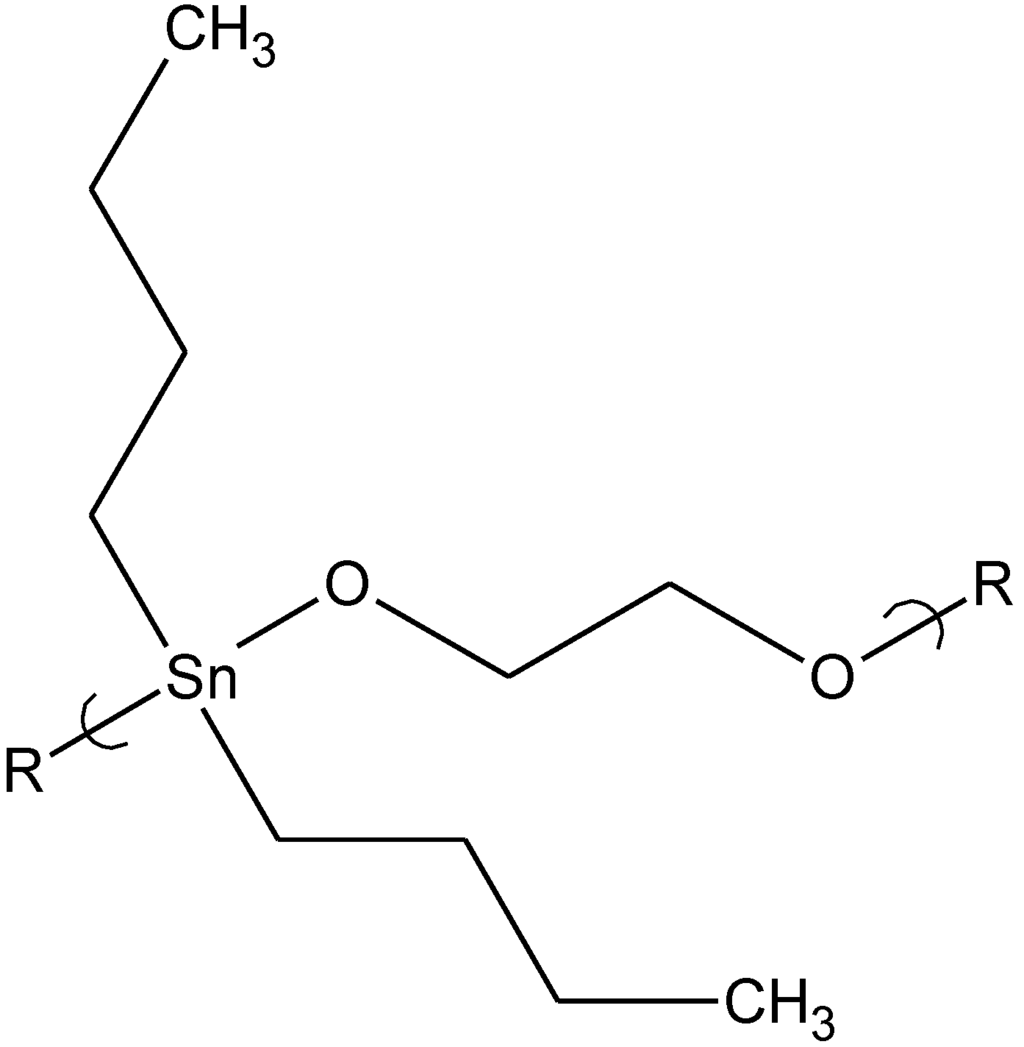
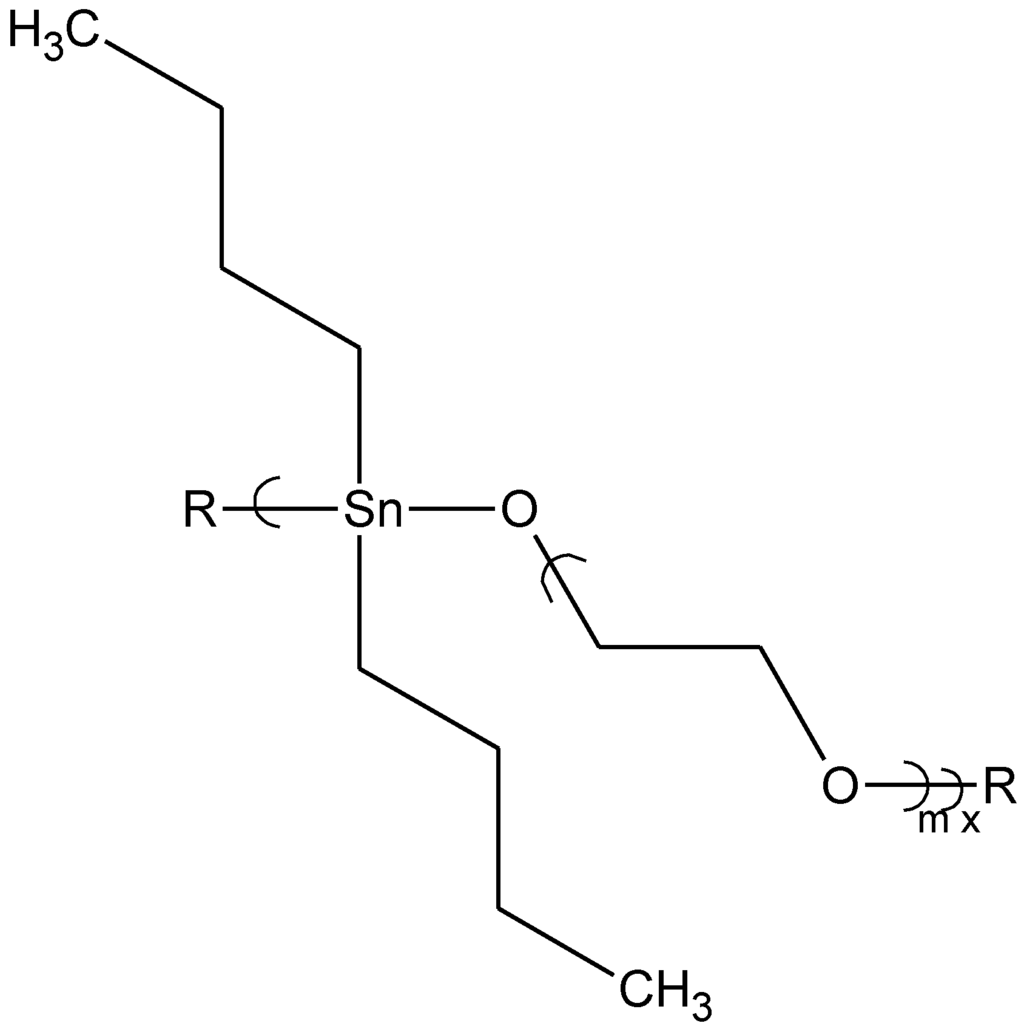
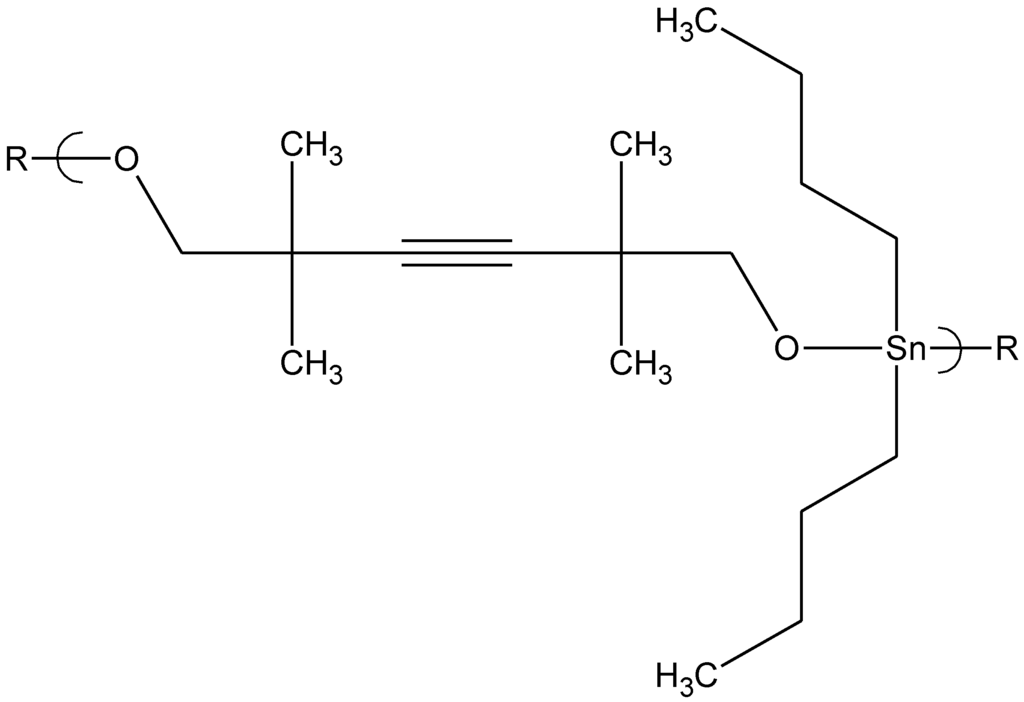
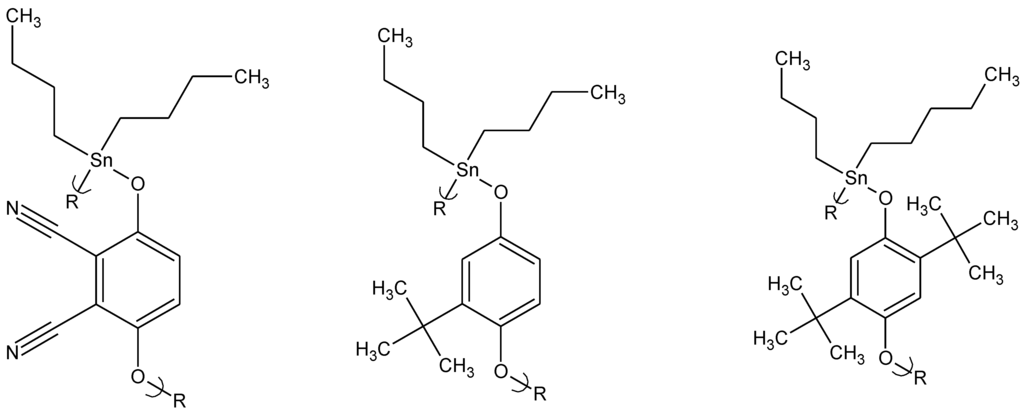
Abstract
Polymers containing platinum and to a lesser extent tin, have repeatedly demonstrated antitumor activity in vitro and in vivo against a variety of cell and tumor types. The mechanisms responsible for the antitumor activity include inducing a delay in cell proliferation and sister chromatid exchanges blocking tumor growth. As most DNA and some RNA viruses require, and even induce, infected cells to initiate DNA replication and subsequent cell division, compounds with antitumor activity will very likely also possess antiviral activity. This article examines the use of metal-containing polymers as a novel class of antivirals.
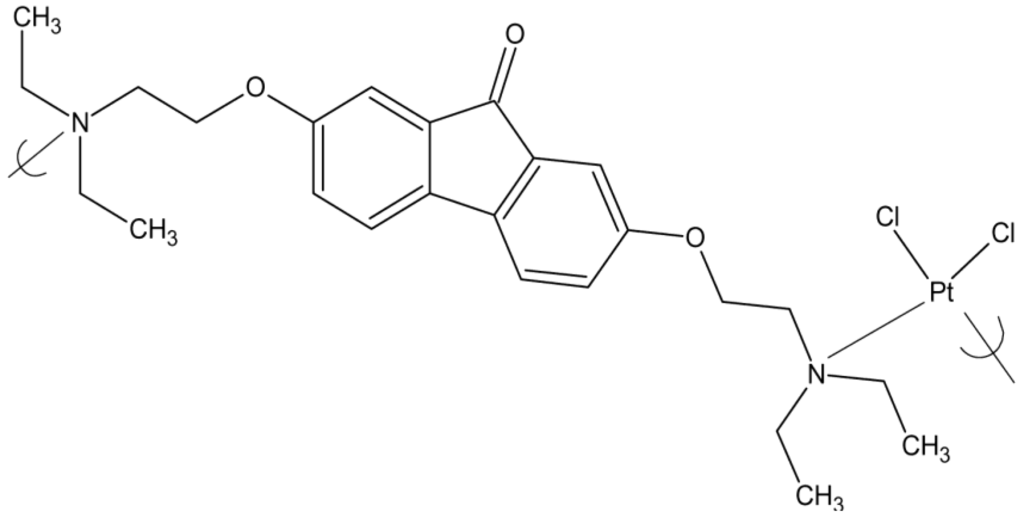
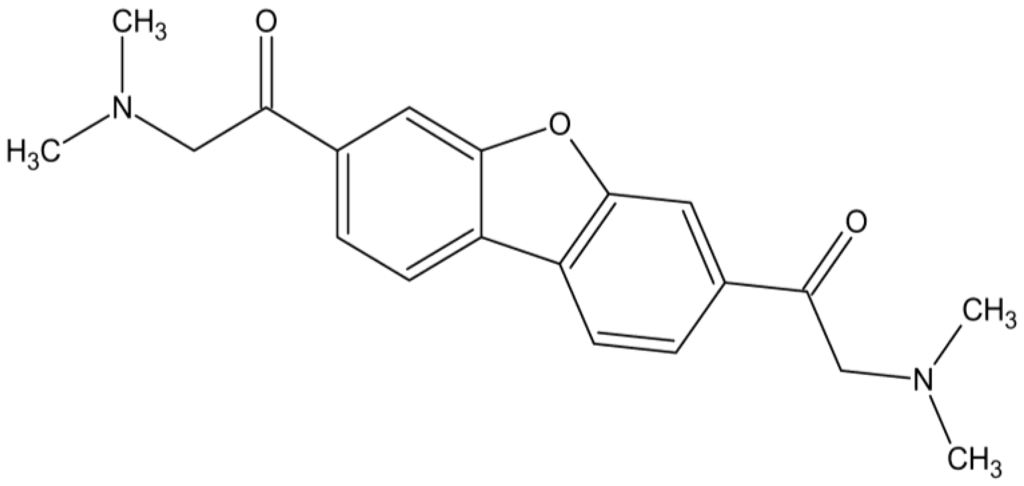
Repeat unit of the product of potassium tetrachloroplatinate II and tilorone
1. Introduction
This paper brings together results from a number of studies of ours over the past few years describing results from investigating the antiviral activity of organotin and cisplatin-like polymers that represent two new groups of compounds that offer antiviral activity. While the studies demonstrate that some of these products exhibit good antiviral activities, they represent just a beginning and much needs to be done before coherent trends are known.
It is not surprising that metal-containing moieties exhibit an effect on many of the essential units within our biosphere. Most metals and metal-containing units possess vacate p, d, and f orbitals that can interact with a variety of biologically entities. The site(s), extent and biological result of these interactions varies widely.
