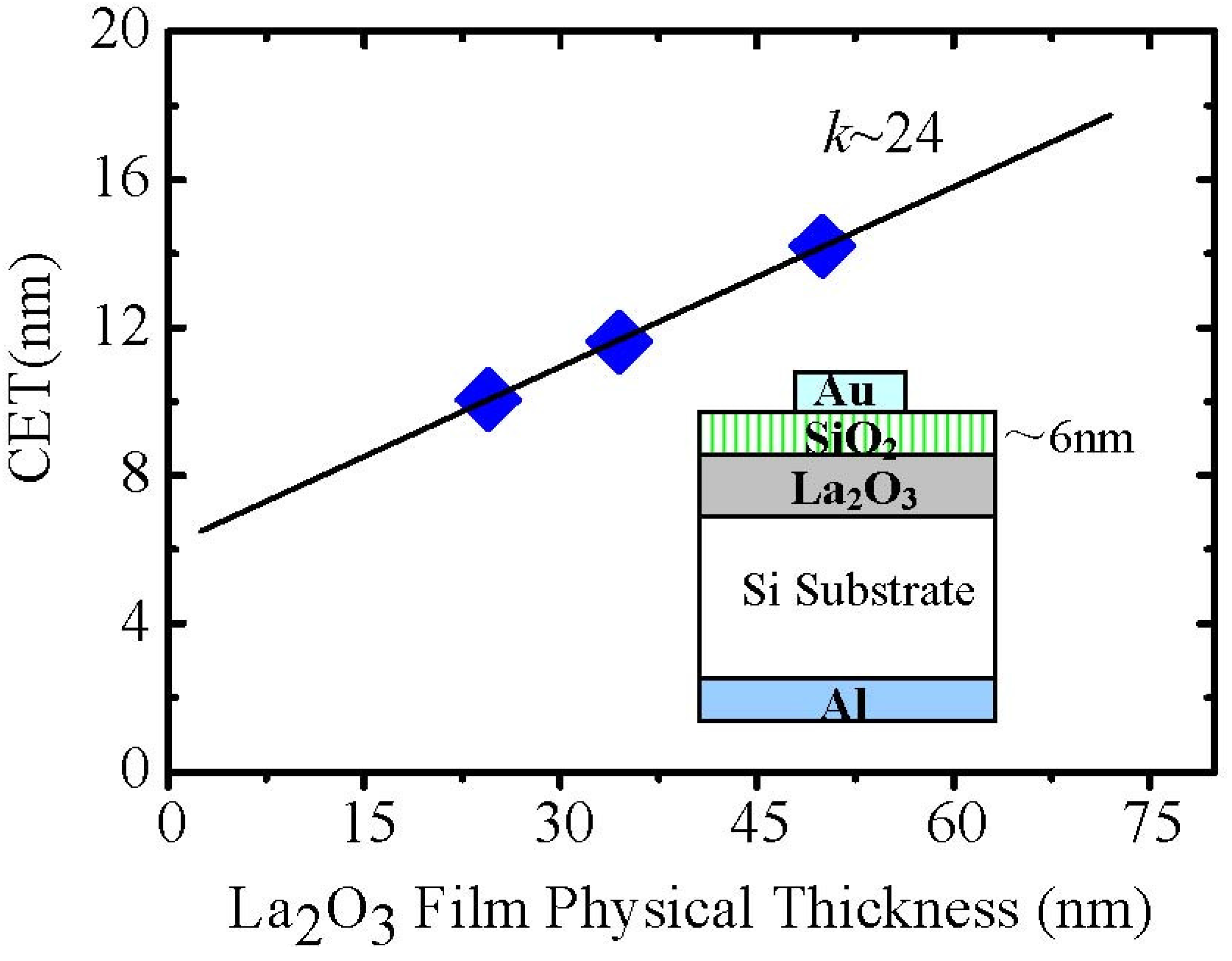
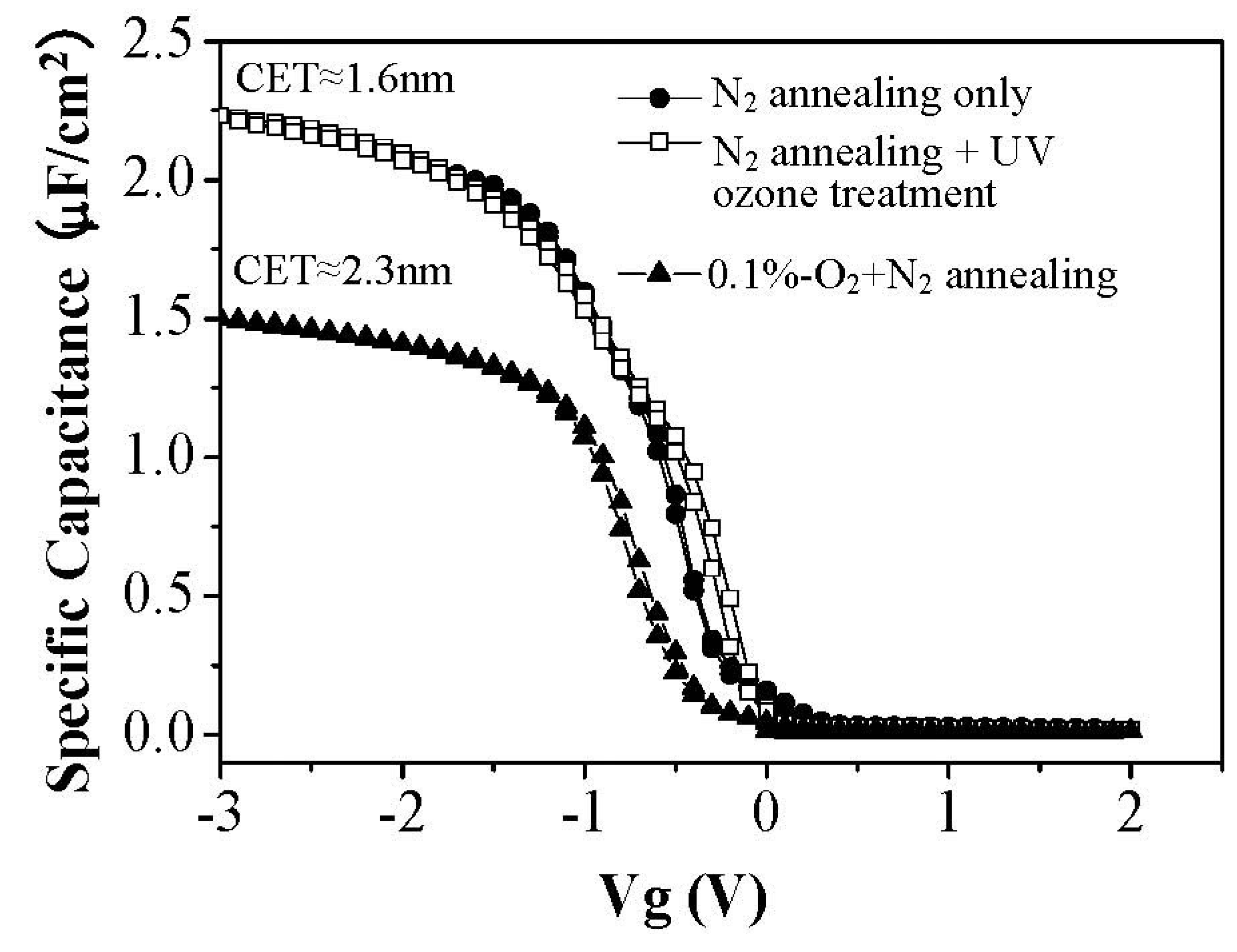
On the other hand, for ambient oxygen annealing to heal oxygen vacancies, a high temperature process is generally necessary. Although ambient oxygen annealing shows similar effects as the UV ozone treatment in terms of the moisture absorption suppression, compared to the UV ozone post treatment, ambient oxygen annealing enhanced the capacitance equivalent thickness (CET) of the film (
Figure 11) due to the formation of a thicker interface layer between silicon substrate and La
2O
3 film. Therefore, the UV ozone post treatment is a good method to suppress the moisture absorption suppression of La
2O
3 films with the merit of no interface layer thickness enhancement.
Figure 11.
C–V curve (100 kHz) of Au/La2O3/Si MIS capacitors with and without UV ozone post treatment after N2 annealing.
Figure 11.
C–V curve (100 kHz) of Au/La2O3/Si MIS capacitors with and without UV ozone post treatment after N2 annealing.
3.3. Design for Higher-k of HfO2 and La2O3 Gate Dielectric Films
One very important reason for lanthanum oxide (La
2O
3) as a promising high-
k gate dielectric to replace SiO
2 is its high permittivity. However, many low permittivity La
2O
3 films have been reported [
4,
5,
6]. In terms of the reasons for the low permittivity of La
2O
3 films, two very possible ones are being considered as mentioned earlier. The first is moisture absorption which degrades the permittivity of La
2O
3 films due to the formation of low permittivity lanthanum hydroxide as discussed above [
25]. The second is the low density of amorphous La
2O
3 films. In fact, the permittivity of La
