{Fe6O2}-Based Assembly of a Tetradecanuclear Iron Nanocluster
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Preliminary Characterization
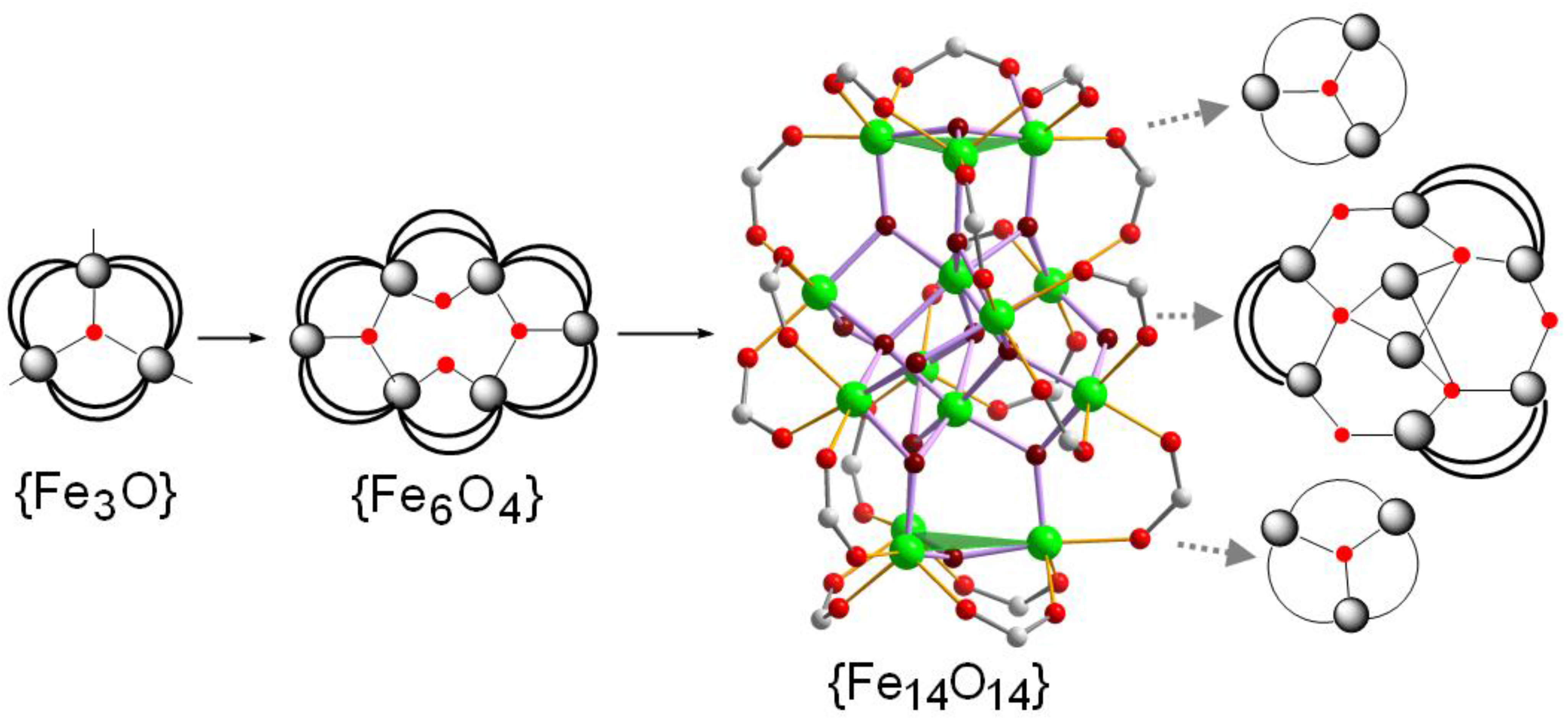
2.2. X-ray Structure

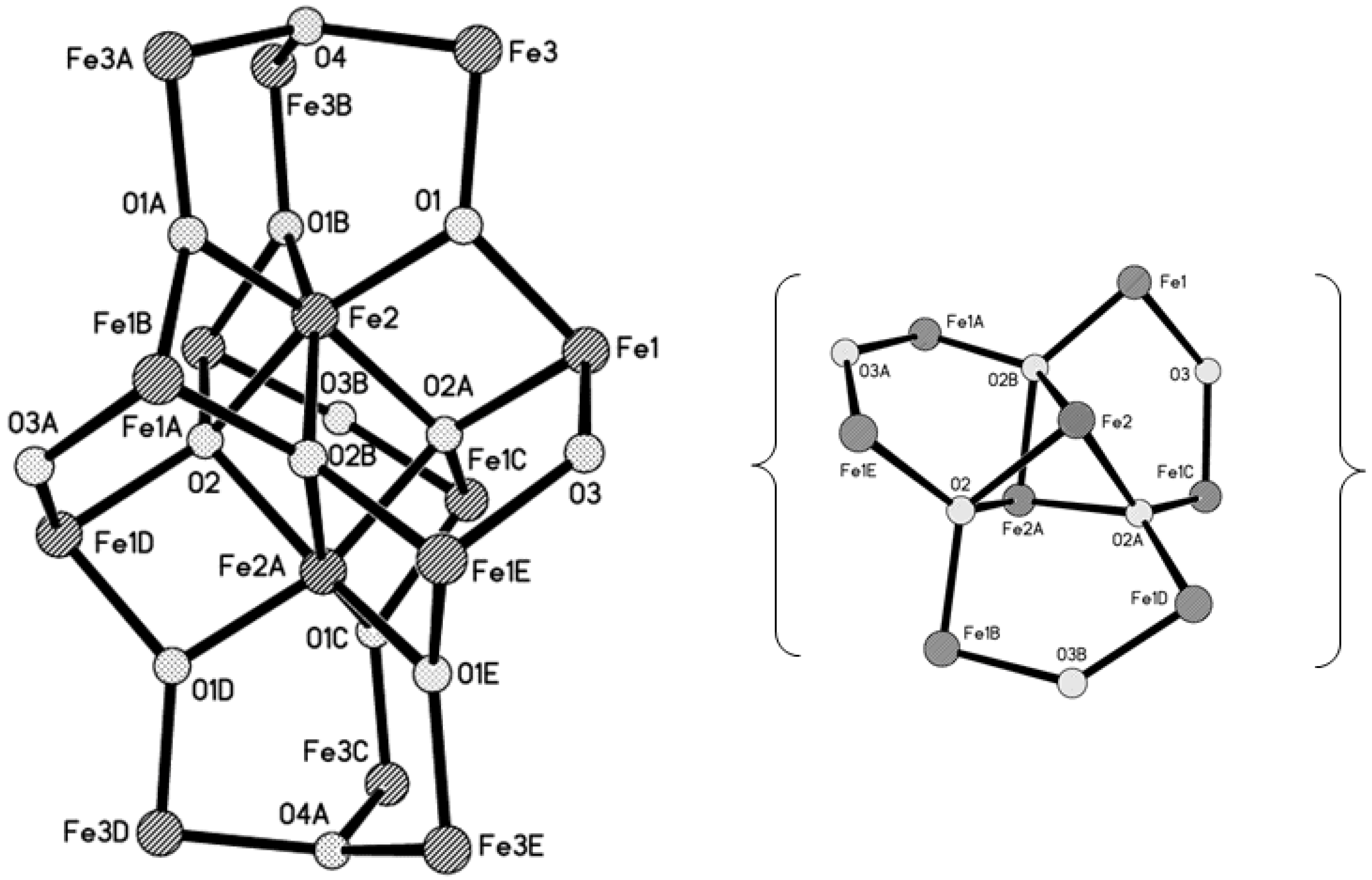
| Fe1−O2A | 1.965(2) | Fe2−O1B | 1.951(3) |
| Fe1−O3 | 1.966(2) | Fe2−O2B | 2.090(3) |
| Fe1−O1 | 1.970(3) | Fe2−O2A | 2.090(3) |
| Fe1−O10 | 2.021(3) | Fe3−O1 | 1.856(3) |
| Fe1−O5 | 2.033(4) | Fe3−O4 | 1.934(1) |
| Fe1−O6 | 2.033(4) | Fe3−O7 | 1.958(3) |
| Fe2−O1 | 1.951(3) | Fe3−O8 | 1.975(3) |
| Fe2−O1A | 1.951(3) | Fe3−O9 | 2.004(3) |
| Fe2−O2 | 2.091(3) | ||
| Metal···Metal separations | |||
| Fe1···Fe3 | 3.4638 (11) | Fe2···Fe2C | 2.799(2) |
| Fe2···Fe1 | 2.962(7) | Fe2···Fe3 | 3.3756 (11) |
| Fe3···Fe3A | 3.2983 (11) | ||
| O2A−Fe1 −O3 | 93.01(13) | O1−Fe2−O2B | 98.78(10) |
| O2A−Fe1 −O1 | 85.08(11) | O1A−Fe2−O2B | 82.26(10) |
| O3 −Fe1 −O1 | 94.58(11) | O1B−Fe2−O2B | 162.24(10) |
| O2A−Fe1 −O10 | 177.44(12) | O1−Fe2−O2A | 82.26(10) |
| O3−Fe1−O10 | 87.04(15) | O1A−Fe2−O2A | 162.24(10) |
| O1−Fe1−O10 | 92.37(12) | O1B−Fe2−O2A | 98.78(10) |
| O2A−Fe1−O5 | 96.96(14) | O2B−Fe2−O2A | 80.09(11) |
| O3−Fe1−O5 | 88.05(16) | O1−Fe2−O2 | 162.24(10) |
| O1−Fe1−O5 | 176.60(17) | O1−Fe3−O4 | 93.91(16) |
| O10−Fe1−O5 | 85.60(14) | O1−Fe3−O7 | 122.30(15) |
