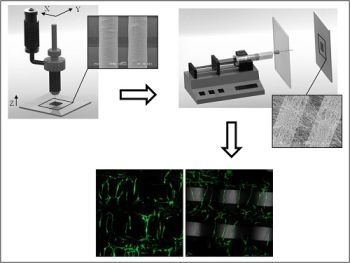
Dual-Scale Polymeric Constructs as Scaffolds for Tissue Engineering
Abstract
:1. Introduction
2. Results and Discussion
2.1. Development of Dual-Scale Scaffolds
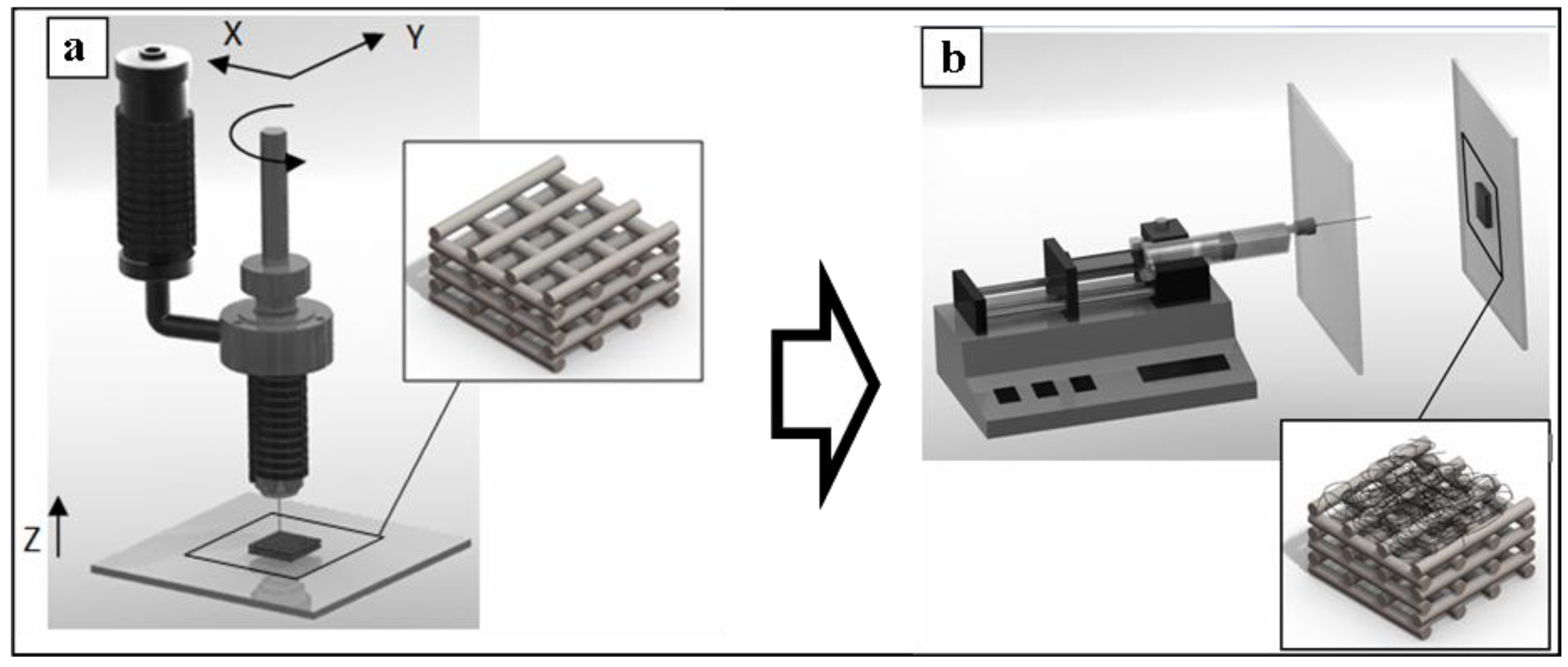
2.1.1. Fabrication and Morphological Characterization of 3D PCL Structures
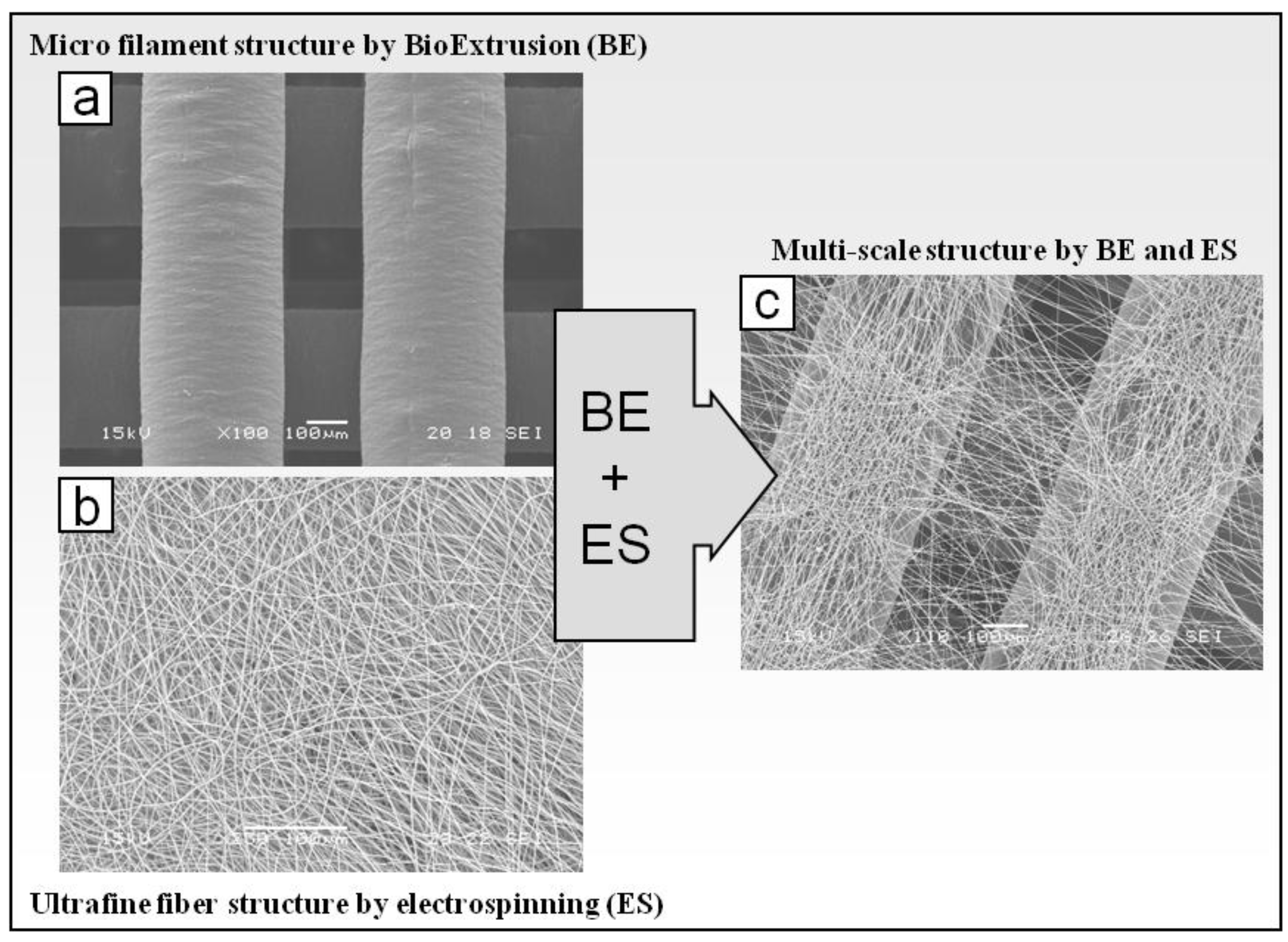
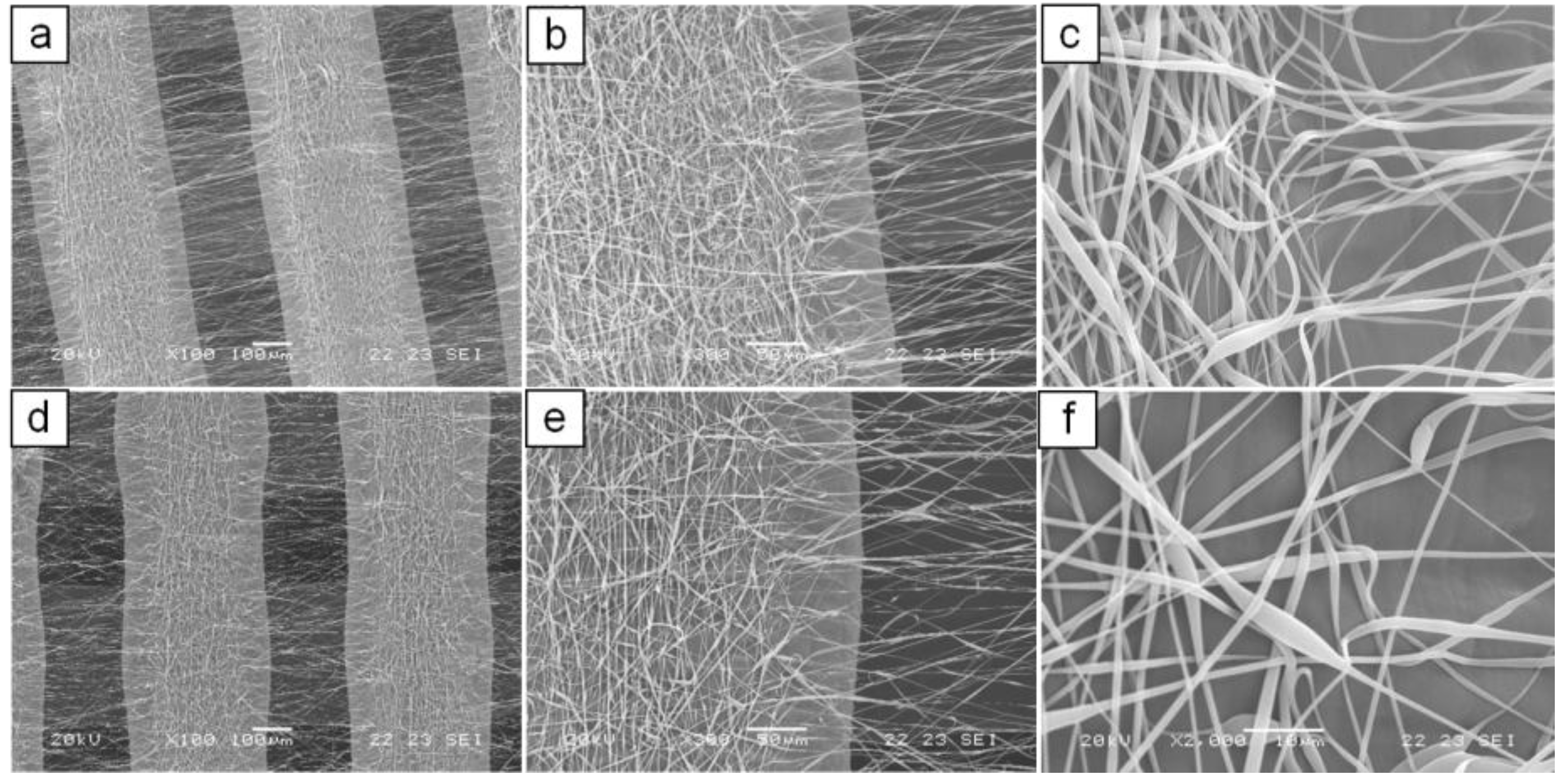
2.1.2. Fabrication and Morphological Characterization of Dual-Scale Scaffolds
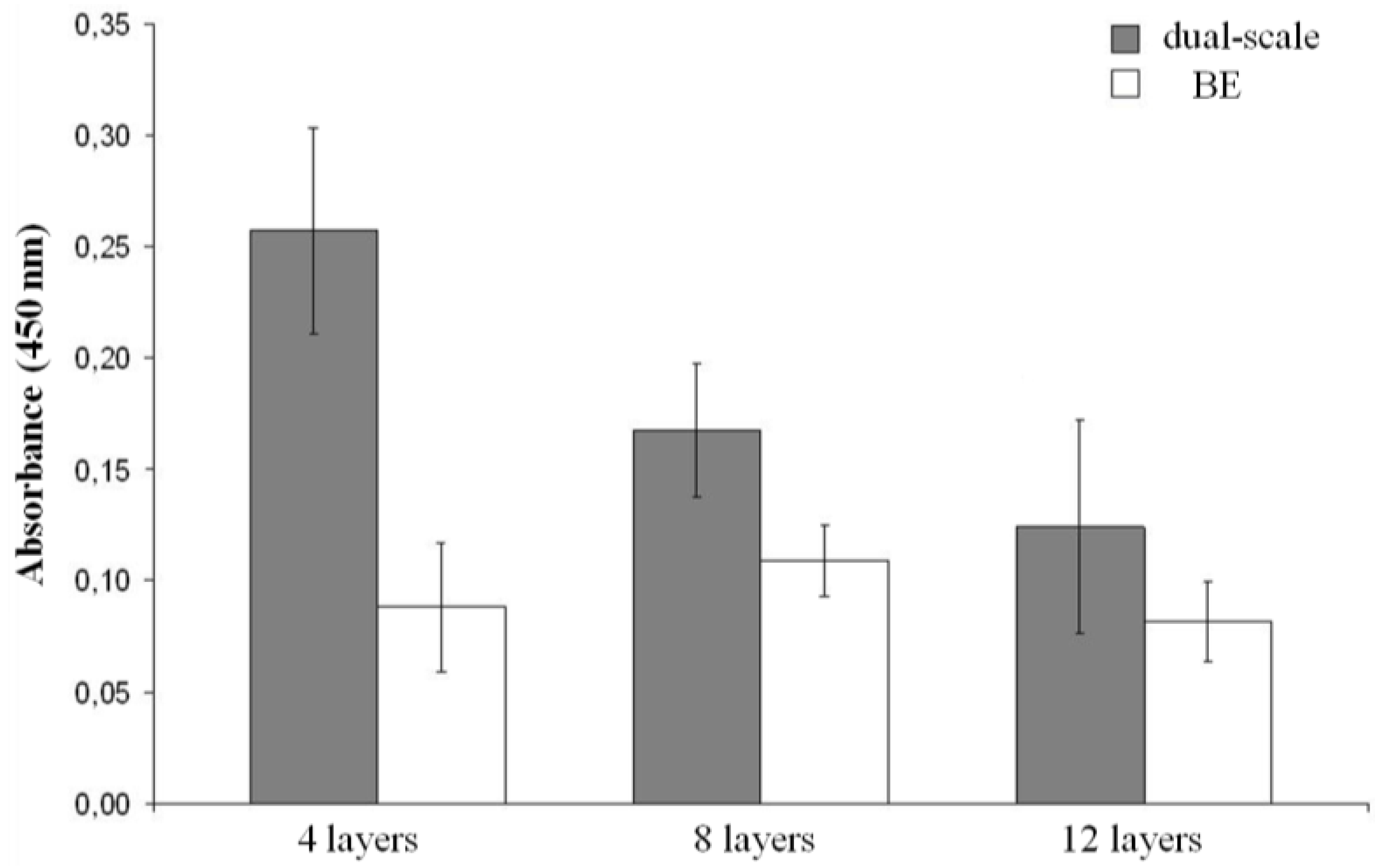
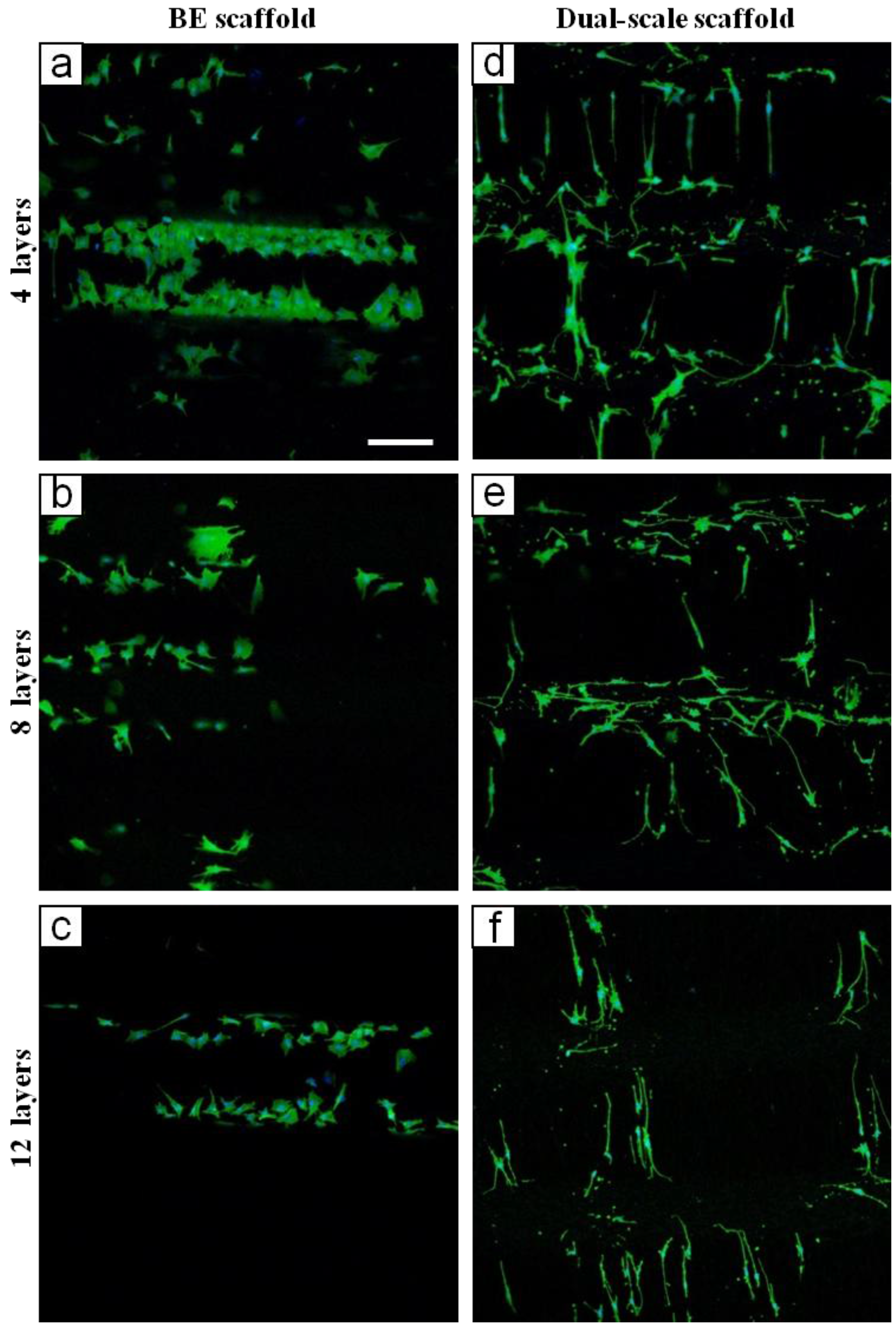
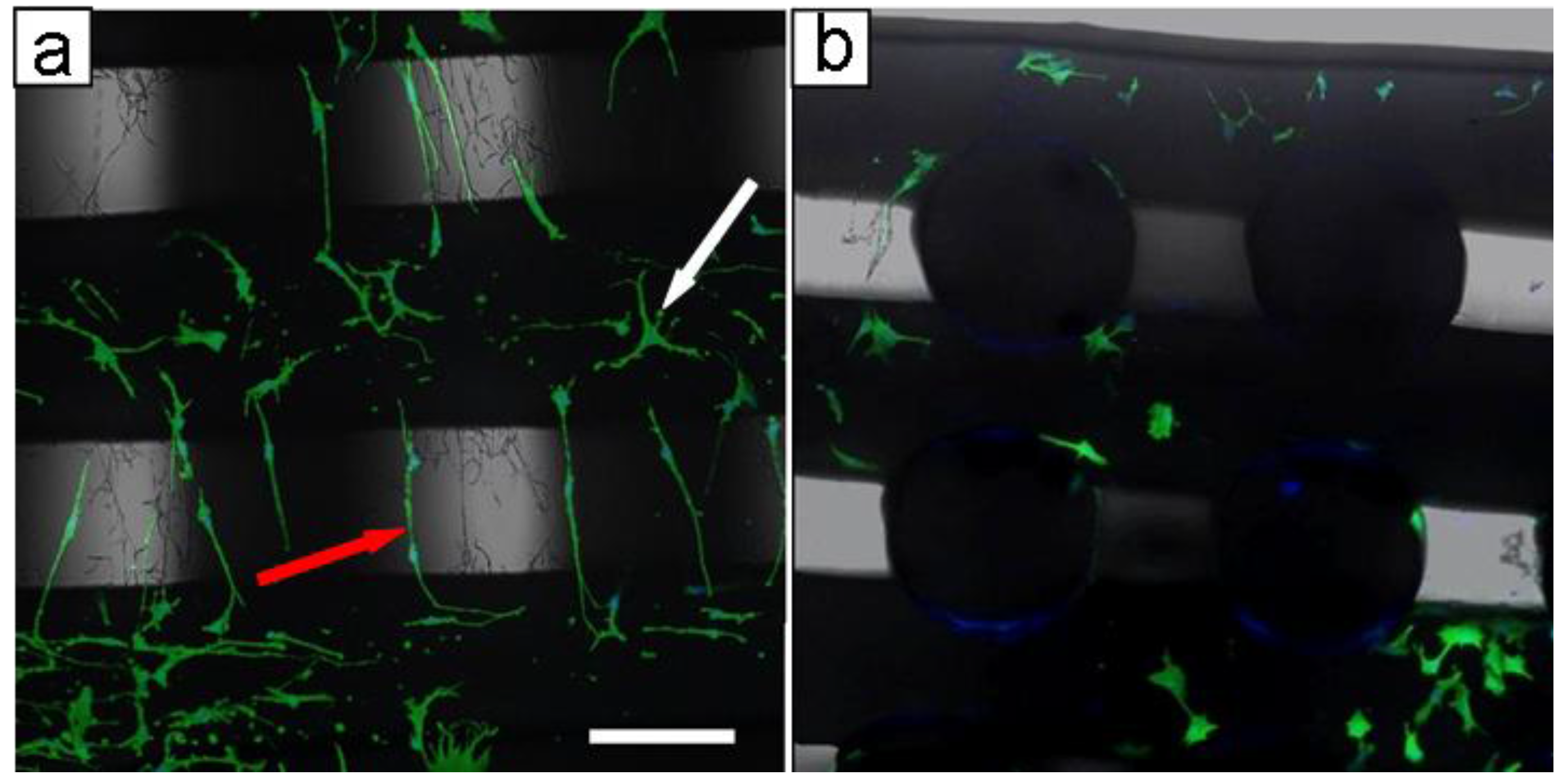
2.2. Biological Results
3. Experimental Section
3.1. Materials
3.2. Fabrication of Dual-Scale Fibrous Scaffolds
3.2.1. Production of 3D Scaffolds by Bioextrusion
3.2.2. Electrospinning of Polymer Solution
3.3. Morphological Characterization
3.4. Biological Evaluation
3.4.1. Cell Culture onto PCL Scaffolds
3.4.2. WST-1 Cell Proliferation Assay
3.4.3. Confocal Laser Scanning Microscopy (CLSM)
3.5. Statistical Evaluation
4. Conclusions
Acknowledgements
References
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef] [PubMed]
- Mouriño, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface 2010, 7, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric materials for bone and cartilage repair. Prog. Polym. Sci. 2010, 35, 403–440. [Google Scholar] [CrossRef]
- Gomes, M.E.; Holtorf, H.L.; Reis, R.L.; Mikos, A.G. Influence of the porosity of starch-based fiber mesh scaffolds on the proliferation and osteogenic differentiation of bone marrow stromal cells cultured in a flow perfusion bioreactor. Tissue Eng. 2006, 12, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Tuzlakoglu, K.; Bolgen, N.; Salgado, A.; Gomes, M.; Piskin, E.; Reis, R. Nano- and micro-fiber combined scaffolds: A new architecture for bone tissue engineering. J. Mater. Sci. Mater. Med. 2005, 16, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Detta, N.; Piras, A.M.; Chiellini, F.; Clarke, D.A.; Reilly, G.C.; Chiellini, E. Development of electrospun three-arm star poly(ε-caprolactone) meshes for tissue engineering application. Macromol. Biosci. 2010, 10, 887–897. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Piras, A.M.; Detta, N.; Ylikauppila, H.; Nikkola, L.; Ashammakhi, N.; Chiellini, F.; Chiellini, E. Poly(vinyl alcohol)-based electrospun meshes as potential candidate scaffolds in regenerative medicine. J. Bioact. Compat. Polym. 2011, 26, 20–34. [Google Scholar] [CrossRef]
- Puppi, D.; Piras, A.M.; Chiellini, F.; Chiellini, E.; Martins, A.; Leonor, I.B.; Neves, N.; Reis, R. Optimized electro- and wet-spinning techniques for the production of polymeric fibrous scaffolds loaded with bisphosphonate and hydroxyapatite. J. Tissue Eng. Regen. Med. 2010. [Google Scholar] [CrossRef]
- Leong, K.F.; Cheah, C.M.; Chua, C.K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials 2003, 24, 2363–2378. [Google Scholar] [CrossRef] [PubMed]
- Bártolo, P.J.; Almeida, H.A.; Rezende, R.A.; Laoui, T.; Bidanda, B. Advanced Processes to Fabricate Scaffolds for Tissue Engineering. In Virtual Prototyping & Bio Manufacturing in Medical Applications; Bidanda, B., Bártolo, P., Eds.; Springer: New York, NY, USA, 2008; pp. 149–170. [Google Scholar]
