Calcium Phosphate Bone Cements Including Sugar Surfactants: Part Two—Injectability, Adhesive Properties and Biocompatibility
Abstract
:1. Introduction
2. Results and Discussion
2.1. Injectability
| surfactant | 0% | 1% | |
|---|---|---|---|
| Cementek | SE11S | 44 | 91 |
| SE16P | 95 | ||
| SE16L | 90 | ||
| M68EC | 85 | ||
| M14 | 67 | ||
| ONS10 | 93 | ||
| Cementek LV | SE11S | 84 | - |
| SE16P | - | ||
| M68EC | - | ||
| M14 | - |
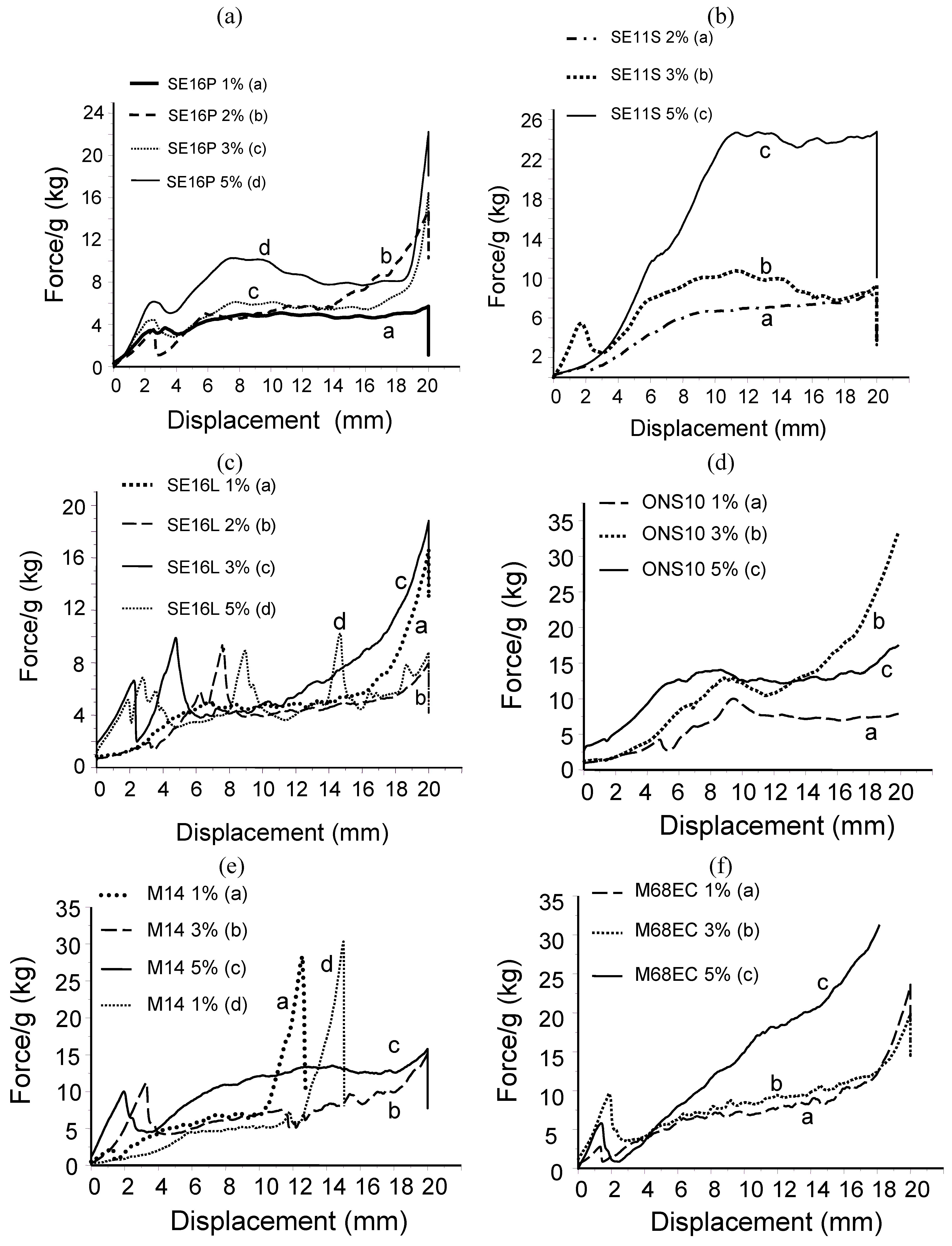
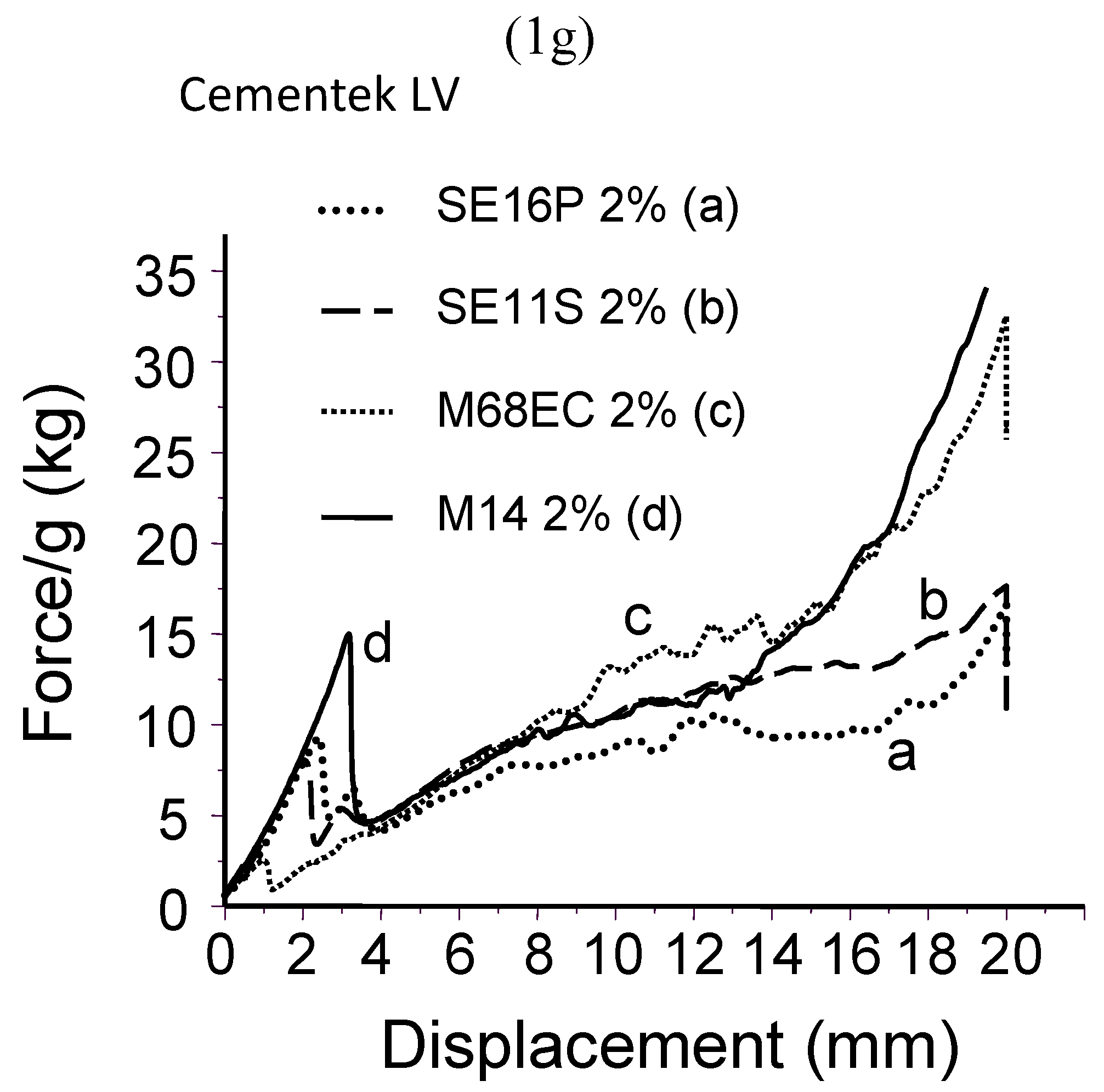
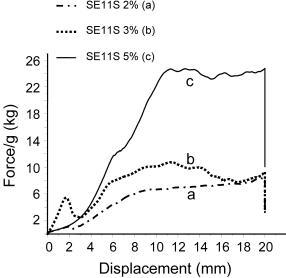
2.2. Probe-tack adhesion measurements
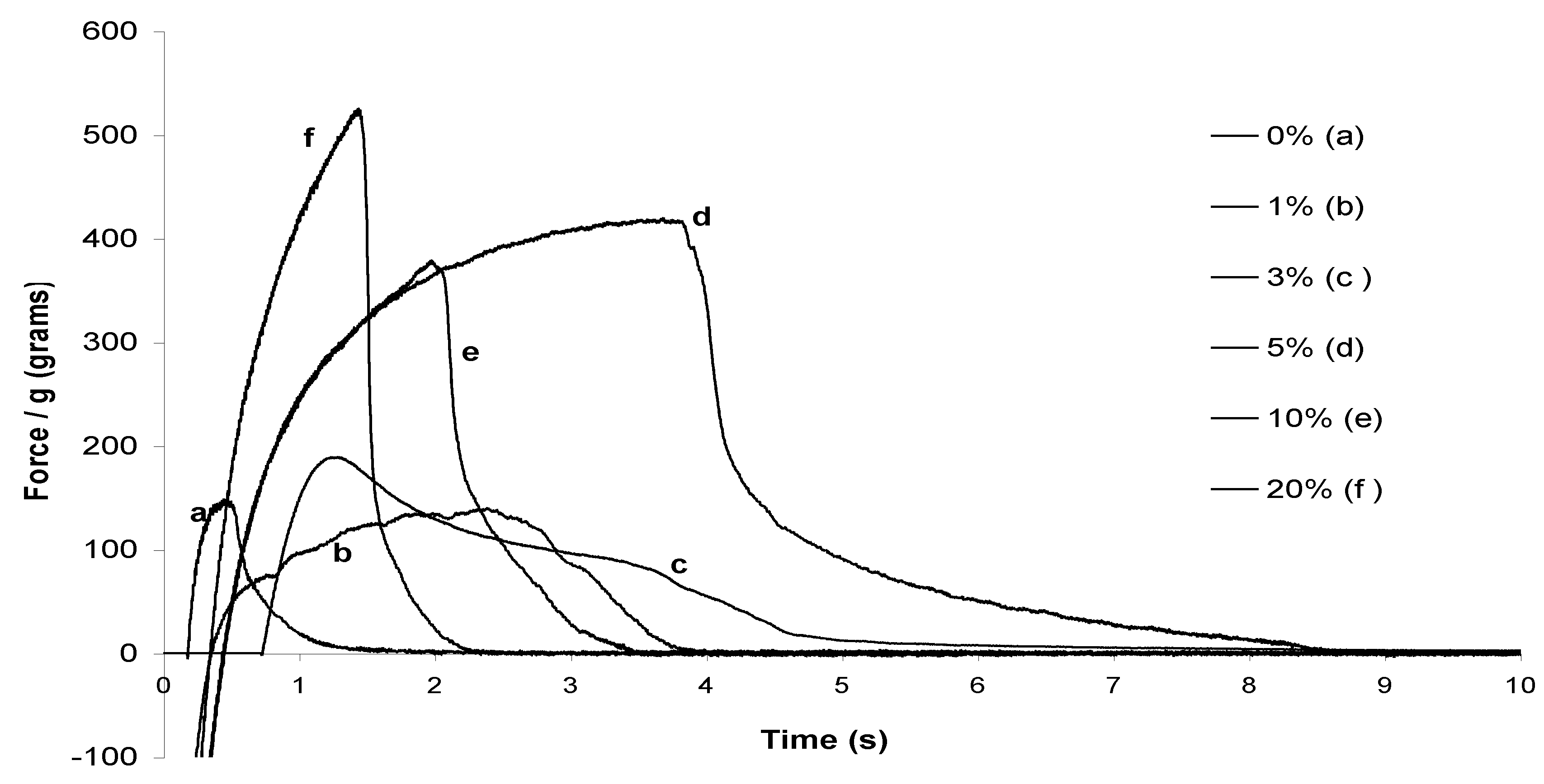
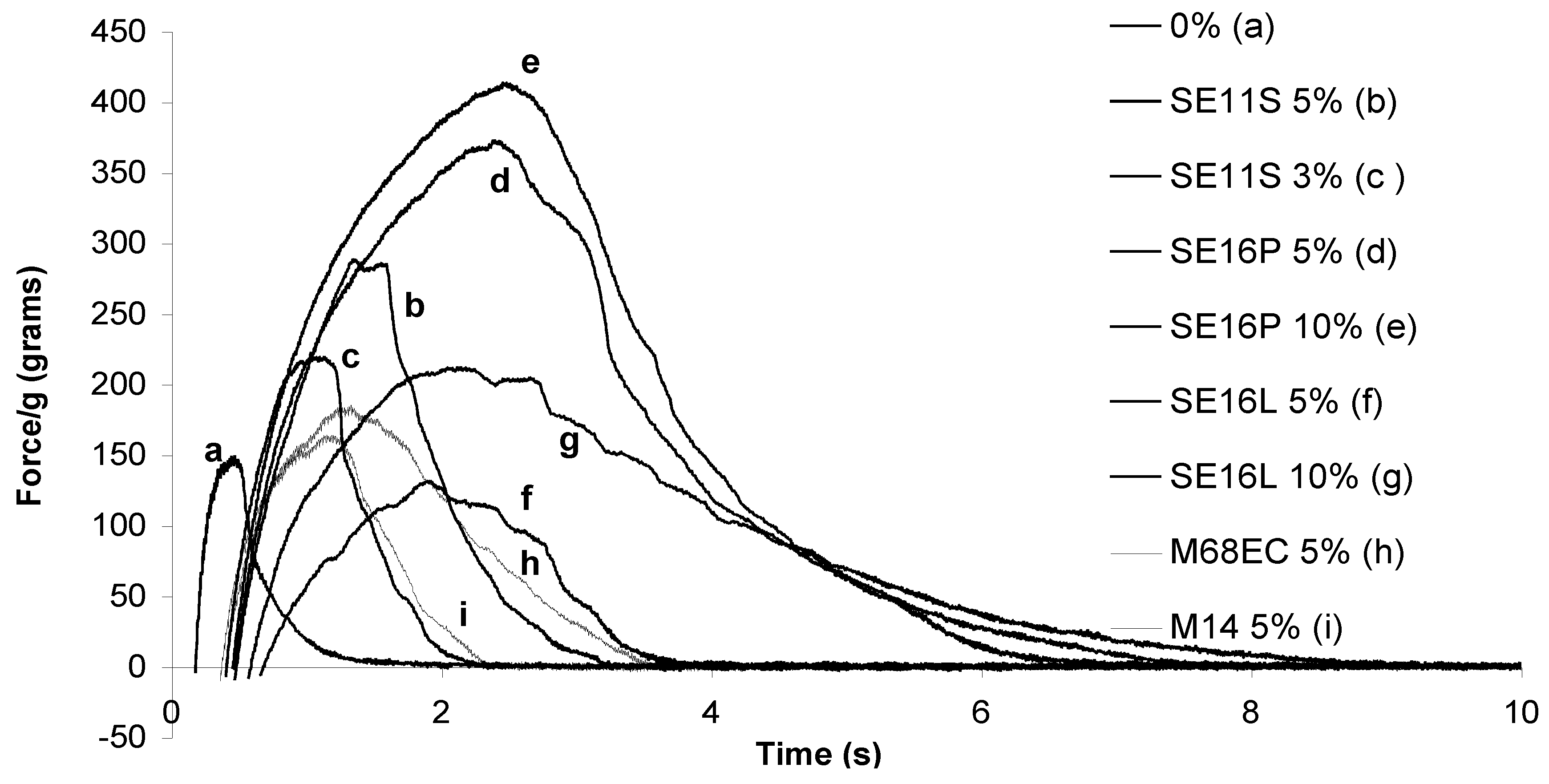
| Sugar surfactant | Surfactant amount (%) | Adhesion Energy (J/m2 ) |
|---|---|---|
| none | 0 | 0.6 |
| SE16P | 1 | 2.0 |
| SE16P | 3 | 2.4 |
| SE16P | 5 | 9.3 |
| SE16P | 10 | 3.4 |
| SE16P | 20 | 2.8 |
| SE16L | 5 | 1.7 |
| SE11S | 3 | 2.3 |
| SE11S | 5 | 0.5 |
| SE5S | 1 | 0.9 |
| SE5S | 3 | 1.6 |
| SE5S | 5 | 1.4 |
| SE5S | 10 | 1.8 |
| M68EC | 5 | 1.4 |
| M14 | 5 | 0.9 |
| ONS10 | 5 | 0.08 |
| Sugar Surfactant | Surfactant amount (%) | Adhesion Energy to Bone (J/m2 ) |
|---|---|---|
| none | 0 | 0.4 |
| SE16P | 5 | 6.5 |
| SE16P | 10 | 7.4 |
| SE16L | 5 | 1.4 |
| SE16L | 10 | 4.6 |
| SE11S | 3 | 1.2 |
| SE11S | 5 | 2.2 |
| M68EC | 5 | 1.9 |
| M14 | 5 | 1.1 |
| ONS10 | 5 | 0.01 |
2.3. Gentamicine release
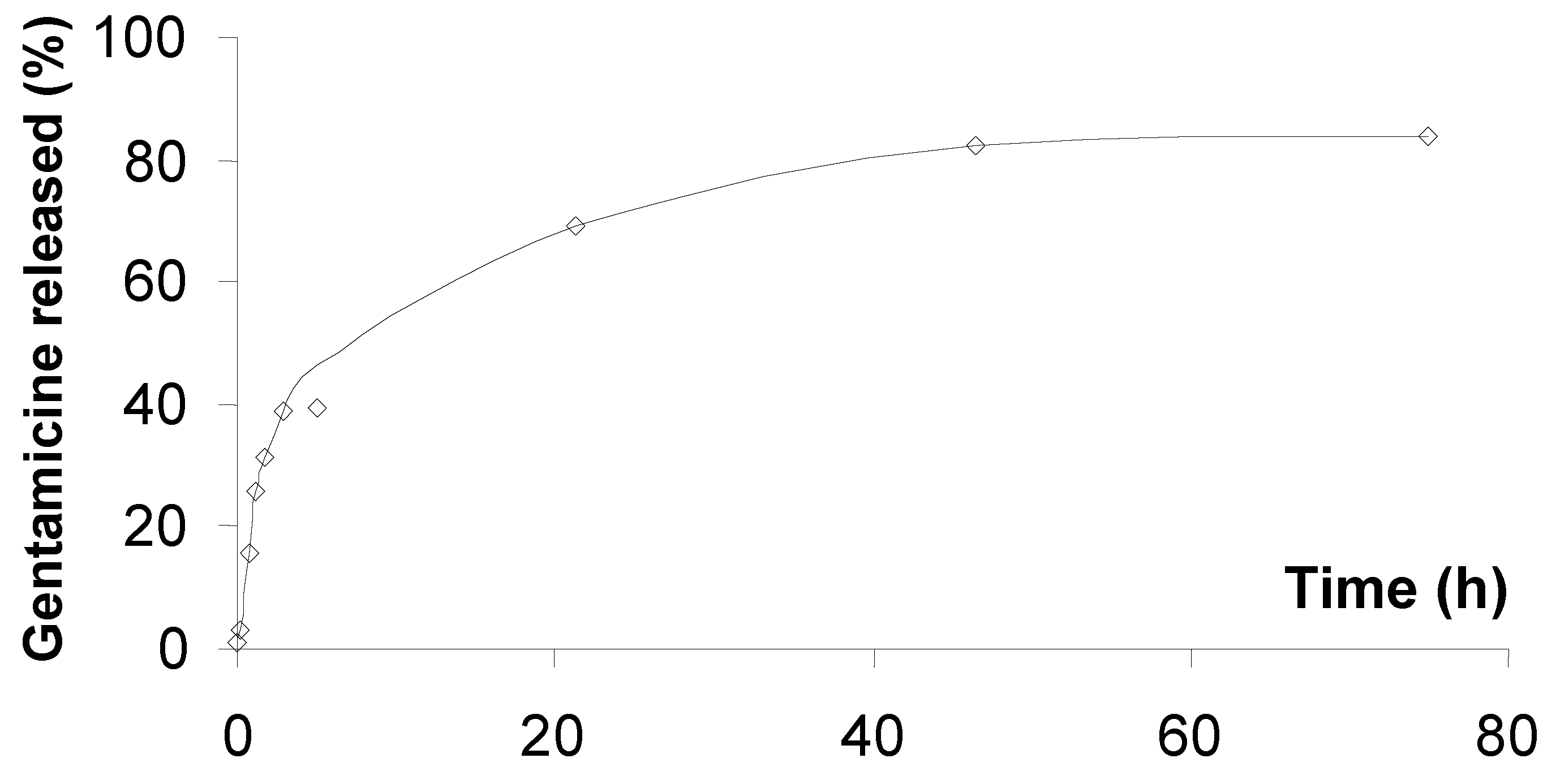
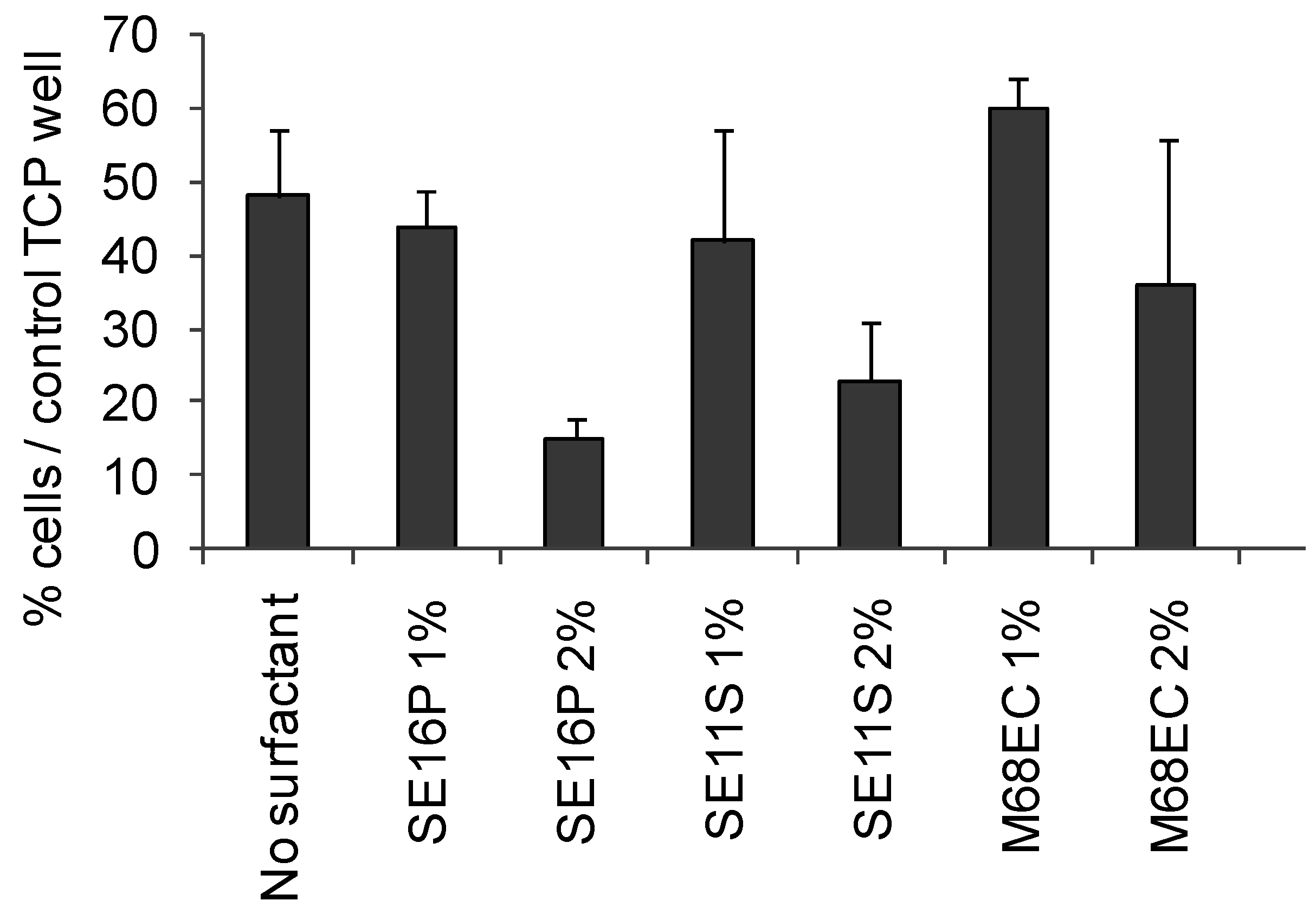
2.4. Osteoblast viability and morphology

3. Experimental Section