| O2A−Fe1−O6 | 93.15(11) | O4−Fe3−O7 | 92.93(13) |
| O3−Fe1−O6 | 172.60(16) | O1−Fe3−O8 | 132.27(15) |
| O1−Fe1−O6 | 90.03(14) | O4−Fe3−O8 | 90.90(13) |
| O10−Fe1−O6 | 86.98(14) | O7−Fe3−O8 | 104.78(16) |
| O5−Fe1−O6 | 87.14(18) | O1−Fe3−O9 | 92.77(13) |
| O1−Fe2−O1A | 98.62(11) | O4−Fe3−O9 | 173.22(17) |
| O1−Fe2−O1B | 98.62(11) | O7−Fe3−O9 | 84.43(14) |
| O1A−Fe2−O1B | 98.62(11) | O8−Fe3−O9 | 83.76(14) |
A: –y+1, x–y, z B: –x+y+1, –x+1, z C: y+1/3, x–1/3, –z+1/6 | |||
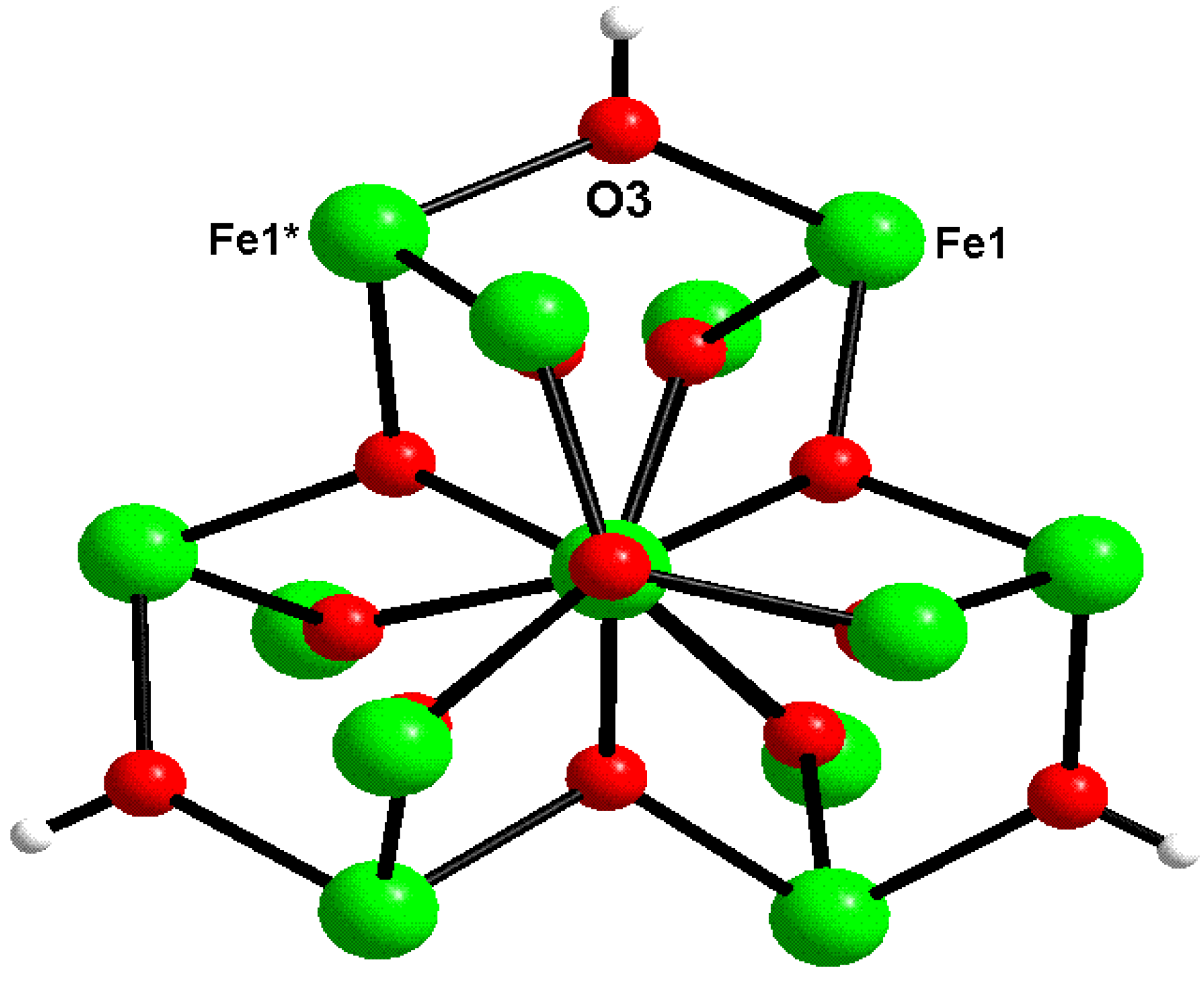
| Atom | Value | Assigned state |
|---|---|---|
| Fe1 | 3.14 | Fe3+ |
| Fe2 | 3.02 | Fe3+ |
| Fe3 | 3.06 | Fe3+ |
| O1 | 1.93 | O2− |
| O2 | 1.96 | O2− |
| O3 | 1.12 | HO− |
| O4 | 1.87 | O2− |
| O5 | 1.92 | O2− |
| O6 | 1.93 | O2− |
| O7 | 1.94 | O2− |
| O8 | 2.04 | O2− |
| O9 | 1.95 | O2− |
| O10 | 1.99 | O2− |
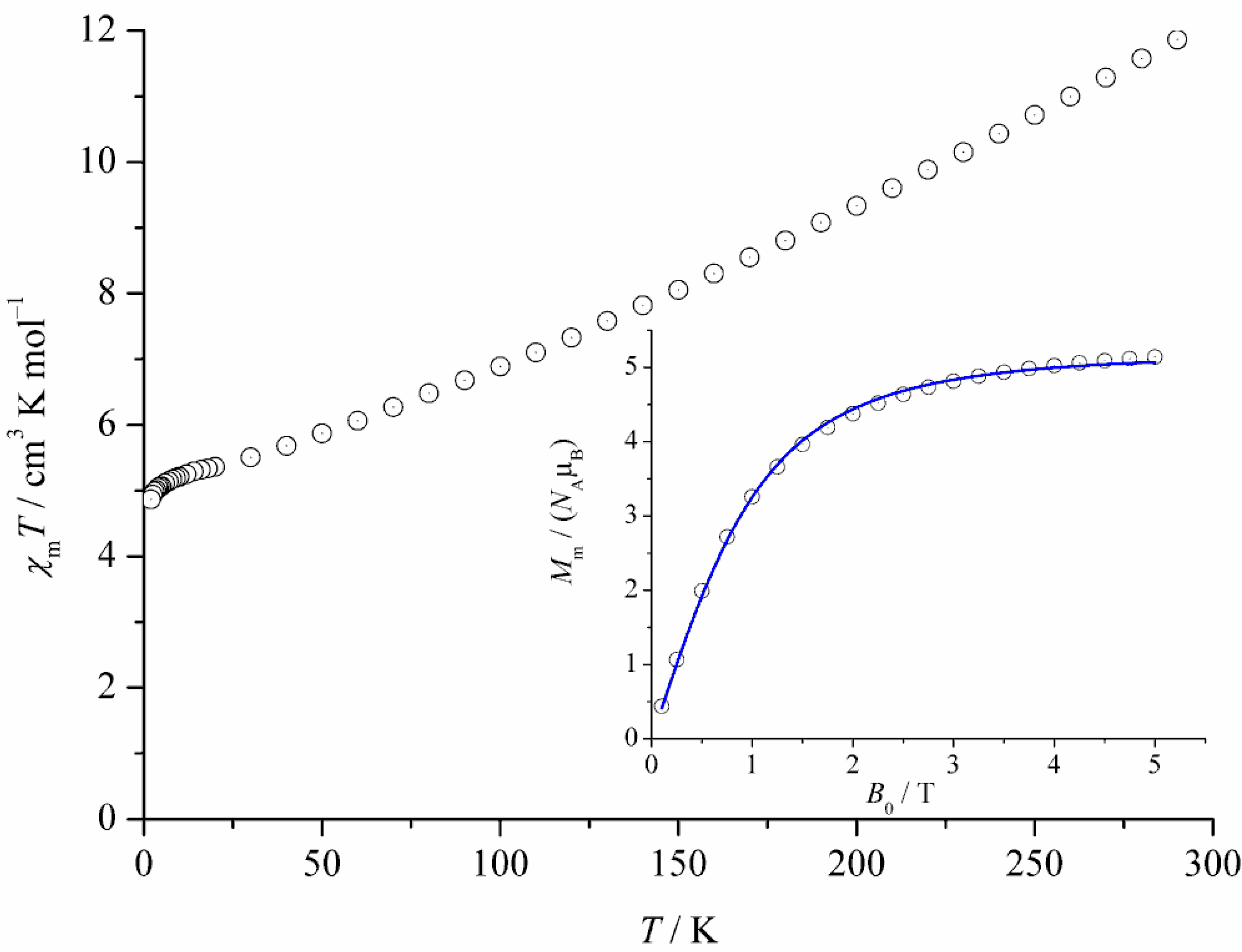
2.3. Magnetic Properties
3. Experimental Section
3.1. Materials and Physical Measurements
3.2. X-ray Crystallography
3.3. Synthesis of [Fe14O10(OH)4(O2CCMe3)18]
4. Conclusions
Acknowledgements
References and Notes
- Loehr, T.M. Iron Carriers and Iron Proteins; VCH: Weinheim, Germany, 1989; pp. 1–534. [Google Scholar]