- Yeong, W.-Y.; Chua, C.-K.; Leong, K.-F.; Chandrasekaran, M. Rapid prototyping in tissue engineering: challenges and potential. Trends Biotechnol. 2004, 22, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Erol, M.; Boccaccini, A.R. Processing technologies for 3D nanostructured tissue engineering scaffolds. Adv. Eng. Mater. 2010, 12, B467–B487. [Google Scholar] [CrossRef]
- Stevens, M.M.; George, J.H. Exploring and Engineering the Cell Surface Interface. Science 2005, 310, 1135–1138. [Google Scholar] [CrossRef] [PubMed]
- Detta, N.; Puppi, D.; Errico, C.; Chiellini, F.; Piras, A.M.; Chiellini, E. Polymeric nanofiber constructs in drug delivery and tissue engineering. In Nanoparticles: Synthesis, Characterization and Applications; Chaughule, R.S., Ramanujan, R., Eds.; American Scientific Publishers: Stevenson Ranch, CA, USA, 2010; pp. 271–291. [Google Scholar]
- Price, R.L.; Gutwein, L.G.; Kaledin, L.; Tepper, F.; Webster, T.J. Osteoblast function on nanophase alumina materials: Influence of chemistry, phase, and topography. J. Biomed. Mater. Res. A 2003, 67A, 1284–1293. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Siegel, R.W.; Bizios, R. Osteoblast adhesion on nanophase ceramics. Biomaterials 1999, 20, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.W.; Cui, F.Z.; Hou, S.P.; Xu, Q.Y.; Chen, L.N.; Lee, I.S. Culture of neural cells on silicon wafers with nano-scale surface topograph. J. Neurosci. Methods 2002, 120, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Kotaki, M.; Inai, R.; Ramakrishna, S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005, 11, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospinning of polymeric nanofibers for tissue engineering applications: A review. Tissue Eng. 2006, 12, 1197. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.; Dinucci, D.; Cometa, S.; Alderighi, M.; Bártolo, P.J.; Chiellini, F. Polycaprolactone scaffolds fabricated via bioextrusion for tissue engineering applications. Int. J. Biomater. 2009, 2009, 239643:1–239643:9. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Leong, M.F.; Chan, W.Y.; Chian, K.S.; Rasheed, M.Z.; Anderson, J.M. Fabrication and in vitro and in vivo cell infiltration study of a bilayered cryogenic electrospun poly(D,L-lactide) scaffold. J. Biomed. Mater. Res. A 2010, 94A, 1141–1149. [Google Scholar] [PubMed]
- Heydarkhan-Hagvall, S.; Schenke-Layland, K.; Dhanasopon, A.P.; Rofail, F.; Smith, H.; Wu, B.M.; Shemin, R.; Beygui, R.E.; MacLellan, W.R. Three-dimensional electrospun ECM-based hybrid scaffolds for cardiovascular tissue engineering. Biomaterials 2008, 29, 2907–2914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ouyang, H.; Lim, C.T.; Ramakrishna, S.; Huang, Z.-M. Electrospinning of gelatin fibers and gelatin/PCL composite fibrous scaffolds. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72B, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Sill, T.J.; Von Recum, H.A. Electrospinning: Applications in drug delivery and tissue engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Pham, Q.P.; Sharma, U.; Mikos, A.G. Electrospun poly(ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules 2006, 7, 2796–2805. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Son, J.; Park, S.; Kim, W. Hybrid process for fabricating 3D hierarchical scaffolds combining rapid prototyping and electrospinning. Macromol. Rapid Commun. 2008, 29, 1577–1581. [Google Scholar] [CrossRef]