3.1. Materials
3.2. Sample preparation
3.3. Injectability
3.4. Probe-Tack Measurements
3.5. Gentamicine release
3.6. Osteoblast culture experiments
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgements
References and Notes
- Dorozhkin, S.V. Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 2008, 43, 3028–3057. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Bohner, M.; Baroud, G. Injectability of calcium phosphate pastes. Biomaterials 2005, 26, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G. Injectable bone cements for use in vertebroplasty and kyphoplasty: State-of-the-art review. J. Biomed. Mater. Res., Part B 2006, 76, 456–468. [Google Scholar] [CrossRef]
- Habib, M.; Baroud, G.; Gitzhofer, F.; Bohner, M. Mechanisms underlying the limited injectability of hydraulic calcium phosphate paste. Acta Biomater. 2008, 4, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Biresaw, G. Surfactants in lubrication. In Lubricant Additives: Chemistry and Applications, 2nd ed.; Rudnick, L.R., Ed.; CRC Press: Boca Raton, FL, USA, 2009; p. 407. [Google Scholar]
- Ginebra, M.P.; Delgado, J.A.; Harr, I.; Almirall, A.; Del Valle, S.; Planell, J.A. Factors affecting the structure and properties of an injectable self-setting calcium phosphate foam. J. Biomed. Mater. Res. Part A 2007, 80, 351–361. [Google Scholar] [CrossRef]
- Ginebra-Molins, M.P.; Planell-Estany, J.A.; Gil-Mur, F.X. Injectable, self-setting calcium phosphate foam. Patent WO 2006/030054, 2006. [Google Scholar]
