Dyes as Photoinitiators or Photosensitizers of Polymerization Reactions
Abstract
:1. Introduction
2. Overview of the Available Dye Based Radical Photoinitiators
- (i)
- one-component (or Type I) systems where radicals are readily produced through a homolytic bond cleavage (3a),
- (ii)
- two-component (Type II) systems that involve energy (2b) or electron (2c) transfer in PS/PI combination or electron/proton or direct hydrogen transfer in PI/coI where coI is a hydrogen donating compound AH (3b),
- (iii)
- multi-component photoinitiating systems containing three or more compounds and exhibiting a much more complex mechanism, e.g. in (3b-c) in the case of a PI/AH/B system (where it very often appears that A● is the initiating radical whereas PIH● is a terminating radical of the growing polymer chain; B is an additive being able to scavenge the detrimental radicals).
2.1. One-component photoinitiating systems
- (i)
- bisacylphosphine oxides (absorbing up to 480 nm and working through a C-P cleavage);
- (ii)
- organoborates (composed of a borate anion and a visible light absorbing counter cation which allows to tune the absorption);
- (iii)
- organometallic compounds (the most widely used derivatives are the titanocenes which exhibit an excellent absorption around 500 nm);
- (iv)
- metal carbonyl derivatives centered on Mn, Fe, Mo, Cr, Os, Re, Ru;
- (v)
- metal salts and metallic salt complexes based on Co, Fe, Ni, Cu, Cr;
- (vi)
- light absorbing amines;
- (vii)
- difunctional compounds containing two cleavable moieties (which lead to a light absorption better than that of the parent monofunctional compound);
- (viii)
- mono-and di-benzoyl germanes (that absorb around 400 nm);
- (ix)
- bis germyl ketones (the absorption at the Ar laser line is quite good);
- (x)
- group 14 di-metal compounds;
- (xi)
- new transition metal carbonyls.
2.2. Two-component photoinitiating systems
- (i)
- carefully selected α-diketones (which allow an excellent efficiency in the near UV-visible region for the photopolymerization of clear thick molded objects or coatings under visible light and sun exposure);
- (ii)
- substituted benzophenone, camphorquinone or thioxanthone and related amino (or thiol) derivatives and thioxanthone-fluorenes;
- (iii)
- various ketone families such as anthraquinone, fluorenone, flavone, anthrone, quinone, naphtacenequinone, quinoline, benzodioxinone;
- (iv)
- xanthenic dyes…, thiazines (methylene blue…), acridines, N-methylacridone, phenosafranines, thiopyronines, riboflavines, phenoxazines, pyrromethenes, polymethines, fluorones, squarylium, julolidine dyes, phenoxazones, quinolinones, phtalocyanines, benzopyranones, rhodanines, crystal violet/benzofuranone derivatives, dimethyl aminostyryl benzothiazolinium iodides. The mechanisms involved in the different systems and the various encountered or promising applications have been reported;
- (v)
- bis-arylimidazole derivatives (for example, Cl-bis-imidazole derivatives HABI are largely used in the laser imaging area; because of the low bond energy between the two imidazoyl moieties, a very fast cleavage occurs leading to two lophyl radicals which then react with electron/hydrogen donors such as mercaptans or N-phenyl glycine);
- (vi)
- coumarin and ketocoumarin derivatives;
- (vii)
- pyrylium and thiopyrylium salts (that can decompose peresters; addition of a diphenyl iodonium salt or a bromo compound such as CBr4 to a thiopyrylium salt leads to the generation of radicals);
- (viii)
- ketone/ketone based systems (a typical energy transfer is encountered between a thioxanthone derivative and a morpholino ketone derivative or between a mercaptothioxanthone and a bis phosphineoxide derivative);
- (ix)
- ketone/cleavable compounds (hydroxamic esters, thiohydroxamic esters, etc.);
- (x)
- organometallic compound/ketone based systems (e.g., in ruthenium tris bipyridine/morpholinoketone);
- (xi)
- photosensitizer linked photoinitiator or co-initiator based systems (e.g., a dye linked to a triazine moiety or eosin linked to a O-acyloxime);
- (xii)
- miscellaneous systems (xanthene/thiol, carbazole/triazine, photosensitizer/ketone, etc.)