- Yoon, H.; Ahn, S.; Kim, G. Three-dimensional polycaprolactone hierarchical scaffolds supplemented with natural biomaterials to enhance mesenchymal stem cell proliferation. Macromol. Rapid Commun. 2009, 30, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Morsi, Y.; Patel, S.; Ke, Q.-F.; Mo, X.-M. A novel approach via combination of electrospinning and FDM for tri-leaflet heart valve scaffold fabrication. Front. Mater. Sci. China 2009, 3, 359–366. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, T.G.; Kim, H.C.; Yang, D.-Y.; Park, T.G. Development of dual scale scaffolds via direct polymer melt deposition and electrospinning for applications in tissue regeneration. Acta Biomater. 2008, 4, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Chung, S.; Pedro, A.J.; Sousa, R.A.; Marques, A.P.; Reis, R.L.; Neves, N.M. Hierarchical starch-based fibrous scaffold for bone tissue engineering applications. J. Tissue Eng. Regen. Med. 2009, 3, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.I.; Tuzlakoglu, K.; Fuchs, S.; Gomes, M.E.; Peters, K.; Unger, R.E.; Piskin, E.; Reis, R.L.; Kirkpatrick, C.J. Endothelial cell colonization and angiogenic potential of combined nano- and micro-fibrous scaffolds for bone tissue engineering. Biomaterials 2008, 29, 4306–4313. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.; Küffer, J.; Ströbel, S.; Dubini, G.; Martin, I.; Wendt, D. Computational evaluation of oxygen and shear stress distributions in 3D perfusion culture systems: Macro-scale and micro-structured models. J. Biomech. 2008, 41, 2918–2925. [Google Scholar] [CrossRef] [PubMed]