2O
3 film without moisture absorption (0 hour in the air) still shows some low permittivity (~20). This indicates that the low permittivity could be an intrinsic property of La
2O
3 films, which could be partly attributed to poor cystallinity,
i.e., not totally attributed to moisture absorption.
Therefore, it is necessary to prepare well-crystallized La-based films to enhance and stabilize the permittivity of La
2O
3 films. From the phase diagram of the La
2O
3-Y
2O
3 system, a high melting point of La
2−xY
xO
3 could be observed which indicates a low crystallization temperature of La
2−xY
xO
3. On the other hand, Y
2O
3 shows a much lower crystallization temperature than La
2O
3 (
Figure 12). It is very possible that La
2−xY
xO
3 films could also exhibit a low crystallization temperature. Furthermore, Y is in the same element group in the elements table as La and is the nearest element to La. It can be expected that La
2−xY
xO
3 can show similar properties as La
2O
3, for example permittivity, band gaps and so on, except for moisture absorption phenomena. On the other hand, a very common viewpoint is that amorphous film (high crystallization temperature) is better than crystallized film as high-
k gate insulators. It is believed that grain boundaries in polycrystalline films might constitute electrical leakage paths, giving rise to dramatically increased gate leakage currents. However, there are few reports about the grain boundary induced leakage current in high-
k gate dielectrics; currently, expitaxial (crystalline) film are also technologically feasible.
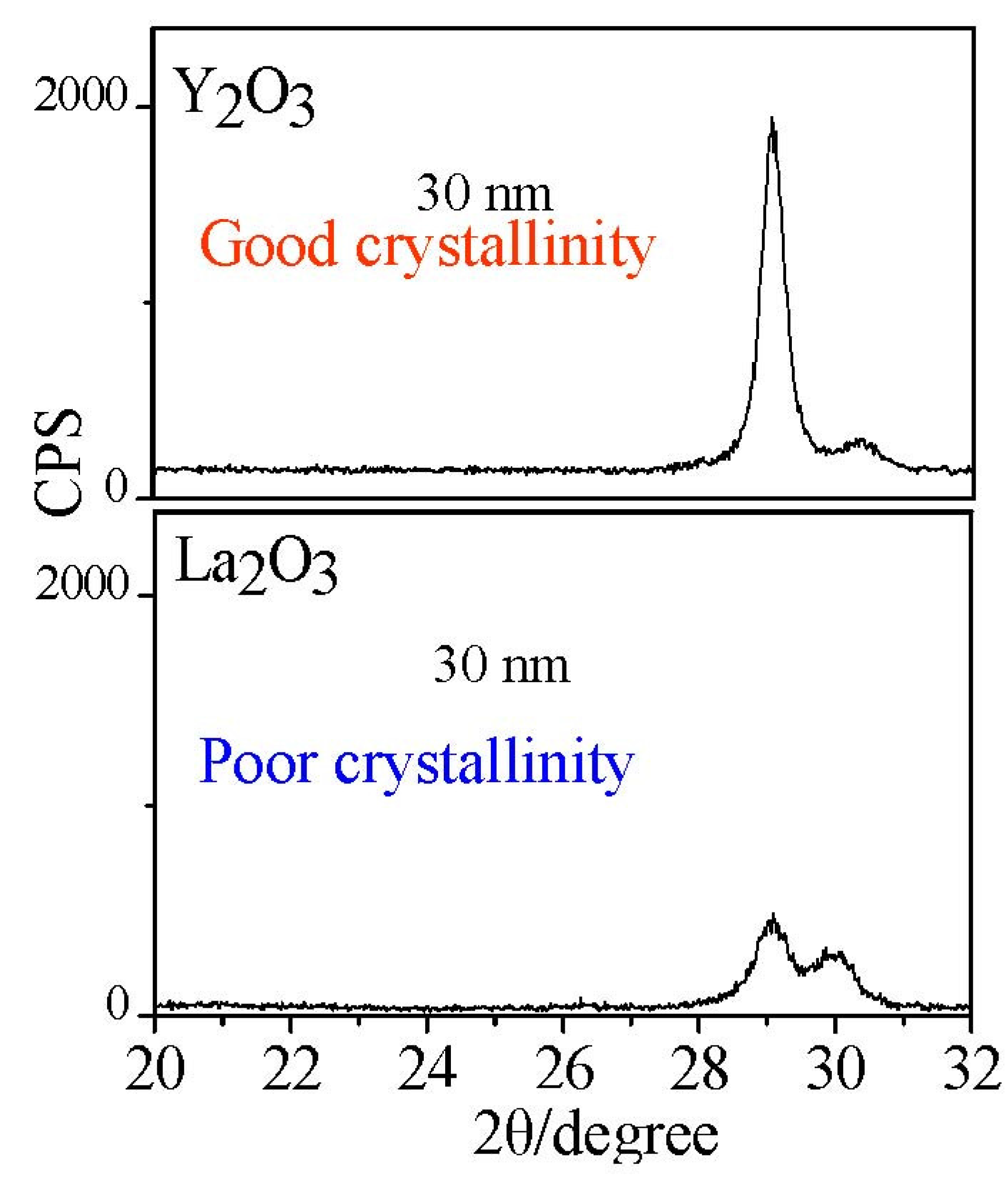
Figure 12.
