Immobilization of Lead and Zinc in Tailings Sand Using a Stabilizer Synthesized from Granite Sawdust for Mine Remediation
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Methods
2.2.1. Leaching Experiment of Tailings Waste
2.2.2. Physical and Chemical Analysis of Tailings Waste
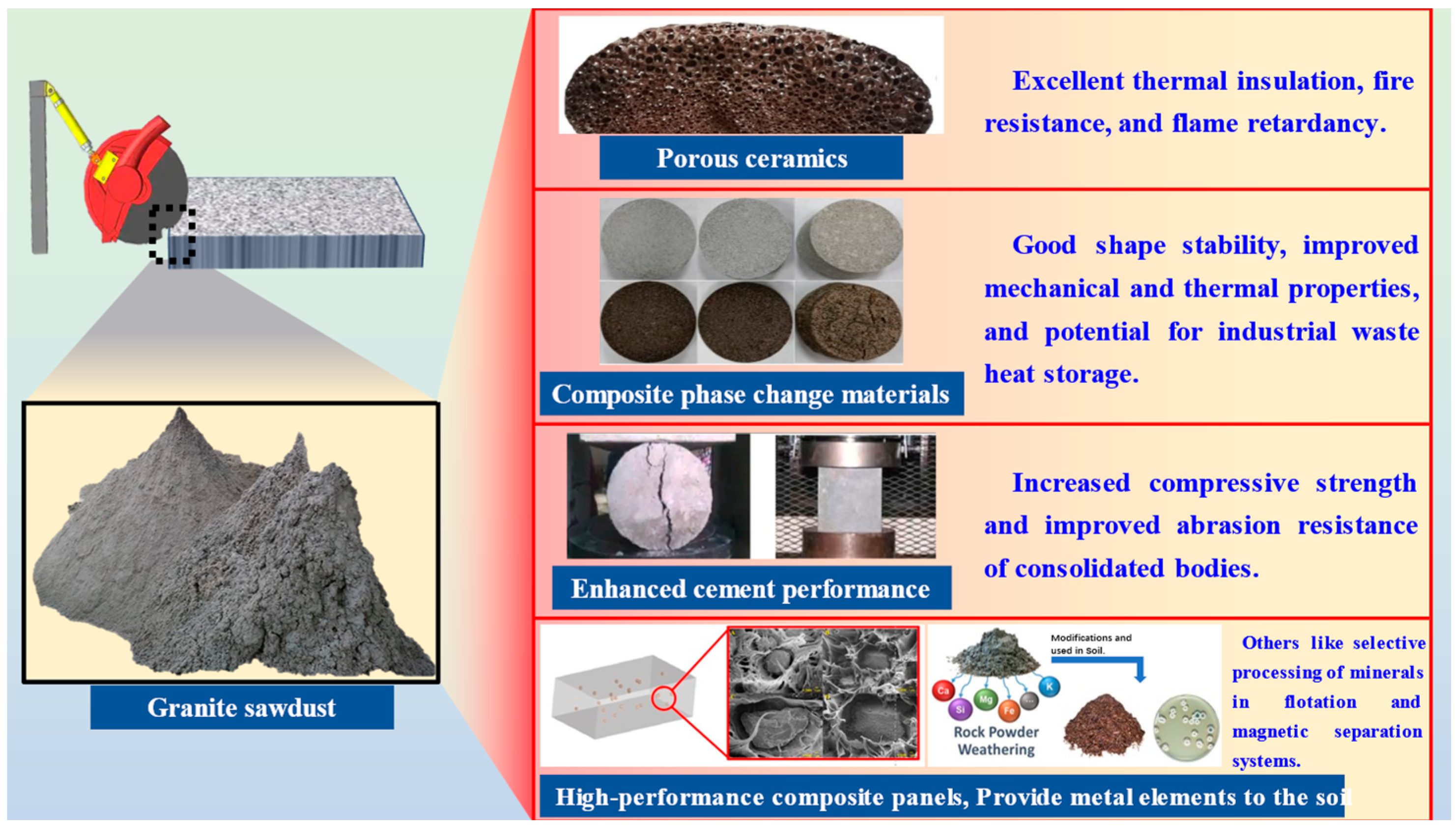
2.2.3. Basic Characterization Analysis of Granite Sawdust
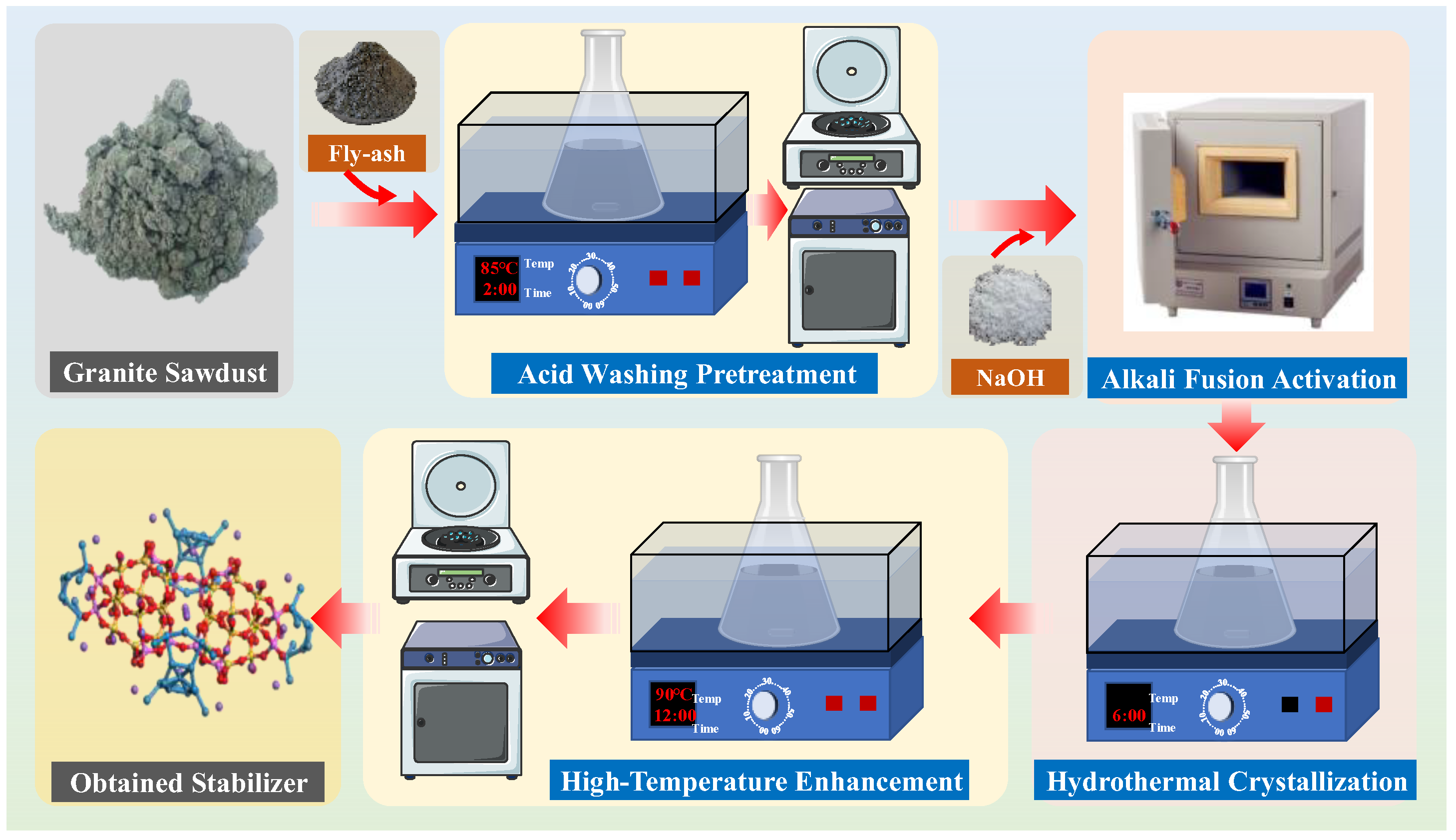
2.2.4. Preliminary Synthesis of Granite Sawdust-Based Stabilizer
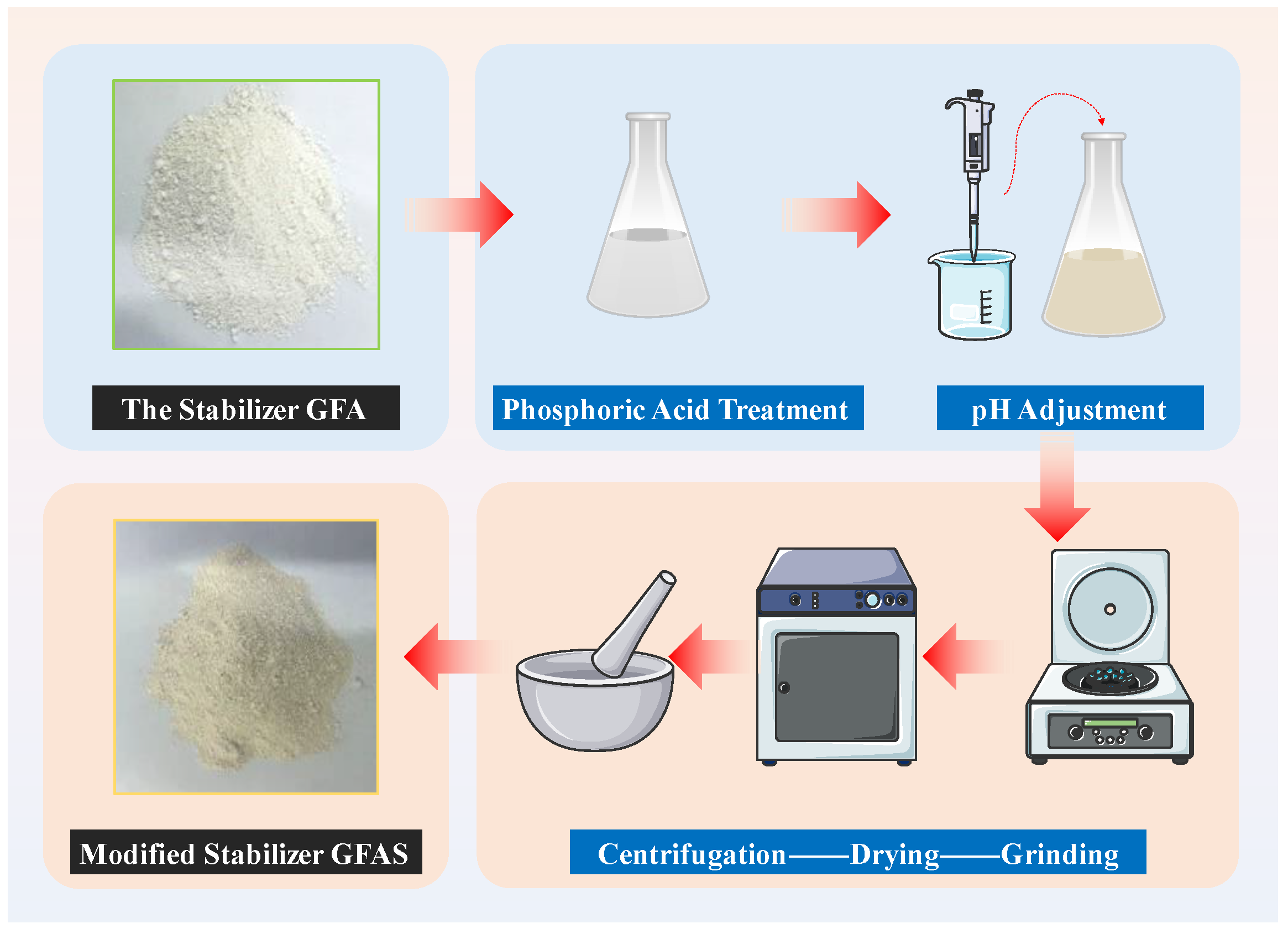
2.2.5. Modification and Optimization of Synthetic Products
2.2.6. Phase and Structural Testing of GFAS
2.2.7. Cation Exchange Capacity and Specific Surface Area Testing of GFAS
2.2.8. Adsorption Isotherms and Adsorption Kinetics of GFAS
3. Results and Discussion
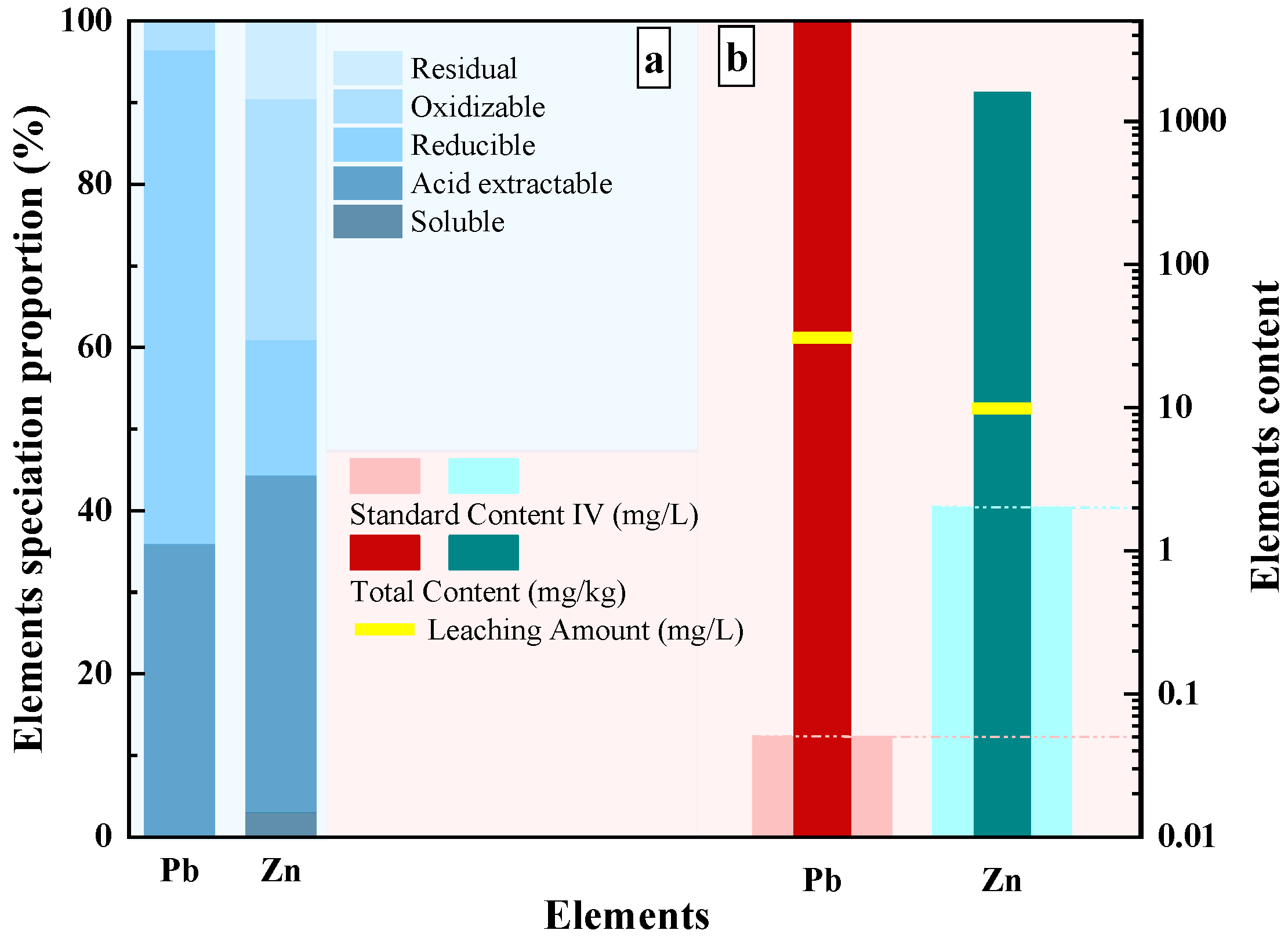
3.1. Physicochemical Characterization of Tailings Waste
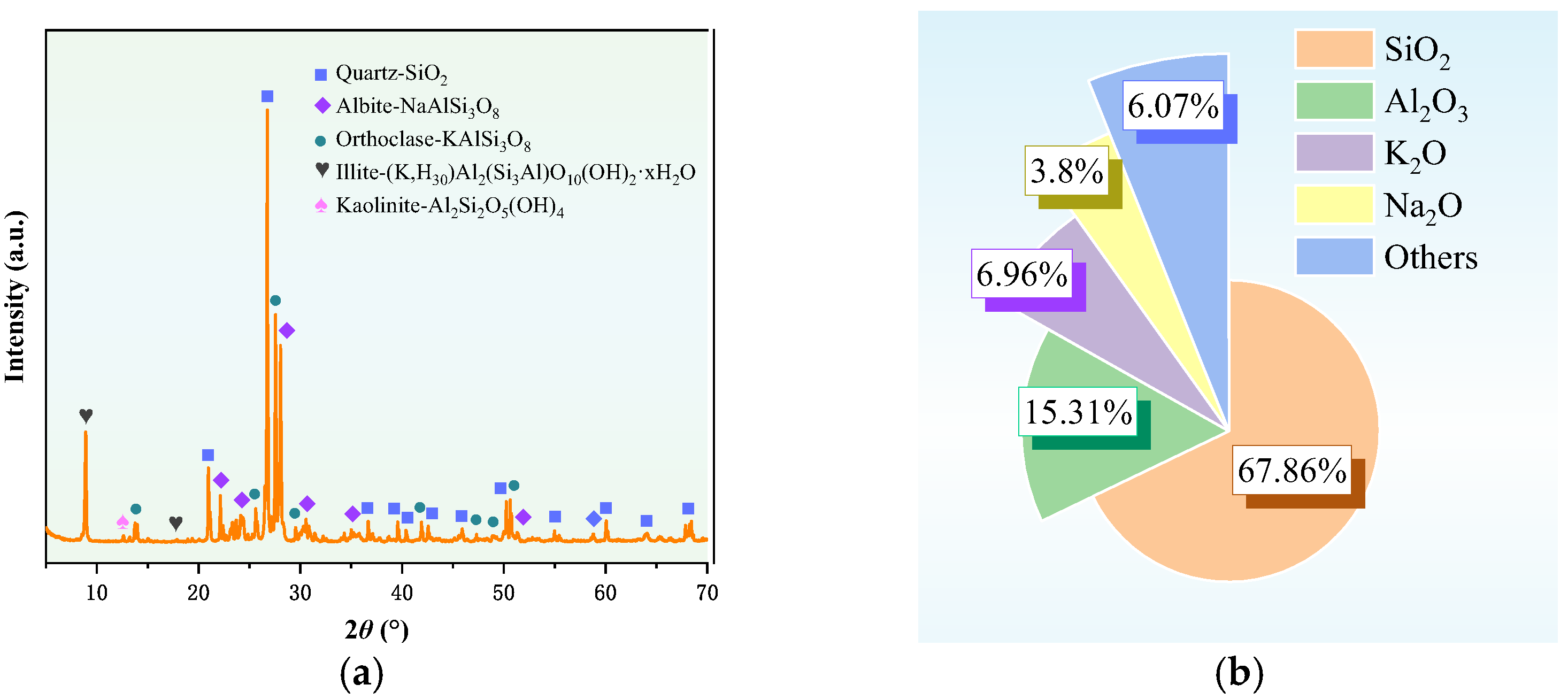
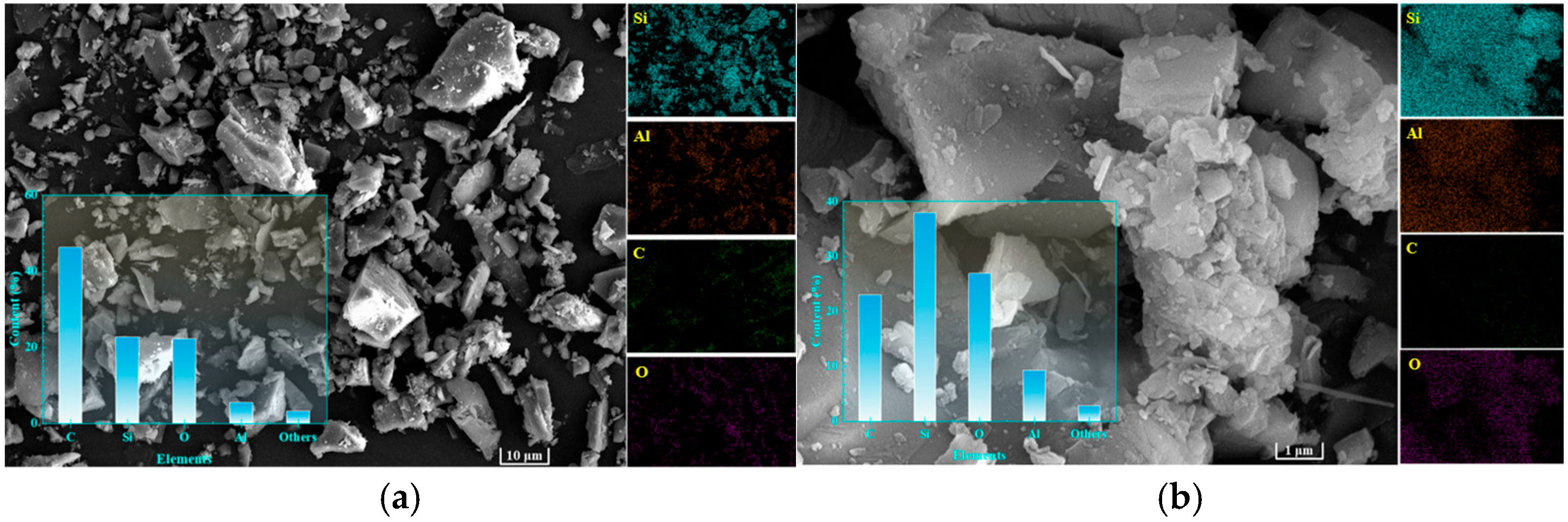
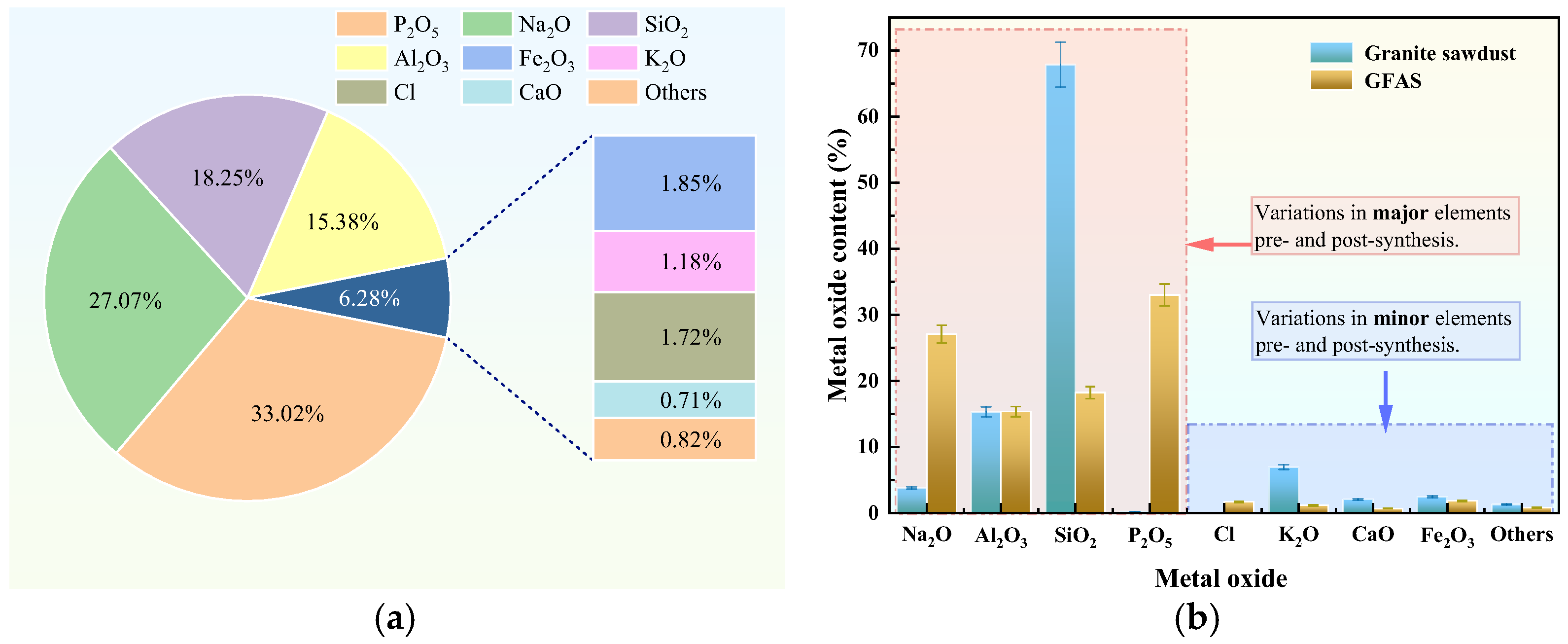
3.2. Characterization of Granite Sawdust Properties
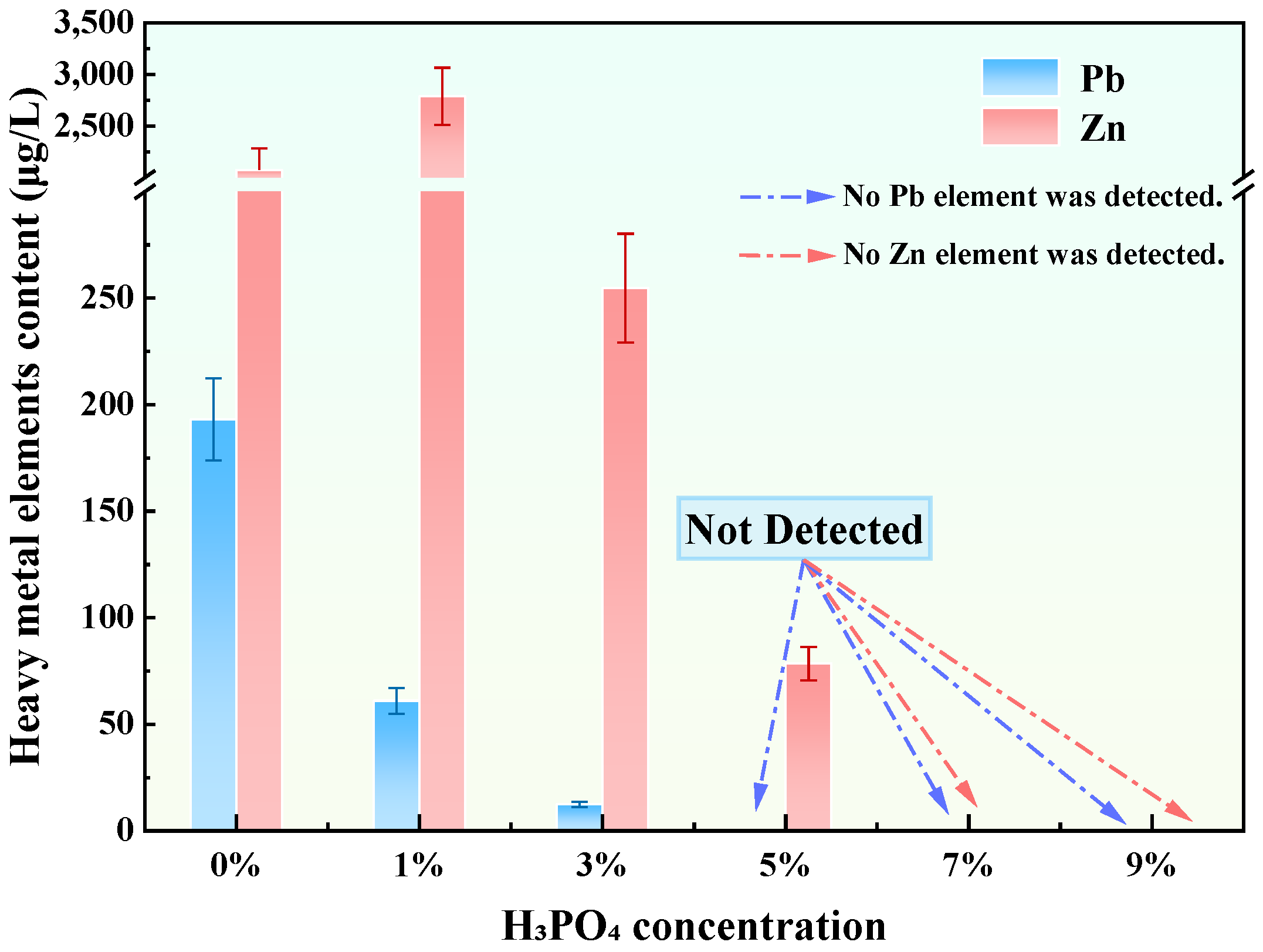
3.3. Analysis of the Preliminary Synthesis of Granite Sawdust-Based Stabilizer
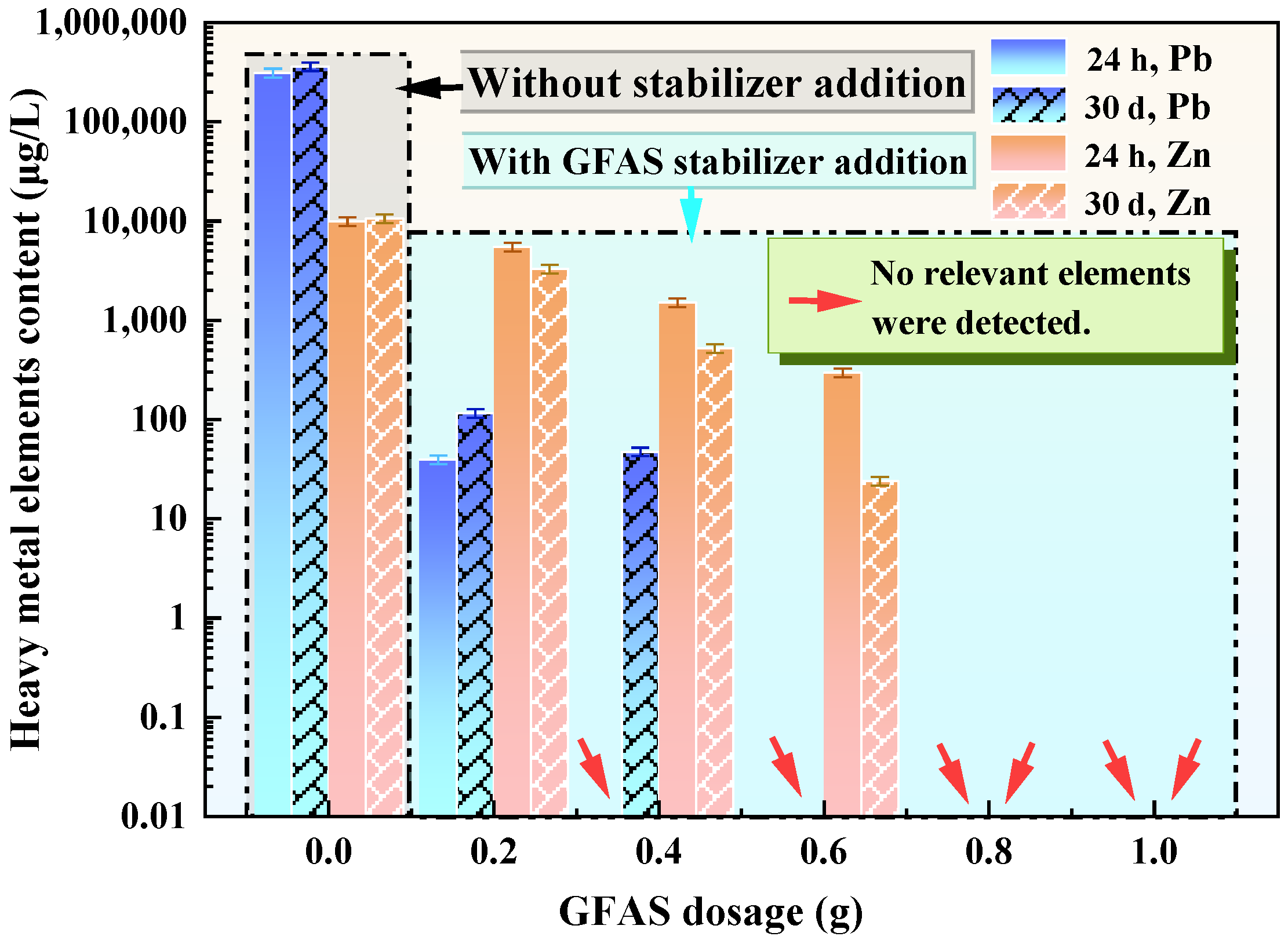
3.4. Modification of Synthesized Granite Sawdust-Based Stabilizer
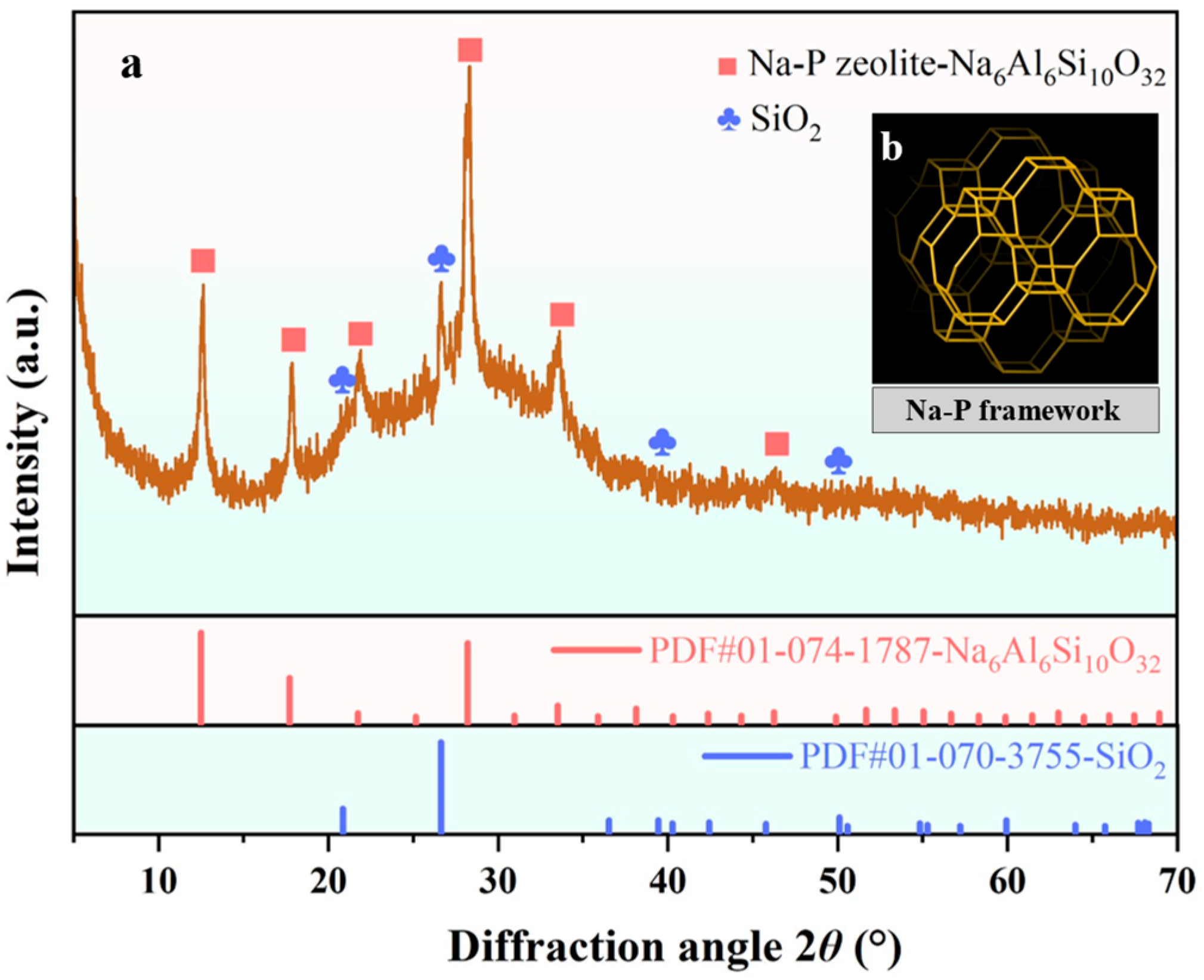
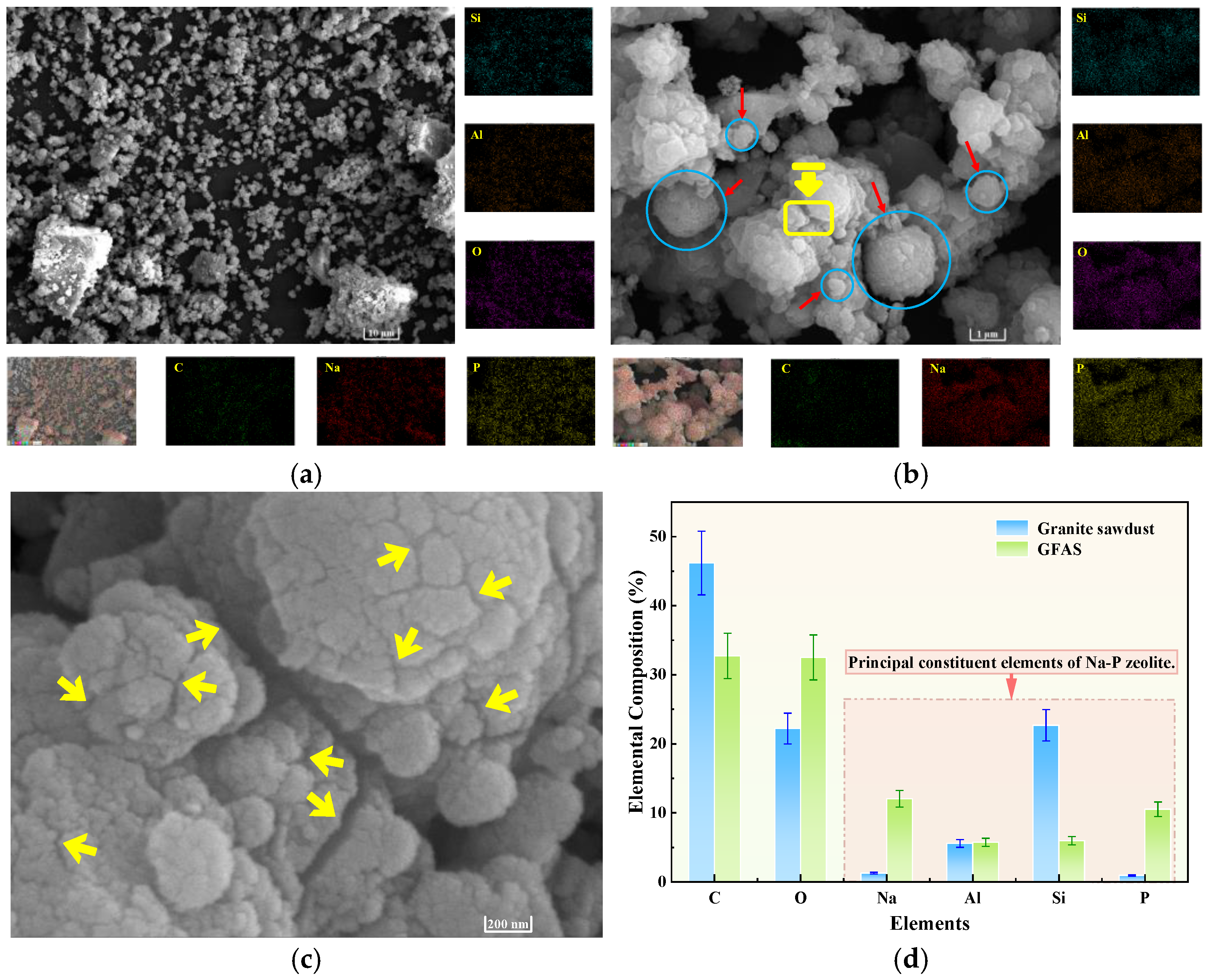
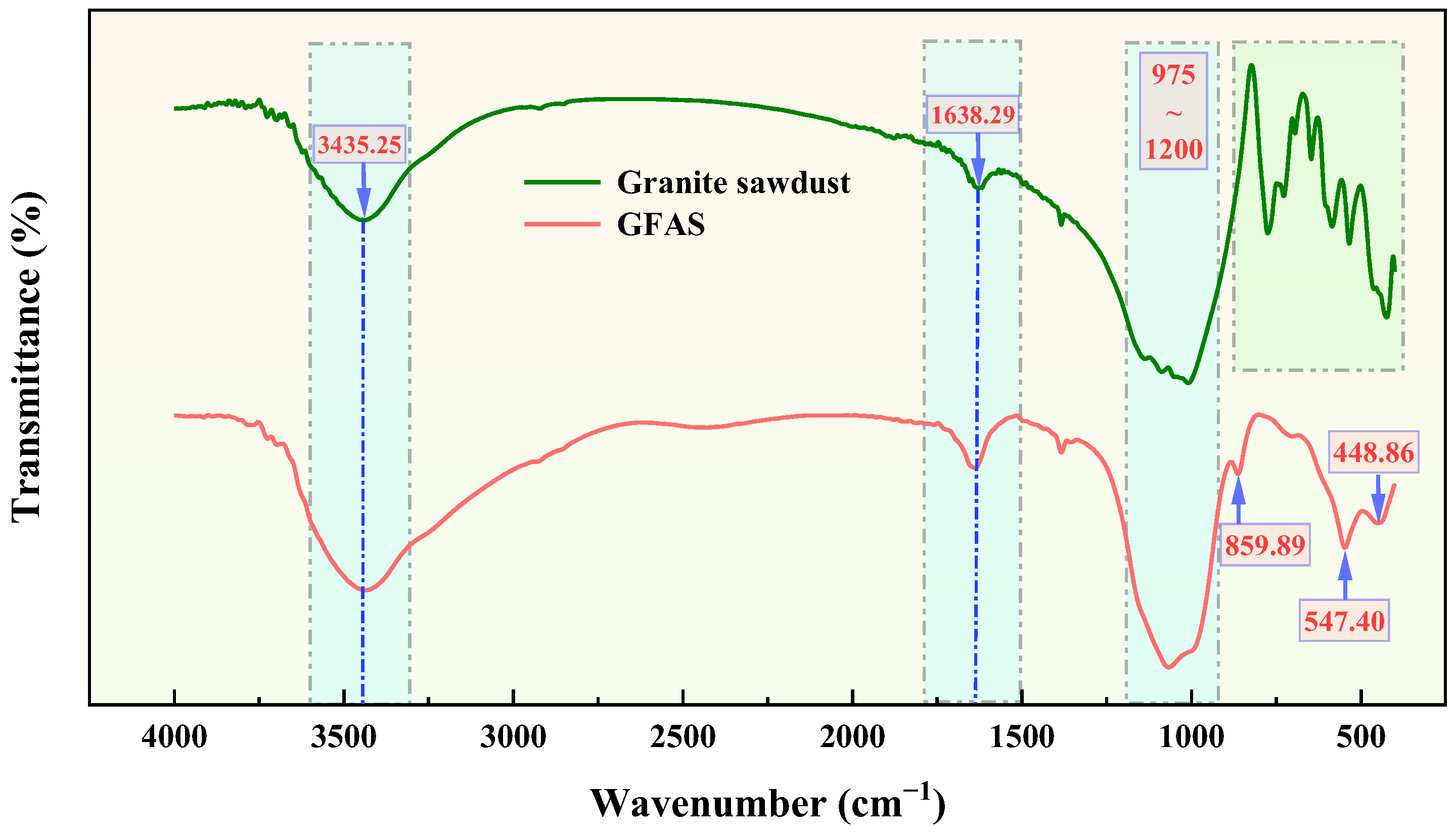
3.5. Phase and Structural Analysis of GFAS
3.6. Cation Exchange Capacity and Specific Surface Area Analysis of GFAS
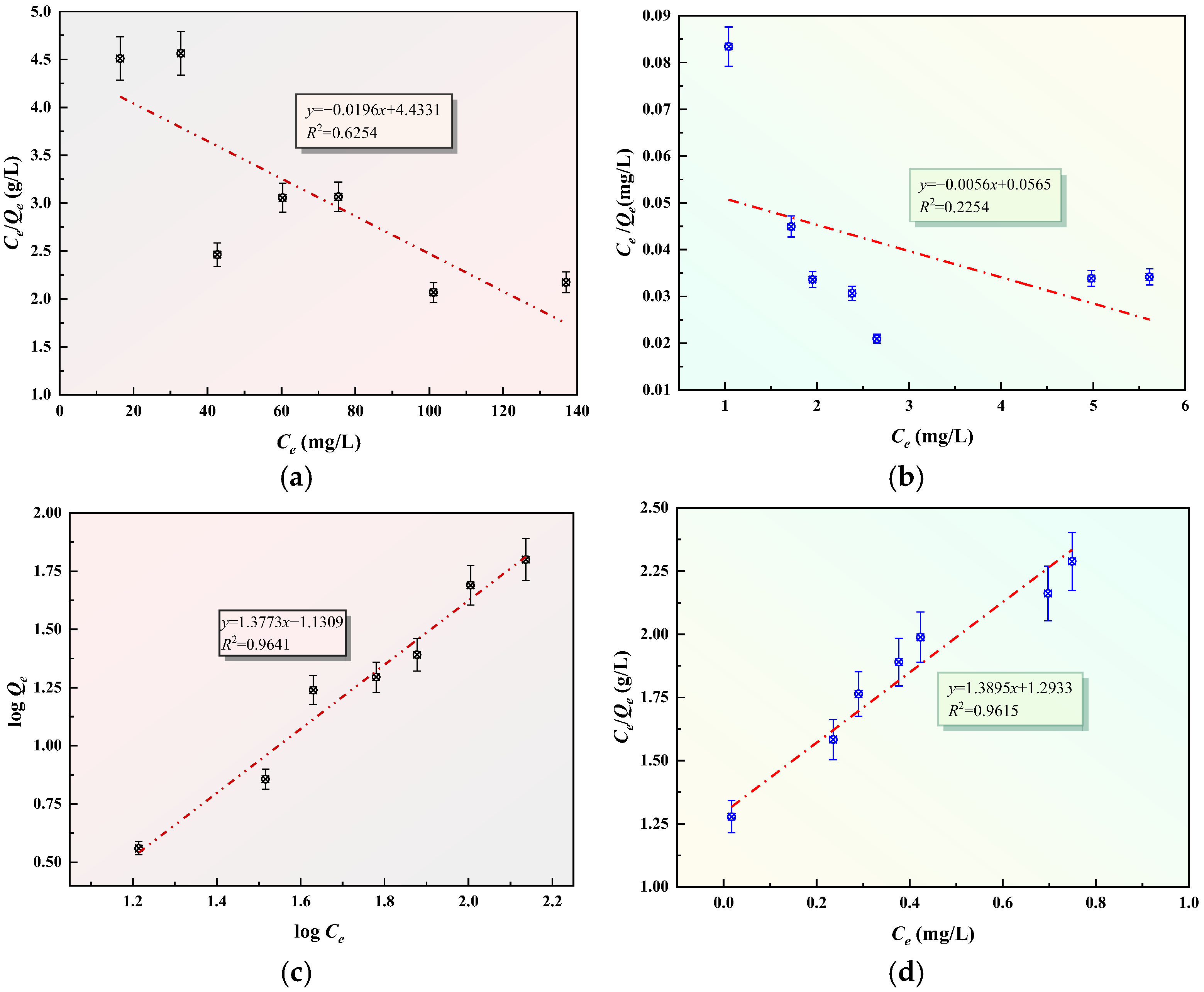
3.7. Analysis of Adsorption Isotherms and Adsorption Kinetics of GFAS
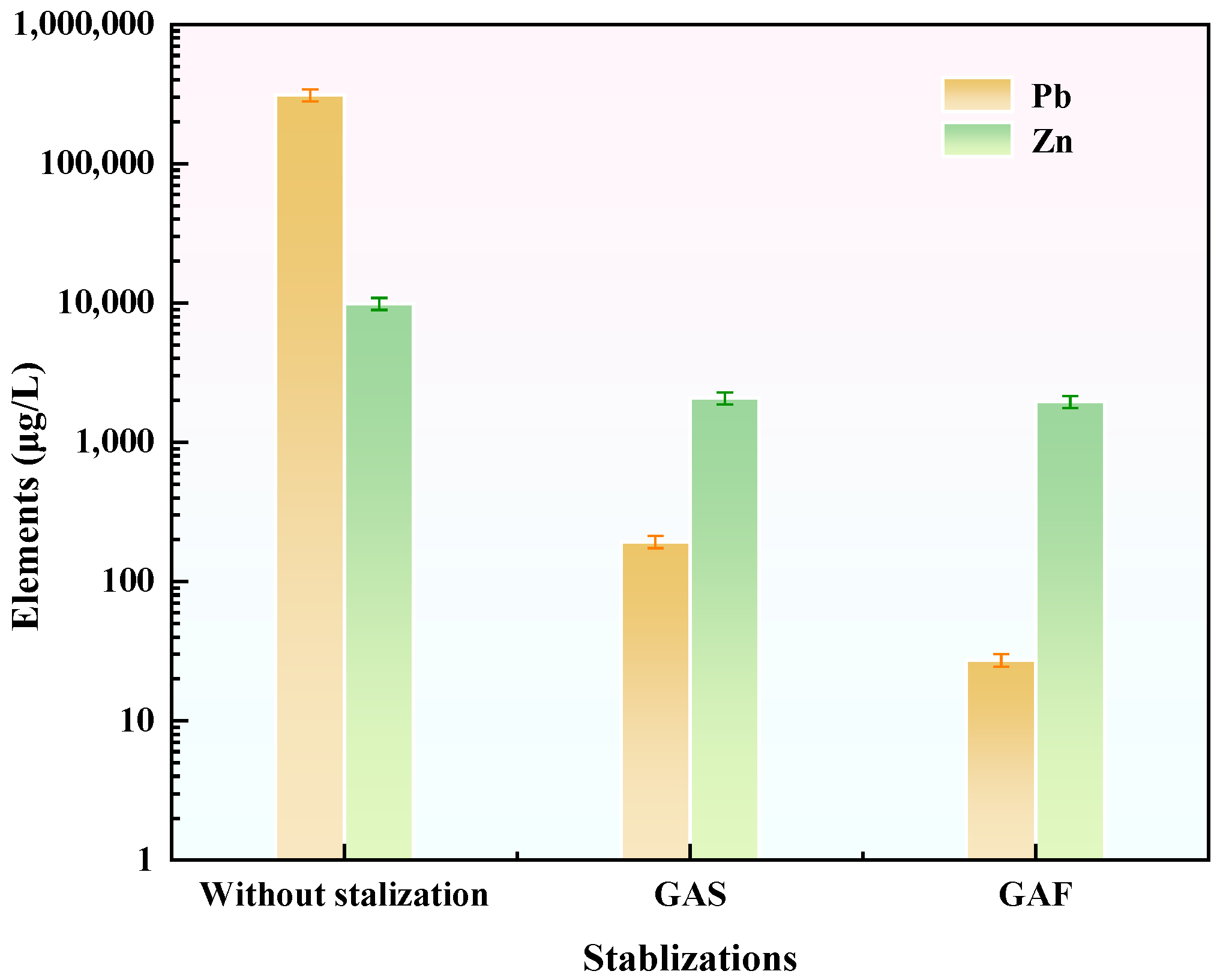
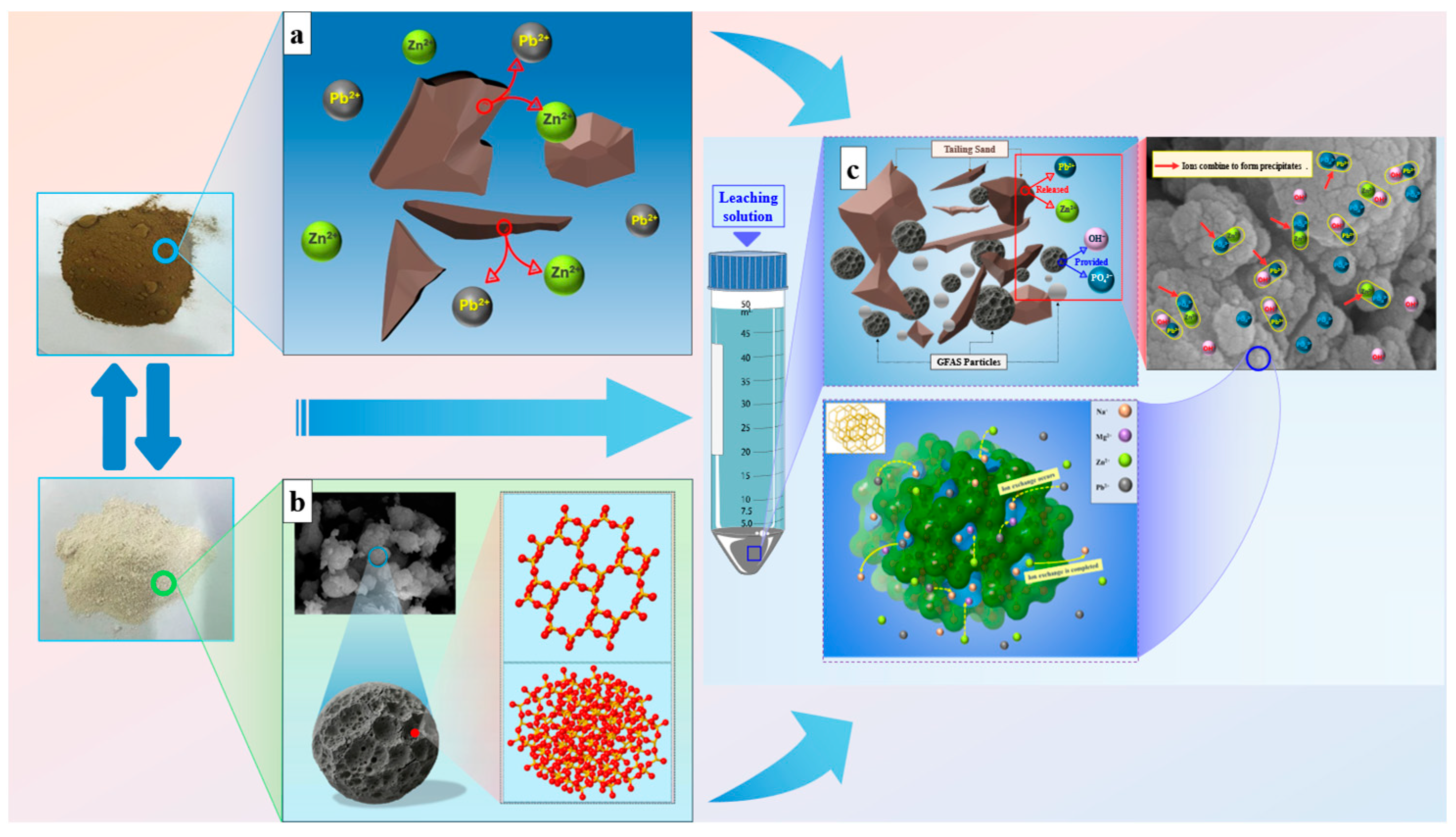