- Woodfield, T.B.F.; Malda, J.; De Wijn, J.; Péters, F.; Riesle, J.; Van Blitterswijk, C.A. Design of porous scaffolds for cartilage tissue engineering using a three-dimensional fiber-deposition technique. Biomaterials 2004, 25, 4149–4161. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Shor, L.; Darling, A.; Khalil, S.; Sun, W.; Güçeri, S.; Lau, A. Precision extruding deposition and characterization of cellular poly-ε-caprolactone tissue scaffolds. Rapid Prototyping J. 2004, 10, 42–49. [Google Scholar] [CrossRef]
- Domingos, M.; Chiellini, F.; Gloria, A.; Ambrosio, L.; Bartolo, P.J.; Chiellini, E. BioExtruder: Study of the influence of process parameters on PCL scaffolds properties. In Innovative Developments in Design and Manufacturing. Advanced Research in virtual and Rapid Prototyping; Taylor & Francis: Oxford, UK, 2009; pp. 67–73. [Google Scholar]
- Mota, C.; Almeida, H.A.; Mateus, A.; Bártolo, P.J.; Ferreira, N.; Domingos, M.; Alves, N.M. Processo e equipamento de fabrico rápido por bioextrusão. Portuguese Patent No. 104247, 2008. [Google Scholar]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef] [PubMed]
- Puppi, D.; Piras, A.M.; Detta, N.; Dinucci, D.; Chiellini, F. Poly(lactic-co-glycolic acid) electrospun fibrous meshes for the controlled release of retinoic acid. Acta Biomater. 