Cystallinity comparison of Y2O3 and La2O3 films.
Figure 12.
Cystallinity comparison of Y2O3 and La2O3 films.
Among La-based high-
k materials, La
1−xHf
xO
y and LaAlO
3 are two attractive ones because La
1−xHf
xO
y is a good amorphous insulator up to 900 °C [
26] and LaAlO
3 shows a high permittivity and a large band gap. [
19] However, La
1−xHf
xO
y film crystallizes in the pylochlore La
2H
2fO
7 after annealing at 1000 °C [
27], while in the conventional complementary metal-oxide semiconductor (CMOS) process, annealing higher than 1000 °C is necessary to activate the source and drain dopant. In terms of LaAlO
3 film, as low permittivity LaAlO
3 films (<20) are always reported, [
28] it might be very difficult to prepare high permittivity LaAlO
3 films. A very possible reason for the low permittivity of LaAlO
3 films is the poor crystallinity which induces the low density of films. These results indicate that it is very difficult to prepare an amorphous high permittivity dielectric film as an alternative gate insulator. Although Ta
2O
5 film shows a high permittivity even in the amorphous state, [
29] due to its very small conduction band offset with silicon, it cannot be used as a high-
k gate dielectric. It is well known that La
2O
3 has a large conduction band offset with silicon of about 2.3 eV and a high permittivity. Therefore, La
1−xTa
xO
y film with an appropriate Ta concentration might be suitable as a gate dielectric which exhibits a medium conduction band offset with silicon, due to the introduction of La
2O
3. At the same time, a high permittivity of La
1−xTa
xO
y film can be expected thanks to the high permittivity of La
2O
3 and Ta
2O
5. In terms of crystallization temperature, due to the low melting point of La
1−xTa
xO
y from the La
2O
3-Ta
2O
5 phase diagram [
30], La
1−xTa
xO
y films might show a high crystallization temperature. Therefore, we also investigated La
1−xTa
xO
y films with different Ta concentrations as high-
k gate insulators in terms of the crystallization temperature, permittivity, band gap and electrical properties. The above discussion indicates that, theoretically and practically, both amorphous and well-crystallized high-
k films might also be possible choices as gate insulators.
The La2−xYxO3 and La1-xTaxOy films with different Y or Ta atomic concentrations were deposited on the HF-last Si (100) substrates or thick Pt films deposited on SiO2/Si substrates by RF co-sputtering of La2O3 and Y2O3 or Ta2O5 targets (provided by Kojundo Chemical, Japan) in Ar ambient at room temperature. The Y and Ta concentrations were determined by x-ray photoelectron spectroscopy (XPS) measurement. The physical thicknesses of the films were determined with spectroscopic ellipsometry (SE) and glazing incident x-ray reflectivity (GIXR) measurements. The crystallinity of films was investigated by x-ray diffraction (XRD) measurement. The MIM (metal-insulator-metal) capacitors on thick Pt films deposited on SiO2/Si substrates were prepared by depositing the Au film on the La2−xYxO3 or La1−xTaxOy films to evaluate the permittivities. Au was also deposited on some La2−xYxO3 and La1−xTaxOy films on silicon to form Au/La2−xYxO3 or La1−xTaxOy/Si metal insulator semiconductor (MIS) capacitors. The capacitance-voltage (C-V) with a frequency of 100 kHz and gate current density-gate voltage (J-V) measurements were performed for the Au/La2−xYxO3 or La1−xTaxOy/Si MIS capacitors.