2.3. Multi-component photoinitiating systems
- (i)
- ketone (e.g., isopropylthioxanthone)/amine/bromo compound or onium salt;
- (ii)
- ketocoumarin/amine/onium salt;
- (iii)
- eosin/amine/onium salt, bromo compound, THF or keto-oxime derivative PDO; methylene blue/amine/onium salt;
- (iv)
- dye (e.g., merocyanine, safranine)/amine/bromo compound or onium salt;
- (v)
- thioxanthene dye/amine/onium salt or bromo compound;
- (vi)
- acridinium cation/dihydropyridine/onium salt;
- (vii)
- ketone/amine/maleimide or maleic anhydride;
- (viii)
- ketone (isopropylthioxanthone/benzophenone sulfonylketone/amine);
- (ix)
- dye/amine/triazine derivatives;
- (x)
- dye (crystal violet, phenosafranine, methylene blue, thiopyronine/amine/ketone) (acetophenone, benzophenone, thioxanthone, 4,4’-bis-dimethylamino benzophenone);
- (xi)
- (keto)coumarin/amine/ferrocenium salt;
- (xii)
- coumarin, ketocoumarin or titanocene derivative/ bis-aryl imidazole Cl-HABI/mercaptobenzoxazole;
- (xiii)
- dye (methylene blue)/amine/cobalt salts;
- (xiv)
- dye (Rose Bengal, eosin)/ferrocenium salt/amine/hydroperoxide;
- (xv)