- Muller, A.S.; Gagnaire, J.; Queneau, Y.; Karaoglanian, M.; Maître, J.P.; Bouchu, A. Winsor behaviour of sucrose fatty acid esters: Choice of the cosurfactant and effect of the surfactant composition. Colloids Surf. A 2002, 203, 55–66. [Google Scholar] [CrossRef]
- Fitremann, J.; Queneau, Y.; Maître, J.P.; Bouchu, A. Co-melting of solid sucrose and multivalent cations soaps for solvent-free synthesis of sucrose esters. Tetrahedron Lett. 2007, 48, 4111–4114. [Google Scholar] [CrossRef]
- Datsyuk, V.; Landois, P.; Fitremann, J.; Peigney, A.; Galibert, A.M.; Soula, B.; Flahaut, E. Double-walled carbon nanotubes dispersion via surfactant substitution. J. Mater. Chem. 2009, 19, 2729–2736. [Google Scholar] [CrossRef]
- Von Rybinski, W.; Hill, K. Alkyl polyglycosides: Properties and applications of a new class of surfactants. Angew. Chem. Int. Ed. 1998, 37, 1328–1345. [Google Scholar] [CrossRef]
- Bercier, A.; Gonçalves, S.; Lignon, O.; Fitremann, J. Calcium phosphate bone cement including sugar surfactants: part one—Porosity, setting times and compressive strength. Materials 2010, 3, 4695–4709. [Google Scholar] [CrossRef]
- Ratier, A.; Freche, M.; Lacout, J.L.; Rodriguez, F. Behaviour of an injectable calcium phosphate cement with added tetracycline. Int. J. Pharm. 2004, 274, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Khairoun, I.; Boltong, M.G.; Driessens, F.C.M.; Planell, J.A. Some factors controlling the injectability of calcium phosphate bone cements. J. Mater. Sci.: Mater. Med. 1998, 9, 425–428. [Google Scholar] [CrossRef]