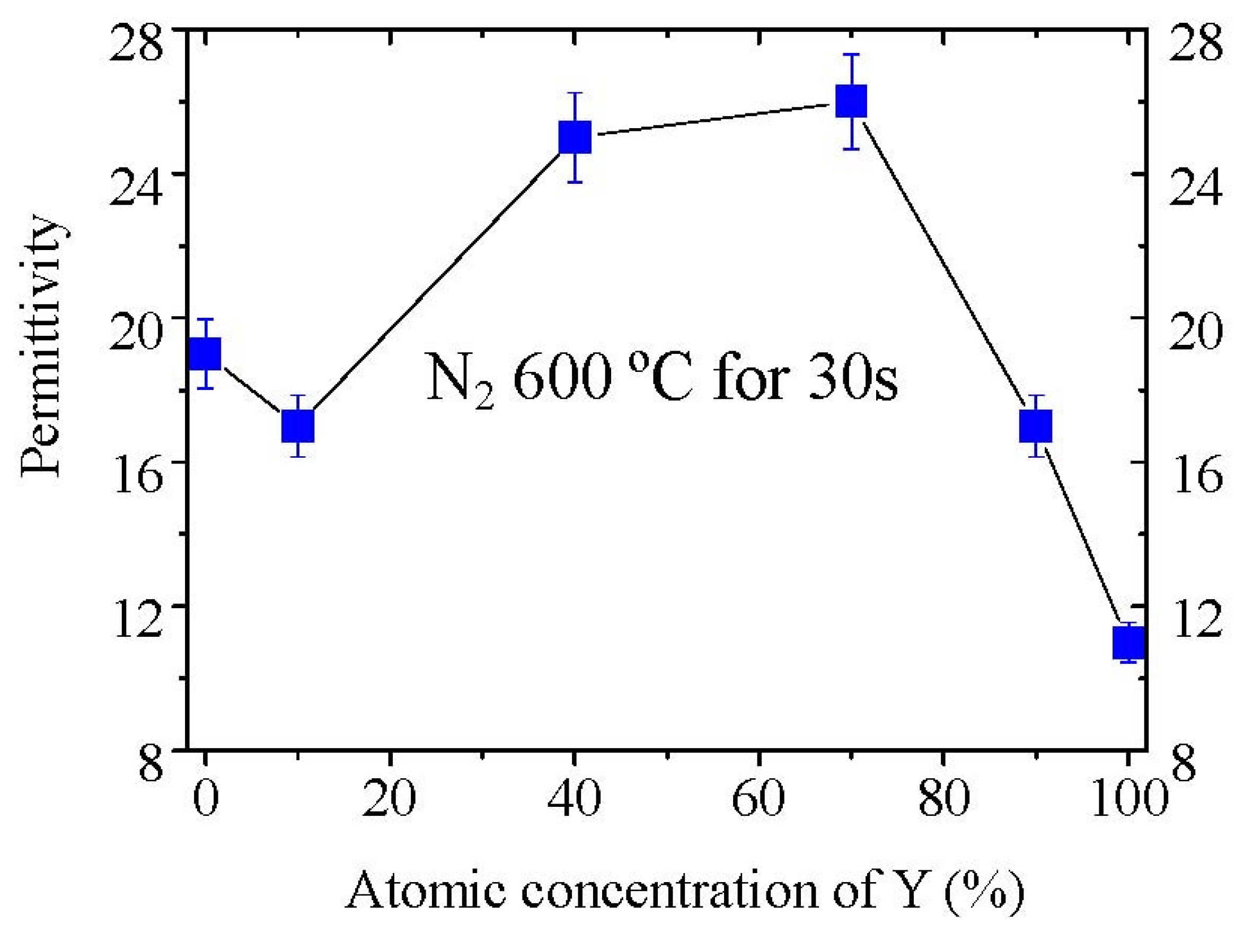
Figure 13 shows the permittivity variation of La
2−xY
xO
3 films annealed at 600 °C in pure N
2 ambient with the Y concentration. It is noticed that the permittivity of La
2O
3 film is low compared with the large value of 27 reported previously. The low permittivity of La
2O
3 film might be attributed to the poor crystallization of the film and to the moisture absorption because we did not intentionally exclude the sample from moisture. In our study, the Y
2O
3 film has a permittivity of 12 as reported [
31]. The permittivity of La
2−xY
xO
3(
x = 0.2) film is a little smaller than that of the La
2O
3 film, whereas the La
2−xY
xO
3(
x = 0.8) and La
2−xY
xO
3(
x = 1.4) films show much higher permittivity (~26) than La
2O
3 film in our study. This value is also very close to the high permittivity value of La
2O
3 film as reported [
9]. When the Y concentration is as high as 90% (
x = 1.8), the permittivity of La
2−xY
xO
3 film decreases to 15. But this value is still higher than the permittivity of Y
2O
3.
Figure 13.
Variation of the permittivities of La2−xYxO3 films with the Y concentration. Permittivities were determined by MIM capacitors. The films were thought to be exposed to the air for 0 hour rather than moisture prevented because we did not exclude the films from moisture on purpose, and just deposited the films with the Au electrode as quickly as possible after annealing in the RTA furnace.
Figure 13.
Variation of the permittivities of La2−xYxO3 films with the Y concentration. Permittivities were determined by MIM capacitors. The films were thought to be exposed to the air for 0 hour rather than moisture prevented because we did not exclude the films from moisture on purpose, and just deposited the films with the Au electrode as quickly as possible after annealing in the RTA furnace.
To explain the reason for high permittivity of La
2−xY
xO
3 films, it is necessary to discuss the
Clausius-Mosotti equation for the theory calculation of permittivity [
32].
Put simply, the
Clausius-Mosotti equation tells us that the permittivity of a well crystallized film is determined by the molar volume and total polarizability which is given as Equation (1). We can understand easily from Equation (1) that if α
T is assumed to be a constant in spite of the V
m change, the smaller V
m will induce a larger permittivity. For rare earth oxides (R
2O
3), the hexagonal phase exhibits much smaller molar volumes, as shown in
Figure 14. For hexagonal La
2O
3, α
T is 18.17Å from the Shannon’s additivity rule (α
T(La
2O
3) = 2α(La
3+) + 3α(O
−2)) and V
m is 82.7 Å
3 from Reference [
33]. With the above values, we can estimate that the permittivity of hexagonal La
2O
3 is about 35, which is larger than the reported permittivities of La
2O
3 films. This difference comes from the poor crystallinity of the reported La
2O
3 films and the low permittivity cubic phase of some La
2O
3 films. The same method is applied to hexagonal Y
2O
3 to estimate the permittivity. The V
m of hexagonal Y
2O
3 is assumed to be 90% to that of cubic Y
2O
3 [
34], as in the case of La
2O
3, because no XRD pattern of the hexagonal Y
2O
3 has been reported. We can then estimate that the permittivity of hexagonal Y
2O
3 is 22, which is much larger than the permittivity of the cubic phase Y
2O
3 in our study (
k~11). In summary, for rare earth oxides, hexagonal phase (and well crystallized) is preferred in order to achieve high permittivity. Next, let me explain the reason for the high permittivity of La
2−xY
xO
3 films.