A number of classic chemotherapeutic compounds have demonstrated not only antitumor but unexpected antiviral activities [1,2]. The hypothesis is that cells infected with most DNA viruses and some RNA viruses normally demonstrate increased cellular DNA replication, a condition also seen in transformed cells. A wide variety of metal-containing compounds, including those contained within a polymer, are known to offer antitumor activity. These include gold, ruthenium, vanadanium, copper, titanium, iron (ferrocene), lanthanides and various metal ions. Here our focus will be on organotin and platinum-containing compounds. Organotins are known to have broad antitumor activity [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54] through different mechanisms including the inhibition of cellular DNA replication [7,55,56]. Cisplatin and related compounds are some of the most widely used anticancer drugs. The main site of activity is believed to be on the DNA itself. The topic of platinum-containing compounds including polymers has been reviewed including their anticancer activity [57,58,59,60,61,62]. Here we describe some of results related to their ability to inhibit virus growth.
2. Results and Discussion
2.2. Acyclovir
The inhibitory activity of acyclovir is highly selective [34,60]. Acyclovir is widely used to inhibit several herpes viruses, particularly HSV-1 and HSV-2. It is also used to treat varicella-zoster virus (VZV), Epstein-Barr virus (EBV), and the cytomegalovirus (CMV). Thus, acyclovir is a first line antiviral drug. We synthesized a variety of products focusing our antiviral efforts on organotin materials (Figure 8) [34].