- Wieghardt, K.; Pohl, K.; Jibril, I.; Huttner, G. Hydrolysis products of the monomeric amine complex (C6H15N3)FeCl3: the structure of the octameric iron(III) cation of {[(C6H15N3)6Fe8(μ3-O)2(μ2-OH)12]Br7(H2O)}Br·8H2O. Angew. Chem. Int. Ed. 1984, 23, 77–78. [Google Scholar] [CrossRef]
- Beneli, C.; Parsons, S.; Solan, G.A.; Winpenny, R.E.P. Ferric wheels and cages: decanuclear iron complexes with carboxylato and pyridonato ligands. Angew. Chem. Int. Ed. 1996, 35, 1825–1828. [Google Scholar] [CrossRef]
- Sangregorio, C.; Ohm, T.; Paulsen, C.; Sessoli, R.; Gatteschi, D. Quantum tunneling of the magnetization in an iron cluster nanomagnet. Phys. Rev. Lett. 1997, 78, 4645–4648. [Google Scholar] [CrossRef]
- Barra, A.-L.; Caneschi, A.; Cornia, A.; de Biani, F.; Gatteschi, D.; Sangregorio, C.; Sessoli, R.; Sorace, L. Single-molecule magnet behavior of a tetranuclear iron(III) complex. The origin of slow magnetic relaxation in iron(III) clusters. J. Am. Chem. Soc. 1999, 121, 5302–5310. [Google Scholar]
- Oshio, H.; Hoshino, N.; Ito, T. Superparamagnetic behavior in an alkoxo-bridged iron(II) cube. J. Am. Chem. Soc. 2000, 122, 12602–12603. [Google Scholar] [CrossRef]
- Goodwin, J.C.; Sessoli, R.; Gatteschi, D.; Wernsdorfer, W.; Powell, A.K.; Heath, S.L. Towards nanostructured arrays of single molecule magnets: new Fe19 oxyhydroxide clusters displaying high ground state spins and hysteresis. J. Chem. Soc., Dalton Trans. 2000, 1835–1840. [Google Scholar]