Figure 15 shows the XRD patterns of all La
2-xY
xO
3 films on Pt film after annealing at 600 °C. It can be observed that the La
2O
3 film is polycrystallized in the hexagonal phase. In the XRD pattern of La
2−xY
xO
3(
x = 0.2) film, both peaks attributed to the cubic phase and hexagonal phase are found. Therefore, the permittivity of La
2−xY
xO
3(
x = 0.2) film is smaller than that of the La
2O
3 film, due to the low permittivity of the cubic phase.
Figure 14.
Molar volume comparison of hexagonal and cubic rare earth oxide (R2O3).
Figure 14.
Molar volume comparison of hexagonal and cubic rare earth oxide (R2O3).
Figure 15.
XRD patterns of La
2−xY
xO
3 films with different Y concentrations after annealing at 600 °C. (
![Materials 05 01413 i002]()
: Hexagonal La
1−xY
xO
3 (002),
![Materials 05 01413 i003]()
: Cubic Y
2O
3 (222),
![Materials 05 01413 i004]()
: Cubic La
2O
3 (222)).
Figure 15.
XRD patterns of La
2−xY
xO
3 films with different Y concentrations after annealing at 600 °C. (
![Materials 05 01413 i002]()
: Hexagonal La
1−xY
xO
3 (002),
![Materials 05 01413 i003]()
: Cubic Y
2O
3 (222),
![Materials 05 01413 i004]()
: Cubic La
2O
3 (222)).
The 40%Y (
x = 0.8) and 70%Y (
x = 1.4) La
2−xY
xO
3 films are well crystallized in the hexagonal phase after annealing at 600 °C. The crystallinity of the film can be estimated with the full-width at half-maximum (FWHM) of the XRD peak. The smaller FWHM indicates better crystallinity. The FWHM of hexagonal (002) peak of 40%Y (
x = 0.8) and 70%Y (
x = 1.4) La
2−xY
xO
3 films’ XRD patterns are only 0.4 degree, while that of La
2O
3 film is about 1.4 degree. It indicates that 40%Y (
x = 0.8) and 70%Y (
x = 1.4) La
2−xY
xO
3 films exhibit a better crystallinity than La
2O
3 film. As reported by R.A.B Devine [
4], the permittivity of amorphous La
2O
3 is very low, due to its low density. Therefore, the better crystallinity results in 40%Y (
x = 0.8) and 70%Y (
x = 1.4) La
2−xY
xO
3 films having a much higher permittivity than La
2O
3 film, even though low polarizibility Y
3+ ions were introduced. Another very important factor is that 40%Y (
x = 0.8) and 70%Y (
x = 1.4) La
2−xY
xO
3 films were both crystallized in the hexagonal phase rather than the cubic phase. As discussed above, the hexagonal rare earth oxides show much larger permittivities than cubic rare earth oxides as expected from the
Clausius-Mossotti relationship. It is reasonable that the 40%Y (
x = 0.8) and 70%Y (
x = 1.4) La
2−xY
xO
3 films which are well crystallized in the hexagonal phase show a high permittivity of 26. In addition, the peak of the hexagonal (002) La
2−xY
xO
3 gradually shifts to a larger 2θ as Y concentration increases. This shift is attributed to the decrease of the lattice parameter due to the smaller ionic radius of Y
3+ than that of La
3+. For the La
2−xY
xO
3 (
x = 1.8) film, it is found from the XRD pattern that the film contains both the cubic and hexagonal phases. Therefore its permittivity is larger than that of the Y
2O
3 with a cubic phase, but smaller than that of 40%Y (
x = 0.8) and 70%Y (
x = 1.4) La
2−xY
xO
3 films due to the low polarizibility Y