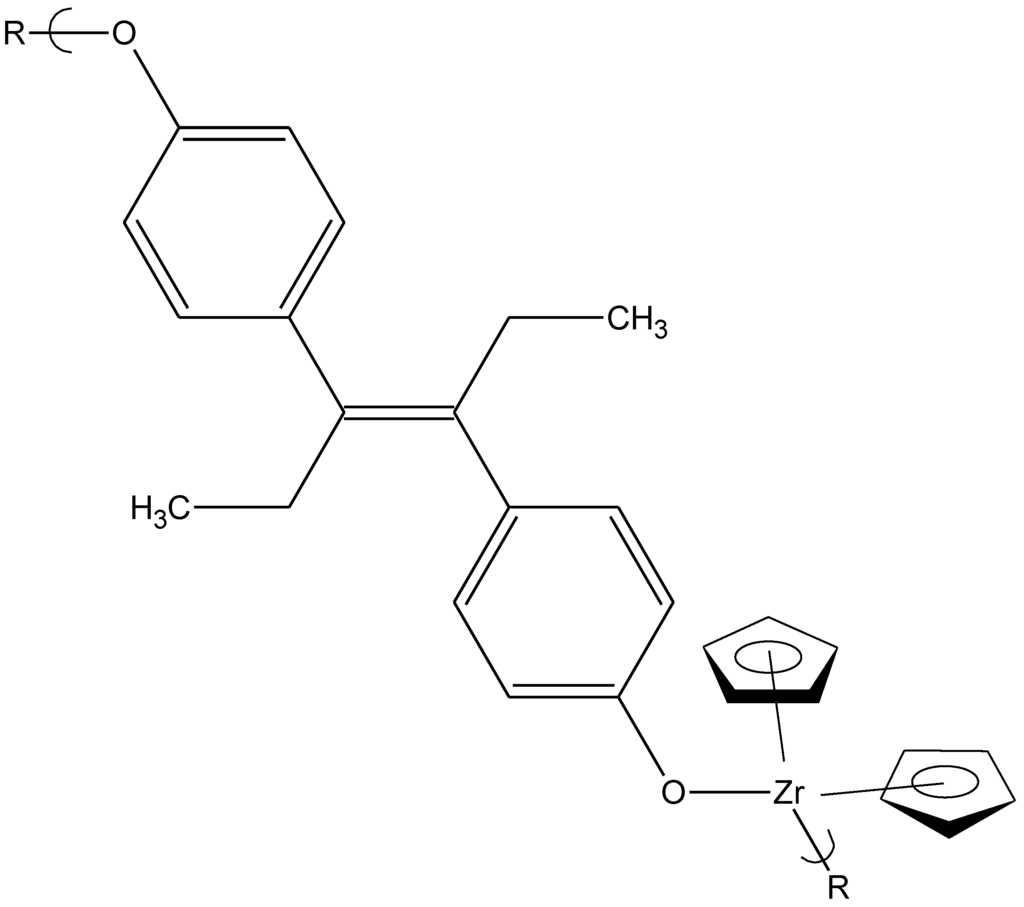
Figure 7.
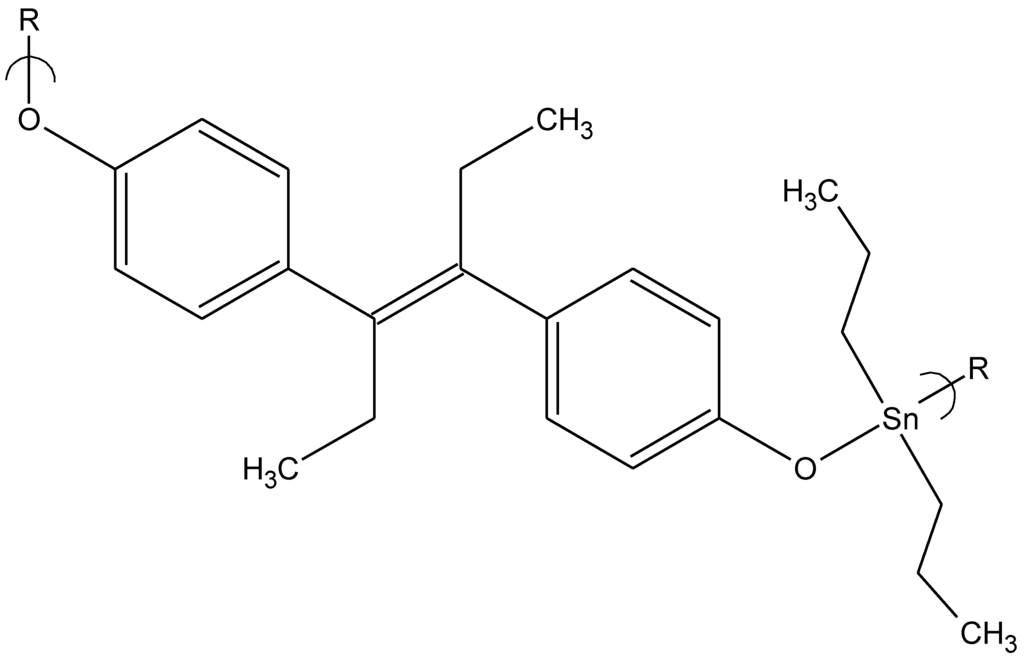
Repeat unit for the product of bis(cyclopentadienyl)zirconium dichloride and diethylstilbestrol.
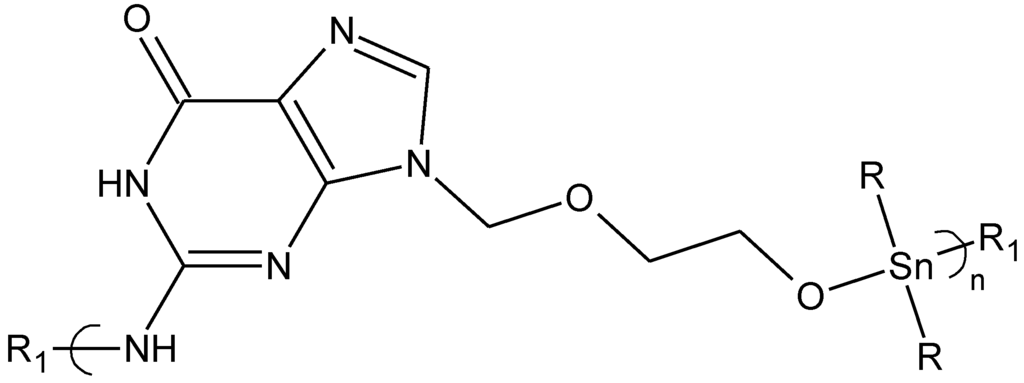
Figure 8.
Repeat unit for the product derived from reaction of acyclovir with organotin dihalides.
Results of antiviral studies showed several things. First, in all cases many of the polymers out performed acyclovir itself. The performance is even greater when compared with the amount of acyclovir present in each sample. In general, the amount of acyclovir within the polymers represents about one half of the weight of the polymer so that all of the polymers out performed acyclovir itself on a total possible amount of acyclovir moiety present. Second, the order of inhibition, based on the concentration needed to effect 50% inhibition is HSV-1 > VZV > Vaccinia WR > Reovirus ST3 with little inhibition found for the reovirus but outstanding inhibition found for HSV-1 and VZV viruses. Third, the order of viral growth inhibition is similar for each of the viruses and also similar to the order of GI values found for the cancer cell lines. The trend with respect to VZV is the most divergent of the trends but it still has dibutyltin and diethyltin inhibiting at the lowest concentrations. For HSV-1 the order is dibutyltin > diethyltin > diphenyltin = dioctyltin > acyclovir > dicyclohexyltin and for VZV the trend is diethyltin > dibutyltin > dioctyltin > diphenyl > dicyclohexyltin > acyclovir.
The minimum inhibition concentration, MIC, values are much lower indicating that for viral infections where the number of viruses are not great, that the polymers and acyclovir itself should demonstrate good antiviral activity. The overall trend is approximately dibutyltin > diethyltin > diphenyltin > dioctyltin > acyclovir > dicyclohexyltin again similar to that found in other parts of the study that focused on cancer.
These studies are consistent with some of the organotin polymers, namely the dibutyltin, diethyltin and diphenyltin polymers, offering superior inhibition in comparison to acyclovir. Again, these trends are accentuated when considering that only about half of the polymers weight is derived from acyclovir. Thus, the activities are not due to the acyclovir alone, but are enhanced either because of the presence of the organotin moiety, presence of the acyclovir within a polymer, through control release of the acyclovir, or some combination of these factors.