- Rocha Alves, H.L.; Dos Santos, L.A.; Bergmann, C.P. Injectability evaluation of tricalcium phosphate bone cement. J. Mater. Sci.: Mater. Med. 2008, 19, 2241–2246. [Google Scholar] [CrossRef]
- Gauthier, O.; Khairoun, I.; Bosco, J.; Obadia, L.; Bourges, X.; Rau, C.; Magne, D.; Bouler, J.M.; Aguado, E.; Daculsi, G.; Weiss, P. Non-invasive bone replacement using a new injectable calcium phosphate biomaterial. J. Biomed. Mater. Res. 2003, 66, 47–54. [Google Scholar] [CrossRef]
- Leroux, L.; Hatim, Z.; Freche, M.; Lacout, J.L. Effect of various adjuvants (lactic acid, glycerol, and chitosan), on the injectability of a calcium phosphate cement. Bone 1999, 25, 31S–34S. [Google Scholar] [CrossRef] [PubMed]
- Morita, S.; Furuya, K.; Ishihara, K.; Nakabayashi, N. Performance of adhesive bone cement containing hydroxyapatite particles. Biomaterials 1998, 19, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Grover, L.M.; Gbureck, U.; Farrar, D.F.; Barralet, J.E. Adhesion of a novel calcium phosphate cement to cortical bone and several common biornaterials. Key Eng. Mater. 2006, 309-311, 849–852. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaître, J.; Van Landuyt, P.; Zambelli, P.Y.; Merkle, H.P.; Gander, B. Gentamicin-loaded hydraulic calcium phosphate bone cement as antibiotic delivery system. J. Pharm. Sci. 1997, 86, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Ratier, A.; Gibson, I.R.; Best, S.M.; Freche, M.; Lacout, J.L.; Rodriguez, F. Setting characteristics and mechanical behaviour of a calcium phosphate bone cement containing