3+ ion and the low permittivity cubic phase.
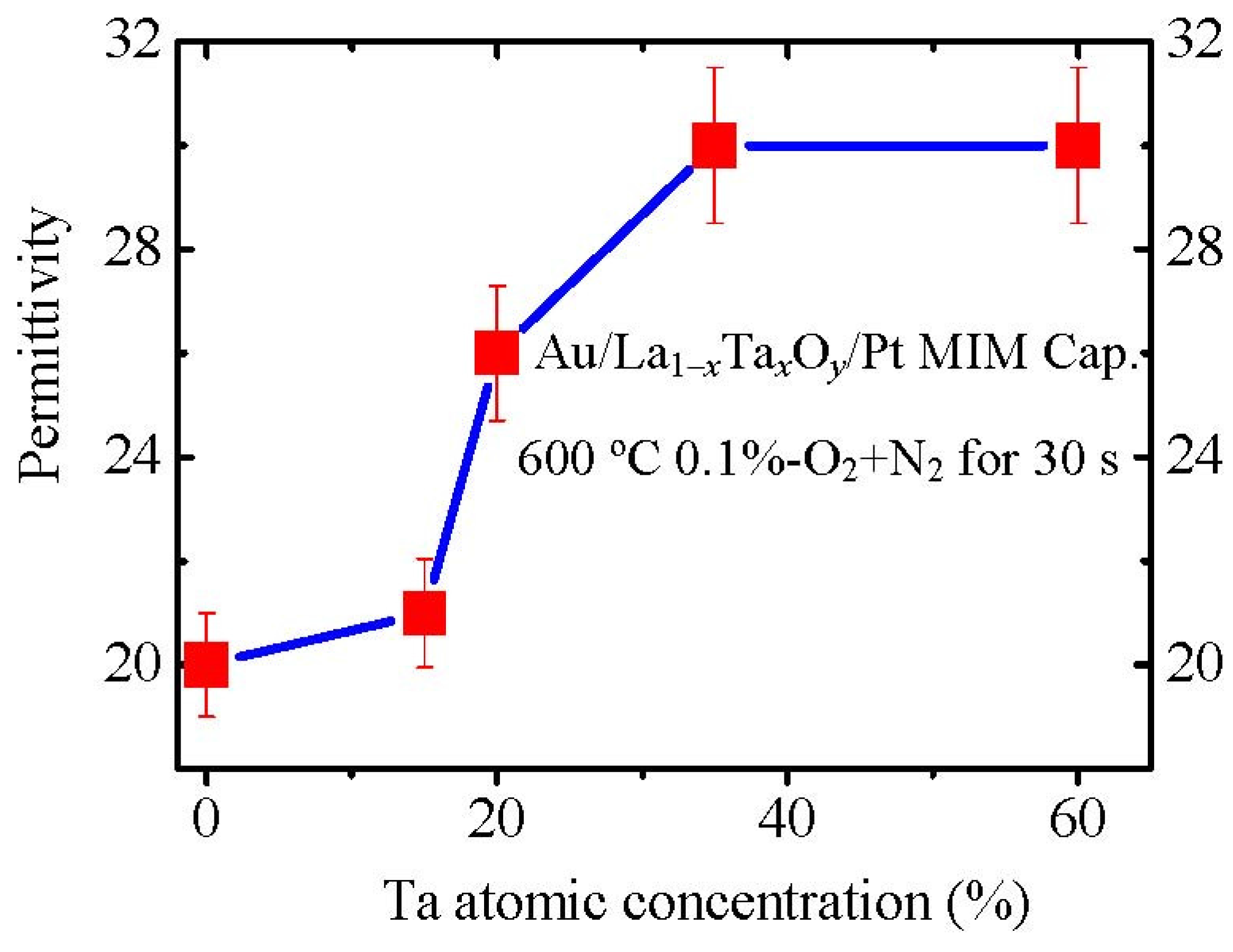
We also prepared La
1−xTa
xO
y films with different Ta concentrations. The permittivities were measured with Au/La
1−xTa
xO
y/Pt MIM capacitors. La
1−xTa
xO
y(
x = 0.35) film shows a high permittivity of about 30 (
Figure 16), which is comparable to the largest reported permittivity of La
2O
3 [
35] and amorphous Ta
2O
5. This permittivity value is also much larger than that of amorphous La
1−xHf
xO
y and well crystallized LaAlO
3 films. The very possible reason for the high permittivity of amorphous La
1−xTa
xO
y is a higher density of Ta
2O
5 [
36,
37] than La
2O
3. This higher density could induce a higher permittivity. The main reason is as follows: if we assume that the unit structure of La
2O
3 is not changed by Ta
2O
5 doping, the higher material density would induce a higher dipole density (more dipoles in the unit volume), resulting in a higher permittivity. Therefore, a high density Ta
2O
5 doping will enhance the permittivity of La
2O
3 although the film is amorphous, which will be discussed in more detail later.
Figure 16.
Variation of permittivities of La1−xTaxOy films with Ta concentration.
Figure 16.
Variation of permittivities of La1−xTaxOy films with Ta concentration.
3.4. Design of Crystallization Behavior of High-k Gate Dielectric Films
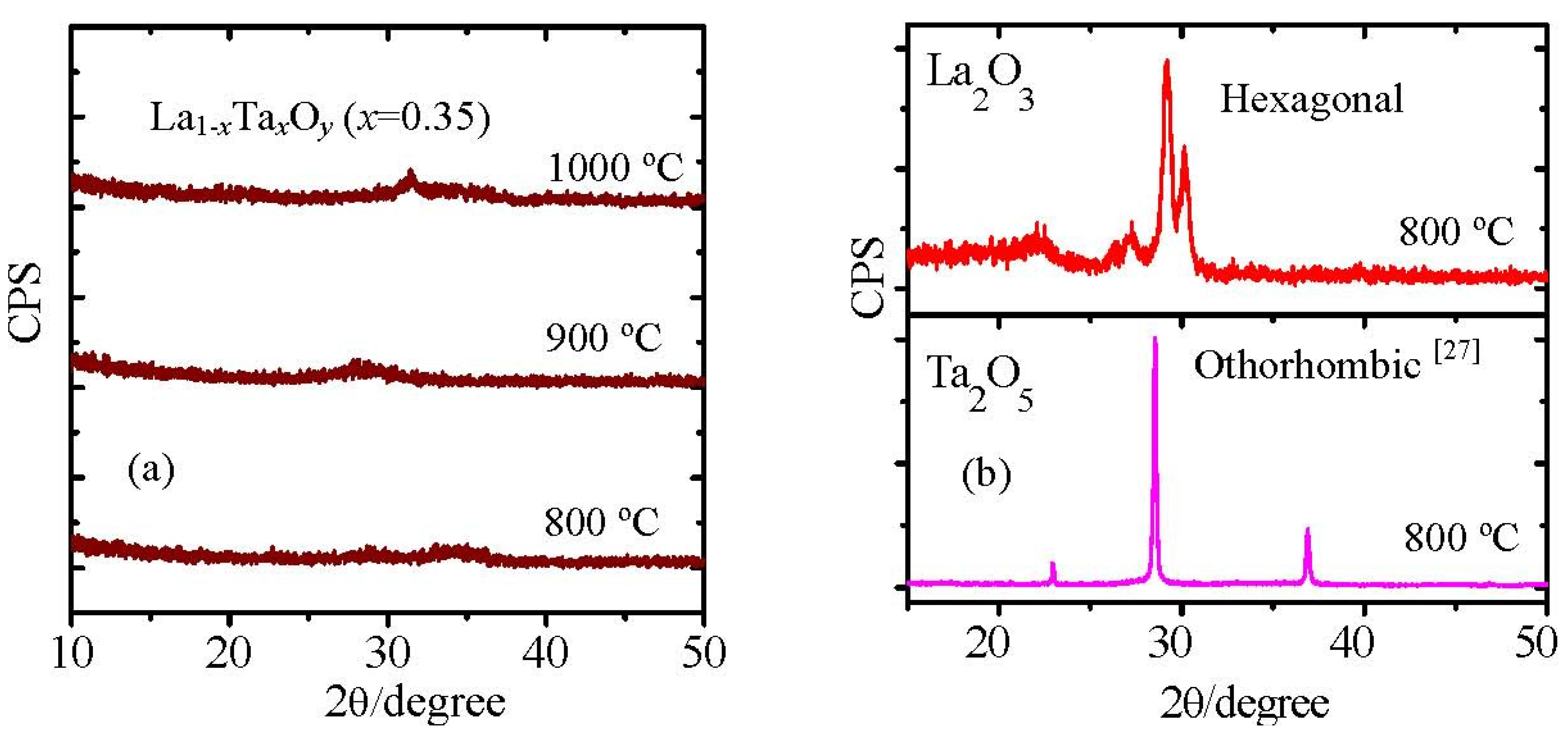
Figure 17(a) shows the XRD patterns of La
1−xTa
xO
y films with a Ta concentration of 35% (
x = 0.35) after they were annealed at 800 °C, 900 °C and 1000 °C in ambient N
2. It can be observed that the film was still in the amorphous state even it was annealed at 1000 °C. This indicates that the crystallization temperature of La
1−xTa
xO
y(
x = 0.35) film is higher than 1000 °C. As a gate dielectric, an amorphous film is preferred rather than a poly-crystallized film because grain boundaries can induce a leakage current through the dielectric [
38]. As La
1−xTa
xO
y(
x = 0.35) film shows a crystallization temperature higher than 1,000 °C, it will be compatible with the conventional CMOS process. On the other hand, both La
2O
3 and Ta
2O
5 films crystallize after annealing at 800 °C (
Figure 17b). Furthermore, crystallization temperatures of La
1−xTa
xO
y(
x = 0.35) and La
1−xTa
xO
y(
x = 0.6) films are about 800 °C and 1000 °C, respectively. Both are higher than that of La
2O
3 [
39] and Ta
2O
5 [
35]. It also indicates that the crystallization temperature of La
1−xTa
xO
y film is sensitive to the Ta concentration, and to prepare the high crystallization temperature La
1−xTa
xO
y film it is crucial to control the Ta concentration. In the case of La
2−xY
xO
3, this shows a very low crystallization temperature [
40]. Why is there so large a difference? To answer this question, I will explain the crystallization mechanism of La-based ternary oxides (La
2−xM
xO
3 or La
1−xM
xO
y) in more detail later. We think there are two main factors to influence the crystallization temperature of La-based ternary oxides. One is the size difference between La and M ions. And the other is the difference of valence state of La and M ions.