3. Experimental Section
3.1. Synthesis of Bioactive Materials
The bioactive materials described in this presentation have been synthesized employing two well known and simple systems. The organotin polymers were synthesized employing the interfacial polycondensation system. Interfacial reactions are carried out under essentially non-equilibrium conditions and as such are not as sensitive to non-equal molar reactant amounts in achieving high polymer. The technique is herterophasic, with two fast-reacting reactants dissolved in a pair of immiscible liquids, one of which is usually water. The aqueous phase typically contains the Lewis base such as diol, diamine, or dithiol. The organic phase contains the Lewis acid, generally an acid halide such as in this case the organotin dihalides, dissolved in a suitable organic solvent such as hexane. Reaction occurs near the interface, hence the name. The interfacial synthetic process is employed industrially to synthesize polycarbonates and aromatic amines (aramids). For the present systems, reactions are rapid occurring generally within less than 30 seconds.
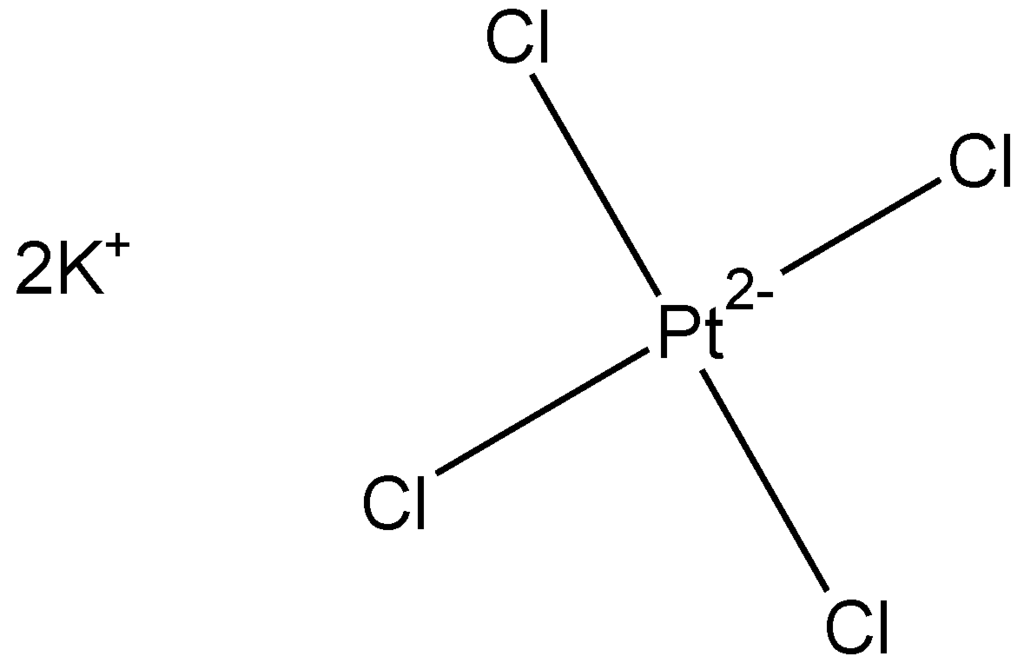
The platinum-containing polymers are synthesized employing a simple aqueous solution system. Briefly, each of the reactants, here the potassium tetrachloroplatinate and diamine, are dissolved in separate aqueous solutions. These solutions are mixed together with mild stirring. Polymer is produced as a precipitate after several hours.
In all cases, the reactants are available commercially. Thus, the polymers discussed in this review can be easily and rapidly synthesized employing commercially available reactants and commercially employed reaction systems.
The two polymer types are synthesized employing two different approaches. For the synthesis of the organotin polymers, the products are produced employing a condensation mechanism where the tin on the organotin dihalide acts as an electrophillic site being attacked by Lewis bases such as diols and diamines, emitting HX for each reaction step (Figure 14).
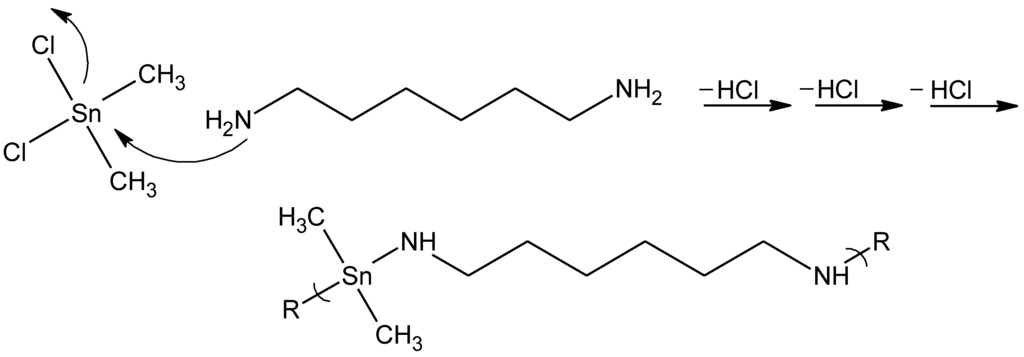
Figure 14.
General reaction scheme for the reaction between dimethyltin dichloride and 1.6-hexamine to form a dimethyltin polyamine.
In the case of the platinum derivatives based on cisplatin, the reaction is a coordination reaction where the diamine simply coordinates with the platinum (Figure 15).
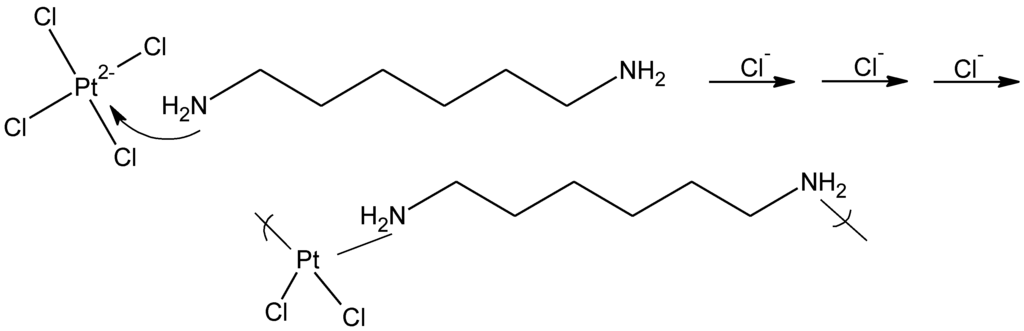
Figure 15.
General reaction scheme for the reaction between tetrachloroplatinate and 1,6-hexanediamine forming the platinum-containing polymer.
On a stability level, under room conditions, the organotin polymers are generally stable for months to years in solution whereas the platinum-containing polymers are generally stable for weeks to months. Both are stable for years as solids. For our studies, the samples are maintained well below zero except when they are in the process of being tested.
3.3. Vaccinia and HSV-1 Plaque Assays
WI-38 cells were plated at 80% confluency in 24 well plates. After an overnight incubation, the media removed and 100 µL of the media without serum containing the polymeric drug and virus (MOI < 1) was added. After a one hour incubation, rocking every 15 min, 500 µL of MEM with 10% FBS and 1X APS was added to the wells. The vaccinia infections were incubated for 10 h, the HSV-1 infections for 96 h. The plates were sonicated to release the entire virus in the cells and the samples stored at −20 °C until the plaque assays performed. The infections were done in duplicate. For the plaque assays, the viral samples were serially diluted from 10−1 to 10−4 in MEM without serum on the day of the infection. 143 (vaccinia) and vero (HSV-1) cells were plated at 80% confluency in 12 wells plates. After an overnight incubation, the media was removed and 125 µL of the serial dilutions viral samples from the WI-38 infections added. After a one hour incubation, rocking every 15 min, one milliliter of MEM with 5% BCS was added to each well. After a 48 h incubation, the media was removed, and crystal violet added to the cells. The viable cells were stained with the crystal violet, allowing the plaques to be counted. The wells that contain between 30 and 300 plaques were counted and the viral titer calculated. The GI50 of the drugs with the WI-38 cells and the concentration of the drug that inhibits 50% of the virus were compared. The plaque assays were done in duplicate.
4. Conclusions
This review of current studies examining the antiviral activity of metal-containing polymers represents only a beginning in the quest for urgently needed antiviral agents. We have focused on organotin and cisplatin-like polymers, an area with the most activity. The initial results indicate that further study is merited and that these two groups of metal-containing polymers may indeed represent two families of potential commercial antiviral drugs. This paper is intended to alert others of the potential use of metal-containing polymers in the war against viruses.