2010, 6, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Chiellini, E.; Chiellini, F.; Errico, C.; Detta, N.; Piras, A.M.; Puppi, D. Procedimento e Dispositivo di Elettrofilatura per la Produzione di Nano/Microfibre Polimeriche Unidirezionate. Italian Patent No. RM2009A00286, 2009. [Google Scholar]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Edmondson, D.; Veiseh, O.; Matsen, F.A.; Zhang, M. Electrospun chitosan-based nanofibers and their cellular compatibility. Biomaterials 2005, 26, 6176–6184. [Google Scholar] [CrossRef] [PubMed]
- Katta, P.; Alessandro, M.; Ramsier, R.D.; Chase, G.G. Continuous Electrospinning of Aligned Polymer Nanofibers onto a Wire Drum Collector. Nano Lett. 2004, 4, 2215–2218. [Google Scholar] [CrossRef]
- Sundaray, B.; Subramanian, V.; Natarajan, T.S.; Xiang, R.-Z.; Chang, C.-C.; Fann, W.-S. Electrospinning of continuous aligned polymer fibers. Appl. Phys. Lett. 2004, 84, 1222–1224. [Google Scholar] [CrossRef]
- Theron, A.; Zussman, E.; Yarin, A.L. Electrostatic field-assisted alignment of electrospun nanofibres. Nanotechnology 2001, 12, 384–390. [Google Scholar] [CrossRef]
- Inai, R.; Kotaki, M.; Ramakrishna, S. Structure and properties of electrospun PLLA single nanofibres. Nanotechnology 2005, 16, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Dalton, P.D.; Klee, D.; Möller, M. Electrospinning with dual collection rings. Polymer 2005, 46, 611–614. [Google Scholar] [CrossRef]
- Kessick, R.; Fenn, J.; Tepper, G. The use of AC potentials in electrospraying and electrospinning processes. Polymer 2004, 45, 2981–2984. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning of polymeric and ceramic nanofibers as uniaxially aligned arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospinning nanofibers as uniaxially aligned arrays and layer-by-layer stacked films. Adv. Mater. 2004, 16, 361–366. [Google Scholar] [CrossRef]