Figure 17.
(a) XRD patterns of La1−xTaxOy(x = 0.35) film annealed at 800 °C, 900 °C and 1000 °C; (b) XRD patterns of La2O3 and Ta2O5 films annealed at 800 °C. The thickness of the films was about 30 nm.
Figure 17.
(a) XRD patterns of La1−xTaxOy(x = 0.35) film annealed at 800 °C, 900 °C and 1000 °C; (b) XRD patterns of La2O3 and Ta2O5 films annealed at 800 °C. The thickness of the films was about 30 nm.
It has been reported that the stability of amorphous ternary metal oxide (A
2−xM
xO
3) is substantially determined by the size difference between metal A ion and metal M ion when A and M has the same valence state [
41]. The close size of metal A ion to metal M ion would induce the formation of a solid solution. When there is a large size difference between metal A ion and metal M ion, such a solid solution is not stable. Then, the oxide can be stabilized in the amorphous state. In the case of La-based ternary oxides, the difference between La
3+ ion size(r(La
3+)) and M
3+ ion size(r(M
3+)) could affect the crystallization temperature of La
2−xM
xO
3.
Table 1 shows the crystallization temperatures of La
2−xAl
xO
3 [
42], La
2−xSc
xO
3 [
43] and La
2−xY
xO
3 films [
21]. This is because r(La
3+) > r(Y
3+) > r(Sc
3+) > r(Al
3+), La
2−xAl
xO
3 has the highest crystallization temperature, and La
2−xY
xO
3 the lowest. Thus, we obtained the well crystallized La
2−xY
xO
3 films as discussed earlier.
Table 1.
Crystallization temperatures of La1−xMxOy ternary metal oxides in the case of M3+.
Table 1.
Crystallization temperatures of La1−xMxOy ternary metal oxides in the case of M3+.
| Ternary Oxide (La2−xMxO3) | Metal Ion Size (La-M)(Å) | Crystallization Temperature (°C) |
|---|
| La2−xAlxO3 | 1.16–0.51 | 800 |
| La2−xScxO3 | 1.16–0.73 | 650 |
| La2−xYxO3 | 1.16–1.02 | 400 |
We think that the valence state of the M ion can also affect the crystallization temperature of La
1−xM
xO
y ternary oxide because the valence state could determine the oxygen ratio in MO
y. When we dope MO
y into La
2O
3 (LaO
1.5), and if
y is larger than 1.5, there will be superfluous oxygen which can distort the oxide network and then enhance the crystallization temperature of La
2O
3. We studied several La
1−xM
xO
y ternary metal oxides with different valence states of M (Y
3+, Hf
4+ and Ta
5+). The crystallization temperatures of these ternary metal oxides are shown in
Table 2. An obvious trend can be found from the table: the ternary metal oxide which exhibits a larger valence state of the M ion (larger
y) shows a higher crystallization temperature. La
1−xTa
xO
y ternary oxide shows the highest crystallization temperature among these ternary metal oxides because of the difference of ion size and valence state of La and M. La
2−xY
xO
3 films show a low crystallization temperature due to the close size of La
3+ to Y
3+ and the same valence state of La
3+ and Y
3+. And La
1−xHf
xO
y film also shows a relatively high crystallization temperature, thanks to the large size difference between La
3+ and Hf
4+. In summary, the high crystallization temperature of La
1−xTa
xO
y film can be attributed to the large size and valence state difference between La
3+ and Ta
5+.
Table 2.
Crystallization temperatures of La1−xMxOy ternary metal oxides in the case of M3+, M4+ and M5+.
Table 2.
Crystallization temperatures of La1−xMxOy ternary metal oxides in the case of M3+, M4+ and M5+.
| Ternary oxide (La1−xMxOy) | Metal ion size(La–M)(Å) | Crystallization temperature (°C) |
|---|
| La1−xTaxOy (M5+) | 1.16–0.74 | >1000 |
| La1−xHfxOy (M4+) | 1.16–0.83 | 1000 |
| La2−xYxO3 (M3+) | 1.16–1.02 | 400 |
3.5. Summary
In this paper, most recent progresses of two most important issues, moisture absorption phenomena and low experimental permittivity, of rare earth oxide films used as high-k gate insulators for advanced CMOS devices, have been reviewed from both experimental and theoretical points of view.
It has been found that moisture absorption degrades the permittivity of La2O3 film annealed in N2 ambient after exposure to air for several hours because of the formation of La(OH)3 with a lower permittivity and it is thus concluded that the moisture absorption could be a possible reason for the scattering k-values of La2O3 films. Furthermore, AFM results indicate that moisture absorption also increases the surface roughness of La2O3 films on silicon. Thus, an in situ gate electrode process would be needed for La2O3 CMOS application.