- coumarin or diethyl amino benzophenone/amine/bis-aryl imidazole/onium salt, pyrromethene/amine/triazine, miscellaneous systems (based on ruthenium derivatives, gold containing molecule, etc.).
3. Overview of the Available Dye Based Cationic Photoinitiating Systems
3.1. Colored onium salts
3.2. Organometallic complexes
3.3. Dye/onium salt systems
3.4. Photoacids PAG
4. Dye Based Anionic Photoinitiators and Photobase Generators (PBG)
5. Recent Developments of Silane Based System for FRP, CP and FRPCP upon Visible Light Excitation under Air
5.1. Silyl radicals for FRPCP processes
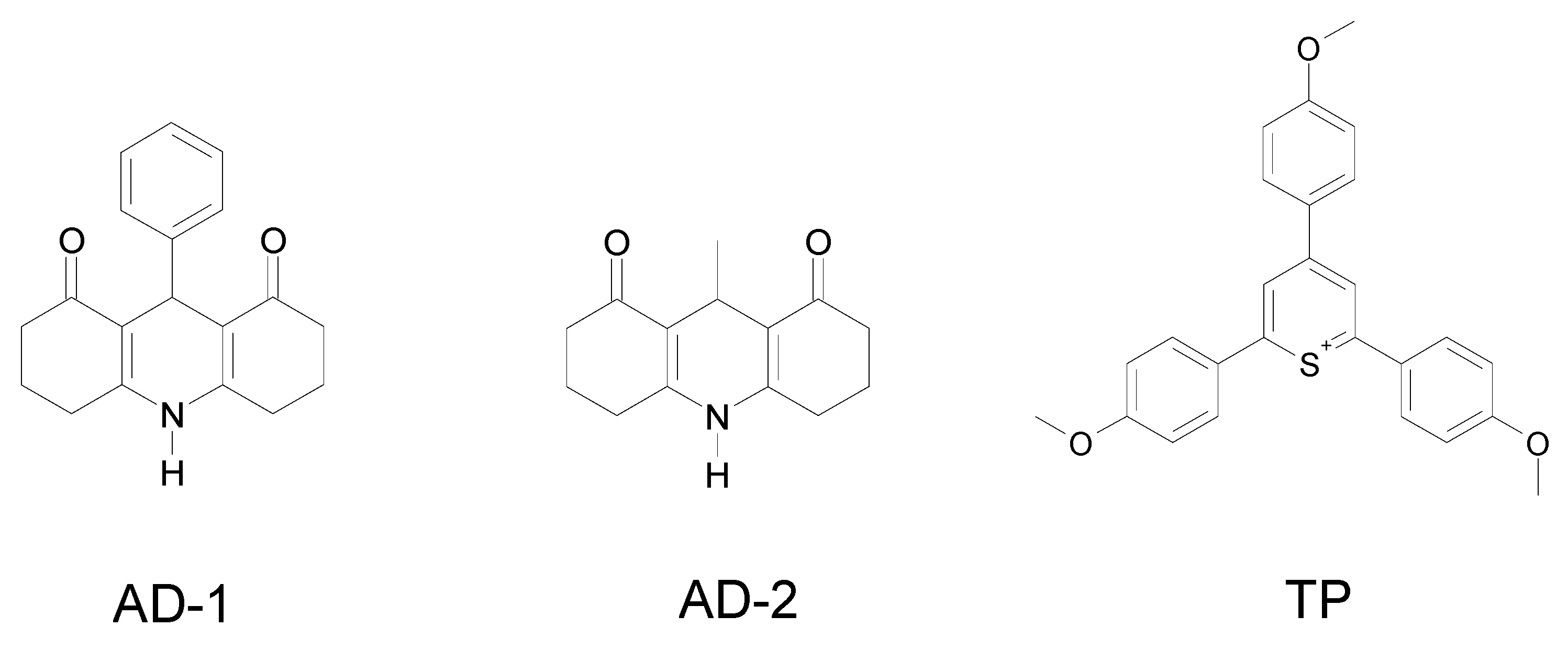
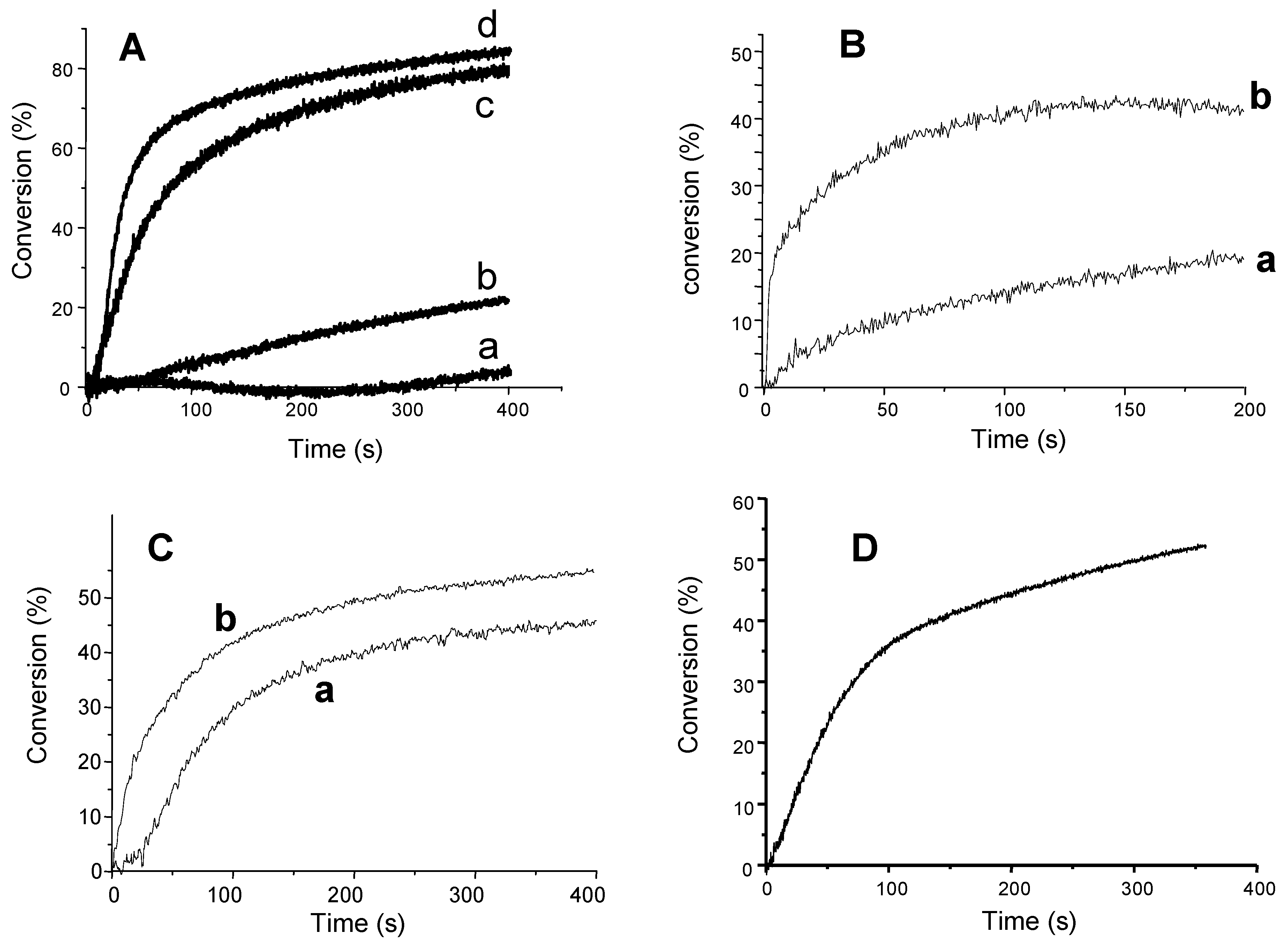
5.2. Silyl radicals for FRP processes

6. Conclusions
References
- Radiation Curing of Polymeric Materials; Hoyle, C.E.; Kinstle, J.F. (Eds.) ACS Publications: Washington, DC, USA, 1990; p. 417.
- Lasers in Polymer Science and Technology; Fouassier, J.P.; Rabek, J.F. (Eds.) CRC Press: Boca Raton, FL, USA, 1990.
- Radiation Curing in Polymer Science and Technology; Fouassier, J.P.; Rabek, J.F. (Eds.) Elsevier Science Publishers LTD: London, UK, 1993.
