Cell-Sized Liposomes and Droplets: Real-World Modeling of Living Cells
Abstract
:1. Introduction
2. Modeling of Living Cells
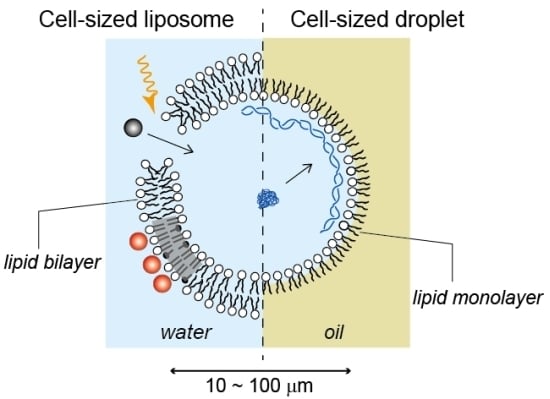
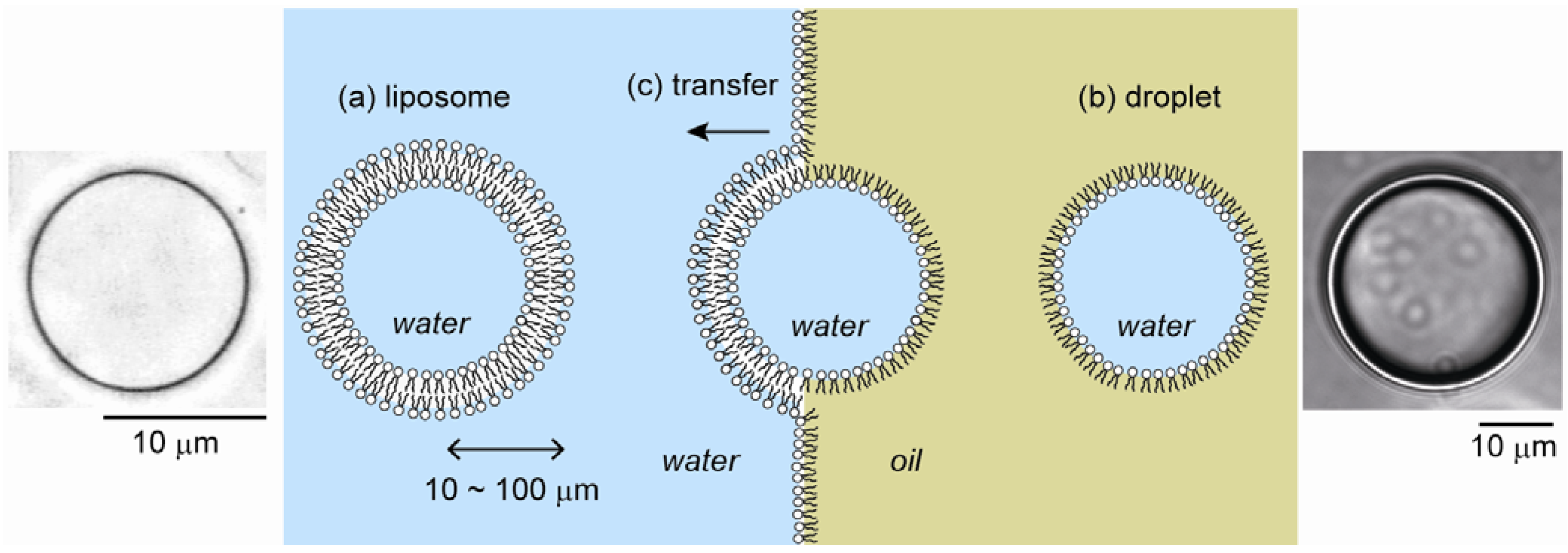
2.1. Cell-Sized Liposomes
2.2. Cell-Sized Droplets
3. Encapsulation of Biopolymers in a Cell-Sized Confined Space
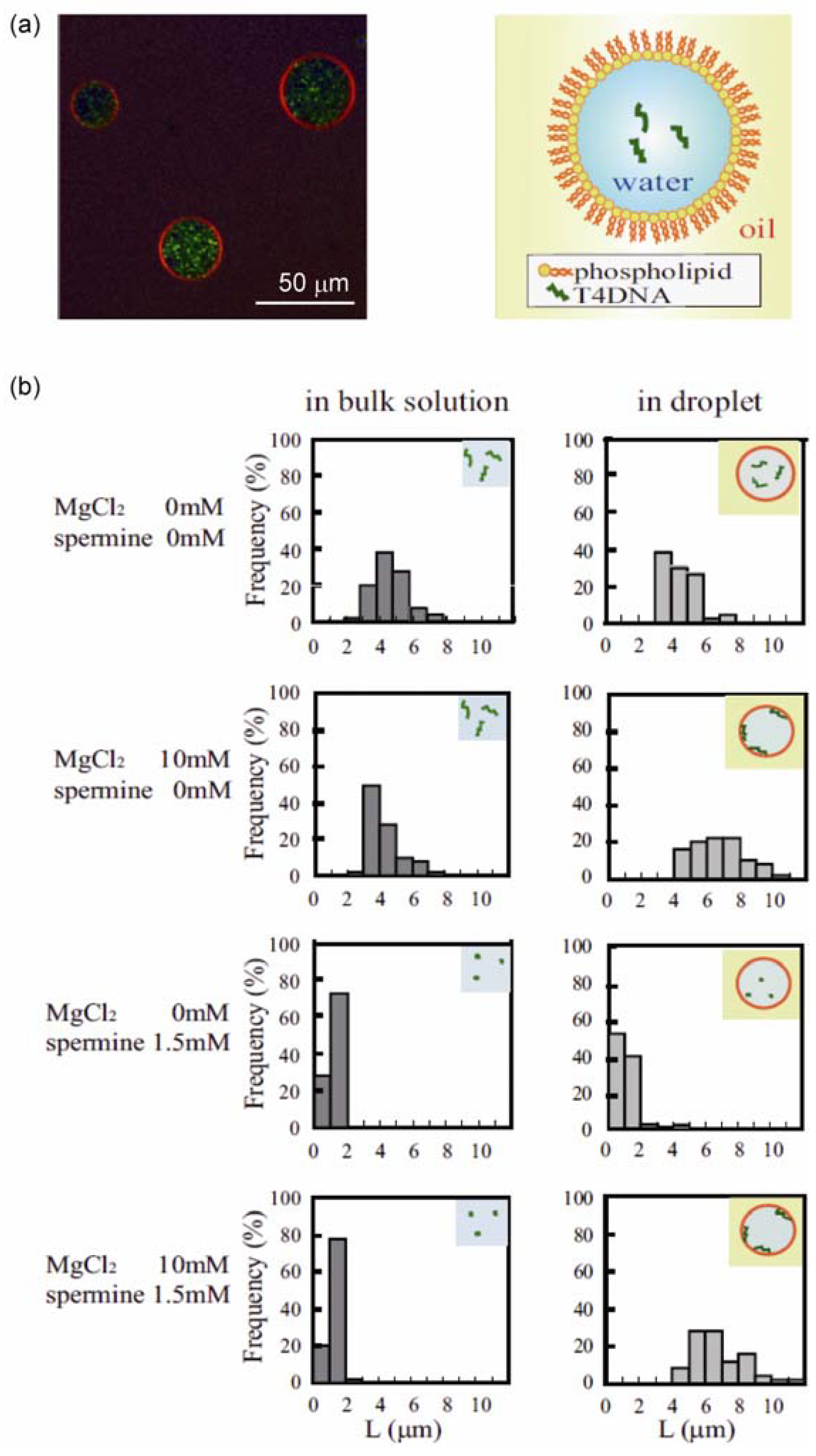
3.1. Conformational Transition of DNA in a Confined Space
3.2. Structural Transition of Actin Filament in a Confined Space
4. Association on Soft Membranes
4.1. Microdomains
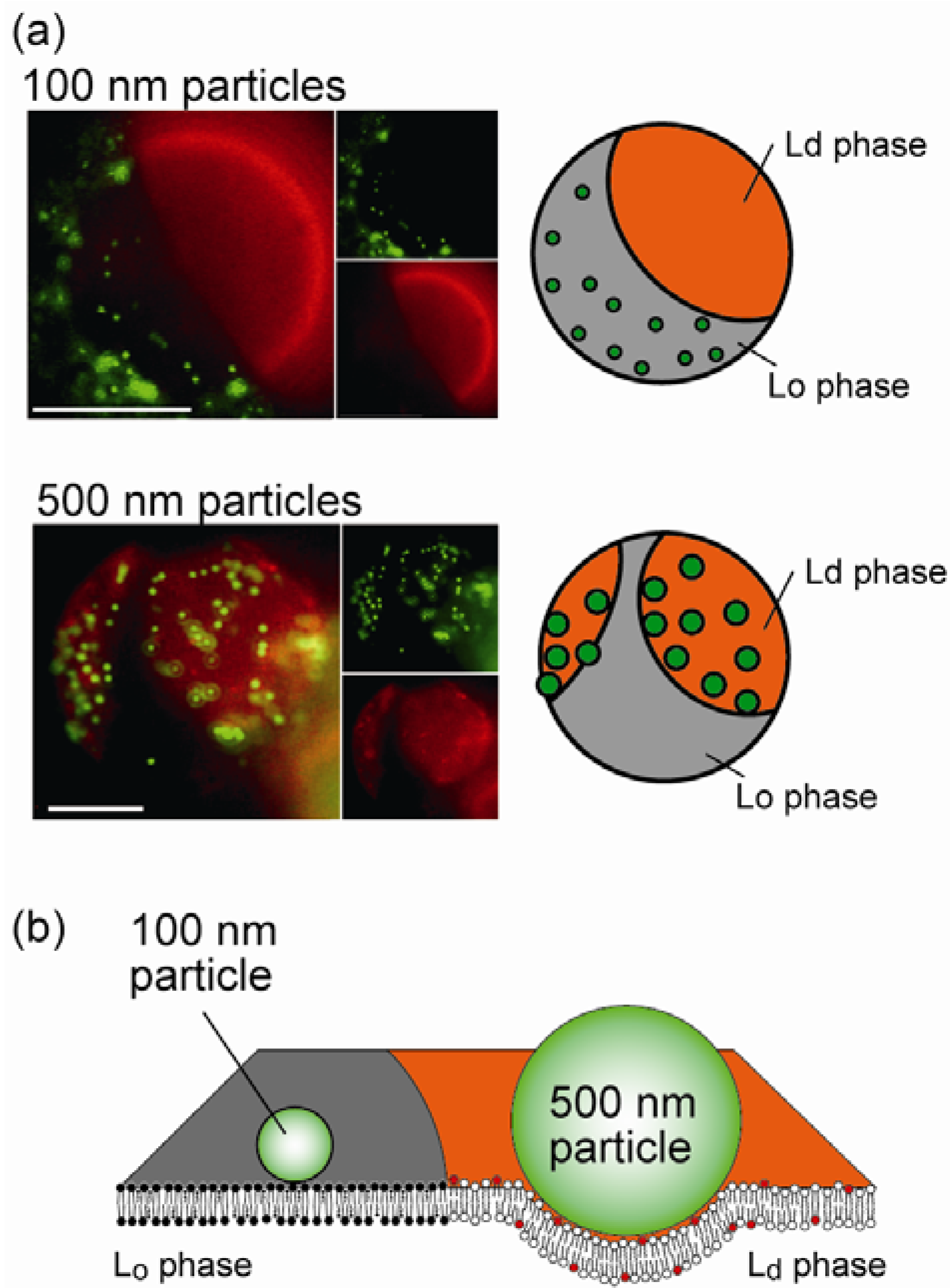
4.2. Size-Dependent Partitioning of Nanoparticles
4.3. Selective Localization of Peptides
5. Transformable Boundary for Cell-Sized Compartments
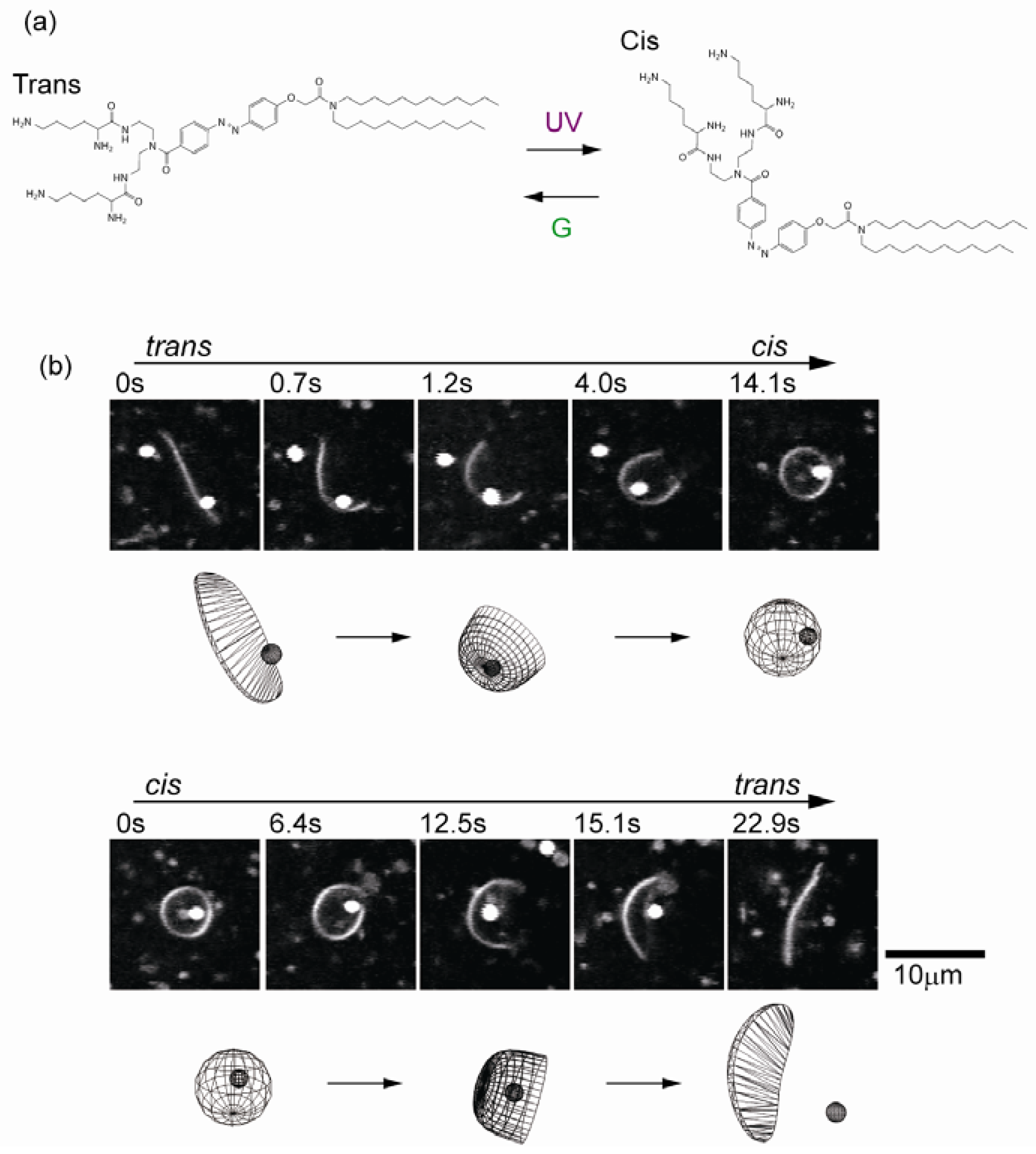
6. Concluding Remarks
Acknowledgments
References
- Fletcher, D.A.; Liu, A.P. Biology under construction: In vitro reconstitution of cellular function. Nat. Rev. Mol. Cell. Biol. 2009, 10, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Luisi, P.L. Chemical aspects of synthetic biology. Chem. Biodivers. 2007, 4, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.D. Novel applications of liposomes. Trends Biotechnol. 1998, 16, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Luisi, P.L.; Walde, P. Giant Vesicles; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Hase, M.; Yamada, A.; Hamada, T.; Baigl, D.; Yoshikawa, K. Manipulation of cell-sized phospholipid-coated microdroplets and their use as biochemical microreactors. Langmuir 2007, 23, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, A.V.; Luisi, P.L. Cell-free protein synthesis through solubilisate exchange in water/oil emulsion compartments. ChemBioChem 2004, 5, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Walde, P.; Cosentino, K.; Engel, H.; Stano, P. Giant vesicles: Preparations and applications. ChemBioChem 2010, 11, 848–865. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Yamanaka, T.; Hamada, T.; Hase, M.; Yoshikawa, K.; Baigl, D. Spontaneous transfer of phospholipid-coated oil-in-oil and water-in-oil micro-droplets through an oil/water interface. Langmuir 2006, 22, 9824–9828. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Miura, Y.; Komatsu, Y.; Kishimoto, Y.; Vestergaard, M.; Takagi, M. Construction of asymmetric cell-sized lipid vesicles from lipid-coated water-in-oil microdroplets. J. Phys. Chem. B 2008, 112, 14678–14681. [Google Scholar] [CrossRef] [PubMed]
- Pautot, S.; Frisken, B.J.; Weitz, D.A. Engineering asymmetric vesicles. Proc. Natl. Acad. Sci. USA 2003, 100, 10718–10721. [Google Scholar] [CrossRef] [PubMed]
- Bloomfield, V.A. DNA condensation. Curr. Opin. Struct. Biol. 1996, 6, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K. Controlling the higher-order structure of giant DNA molecules. Adv. Drug Deliv. Rev. 2001, 52, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Shindo, E.; Sakaue, T.; Tsuji, A.; Yoshikawa, K. Conformational transition of giant DNA in a confined space surrounded by a phospholipid membrane. Biophys. J. 2009, 97, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Yoshikawa, K.; Vasilevskaya, V.V.; Khokhlov, A.R. Discrete coil-globule transition of single duplex DNAs induced by polyamines. J. Phys. Chem. B 1997, 101, 9396–9401. [Google Scholar] [CrossRef]