- Moragues-Canovas, M.; Riviere, P.; Ricard, L.; Paulsen, C.; Wernsdorfer, W.; Rajaraman, G.; Brechin, E.K.; Mallah, T. Resonant quantum tunneling in a new tetranuclear iron(III)-based single-molecule magnet. Adv. Mater. 2004, 16, 1101–1105. [Google Scholar]
- Boudalis, A.K.; Donnadieu, B.; Nastopoulos, V.; Clemente-Juan, M.J.; Mari, A.; Sanakis, Y.; Tuchagues, J.-P.; Perlepes, S.P. A nonanuclear iron(II) single-molecule magnet. Angew. Chem., Int. Ed. 2004, 43, 2266–2270. [Google Scholar] [CrossRef]
- Powell, G.W.; Lancashire, H.N.; Brechin, E.K.; Collison, D.; Heath, S.L.; Mallah, T.; Wernsdorfer, W. Building molecular minerals: all ferric pieces of molecular magnetite. Angew. Chem., Int. Ed. 2004, 43, 5772–5775. [Google Scholar] [CrossRef]
- van Slageren, J.; Rosa, P.; Caneschi, A.; Sessoli, R.; Casellas, H.; Rakitin, Yu.V.; Cianchi, L.; Del Giallo, F.; Spina, G.; Bino, A.; Barra, A.-L.; Guidi, T.; Carretta, S.; Caciuffo, R. Static and dynamic magnetic properties of an [Fe13] cluster. Phys. Rev. B 2006, 73, 014422:1–014422:10. [Google Scholar] [CrossRef]
- Jones, L.F.; Low, D.M.; Helliwell, M.; Raftery, J.; Collison, D.; Aromi, G.; Cano, J.; Mallah, T.; Wernsdorfer, W.; Brechin, E.K.; Mcinnes, E.J.L. Fe(III) clusters built with tripodal alcohol ligands. Polyhedron 2006, 25, 325–333. [Google Scholar] [CrossRef]