- Fouassier, J.P. Photoinitiation, Photopolymerization and Photocuring: Fundamental and Applications; Hanser Publishers: New York, NY, USA, 1995. [Google Scholar]
- Crivello, J.V. Photoinitiators for Free Radical, Cationic and Anionic Photopolymerization, 2nd ed.; Bradley, G., Ed.; Wiley: New York, NY, USA, 1998. [Google Scholar]
- Neckers, D.C.; Jager, W. UV and EB at the Millenium; Sita Technology: London, UK, 1999. [Google Scholar]
- Dietliker, K. A Compilation of Photoinitiators Commercially Available for UV Today; Sita Technology Ltd: Edinburgh, UK, 2002. [Google Scholar]
- Photoinitiated Polymerization; Belfied, K.D.; Crivello, J.V. (Eds.) ACS Publications: Washington, DC, USA, 2003; p. 847.
- Photochemistry and UV Curing; Fouassier, J.P. (Ed.) Research Signpost: Trivandrum, India, 2006.
- Bottcher, H. Technical Applications of Photochemistry; Deutscher Verlag fur grundstoffindustrie: Leipzig, Germany, 1991. [Google Scholar]
- Pappas, S.P. UV-Curing: Science and Technology; Tech. Mark. Corp.: Stamford, 1986; Plenum Press: New York, NY, USA, 1992.
- Photoresponsive Polymers; Krongauz, V.; Trifunac, A. (Eds.) Chapman and Hall: New York, NY, USA, 1994.