- Tsuji, A.; Yoshikawa, K. On-off switching of transcriptional activity of large DNA through a conformational transition in cooperation with phospholipid membrane. J. Am. Chem. Soc. 2010, 132, 12464–12471. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.M.; Tsumoto, K.; Hamada, T.; Akiyoshi, K.; Nakatani, Y.; Yoshikawa, K. Gene expression within cell-sized lipid vesicles. ChemBioChem 2003, 4, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Yanagisawa, M.; Sato, Y.T.; Fujiwara, K.; Yoshikawa, K. Cell-sized confinement in microspheres accelerates the reaction of gene expression. Sci. Rep. 2012. [Google Scholar] [CrossRef] [Green Version]
- Hase, M.; Yoshikawa, K. Structural transition of actin filament in a cell-sized water droplet with a phospholipid membrane. J. Chem. Phys. 2006, 124, 104903:1–104903:6. [Google Scholar] [CrossRef] [Green Version]
- Takiguchi, K.; Negishi, M.; Tanaka-Takiguchi, Y.; Homma, M.; Yoshikawa, K. Transformation of actoHMM assembly confined in cell-sized liposome. Langmuir 2011, 27, 11528–11535. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.C.; Stuhrmann, B.; Koenderink, G.H. Encapsulation of active cytoskeletal protein networks in cell-sized liposomes. Langmuir 2011, 27, 10061–10071. [Google Scholar] [CrossRef] [PubMed]
- Murrell, M.; Pontani, L.L.; Guevorkian, K.; Cuvelier, D.; Nassoy, P.; Sykes, C. Spreading dynamics of biomimetic actin cortices. Biophys. J. 2011, 100, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Montero, I.; Rodriguez-Garcia, R.; Monroy, F. Artificial spectrin shells reconstituted on giant vesicles. J. Phys. Chem. Lett. 2012, 3, 1583–1588. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Hancock, J.F. Lipid rafts: Contentious only from simplistic standpoints. Nat. Rev. Mol. Cell. Biol. 2006, 7, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Veatch, S.L.; Keller, S.L. Seeing spots: Complex phase behavior in simple membranes. Biochim. Biophys. Acta Mol. Cell. Res. 2005, 1746, 172–185. [Google Scholar] [CrossRef]
- Hamada, T.; Kishimoto, Y.; Nagasaki, T.; Takagi, M. Lateral phase separation in tense membranes. Soft Matter 2011, 7, 9061–9068. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell. Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.A.; Stinson, B.M.; Go, M.S.; Carmona, L.M.; Reminick, J.I.; Fang, X.; Baumgart, T. Temperature-dependent phase behavior and protein partitioning in giant plasma membrane vesicles. Biochim. Biophys. Acta 2010, 1798, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.C.; Lamm, M.H. A biophysical perspective of understanding nanoparticles at large. Phys. Chem. Chem. Phys. 2011, 13, 7273–7283. [Google Scholar] [PubMed]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Dimova, R.; Pouligny, B.; Dietrich, C. Pretransitional effects in dimyristoylphosphatidylcholine vesicle membranes: Optical dynamometry study. Biophys. J. 2000, 79, 340–356. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.; Angelova, M.; Pouligny, B. Adhesion of latex spheres to giant phospholipid vesicles: Statics and dynamics. J. Phys. II 1997, 7, 1651–1682. [Google Scholar]