Accordingly, the moisture absorption phenomena in main high-k gate oxides have been theoretically discussed by comparing the Gibbs free energy change of the moisture absorption reactions of these oxides. The results show that moisture absorption could occur in most high-k oxides, especially in rare earth oxides. On the other hand, La2O3 shows the largest moisture-absorption-reaction rate among main high-k oxide candidates. To enhance moisture resistance of La2O3, doping a second oxide, which has a stronger moisture resistance than La2O3, could be an applicable solution.
The moisture absorption and associated leakage current of La2O3 films were suppressed by UV ozone post treatment. The suppression effect by UV ozone treatment has been considered to come from the healing of oxygen vacancies in La2O3 films, since ambient oxygen annealing also shows the same suppression effect. Compared with ambient oxygen annealing, however, UV ozone post treatment can be carried out at low temperatures, which prevents the formation of a thick interface layer.
With the phase control method, the permittivities and the moisture-resistance of La2O3 films have been improved significantly. Higher-k well crystallized lanthanum based oxide films, La2−xYxO3, were prepared, which exhibit a permittivity as high as 28 with an appropriate Y concentration, due to the formation of a high permittivity hexagonal phase, and also show much better resistance to moisture than La2O3 film after annealing at 600 °C. La1−xTaxOy films with different Ta concentrations were investigated. The La1−xTaxOy (x = 0.35) film shows not only a high crystallization temperature (>1000 °C), but also a high permittivity (~30).
Furthermore, a systematic discussion on the crystallization behaviors of lanthanum-based ternary oxide has been given, which provides a possible guideline for preparing amorphous or well crystallized lanthanum-based ternary oxides. This should be also useful for other high-k oxides to prepare well crystallized or amorphous films as new gate insulators.
 : hydroxide).
: hydroxide).
 : hydroxide).
: hydroxide).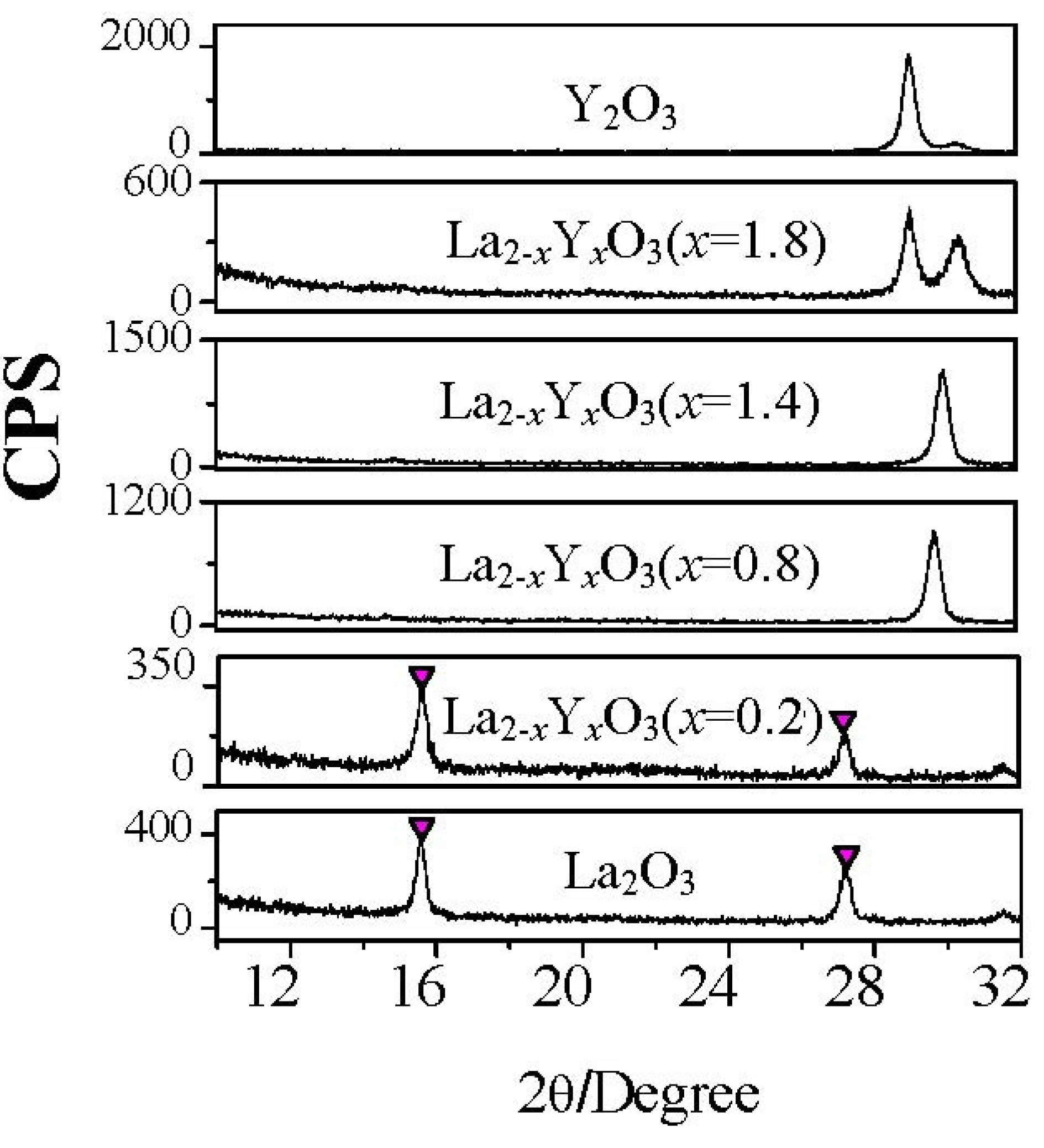
 : Hexagonal La1−xYxO3 (002),
: Hexagonal La1−xYxO3 (002),  : Cubic Y2O3 (222),
: Cubic Y2O3 (222),  : Cubic La2O3 (222)).
: Cubic La2O3 (222)).
 : Hexagonal La1−xYxO3 (002),
: Hexagonal La1−xYxO3 (002),  : Cubic Y2O3 (222),
: Cubic Y2O3 (222),  : Cubic La2O3 (222)).
: Cubic La2O3 (222)).