- Reiser, A. Photoreactive Polymers: The Science and Technology of Resists; Wiley: New York, NY, USA, 1989. [Google Scholar]
- Photopolymerization: Fundamentals and Applications; Scranton, A.B.; Bowman, A.; Peiffer, R.W. (Eds.) ACS Publications: Washington, DC, USA, 1997.
- Davidson, S. Exploring the Science, Technology and Application of UV and EB Curing; Sita Technology Ltd: London, UK, 1999. [Google Scholar]
- Crivello, J.V.; Dietliker, K. Photoinitiators for Free Radical Cationic and Anionic Photopolymerization; Bradley, G., Ed.; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Photosensitive Systems for Photopolymerization Reactions, Trends in Photochemistry & Photobiology; Fouassier, J.P. (Ed.) Research Trends: Trivandrum, India, 1999; Volume 5.
- Light Induced Polymerization Reactions, Trends in Photochemistry & Photobiology; Fouassier, J.P. (Ed.) Research Trends: Trivandrum, India, 2001; Volume 7.
- Schwalm, R. UV Coatings: Basics, Recent Developments and New Applications; Elsevier: Oxford, UK, 2007. [Google Scholar]
- Schnabel, W. Polymers and Light: Fundamentals and Technical Applications; Wiley-VCH: Weinheim, Germany, 2007. [Google Scholar]
- Photochemistry and Photophysics of Polymer Materials; Allen, N.S. (Ed.) Wiley: New York, NY, USA, 2010.
- Basics of Photopolymerization Reactions; Fouassier, J.P.; Allonas, X. (Eds.) Researchsignpost: Trivandrum, India, 2010.
- Lasers in Chemistry; Lackner, M. (Ed.) Wiley-VCH: Weinheim, Germany, 2008.
- Macromolecular Engineering: From Precise Macromolecular Synthesis to Macroscopic Materials Properties and Applications; Matyjaszewski, K.; Gnanou, Y.; Leibler, L. (Eds.) Wiley-VCH: Weinheim, Germany, 2007.
- Eaton, D.F. Dye Sensitized Photopolymerization in Advances in Photochemistry; Hammond, D.H., Gollnick, G.S., Eds.; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Fouassier, J.P. Photoinitiators and photosensitizers for high speed photopolymers in laser imaging. Recent Res. Dev. Polym. Sci. 1999, 4, 131–145. [Google Scholar]
- Fouassier, J.P.; Allonas, X.; Lalevée, J. Macromolecular Engineering: From Precise Macromolecular Synthesis to Macroscopic Materials Properties and Applications; Matyjaszewski, K., Gnanou, Y., Leibler, L., Eds.; Wiley-VCH: Weinheim, Germany, 2007; Volume 1, pp. 643–672. [Google Scholar]
- Allonas, X.; Crouxte-Barghorn, C.; Fouassier, J.P.; Lalevée, J.; Malval, J.P.; Morlet-Savary, F. Lasers in Chemistry; Lackner, M., Ed.; Wiley-VCH: Weinheim, Germany, 2008; Volume 2, pp. 1001–1027. [Google Scholar]
- Fouassier, J.P.; Allonas, X.; Lalevée, J.; Dietlin, C. Handbook on Photochemistry and Photophysics of Polymer Materials; Allen, N.S., Ed.; Wiley: New York, NY, USA, 2010; pp. 350–417. [Google Scholar]
- Kahveci, M.U.; Gilmaz, A.G.; Yagci, Y. Handbook on Photochemistry and Photophysics of Polymer Materials; Allen, N.S., Ed.; Wiley: New York, NY, USA, 2010; pp. 421–478. [Google Scholar]
- Ivan, M.G.; Scaiano, J.C. Handbook on Photochemistry and Photophysics of Polymer Materials; Allen, N.S., Ed.; Wiley: New York, NY, USA, 2010; pp. 479–508. [Google Scholar]