tetracycline. Biomaterials 2001, 22, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Takechi, M.; Miyamoto, Y.; Momota, Y.; Yuasa, T.; Tatehara, S.; Nagayama, M.; Ishikawa, K.; Suzuki, K. The in vitro antibiotic release from anti-washout apatite cement using chitosan. J. Mater. Sci.: Mater. Med. 2002, 13, 973–978. [Google Scholar] [CrossRef]
- Stallmann, H.P.; Faber, C.; Bronckers, A.L.J.J.; Amerongen, A.V.N.; Wuisman, P.I.J.M. Osteomyelitis prevention in rabbits using antimicrobial peptide hLF-11 or gentamicin-containing calcium phosphate cement. J. Antimicrob. Chemother. 2004, 54, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Matsuda, Y.; Wang, Z.; Fox, J.L.; Higuchi, W.I. Effect of sodium bicarbonate amount on in vitro indomethacin release from self-setting carbonated-apatite cement. Pharm. Res. 1997, 14, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Blom, E.J.; Klein-Nulend, J.; Wolke, J.G.C.; Kurashina, K.; van Waas, M.A.J.; Burger, E.H. Transforming growth factor-beta1 incorporation in an alpha-tricalcium phosphate/dicalcium phosphate dihydrate/tetracalcium phosphate monoxide cement: Release characteristics and physicochemical properties. Biomaterials 2002, 23, 1261–1268. [Google Scholar] [CrossRef]
- Autefage, H.; Briand-Mésange, F.; Cazalbou, S.; Drouet, C.; Fourmy, D.; Gonçalvès, S.; Salles, J.P.; Combes, C.; Swider, P.; Rey, C. Adsorption and release of BMP-2 on nanocrystalline apatite-coated and uncoated hydroxyapatite/beta-tricalcium phosphate porous ceramics. J. Biomed. Mater. Res. Part B 2009, 91, 706–715. [Google Scholar] [CrossRef]