In general there needs to be a better correlation between the particular viruses inhibited and the structures of the compounds responsible for this inhibition. Further, after appropriate screening the compounds need to undergo in vivo testing. As this is occurring, mechanistic and site(s) of activity need to be investigated. Towards these ends the following need to be accomplished.
A number of studies will need to be carried out to demonstrate the ability of metal-containing biologically active materials to combat particular viruses. Along with continued screening, additional viruses should be studied. Such studies will not only identify additional candidates, but they may assist in identifying the site and mode of activity.
From the current studies with organotins and Group IVB metallocenes, the products from the Lewis bases hydroquinone and diethylstilbestrol exhibit the best promise as antiviral materials. In fact, with the exception of the organotin polyethers derived from ethylene glycol and PEG, all of the products that exhibit an ability to inhibit viral growth are derived from Lewis bases that contain pi-bonding that offer the ability to pi-bond with elements within the cell or viral organism to interact with and to effect their inhibition and/or destruction. By themselves, these Lewis bases exhibit little or no antiviral activity but combined with the organotin moiety they exhibit a range of activities. These products, along with the ethylene glycol and water soluble PEG products, merit further testing against other viruses and in vivo evaluation against the particular viruses where they exhibit good inhibition.
Along with the materials that have already shown promise, other compounds that exhibit good activity against particular viruses should be tested in vivo to determine if they continue to show antiviral activity.
The future for these compounds rests on the ability of the chemists and biochemists to convince the biologists and virologists, and more importantly the funding agencies, that organometallic polymers are viable as drugs to treat human infections. Laboratory and cell culture data continues to accumulate, or in some cases remain buried in the literature, but little work has been done to define the toxicity and possible efficacy of these compounds in humans.
In the next 10 years, in preparation for phase I clinical trials in humans, we expect/hope to see safety pharmacology studies conducted in rats and extended to dogs. Pharmacokinetic and metabolism studies, as well as general toxicity studies, need to be also undertaken in rats followed by reproduction toxicity studies. A strong record of compound discovery and cell testing exist but to move these compounds forward the absorption, distribution, metabolic and excretion profiles of these compounds in animals, must be produced. Further, incorporation of dyes such as fluorescein into these polymer backbones can be easily accomplished allowing for additional identification of specific sites and organs where these polymers concentrate.
These potential antiviral drugs are all synthesized rapidly from commercially available reactants employing techniques that are already industrially utilized to synthesize millions of tons of materials. Thus, the ready availability of these agents is apparent.
There exists a correlation between ability to inhibit cancer cell growth and ability to inhibit viral replication. Further, compounds derived from known antibacterial agents show the ability to inhibit bacteria, cancer cell lines, and viral replication contributing to the ongoing increase in data that demonstrates the common cellular pathways utilized during tumor growth and bacterial/viral infections [88,89,90,91,92,93,94].
In summary, organotin polymers exhibit viral reduction specific to the nature of the organotin and Lewis base nucleophiles. With the exception of the organotin polyethers derived from ethylene glycol and PEG, the first water soluble organotin polymer, the Lewis base contains a site of unsaturation allowing the polymers to interact with various biological agents that may interfere with viral replication.
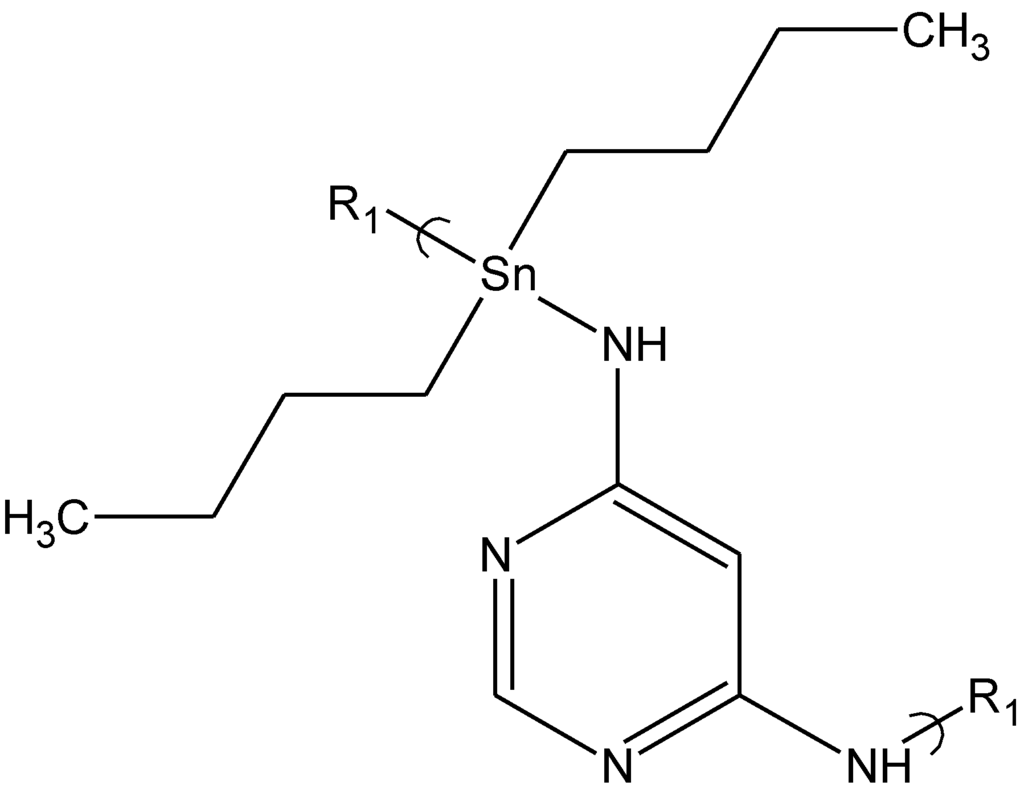
The cisplatin polymers inhibit viral replication at low concentrations and the tilorone polymers demonstrate viral inhibition at lower concentrations and with a greater variety of virus than the known antiviral drug acyclovir.
References
- Takimoto, C.H.; Wright, J.; Arbuck, S.G. Clinical applications of the camptothecins. Biochim. Biophys. Acta 1998, 1400, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Gasperi-Campani, A.; Roncuzzi, L.; Zoli, W.; Amadori, D. Saporin 6 and lonidamine in primary cell cultures from human breast carcinomas: a synergistic effect. Anticancer Drug Des. 1997, 12, 91–98. [Google Scholar] [PubMed]
- Alama, A.; Tasso, B.; Novelli, F.; Sparatore, F. Organometallic compounds in oncology: implications of novel organotins as antitumor agents. Drug Discov. Today 2009, 14, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, Y. Biological activity of tin and immunity. Sangyo Eiseigaku Zasshi 1997, 39, 1–20. [Google Scholar] [PubMed]
- Abdellah, M.A.; Hadjikakou, S.K.; Hadjiliadis, N.; Kubicki, M.; Bakas, T.; Kourkoumelis, N.; Simos, Y.V.; Karkabounas, S.; Barsan, M.M.; Butler, I.S. Synthesis, Characterization, and Biological Studies of Organotin(IV) Derivatives with o- or p-hydroxybenzoic Acids. Bioinorg. Chem. Appl. 2009, 2009, 542979:1–542979:12. [Google Scholar]
- Bara, A.; Socaciu, C.; Silvestru, C.; Haiduc, I. Antitumor organometallics. I. Activity of some diphenyltin (IV) and diphenylantimony (III) derivatives on in vitro and in vivo Ehrlich ascites tumor. Anticancer Res. 1991, 11, 1651–1655. [Google Scholar] [PubMed]
- Alama, A.; Viale, M.; Cilli, M.; Bruzzo, C.; Novelli, F.; Tasso, B.; Sparatore, F. In vitro cytotoxic activity of tri-n-butyltin(IV)lupinylsulfide hydrogen fumarate (IST-FS 35) and preliminary antitumor activity in vivo. Invest. New Drug. 2009, 27, 124–130. [Google Scholar] [CrossRef]
- Barbieri, F.; Sparatore, F.; Cagnoli, M.; Bruzzo, C.; Novelli, F.; Alama, A. Antiproliferative activity and interactions with cell-cycle related proteins of the organotin compound triethyltin(IV)lupinylsulfide hydrochloride. Chem. Biol. Interact 2001, 134, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, F.; Viale, M.; Sparatore, F.; Favre, A.; Cagnoli, M.; Bruzzo, C.; Novelli, F.; Alama, A. Cytotoxicity in vitro and preliminary antitumor activity in vivo of a novel organotin compound. Anticancer Res. 2000, 20, 977–980. [Google Scholar] [PubMed]