- Bagai, R.; Wernsdorfer, W.; Abboud, K.A.; Christou, G. Exchange-biased dimers of single-molecule magnets in OFF and ON states. J. Am. Chem. Soc. 2007, 129, 12918–12919. [Google Scholar] [CrossRef] [PubMed]
- Ako, A.M.; Mereacre, V.; Lan, Y.; Wernsdorfer, W.; Clerac, R.; Anson, C.E.; Powell, A.K. An undecanuclear FeIII single-molecule magnet. Inorg. Chem. 2010, 49, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Winpenny, R.E.P. Serendipitous assembly of polynuclear cage compounds. J. Chem. Soc. Dalton Trans. 2002, 1–10. [Google Scholar] [CrossRef]
- Canada-Vilalta, C.; Rumberger, E.; Brechin, E.K.; Wernsdorfer, W.; Folting, K.; Davidson, E.R.; Hendrickson, D.N.; Christou, G. Two new hexanuclear iron(III) complexes with S = 5 ground states. J. Chem. Soc. Dalton Trans. 2002, 21, 4005–4010. [Google Scholar] [CrossRef]
- Gerbeleu, N.V.; Batsanov, A.S.; Timko, G.A.; Struchkov, Iu.T.; Indrichan, K.M.; Popovich, G.A. Synthesis and structure of tri- and hexanuclear μ3-oxopivalates of iron(III). Dokl. Akad. Nauk SSSR (Russ.) 1987, 293, 364–367. [Google Scholar]