- Kroese-Deutman, H.C.; Quinten Ruhé, P.; Spauwen, P.H.M.; Jansen, J.A. Bone inductive properties of rhBMP-2 loaded porous calcium phosphate implants inserted at an ectopic site in rabbits. Biomaterials 2005, 26, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Sarda, S.; Nilsson, M.; Balcells, M.; Fernandez, E. Influence of surfactant molecules as air-entraining agent for bone cement macroporosity. J. Biomed. Mater. Res., Part A 2003, 65, 215–221. [Google Scholar] [CrossRef]
- Werner, S.R.L.; Jones, J.R.; Paterson, A.H. Stickiness of maltodextrins using probe tack test during in-situ drying. J. Food Eng. 2007, 80, 859–868. [Google Scholar] [CrossRef]
- Bosc, V.; Ferrari, I.; Michon, C. Adhesion to solid surfaces of gels of iota-carrageenan alone or in mixture with casein. Colloids Surf. A 2008, 331, 2–7. [Google Scholar] [CrossRef]
- Nowakowski, C.M.; Hartel, R.W. Moisture sorption of amorphous sugar products. J. Food Sci. 2002, 67, 1419–1425. [Google Scholar] [CrossRef]
- Lakrout, H.; Creton, C.; Ahn, D.; Shull, K.R. Influence of molecular features on the tackiness of acrylic polymer melts. Macromolecules 2001, 34, 7448–7458. [Google Scholar] [CrossRef]
- Meseguer-Olmo, L.; Ros-Nicolas, M.J.; Clavel-Sainz, M.; Vicente-Ortega, V.; Alcaraz-Baños, M.; Lax-Pérez, A.; Arcos, D.; Ragel, C.V.; Vallet-Regi, M. Biocompatibility and in vivo gentamicin release from bioactive sol-gel glass implants. J. Biomed. Mater. Res. 2002, 61, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Baro, M.; Sanchez, E.; Delgado, A.; Perera, A.; Evora, C. In