3.8. Mechanism of Lead and Zinc Stabilization by GFAS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Wang, X.; Zhang, J.; Zhang, H.; Li, L.; Wu, J. Preparation of foam ceramics from granite sawing dust: Effect of sodium phosphate addition on the properties and pore structure of foam ceramics. Ceram. Int. 2025, 51, 45437–45447. [Google Scholar] [CrossRef]
- Bai, S.; Elwert, T.; Jia, S.; Wang, Q.Y.; Liu, T.; Yao, R. Methodologies for evaluating sawability of ornamental granite and relation modeling combining sawability with environmental impacts: An application in a stone industrial park of China. J. Clean. Prod. 2020, 246, 119004. [Google Scholar] [CrossRef]
- Jalalian, M.H.; Bagherpour, R.; Khoshouei, M. Environmentally sustainable mining in quarries to reduce waste production and loss of resources using the developed optimization algorithm. Sci. Rep. 2023, 13, 22183. [Google Scholar] [CrossRef] [PubMed]
- Hashem, F.S.; Salam, A.T.A.; Monir, D. Mechanical properties and durability of slag granite geopolymer cement incorporated zirconium aluminum layered double hydroxide. Sci. Rep. 2025, 15, 17824. [Google Scholar] [CrossRef]
- Strzałkowski, P.; Duchnowska, M.; Bakalarz, A.; Guzik, K.; Ratajczak, T.; Kaźmierczak, U.; Galos, K. Properties of fine-grained rock waste from the production of granite elements in the Strzegom region, Poland—A case study. Physicochem. Probl. Mi. 2025, 61, 204398. [Google Scholar] [CrossRef]
- Bakalarz, A.; Duchnowska, M. Analysis of the Possibility of Copper Recovery from Flotation Stratiform Copper Ore Tailings. Miner. Process. Extr. Metall. Rev. 2024, 45, 943–949. [Google Scholar] [CrossRef]
- Muraleedharan, M.; Nadir, Y. Factors affecting the mechanical properties and microstructure of geopolymers from red mud and granite waste powder: A review. Ceram. Int. 2021, 47, 13257–13279. [Google Scholar] [CrossRef]