- Manole, O.S.; Batsanov, A.S.; Struchkov, Yu.T.; Timko, G.A.; Synzheryan, L.D.; Gerbeleu, N.V. Synthesis and crystal structure of pentanuclear heterometallic pivalato acetylacetonate complexes. Koord. Khim. (Russ.) 1994, 20, 231–237. [Google Scholar]
- Smith, A.A.; Coxall, R.A.; Harrison, A.; Helliwell, M.; Parsons, S.; Winpenny, R.E.P. High-temperature synthesis of polynuclear iron oxo-hydroxy complexes. Polyhedron 2004, 23, 1557–1565. [Google Scholar] [CrossRef]
- Canada-Vilalta, C.; O’Brien, T.A.; Pink, M.; Davidson, E.R.; Christou, G. Methanolysis and phenolysis routes to Fe6, Fe8, and Fe10 complexes and their magnetic properties: a new type of Fe8 ferric wheel. Inorg. Chem. 2003, 42, 7819–7829. [Google Scholar] [CrossRef] [PubMed]
- Canada-Vilalta, C.; Pink, M.; Christou, G. A phenolysis route to a new type of octanuclear iron(III) wheel: [Fe8(OH)4(OPh)8(O2CBut)12]. Chem. Commun. 2003, 11, 1240–1241. [Google Scholar] [CrossRef]
- Low, D.M.; Jones, L.F.; Bell, A.; Brechin, E.K.; Mallah, T.; Rivire, E.; Teat, S.J.; McInnes, E.J. L. Solvothermal synthesis of a tetradecametallic FeIII cluster. Angew. Chem., Int. Ed. 2003, 42, 3781–3784. [Google Scholar] [CrossRef]
- Gass, I.A.; Milios, C.J.; Gavin Whittaker, A.; Fabiani, F.P.A.; Parsons, S.; Murrie, M.; Perlepes, S.P.; Brechin, E.K. A cube in a tetrahedron: microwave-assisted synthesis of an octametallic FeIII cluster. Inorg. Chem. 2006, 45, 5281–5283. [Google Scholar]