- Hamada, T.; Morita, M.; Miyakawa, M.; Sugimoto, R.; Hatanaka, A.; Vestergaard, M.C.; Takagi, M. Size-dependent partitioning of nano/microparticles mediated by membrane lateral heterogeneity. J. Am. Chem. Soc. 2012, 134, 13990–13996. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Gerl, M.J. Revitalizing membrane rafts: New tools and insights. Nat. Rev. Mol. Cell. Biol. 2010, 11, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Miura, Y.; Ishii, K.; Araki, S.; Yoshikawa, K.; Vestergaard, M.; Takagi, M. Dynamic processes in endocytic transformation of a raft-exhibiting giant liposome. J. Phys. Chem. B 2007, 111, 10853–10857. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Kayed, R.; Head, E.; Thompson, J.L.; McIntire, T.M.; Milton, S.C.; Cotman, C.W.; Glabe, C.G. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science 2003, 300, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Bleiholder, C.; Dupuis, N.F.; Wyttenbach, T.; Bowers, M.T. Ion mobility-mass spectrometry reveals a conformational conversion from random assembly to beta-sheet in amyloid fibril formation. Nat. Chem. 2011, 3, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Hamada, T.; Tendo, Y.; Hata, T.; Vestergaard, M.C.; Takagi, M. Selective localization of Alzheimer's amyloid beta in membrane lateral compartments. Soft Matter 2012, 8, 2816–2819. [Google Scholar] [CrossRef]
- Hamada, T.; Hagihara, H.; Morita, M.; Vestergaard, M.C.; Tsujino, Y.; Takagi, M. Physicochemical profiling of surfactant-induced membrane dynamics in a cell-sized liposome. J. Phys. Chem. Lett. 2012, 3, 430–435. [Google Scholar] [CrossRef]
- Hamada, T.; Hirabayashi, Y.; Ohta, T.; Takagi, M. Rhythmic pore dynamics in a shrinking lipid vesicle. Phys. Rev. E 2009, 80, 051921:1–051921:7. [Google Scholar] [CrossRef]