- Barbieri, F.; Viale, M.; Sparatore, F.; Schettini, G.; Favre, A.; Bruzzo, C.; Novelli, F.; Alama, A. Antitumor activity of a new orally active organotin compound: A preliminary study in murine tumor models. Anticancer Drugs 2002, 13, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Barot, G.; Shahi, K.R.; Roner, M.R.; Carraher, C.E. Synthesis, structural characterization, and ability to inhibit cancer growth of a series of organotin poly(ethylene glycols). J. Inorg. Organomet. Polym. Mater. 2007, 17, 595–603. [Google Scholar] [CrossRef]
- Barot, G.; Shahi, K.R.; Roner, M.R.; Carraher, C.E. Synthesis, anomalous fiber formation, and preliminary anticancer study of the organotin polyether derived from 2-butyne-1,4-diol. J. Polym. Mater. 2006, 23, 423–436. [Google Scholar]
- Carraher, C.E.; Barot, G.; Battin, A. Reactions Between the Matrix and Ion Fragments Created from the MALDI MS of Organotin-Containing Polymers. J. Polym. Mater. 2009, 26, 17–31. [Google Scholar]
- Carraher, C.E.; Roner, M.R.; Shahi, K.; Ashida, Y.; Barot, G. Synthesis and initial cell line results of organotin polyethers containing diethylstilbestrol. J. Inorg. Organomet. Polym. Mater. 2008, 18, 180–188. [Google Scholar] [CrossRef]
- Chasapis, C.T.; Hadjikakou, S.K.; Garoufis, A.; Hadjiliads, N.; Bakas, T.; Kubicki, M.; Ming, Y. Organotin(IV) derivatives of L-Cysteine and their in vitro anti-tumor properties. Bioinorg. Chem. Appl. 2004, 2, 43–54. [Google Scholar] [CrossRef]
- Choudhuri, S.K.; Das Dutta, S.; Chatterjee, R.; Chowdhury, J.R. Antitumor activity of some organotin complexes of hydroxamic acids derived from dibasic carboxylic acid. Chemotherapy 1991, 37, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Crowe, A.J.; Smith, P.J.; Cardin, C.J.; Parge, H.E.; Smith, F.E. Possible pre-dissociation of diorganotin dihalide complexes: relationship between antitumour activity and structure. Cancer Lett. 1984, 24, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Gielen, M.; Kayser, F.; Zhidkova, O.B.; Kampel, V.T.; Bregadze, V.L.; de Vos, D.; Biesemans, M.; Mahieu, B.; Willem, R. Synthesis, characterization and in vitro antitumour activity of novel organotin derivatives of 1,2- and 1,7-Dicarba-Closo-dodecaboranes. Met. Based Drugs 1995, 2, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Hadjikakou, S. K.; Ozturk, II; Xanthopoulou, M. N.; Zachariadis, P. C.; Zartilas, S.; Karkabounas, S.; Hadjiliadis, N. Synthesis, structural characterization and biological study of new organotin(IV), silver(I) and antimony(III) complexes with thioamides. J. Inorg. Biochem. 2008, 102, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Holloway, L.N.; Pannell, K.H.; Whalen, M.M. Effects of a series of triorganotins on atp levels in human natural killer cells. Environ. Toxicol. Pharmacol. 2008, 25, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Koch, B.; Baul, T.S.; Chatterjee, A. Cell proliferation inhibition and antitumor activity of novel alkyl series of diorganotin(IV) compounds. J. Appl. Toxicol. 2008, 28, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.; Sarma, K.D.; Antony, A. Differential effects of tri-n-butylstannyl benzoates on induction of apoptosis in K562 and MCF-7 cells. IUBMB Life 2000, 49, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Roner, M.R.; Carraher, C.E., Jr.; Shahi, K.; Ashida, Y.; Barot, G. Ability of group IVB metallocene polyethers containing dienestrol to arrest the growth of selected cancer cell lines. BMC Cancer 2009, 9, 358. [Google Scholar] [CrossRef] [PubMed]
- Roner, M.R.; Shahi, K.R.; Barot, G.; Battin, A.; Carraher, C.E. Preliminary results for the inhibition of pancreatic cancer cells by organotin polymers. J. Inorg. Organomet. Polym. Mater. 2009, 19, 410–414. [Google Scholar] [CrossRef]
- Samuel, P.M.; de Vos, D.; Raveendra, D.; Sarma, J.A.; Roy, S. 3-D QSAR studies on new dibenzyltin(IV) anticancer agents by comparative molecular field analysis (CoMFA). Bioorg Med. Chem. Lett. 2002, 12, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Syng-Ai, C.; Basu Baul, T.S.; Chatterjee, A. Inhibition of cell proliferation and antitumor activity of a novel organotin compound. J. Environ. Pathol. Toxicol. Oncol. 2001, 20, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Xanthopoulou, M.N.; Hadjikakou, S.K.; Hadjiliadis, N.; Schurmann, M.; Jurkschat, K.; Michaelides, A.; Skoulika, S.; Bakas, T.; Binolis, J.; Karkabounas, S.; Charalabopoulos, K. Synthesis, structural characterization and in vitro cytotoxicity of organotin(IV) derivatives of heterocyclic thioamides, 2-mercaptobenzothiazole, 5-chloro-2-mercaptobenzothiazole, 3-methyl-2-mercaptobenzothiazole and 2-mercaptonicotinic acid. J. Inorg. Biochem. 2003, 96, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Yamabe, Y.; Hoshino, A.; Imura, N.; Suzuki, T.; Himeno, S. Enhancement of androgen-dependent transcription and cell proliferation by tributyltin and triphenyltin in human prostate cancer cells. Toxicol. Appl. Pharmacol. 2000, 169, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Zhao, A.; Shahi, K.R.; Roner, M.R.; Barot, G.; Fiore, T.; Pellerito, C.; Scopelliti, M.; Pellerito, L.; Carraher, C.E. Ciprofloxacin Polymers Derived from Diallyltin and Divinyltin Dihalides. J. Polym. Mater. 2008, 25, 213–236. [Google Scholar]
- Barbieri, R.; Ruisi, G.; Atassi, G. The antitumor activity and the structure of bis(adeninato-N9) diphenyltin(IV). J. Inorg. Biochem. 1991, 41, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Barot, G.; Roner, M.; Shahi, K.; Carraher, C. Synthesis and structural and preliminary anticancer characterization of the organotin polyether from dibutyltin dichloride and 2,4-dimethyl-3-hexyne-2,5-diol. Polym. Mater. Sci. Eng. 2008, 99, 383–385. [Google Scholar]
- Barot, G.; Roner, M.R.; Naoshima, Y.; Nagao, K.; Shahi, K.; Carraher, C.E. Synthesis, structural characterization, and preliminary biological characterization of organotin polyethers derived from hydroquinone and substituted hydroquinones. J. Inorg. Organomet. Polym. Mater. 2009, 19, 12–27. [Google Scholar] [CrossRef]
- Barot, G.; Shahi, K.; Roner, M.; Carraher, C. Synthesis, structural characterization, and ability to inhibit cancer growth of a series of organotin poly(ethylene glycols). Journal J. Inorg. Organomet. Polym. Mater. 2007, 17, 595–603. [Google Scholar] [CrossRef]
- Carraher, C.; Sabir, T.; Roner, M.; Shahi, K.; Bleicher, R.; Roehr, J.; Bassett, K. Synthesis of organotin polyamine ethers containing acyclovir and their preliminary anticancer and antiviral activity. J. Inorg. Organomet. Polym. Mater. 2006, 16, 249–257. [Google Scholar] [CrossRef]
- Carraher, C.; Siegmann-Louda, D. Organotin macromolecules as anticancer drugs. In Macromolecules Containing Metal and Metal-Like Elements, Vol 3. Biomedical Applications; Wiley: Hoboken, NJ, USA, 2004; Volume 3, pp. 57–74. [Google Scholar]