- Muftuogli, A.E.; Tasdelen, M.A.; Yagci, Y. Handbook on Photochemistry and Photophysics of Polymer Materials; Allen, N.S., Ed.; Wiley: New York, NY, USA, 2010; pp. 509–540. [Google Scholar]
- Lalevée, J.; El-Roz, M.; Allonas, X.; Fouassier, J.-P. Organosilanes: Properties, Performance and Applications; Wyman, E.B., Skief, M.C., Eds.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2009. [Google Scholar]
- Lalevée, J.; Tehfe, M.-A.; Allonas, X.; Fouassier, J.-P. Polymer Initiators; Ackrine, W.J., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010. [Google Scholar]
- Lalevée, J.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Allonas, X.; Fouassier, J.P. Oxygen mediated and wavelength tunable cationic photopolymerization reactions under air and low intensity: A new concept. Prog. Org. Coating. 2010, 70, 23–31. [Google Scholar] [CrossRef]
- Lalevée, J.; Tehfe, M.A.; Morlet-Savary, F.; Graff, B.; Allonas, X.; Fouassier, J.P. Radical photopolymerization reactions under air upon lamp and diode laser exposure: The input of the organosilane radical chemistry. Prog. Org. Coating. 2011, in press. [Google Scholar] [CrossRef]
- Timpe, H.J. Radiation Curing in Polymer Science and Technology; Fouassier, J.P., Ed.; Elsevier: Barking, UK, 1993; Volume 2. [Google Scholar]
- Rabek, J.F.; Linden, L.A.; Fouassier, J.F.; Nie, J.; Andrzejewska, E.; Paczkowski, J.; Jakubiak, J.; Wrzyszczynski, A.; Sionkowska, A. Trends in Photochemistry and Photobiology: Photosensitive Systems for Photopolymerization Reactions; Fouassier, J.P., Ed.; Research Trends: Trivandum, India, 1999; Volume 5, pp. 51–62. [Google Scholar]
- Cunningham, A.F.; Desobry, V. Radiation Curing in Polymer Science and Technology; Fouassier, J.P., Rabek, J.F., Eds.; Elsevier: Barking, UK, 1993; Volume 2, pp. 323–374. [Google Scholar]
- Visconti, M. Photochemistry and UV Curing: New Trends; Fouassier, J.P., Ed.; Research Signpost: Trivandrum, India, 2006. [Google Scholar]
- Moszner, N.; Liska, R. Basics of Photopolymerization Reactions; Fouassier, J.P., Allonas, X., Eds.; Researchsignpost: Trivandrum, India, 2010. [Google Scholar]
- Durmaz, Y.Y.; Yilmaz, G.; Atasdelen, M.A.; Aydogan, B.; Koz, B.; Yagci, Y. Basics of Photopolymerization Reactions; Fouassier, J.P., Allonas, X., Eds.; Researchsignpost: Trivandrum, India, 2010. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fouassier, J.-P.; Morlet-Savary, F.; Lalevée, J.; Allonas, X.; Ley, C. Dyes as Photoinitiators or Photosensitizers of Polymerization Reactions. Materials 2010, 3, 5130-5142. https://doi.org/10.3390/ma3125130
Fouassier J-P, Morlet-Savary F, Lalevée J, Allonas X, Ley C. Dyes as Photoinitiators or Photosensitizers of Polymerization Reactions. Materials. 2010; 3(12):5130-5142. https://doi.org/10.3390/ma3125130
Chicago/Turabian StyleFouassier, Jean-Pierre, Fabrice Morlet-Savary, Jacques Lalevée, Xavier Allonas, and Christian Ley. 2010. "Dyes as Photoinitiators or Photosensitizers of Polymerization Reactions" Materials 3, no. 12: 5130-5142. https://doi.org/10.3390/ma3125130
APA StyleFouassier, J.-P., Morlet-Savary, F., Lalevée, J., Allonas, X., & Ley, C. (2010). Dyes as Photoinitiators or Photosensitizers of Polymerization Reactions. Materials, 3(12), 5130-5142. https://doi.org/10.3390/ma3125130