- Saitoh, A.; Takiguchi, K.; Tanaka, Y.; Hotani, H. Opening-up of liposomal membranes by talin. Proc. Natl. Acad. Sci. USA 1998, 95, 1026–1031. [Google Scholar] [PubMed]
- Ishii, K.; Hamada, T.; Hatakeyama, M.; Sugimoto, R.; Nagasaki, T.; Takagi, M. Reversible control of exo- and endo-budding transitions in a photosensitive lipid membrane. ChemBioChem 2009, 10, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Sugimoto, R.; Nagasaki, T.; Takagi, M. Photochemical control of membrane raft organization. Soft Matter 2011, 7, 220–224. [Google Scholar] [CrossRef]
- Hamada, T.; Sato, Y.T.; Yoshikawa, K.; Nagasaki, T. Reversible photoswitching in a cell-sized vesicle. Langmuir 2005, 21, 7626–7628. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Sugimoto, R.; Vestergaard, M.C.; Nagasaki, T.; Takagi, M. Membrane disk and sphere: Controllable mesoscopic structures for the capture and release of a targeted object. J. Am. Chem. Soc. 2010, 132, 10528–10532. [Google Scholar] [CrossRef] [PubMed]
- Stottrup, B.L.; Heussler, A.M.; Bibelnieks, T.A. Determination of line tension in lipid monolayers by Fourier analysis of capillary waves. J. Phys. Chem. B 2007, 111, 11091–11094. [Google Scholar] [CrossRef] [PubMed]
- Negishi, M.; Ichikawa, M.; Nakajima, M.; Kojima, M.; Fukuda, T.; Yoshikawa, K. Phase behavior of crowded like-charged mixed polyelectrolytes in a cell-sized sphere. Phys. Rev. E 2011, 83, 061921:1–061921:5. [Google Scholar] [CrossRef]
- Helfrich, M.R.; Mangeney-Slavin, L.K.; Long, M.S.; Djoko, Y.; Keating, C.D. Aqueous phase separation in giant vesicles. J. Am. Chem. Soc. 2002, 124, 13374–13375. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Lipowsky, R.; Dimova, R. Transition from complete to partial wetting within membrane compartments. J. Am. Chem. Soc. 2008, 130, 12252–12253. [Google Scholar] [CrossRef] [PubMed]