- Carraher, C.E., Jr.; Roner, M.R.; Barot, G. Organotin-containing polyethers as potential anticancer drugs. Cancer Res. J. 2010, 3, 207–232. [Google Scholar]
- Fitzner, B.; Brock, P.; Holzhuter, S.A.; Nizze, H.; Sparmann, G.; Emmrich, J.; Liebe, S.; Jaster, R. Synergistic growth inhibitory effects of the dual endothelin-1 receptor antagonist bosentan on pancreatic stellate and cancer cells. Dig. Dis. Sci. 2009, 54, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Gielen, M.; Willem, R. Cytotoxic activity of bis-[di-n-butyl(4-aminosalicylato)tin] oxide, NSC: 628561, bis-[diphenyl(4-aminosalicylato)tin] oxide, NSC: 628562, and some related compounds, against a series of human tumour cell lines. Anticancer Res. 1992, 12, 257–268. [Google Scholar] [PubMed]
- Gielen, M.; Willem, R. Cytotoxic activity against a series of human tumour cell lines of some diorganotin(iv) 1,2-ethylenediamine N,N′-diacetates, N-(2-hydroxyethyl)- and N-(carbamoylmethyl)-iminodiacetates, and ortho-aminobenzoates. Anticancer Res. 1992, 12, 269–271. [Google Scholar] [PubMed]
- Gielen, M.; Willem, R. In vitro cytotoxicity of diorganotin (IV) trimethoxy-benzoates against sixty human NCl tumor cell lines. Anticancer Res. 1992, 12, 1323–1325. [Google Scholar] [PubMed]
- Hoti, N.; Ma, J.; Tabassum, S.; Wang, Y.; Wu, M. Triphenyl tin benzimidazolethiol, a novel antitumor agent, induces mitochondrial-mediated apoptosis in human cervical cancer cells via suppression of HPV-18 encoded E6. J. Biochem. 2003, 134, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Jan, C.; Jiann, B.; Lu, Y.; Chang, H.; Su, W.; Chen, W.; Yu, C.; Huang, J. Effect of the organotin compound triethyltin on Ca2+ handling in human prostate cancer cells. Life Sci. 2000, 70, 1337–1345. [Google Scholar] [CrossRef]
- Jan, C.R.; Jiann, B.P.; Lu, Y.C.; Chang, H.T.; Su, W.; Chen, W.C.; Yu, C.C.; Huang, J.K. Effect of the organotin compound triethyltin on Ca2+ handling in human prostate cancer cells. Life Sci. 2002, 70, 1337–1345. [Google Scholar] [CrossRef] [PubMed]
- Kopf-Maier, P.; Janiak, C.; Schumann, H. Antitumor properties of organometallic metallocene complexes of tin and germanium. J. Cancer Res. Clin. Oncol. 1988, 114, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Roner, M.; Carraher, C.; Roehr, J.; Bassett, K. Antiviral and anticancer activity of organotin polymers and reactants derived from norfloxacin and ampicillin. J. Polym. Mater. 2006, 23, 153–159. [Google Scholar]
- Saxena, A.; Tandon, J.P. Antitumor activity of some diorganotin and tin (IV) complexes of Schiff bases. Cancer Lett. 1983, 19, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.K.; Huber, F. Organotin compounds and cancer chemotherapy. Coord. Chem. Revs. 1989, 95, 109–123. [Google Scholar] [CrossRef]
- Shahi, K.; Roner, M.; Ashida, Y.; Barot, G.; Carraher, C. Ability of organotin polyethers derived from dienestrol to inhibit ovarian, colon, lung, and breast cancer cells. Polym. Mater. Sci. Eng. 2009, 100, 22–25. [Google Scholar]
- Shahi, K.; Roner, M.; Barot, G.; Carraher, C. Ability of a series of organotin polyethers containing methylene spacers to inhibit prostrate, breast, colon, and lung cancer cell lines. Polym. Mater. Sci. Eng. 2008, 98, 375–377. [Google Scholar]
- Shahi, K.; Roner, M.R.; Carraher, C.E.; Barot, G. Selected organotin polyethers as potential anti-cancer drugs. Polym. Mater. Sci. Eng. 2006, 94, 466–468. [Google Scholar]
- Siegmann-Louda, D.; Carraher, C.; Nagy, D.; Snedden, D.; Ross, J. Simple organotin polyethers as potential anti-cancer drugs. Polym. Mater. Sci. Eng. 2003, 89, 487–488. [Google Scholar]
- Spencer, P.; Holt, W. Anticancer Drugs: Design, Delivery and Pharmacology; Nova Science Publishers: Hauppauge, NY, USA, 2009. [Google Scholar]
- Whalen, M.M.; Loganathan, B.G. Butyltin exposure causes a rapid decrease in cyclic AMP levels in human lymphocytes. Toxicol. Appl. Pharmacol. 2001, 171, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Carraher, C.; Roner, M.R.; Barot, G. Anticancer Drugs: Design, Delivery and Pharmacology; Nova Science: Hauppauge, NY, USA, 2009. [Google Scholar]
- Koch, B.; Basu Baul, T.S.; Chatterjee, A. p53-dependent antiproliferative and antitumor effect of novel alkyl series of diorganotin(IV) compounds. Invest. New Drugs 2009, 27, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Zucker, R.M.; Elstein, K.H.; Easterling, R.E.; Massaro, E.J. Flow cytometric analysis of the cellular toxicity of tributyltin. Toxicol. Lett. 1988, 43, 201–218. [Google Scholar] [CrossRef] [PubMed]
- Siegmann-Louda, D.; Carraher, C. Polymeric platinum-containing drugs in the treatment of cancer. In Macromolecules Containing Metal and Metal-Like Elements. Vol. 3. Biomedical Applications; Wiley: Hoboken, NY, USA, 2004; Volume 3, pp. 119–192. [Google Scholar]
- Gerth, H.U.; Rompel, A.; Krebs, B.; Boos, J.; Lanvers-Kaminsky, C. Cytotoxic effects of novel polyoxotungstates and a platinum compound on human cancer cell lines. Anticancer Drugs 2005, 16, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Wheate, N.J.; Collins, J.G. Multi-nuclear platinum drugs: a new paradigm in chemotherapy. Curr. Med. Chem. Anticancer Agents 2005, 5, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.J.; Friedlos, F.; Lydall, D.A.; Roberts, J.J. Mechanism of cytotoxicity of anticancer platinum drugs: Evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986, 46, 1972–1979. [Google Scholar] [PubMed]
- Arandjelovic, S.; Tesic, Z.; Juranic, Z.; Radulovic, S.; Vrvic, M.; Potkonjak, B.; Ilic, Z. Antiproliferative activity of some cis-/trans-platinum(II) complexes on HeLa cells. J. Exp. Clin. Cancer Res. 2002, 21, 519–526. [Google Scholar] [PubMed]
- Radulovic, S.; Tesic, Z.; Manic, S. Trans-platinum complexes as anticancer drugs: recent developments and future prospects. Curr. Med. Chem. 2002, 9, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Fricker, S.P. A Screening Strategy for Metal Antitumor Agents as Exemplified by Gold(III) Complexes. Met. Based Drugs 1999, 6, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Milacic, V.; Fregona, D.; Dou, Q.P. Gold complexes as prospective metal-based anticancer drugs. Histol. Histopathol. 2008, 23, 101–108. [Google Scholar] [PubMed]
- Shahi, K.; Roner, M.; Barot, G.; Carraher, C. Ability of dibutyltin poly(ethylene oxides) to inhibit the viruses associated with small pox and herpes. Polym. Mater. Sci. Eng. 2008, 99, 51–53. [Google Scholar]
- Shahi, K.; Roner, M.; Battin, A.; Carraher, C. Ability of organotin pyrimidine polyamines to hinhibit HSV-1 (Herpes simplex virus) and Vaccinia (small pox virus) viruses. Polym. Mater. Sci. Eng. 2008, 99, 365–367. [Google Scholar]