- Mendoza, J.-M.F.; Capitano, C.; Peri, G.; Josa, A.; Rieradevall, J.; Gabarrell, X. Environmental management of granite slab production from an industrial ecology standpoint. J. Clean. Prod. 2014, 84, 619–628. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xue, L.; Wu, X.; Jian, S.; Lv, Y. Research Progress on Preparation of Building Materials from Granite Saw Mud. Bull. Chin. Ceram. Soc. 2023, 42, 554–564. [Google Scholar] [CrossRef]
- Rashwan, M.A.; Al -Basiony, T.M.; Mashaly, A.O.; Khalil, M.M. Behaviour of fresh and hardened concrete incorporating marble and granite sludge as cement replacement. J. Build. Eng. 2020, 32, 101697. [Google Scholar] [CrossRef]
- Wang, W.; Wang, K.; Zhang, J.; Zhang, H.; Li, L.; Wu, J. Effect of process parameters on characteristics and pore structure of foam ceramics prepared from granite sawing dust. Ceram. Int. 2024, 50, 8378–8389. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, N.; Zhang, Y.; Ding, C.; Zhang, Y. Modification of granite sawdust with aluminum ester coupling agent and its novel application in high-density polyethylene composite plate. J. Build. Eng. 2023, 76, 107364. [Google Scholar] [CrossRef]
- Assiamah, S.; Agyeman, S.; Adinkrah-Appiah, K.; Danso, H. Utilization of sawdust ash as cement replacement for landcrete interlocking blocks production and mortarless construction. Case Stud. Constr. Mater. 2022, 16, e00945. [Google Scholar] [CrossRef]
- Hegazy, A.A.; Shalaby, B.N.; Mashaly, A.O.; Rashwan, M.A. Valorization of granite sawing sludge in producing enhanced clay bricks. J. Build. Eng. 2025, 113, 113941. [Google Scholar] [CrossRef]
- Duchnowska, M.; Bakalarz, A.; Luszczkiewicz, A. Properties of Fine-Grained Rock Waste from the Pilawa Gorna Amphibolite and Migmatite Aggregates Production Plant. Minerals 2023, 13, 345. [Google Scholar] [CrossRef]