- Pradeep, C.P.; Long, D.-L.; Kögerler, P.; Cronin, L. Controlled assembly and solution observation of a 2.6 nm polyoxometalate ‘super’ tetrahedron cluster: [KFe12(OH)18(α-1,2,3-P2W15O56)4]29–. Chem. Commun. 2007, 4254–4256. [Google Scholar]
- Kögerler, P.; Tsukerblat, B.; Müller, A. Structure-related frustrated magnetism of nanosized polyoxometalates: aesthetics and properties in harmony. Dalton Trans. 2010, 39, 21–36. [Google Scholar] [CrossRef]
- Batsanov, A.S.; Struchkov, Y.T.; Timko, G.A. Crystal structure of hexanuclear iron(III) pivalate, Fe6(μ3-O)2(μ-OH)2(μ-Me3CCO2)12. Koord. Khim. (Russ.) 1988, 142, 266–270. [Google Scholar]
- Shaw, R.; Laye, R.H.; Jones, L.F.; Low, D.M.; Talbot-Eeckelaers, C.; Wei, Q.; Milios, C.J.; Teat, S.; Helliwell, M.; Raftery, J.; Evangelisti, M.; Affronte, M.; Collison, D.; Brechin, E.K.; McInnes, E.J.L. 1,2,3-Triazole-bridged tetradecametallic transition metal clusters [M14(L)O6(OMe)18X6] (M = FeIII, CrIII and VIII/IV) and related compounds: ground-state spins ranging from S = 0 to S = 25 and spin-enhanced magnetocaloric effect. Inorg. Chem. 2007, 46, 4968–4978. [Google Scholar] [CrossRef] [PubMed]