- Long, M.S.; Jones, C.D.; Helfrich, M.R.; Mangeney-Slavin, L.K.; Keating, C.D. Dynamic microcompartmentation in synthetic cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5920–5925. [Google Scholar] [CrossRef] [PubMed]
- Stano, P. New aspects of interactions among vesicles. Orig. Life Evol. Biosph. 2007, 37, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Faris, M.D.E.; Lacoste, D.; Pecreaux, J.; Joanny, J.F.; Prost, J.; Bassereau, P. Membrane tension lowering induced by protein activity. Phys. Rev. Lett. 2009, 102, 038102:1–038102:4. [Google Scholar] [CrossRef]
- Rodriguez, N.; Cribier, S.; Pincet, F. Transition from long- to short-lived transient pores in giant vesicles in an aqueous medium. Phys. Rev. E 2006, 74, 061902:1–061902:10. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hamada, T.; Yoshikawa, K. Cell-Sized Liposomes and Droplets: Real-World Modeling of Living Cells. Materials 2012, 5, 2292-2305. https://doi.org/10.3390/ma5112292
Hamada T, Yoshikawa K. Cell-Sized Liposomes and Droplets: Real-World Modeling of Living Cells. Materials. 2012; 5(11):2292-2305. https://doi.org/10.3390/ma5112292
Chicago/Turabian StyleHamada, Tsutomu, and Kenichi Yoshikawa. 2012. "Cell-Sized Liposomes and Droplets: Real-World Modeling of Living Cells" Materials 5, no. 11: 2292-2305. https://doi.org/10.3390/ma5112292
APA StyleHamada, T., & Yoshikawa, K. (2012). Cell-Sized Liposomes and Droplets: Real-World Modeling of Living Cells. Materials, 5(11), 2292-2305. https://doi.org/10.3390/ma5112292