- Zhang, D.; Chang, J. Patterning of Electrospun Fibers Using Electroconductive Templates. Adv. Mater. 2007, 19, 3664–3667. [Google Scholar] [CrossRef]
- Murshid, S.A.; Kamioka, H.; Ishihara, Y.; Ando, R.; Sugawara, Y.; Takano-Yamamoto, T. Actin and microtubule cytoskeletons of the processes of 3D-cultured MC3T3-E1 cells and osteocytes. J. Bone Miner. Metab. 2007, 25, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.M.; Hohman, M.M.; Brenner, M.P.; Rutledge, G.C. Experimental characterization of electrospinning: The electrically forced jet and instabilities. Polymer 2001, 42, 09955–09967. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mota, C.; Puppi, D.; Dinucci, D.; Errico, C.; Bártolo, P.; Chiellini, F. Dual-Scale Polymeric Constructs as Scaffolds for Tissue Engineering. Materials 2011, 4, 527-542. https://doi.org/10.3390/ma4030527
Mota C, Puppi D, Dinucci D, Errico C, Bártolo P, Chiellini F. Dual-Scale Polymeric Constructs as Scaffolds for Tissue Engineering. Materials. 2011; 4(3):527-542. https://doi.org/10.3390/ma4030527
Chicago/Turabian StyleMota, Carlos, Dario Puppi, Dinuccio Dinucci, Cesare Errico, Paulo Bártolo, and Federica Chiellini. 2011. "Dual-Scale Polymeric Constructs as Scaffolds for Tissue Engineering" Materials 4, no. 3: 527-542. https://doi.org/10.3390/ma4030527
APA StyleMota, C., Puppi, D., Dinucci, D., Errico, C., Bártolo, P., & Chiellini, F. (2011). Dual-Scale Polymeric Constructs as Scaffolds for Tissue Engineering. Materials, 4(3), 527-542. https://doi.org/10.3390/ma4030527