vitro-in vivo characterization of gentamicin bone implants. J. Control. Release 2002, 83, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Specchia, N.; Pagnotta, A.; Cappella, M.; Tampieri, A.; Greco, F. Effect of hydroxyapatite porosity on growth and differentiation of human osteoblast-like cells. J. Mater. Sci. 2002, 37, 577–584. [Google Scholar] [CrossRef]
- Bignon, A.; Chouteau, J.; Chevalier, J.; Fantozzi, G.; Carret, J.P.; Chavassieux, P.; Boivin, G.; Melin, M.; Hartmann, D. Effect of micro- and macroporosity of bone substitutes on their mechanical properties and cellular response. J. Mater. Sci.: Mater. Med. 2003, 14, 1089–1097. [Google Scholar] [CrossRef]
- Evans, E.J. Toxicity of hydroxyapatite in vitro: The effect of particle size. Biomaterials 1991, 12, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.S.; Liu, H.C.; Chang, W.H.S.; Li, J.; Lin, F.H.; Tai, H.C. Influence of hydroxyapatite particle size on bone cell activities: An in vitro study. J. Biomed. Mater. Res. 1998, 39, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Pioletti, D.P.; Takei, H.; Lin, T.; Van Landuyt, P.; Ma, Q.J.; Kwon, S.Y.; Paul Sung, K.L. The effects of calcium phosphate cement particles on osteoblast functions. Biomaterials 2000, 21, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Annaz, B.; Hing, K.A.; Kayser, M.; Buckland, T.; Di Silvio, L. Porosity variation in hydroxyapatite and osteoblast morphology: A scanning electron microscopy study. J. Microsc. 