- Kamimoto, Y.; Hagio, T.; Jung, Y.-J.; Ichino, R.; Gil, K. Development of Synthetic Magnetic Zeolite Adsorbents and Application to Ammonium Ion Removal. KSCE J. Civ. Eng. 2020, 24, 1395–1399. [Google Scholar] [CrossRef]
- Ke, G.; Shen, H.; Yang, P. Synthesis of X-Zeolite from Waste Basalt Powder and its Influencing Factors and Synthesis Mechanism. Materials 2019, 12, 3895. [Google Scholar] [CrossRef]
- Akhtar, F.; Andersson, L.; Ogunwumi, S.; Hedin, N.; Bergström, L. Structuring adsorbents and catalysts by processing of porous powders. J. Eur. Ceram. Soc. 2014, 34, 1643–1666. [Google Scholar] [CrossRef]
- Nabavi, M.S.; Mohammadi, T.; Kazemimoghadam, M. Hydrothermal synthesis of hydroxy sodalite zeolite membrane: Separation of H2/CH4. Ceram. Int. 2014, 40, 5889–5896. [Google Scholar] [CrossRef]
- Flores, C.G.; Schneider, H.; Dornelles, J.S.; Gomes, L.B.; Marcilio, N.R.; Melo, P.J. Synthesis of potassium zeolite from rice husk ash as a silicon source. Clean. Eng. Technol. 2021, 4, 100201. [Google Scholar] [CrossRef]
- Swoboda, P.; Döring, T.F.; Hamer, M. Remineralizing soils? The agricultural usage of silicate rock powders: A review. Sci. Total Environ. 2022, 807, 150976. [Google Scholar] [CrossRef]
- Ramos, C.G.; Querol, X.; Dalmora, A.C.; de Jesus Pires, K.C.; Schneider, I.A.H.; Oliveira, L.F.S.; Kautzmann, R.M. Evaluation of the potential of volcanic rock waste from southern Brazil as a natural soil fertilizer. J. Clean. Prod. 2017, 142, 2700–2706. [Google Scholar] [CrossRef]
- Baila, F.; Labbilta, T.; Darmane, Y. Feldspar Purification from Iron Impurities: A Review of Treatment Methods. Miner. Process. Extr. Metall. Rev. 2024, 45, 564–572. [Google Scholar] [CrossRef]