- Grapperhaus, C.A.; O’Toole, M.G.; Mashuta, M.S. Synthesis and structure of the tetradeca-iron(III) oxide-alkoxide cluster [Bu4N]2[Fe14O8(OCH2Me)20Cl8]. Inorg. Chem. Commun. 2006, 9, 1204–1206. [Google Scholar] [CrossRef]
- Tolis, E.I.; Helliwell, M.; Langley, S.; Raftery, J.; Winpenny, R.E.P. Synthesis and characterization of iron(III) phosphonate cage complexes. Angew. Chem., Int. Ed. 2003, 42, 3804–3808. [Google Scholar] [CrossRef]
- Bennett, M.; Holm, R.H. Self-assembly of a tetradecanuclear iron nitride cluster. Angew. Chem. Int. Ed. 2006, 45, 5613–5616. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Baca, S.G.; Speldrich, M.; Ellern, A.; Kögerler, P. {Fe6O2}-Based Assembly of a Tetradecanuclear Iron Nanocluster. Materials 2011, 4, 300-310. https://doi.org/10.3390/ma4010300
Baca SG, Speldrich M, Ellern A, Kögerler P. {Fe6O2}-Based Assembly of a Tetradecanuclear Iron Nanocluster. Materials. 2011; 4(1):300-310. https://doi.org/10.3390/ma4010300
Chicago/Turabian StyleBaca, Svetlana G., Manfred Speldrich, Arkady Ellern, and Paul Kögerler. 2011. "{Fe6O2}-Based Assembly of a Tetradecanuclear Iron Nanocluster" Materials 4, no. 1: 300-310. https://doi.org/10.3390/ma4010300
APA StyleBaca, S. G., Speldrich, M., Ellern, A., & Kögerler, P. (2011). {Fe6O2}-Based Assembly of a Tetradecanuclear Iron Nanocluster. Materials, 4(1), 300-310. https://doi.org/10.3390/ma4010300