2004, 215, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Clarot, I.; Chaimbault, P.; Hasdenteufel, F.; Netter, P.; Nicolas, A. Determination of gentamicin sulfate and related compounds by high-performance liquid chromatography with evaporative light scattering detection. J. Chromatogr., A 2004, 1031, 281–287. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bercier, A.; Gonçalves, S.; Autefage, H.; Briand-Mesange, F.; Lignon, O.; Fitremann, J. Calcium Phosphate Bone Cements Including Sugar Surfactants: Part Two—Injectability, Adhesive Properties and Biocompatibility. Materials 2010, 3, 5111-5129. https://doi.org/10.3390/ma3125111
Bercier A, Gonçalves S, Autefage H, Briand-Mesange F, Lignon O, Fitremann J. Calcium Phosphate Bone Cements Including Sugar Surfactants: Part Two—Injectability, Adhesive Properties and Biocompatibility. Materials. 2010; 3(12):5111-5129. https://doi.org/10.3390/ma3125111
Chicago/Turabian StyleBercier, Ariane, Stéphane Gonçalves, Helène Autefage, Fabienne Briand-Mesange, Olivier Lignon, and Juliette Fitremann. 2010. "Calcium Phosphate Bone Cements Including Sugar Surfactants: Part Two—Injectability, Adhesive Properties and Biocompatibility" Materials 3, no. 12: 5111-5129. https://doi.org/10.3390/ma3125111
APA StyleBercier, A., Gonçalves, S., Autefage, H., Briand-Mesange, F., Lignon, O., & Fitremann, J. (2010). Calcium Phosphate Bone Cements Including Sugar Surfactants: Part Two—Injectability, Adhesive Properties and Biocompatibility. Materials, 3(12), 5111-5129. https://doi.org/10.3390/ma3125111