- Durand, J.F. The impact of gold mining on the Witwatersrand on the rivers and karst system of Gauteng and North West Province, South Africa. J. Afr. Earth Sci. 2012, 68, 24–43. [Google Scholar] [CrossRef]
- Nfor, B.; Fai, P.B.A.; Tamungang, S.A.; Fobil, J.N.; Basu, N. Soil Contamination and Bioaccumulation of Heavy Metals by a Tropical Earthworm Species (Alma nilotica) at Informal E-Waste Recycling Sites in Douala, Cameroon. Environ. Toxicol. Chem. 2022, 41, 356–368. [Google Scholar] [CrossRef]
- Kang, J.; Liu, M.; Qu, M.; Guang, X.; Chen, J.; Zhao, Y.; Huang, B. Identifying the potential soil pollution areas derived from the metal mining industry in China using MaxEnt with mine reserve scales (MaxEnt_MRS). Environ. Pollut. 2023, 329, 121687. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Bhattacharya, T. A review on bioaccessibility and the associated health risks due to heavy metal pollution in coal mines: Content and trend analysis. Environ. Dev. 2023, 46, 100859. [Google Scholar] [CrossRef]
- Uugwanga, M.N.; Kgabi, N.A. Heavy metal pollution index of surface and groundwater from around an abandoned mine site, Klein Aub. Phys. Chem. Earth Parts A/B/C 2021, 124, 103067. [Google Scholar] [CrossRef]
- Zou, H.; Ren, B. Analyzing topsoil heavy metal pollution sources and ecological risks around antimony mine waste sites by a joint methodology. Ecol. Indic. 2023, 154, 110761. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Yin, X.; Wu, Y.; Wang, L.; Tang, S.; Jia, H. Spatial analysis and pollution assessment of heavy metals in the soils of Tongling urban area based on IDW. J. Saf. Environ. 2018, 18, 1989–1996. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Wang, Y.; Chai, L.; Yang, Z.; Liao, Q. High-resolution analyses reveal structural diversity patterns of microbial communities in Chromite Ore Processing Residue (COPR) contaminated soils. Chemosphere 2017, 183, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Wang, W.; Shrivastava, A.K. Cadmium-mediated morphological, biochemical and physiological tuning in three different Anabaena species. Aquat. Toxicol. 2018, 202, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Usman, A.R.A.; Kuzyakov, Y.; Stahr, K. Effect of clay minerals on extractability of heavy metals and sewage sludge mineralization in soil. Chem. Ecol. 2004, 20, 123–135. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, Y.; Wang, M. Remediation of copper polluted red soils with clay materials. J. Environ. Sci. 2011, 23, 461–467. [Google Scholar] [CrossRef]
- Jin, X.; Zha, S.; Li, S.; Chen, Z. Simultaneous removal of mixed contaminants by organoclays—Amoxicillin and Cu(II) from aqueous solution. Appl. Clay Sci. 2014, 102, 196–201. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Zhang, X.-F.; Zhang, X.; Jiang, J.; Yao, J. Alginate-based attapulgite foams as efficient and recyclable adsorbents for the removal of heavy metals. J. Colloid Interface Sci. 2018, 514, 190–198. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Z.; Liang, M.; Hu, H.; Chen, S.; Duan, L.; Chen, Z.; Yang, X.; Cai, J. Experimental study on the stabilization and anti-seepage treatment of lead and zinc elements in heavy metal tailings pond using cement slurry containing heavy metal stabilizing agent. Constr. Build. Mater. 2024, 425, 135964. [Google Scholar] [CrossRef]
- Chang, E.E.; Chiang, P.C.; Lu, P.H.; Ko, Y.W. Comparisons of metal leachability for various wastes by extraction and leaching methods. Chemosphere 2001, 45, 91–99. [Google Scholar] [CrossRef]