- Trombley, M.; Biegley, N.; Carraher, C.; Giron, D. Effect of Tetramisole and Its Platinum Polyamine on Mice Infected with Encephalomyocarditis-Variant-D Virus; Plenum: Hannover, Germany, 1988; pp. 223–238. [Google Scholar]
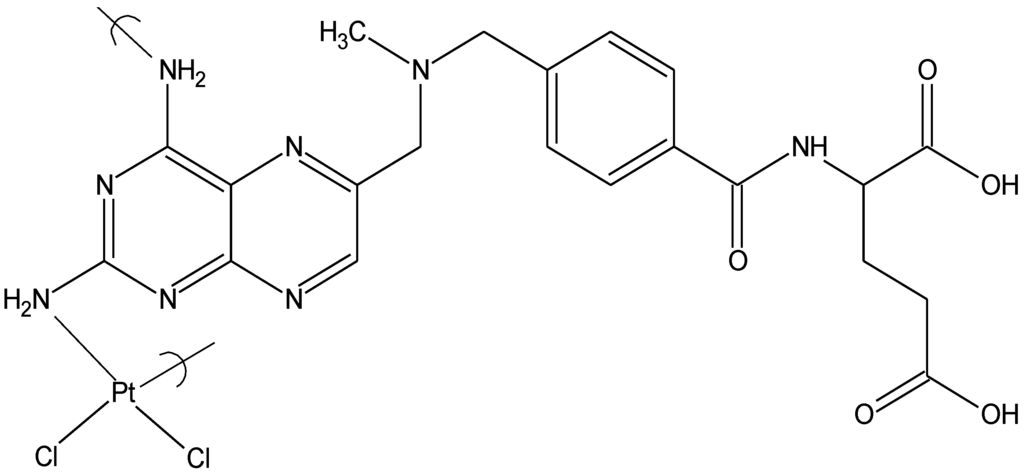
- Carraher, C.; Lopez, I.; Giron, D. Synthesis, structural and biological characterization of the polymeric platinol derivative of methotrexate for the treatment of juvenile diabetes. Polym. Mater. 1985, 53, 644–648. [Google Scholar]
- Roner, M.; Carraher, C.; Dhanji, S. Antiviral activity of cisplatin derivatives of methotrexate against Vaccinia Virus, Varicella Zoster Virus (VZV), Reovirus ST3, and Herpes Simplex Virus (HSV-1). Polym. Mater. Sci. Eng. 2005, 93, 410–413. [Google Scholar]
- Roner, M.; Carraher, C.; Dhanji, S.; Barot, G. Antiviral and anticancer activity of cisplatin derivatives of methotrexate. J. Polym. Mater. 2007, 24, 371–385. [Google Scholar]
- Carraher, C.; Lopez, I.; Giron, D. Polymeric Platinol Derivative of Methotrexate for the Treatment of Virally Related Juvenile Diabetes; Plenum: Hannover, Germany, 1987. [Google Scholar]
- Roner, M.; Carraher, C.; Dhanji, S.; Barot, G. Antiviral and anticancer activity of cisplatin derivatives of tilorone. J. Inorg. Organomet. Polym. Mater. 2008, 18, 374–383. [Google Scholar] [CrossRef]
- Roner, M.R.; Carraher, C.E.; Zhao, A.; Roehr, J.L.; Bassett, K.D.; Siegmann-Louda, D. Activity of acyclovir, ciprofloxacin, and organotin polymers derived from acyclovir and ciprofloxacin against Herpes Simplex virus (HSV-1) and Varicella Zoster virus (VZV). Polym. Mater. Sci. Eng. 2004, 90, 515–518. [Google Scholar]
- Roner, M.R.; Carraher, C.E.; Roehr, J.L.; Bassett, K.D.; Siegmann-Louda, D.W. Anti-viral activity of norfloxacin and ampicillin and dibutyltin polymers derived from norfloxacin and ampicillin against reovirus ST3, vaccinia virus, herpes simplex virus (HSV-1), and varicella zoster virus (VZV). Polym. Mater. Sci. Eng. 2004, 91, 744–746. [Google Scholar]
- Chandra, P.; Wright, G.J. Tilorone hydrochloride: The drug profile. Top Curr. Chem. 1977, 72, 125–148. [Google Scholar] [PubMed]
- Chandra, P.; Will, G.; Gericke, D.; Gotz, A. Inhibition of DNA polymerases from RNA tumor viruses by tilorone and congeners: Site of action. Biochem. Pharmacol. 1974, 23, 3259–3265. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Zunino, F.; Gaur, V.P.; Zaccara, A.; Woltersdorf, M.; Luoni, G.; Gotz, A. Mode of tilorone hydrochloride interaction to DNA and polydeoxyribonucleotides. FEBS Lett. 1972, 28, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Karpov, A.V.; Antonenko, S.V.; Barbasheva, E.V.; Spivak, N. Study of anti-HIV activity of the yeast RNA-tilorone molecular complex. Vopr Virusol. 1997, 42, 17–19. [Google Scholar] [PubMed]
- Karpov, A.V.; Zholobak, N.M.; Spivak, N.Y.; Rybalko, S.L.; Antonenko, S.V.; Krivokhatskaya, L.D. Virus-inhibitory effect of a yeast RNA-tilorone molecular complex in cell cultures. Acta Virol. 2001, 45, 181–184. [Google Scholar] [PubMed]
- Loginova, S.; Koval’chuk, A.V.; Borisevich, S.V.; Kopylova, N.K.; Pashchenko Iu, I.; Khamitov, R.A.; Maksimov, V.A.; Shuster, A.M. Antiviral effectiveness of the combined use of amixine and virasole in experimental hemorrhagic fever with renal syndrome in sucking albino mice. Vopr. Virusol. 2005, 50, 30–32. [Google Scholar] [PubMed]
- Alcaro, S.; Arena, A.; Neri, S.; Ottana, R.; Ortuso, F.; Pavone, B.; Vigorita, M.G. Design and synthesis of DNA-intercalating 9-fluoren-beta-O-glycosides as potential IFN-inducers, and antiviral and cytostatic agents. Bioorg. Med. Chem. 2004, 12, 1781–1791. [Google Scholar] [CrossRef] [PubMed]
- Liakhov, S.A.; Litvinova, L.A.; Andronati, S.A.; Berezina, L.K.; Galkin, B.N.; Osetrov, V.E.; Filippova, T.O.; Golovenko, N. Biochemical mechanisms of realization of antiviral and interferon-inducing activity of amixine and its analogs. Ukr Biokhim Zh 2001, 73, 108–113. [Google Scholar] [PubMed]
- Katz, E.; Margalith, E.; Winer, B. The effect of tilorone hydrochloride on the growth of several animal viruses in tissue cultures. J. Gen. Virol. 1976, 31, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Margalith, E.; Winer, B. Inhibition of vaccinia virus growth by the nucleoside analogue 1-beta-D-ribofuranosyl-1,2,4-triazole-3-carboxamide (virazole, ribavirin). J. Gen. Virol. 1976, 32, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Margalith, E.; Winer, B. Inhibition of herpesvirus deoxyribonucleic acid and protein synthesis by tilorone hydrochloride. Antimicrob. Agents Chemother. 1976, 9, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Roner, M.R.; Cox, D.C. Cellular integrity is required for inhibition of initiation of cellular DNA synthesis by reovirus type 3. J. Virol. 1985, 53, 350–359. [Google Scholar] [PubMed]
- Kapikian, A.Z.; Kim, H.W.; Wyatt, R.G.; Cline, W.L.; Arrobio, J.O.; Brandt, C.D.; Rodriguez, W.J.; Sack, D.A.; Chanock, R.M.; Parrott, R.H. Human reovirus-like agent as the major pathogen associated with “winter” gastroenteritis in hospitalized infants and young children. New Engl. J. Med. 1976, 294, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.; Buneeva, O.; Glover, V. Biological targets for isatin and its analogues: Implications for therapy. Biologics 2007, 1, 151–162. [Google Scholar] [PubMed]
- Fu, G.; Pang, H.; Wong, Y.H. Naturally occurring phenylethanoid glycosides: potential leads for new therapeutics. Curr. Med. Chem. 2008, 15, 2592–2613. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, A.; Owa, T.; Mastrolorenzo, A.; Supuran, C.T. Anticancer and antiviral sulfonamides. Curr. Med. Chem. 2003, 10, 925–953. [Google Scholar] [CrossRef] [PubMed]
- Carballeira, N.M. New advances in the chemistry of methoxylated lipids. Prog. Lipid Res. 2002, 41, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, T.; Utsumi, K.; Tomioka, H.; Kasamoto, M.; Sato, Y.; Anne, T.; De Clercq, E. Synthesis, antiviral, antibacterial and antitumor cell activities of 2′-deoxy-2′-fluoropuromycin. Chem. Pharm. Bull. Tokyo 1995, 43, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L.; Bohlin, L.; Iorizzi, M.; Riccio, R.; Minale, L.; Moreno-Lopez, W. Biological activity of saponins and saponin-like compounds from starfish and brittle-stars. Toxicon 1989, 27, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Krol, W.; Dworniczak, S.; Pietsz, G.; Czuba, Z.P.; Kunicka, M.; Kopacz, M.; Nowak, D. Synthesis and tumoricidal activity evaluation of new morin and quercetin sulfonic derivatives. Acta Pol. Pharm. 2002, 59, 77–79. [Google Scholar] [PubMed]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).