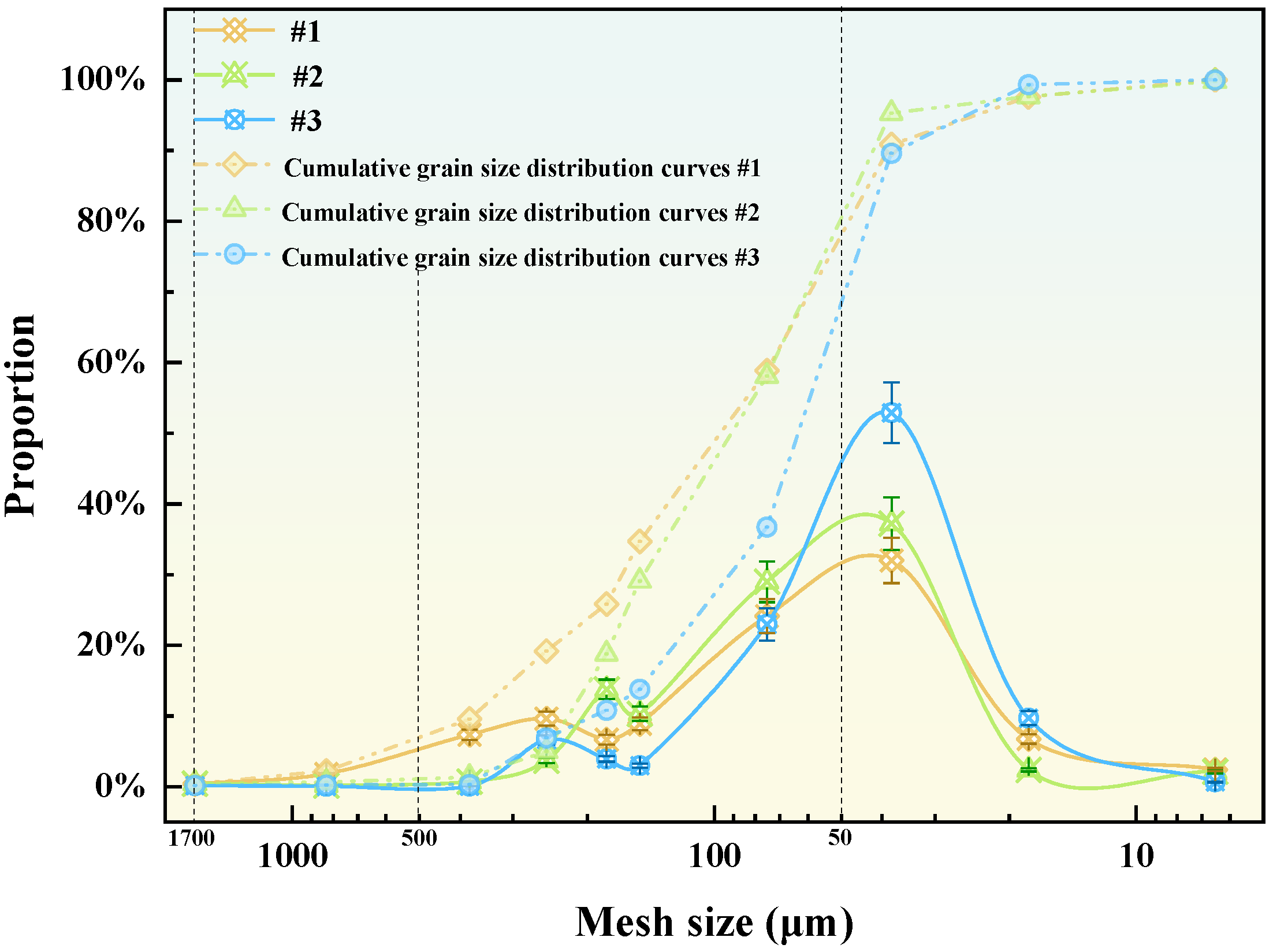
- Ma, S.; Song, Y.; Liu, J.; Kang, X.; Yue, Z.Q. Extended wet sieving method for determination of complete particle size distribution of general soils. J. Rock Mech. Geotech. 2024, 16, 242–257. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, Z.; Hu, Z.; Luo, X. Enhanced anaerobic digestion with the addition of chelator-nickel complexes to improve nickel bioavailability. Sci. Total Environ. 2021, 759, 143458. [Google Scholar] [CrossRef]
- Hadi, J.; Tournassat, C.; Lerouge, C. Pitfalls in using the hexaamminecobalt method for cation exchange capacity measurements on clay minerals and clay-rocks: Redox interferences between the cationic dye and the sample. Appl. Clay Sci. 2016, 119, 393–400. [Google Scholar] [CrossRef]
- Chang, M.-Y.; Juang, R.-S. Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay. J. Colloid Interface Sci. 2004, 278, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, C.; Sparks, D.L.; Levinson, S.; Ravina, I. Kinetics of Soil Chemical Reactions: Relationships between Empirical Equations and Diffusion Models. Soil Sci. Soc. Am. J. 1991, 55, 1307–1312. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, S.; Xia, D.; Shen, Y.; Lv, J.; Liu, X.; Xu, X. Speciation transformation and bioavailability of heavy metals during biogas production from coal slime. Biochem. Eng. J. 2021, 176, 108208. [Google Scholar] [CrossRef]
- Li, R.; Zhao, W.; Li, Y.; Wang, W.; Zhu, X. Heavy metal removal and speciation transformation through the calcination treatment of phosphorus-enriched sewage sludge ash. J. Hazard. Mater. 2015, 283, 423–431. [Google Scholar] [CrossRef]
- GB/T 3838-2002; Environmental Quality Standards for Surface Water. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2002.
- Li, J.; Xia, C.; Cheng, R.; Lan, J.; Chen, F.; Li, X.; Li, S.; Chen, J.; Zeng, T.; Hou, H. Passivation of multiple heavy metals in lead–zinc tailings facilitated by straw biochar-loaded N-doped carbon aerogel nanoparticles: Mechanisms and microbial community evolution. Sci. Total Environ. 2022, 803, 149866. [Google Scholar] [CrossRef]
- Chen, M.; Nong, S.; Zhao, Y.; Riaz, M.S.; Xiao, Y.; Molokeev, M.S.; Huang, F. Renewable P-type zeolite for superior absorption of heavy metals: Isotherms, kinetics, and mechanism. Sci. Total Environ. 2020, 726, 138535. [Google Scholar] [CrossRef] [PubMed]
- Villars, P.; Cenzual, K. (Eds.) Na6Al6Si10O32·12H2O (Na3Al3Si5O16[H2O]6 GIS tet) Crystal Structure; Datasheet from “PAULING FILE Multinaries Edition–2022” in Springer Materials; Springer: Berlin/Heidelberg, Germany; Material Phases Data System (MPDS): Vitznau, Switzerland; National Institute for Materials Science (NIMS): Tsukuba, Japan; Available online: https://materials.springer.com/isp/crystallographic/docs/sd_1005064 (accessed on 5 April 2024).
- Pal, P.; Das, J.K.; Das, N.; Bandyopadhyay, S. Synthesis of NaP zeolite at room temperature and short crystallization time by sonochemical method. Ultrason. Sonochem. 2013, 20, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Küçük, M.E.; Makarava, I.; Kinnarinen, T.; Häkkinen, A. Simultaneous adsorption of Cu(II), Zn(II), Cd(II) and Pb(II) from synthetic wastewater using NaP and LTA zeolites prepared from biomass fly ash. Heliyon 2023, 9, e20253. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D.; Del Vecchio, A. Synthesis and characterization of Na-P1 (GIS) zeolite using a kaolinitic rock. Sci. Rep. 2021, 11, 4872. [Google Scholar] [CrossRef] [PubMed]
- Cai, F.; Ma, F.; Zhang, X.; Reimus, P.; Qi, L.; Wang, Y.; Lu, D.; Thanh, H.V.; Dai, Z. Investigating the influence of bentonite colloids on strontium sorption in granite under various hydrogeochemical conditions. Sci. Total Environ. 2023, 900, 165819. [Google Scholar] [CrossRef]
- Frost, R.L.; Palmer, S.J.; Xi, Y. A Raman spectroscopic study of the mono-hydrogen phosphate mineral dorfmanite Na2(PO3OH)·2H2O and in comparison with brushite. Spectrochim. Acta Part A 2011, 82, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Ghaedrahmat, H.; Masoomi, M.Y.; Zendehdel, M. Synthesize and characterization of ZIF-8/NaP zeolite composites as a stable acid-base catalyst for organic reactions. Polyhedron 2023, 236, 116372. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, H.; Wang, X.; Zhang, M.; Chen, Y.; Zhai, C.; Song, H.; Deng, J.; Sun, J.; Zhang, C. Utilization of NaP zeolite synthesized with different silicon species and NaAlO2 from coal fly ash for the adsorption of Rhodamine B. J. Hazard. Mater. 2021, 415, 125627. [Google Scholar] [CrossRef]
- Tian, W.; Deng, Z.; Wang, H.; Liu, H.; Li, G.; Liu, X.; Chen, Z.; Chen, H.; Li, Y. Negative adsorption in the isotherm adsorption experiments of low-adsorption coal and shale. Nat. Gas Ind. B 2019, 6, 44–50. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, T.; Xiong, X.; Miki, T.; Wu, X.; Yang, L. In- situ synthesis of modified zeolite with high zirconium content using fly ash and its efficient removal for As(V) in solution. J. Environ. Chem. Eng. 2024, 12, 112212. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of heavy metals on natural zeolites: A review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi, H.; Nezamzadeh-Ejhieh, A. An efficient modified zeolite for simultaneous removal of Pb(II) and Hg(II) from aqueous solution. J. Mol. Liq. 2017, 230, 221–229. [Google Scholar] [CrossRef]
- Painer, F.; Baldermann, A.; Gallien, F.; Eichinger, S.; Steindl, F.; Dohrmann, R.; Dietzel, M. Synthesis of Zeolites from Fine-Grained Perlite and Their Application as Sorbents. Materials 2022, 15, 4474. [Google Scholar] [CrossRef] [PubMed]
- Anari-Anaraki, M.; Nezamzadeh-Ejhieh, A. Modification of an Iranian clinoptilolite nano-particles by hexadecyltrimethyl ammonium cationic surfactant and dithizone for removal of Pb(II) from aqueous solution. J. Colloid Interface Sci. 2015, 440, 272–281. [Google Scholar] [CrossRef]
- Barrer, R.M.; Townsend, R.P. Ion-exchange equilibria in zeolites and clay minerals. Different concentration scales and derived thermodynamic functions. J. Chem. Soc. Faraday Trans. 1984, 80, 629–640. [Google Scholar] [CrossRef]
- Abhishek, K.; Shrivastava, A.; Vimal, V.; Gupta, A.K.; Bhujbal, S.K.; Biswas, J.K.; Singh, L.; Ghosh, P.; Pandey, A.; Sharma, P.; et al. Biochar application for greenhouse gas mitigation, contaminants immobilization and soil fertility enhancement: A state-of-the-art review. Sci. Total Environ. 2022, 853, 158562. [Google Scholar] [CrossRef] [PubMed]



| Composition Types | Sample 1# | Sample 2# | Sample 3# |
|---|---|---|---|
| Quartz (%) | 41.77 | 24.02 | 6.18 |
| Siderite (%) | 21.25 | 34.61 | 40.48 |
| Calcite (%) | 10.01 | 18.90 | 24.29 |
| Dolomite (%) | 0 | 13.55 | 0 |
| Olivine (%) | 21.46 | 0 | 0 |
| Illite (%) | 3.72 | 0 | 0 |
| Clinoptilolite (%) | 0 | 8.92 | 0 |
| Ferrous dolomite (%) | 0 | 0 | 29.05 |
| Wollastonite (%) | 1.8 | 0 | 0 |
| Content | Granite Sawdust | GFAS |
|---|---|---|
| BET surface area | 3.68 m2/g | 35.00 m2/g |
| t-Plot micropore area | —— | 6.08 m2/g |
| BJH adsorption cumulative surface area of pores between 1.7 nm and 300.0 nm diameter | —— | 32.81 m2/g |
| BJH Adsorption average pore diameter (4 V/A) | —— | 7.28 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Shi, Y.; Liang, M.; Xue, M.; Li, Z.; Yang, X.; Ma, C.; Duan, L.; Cai, J. Immobilization of Lead and Zinc in Tailings Sand Using a Stabilizer Synthesized from Granite Sawdust for Mine Remediation. Materials 2026, 19, 199. https://doi.org/10.3390/ma19010199
Shi Y, Liang M, Xue M, Li Z, Yang X, Ma C, Duan L, Cai J. Immobilization of Lead and Zinc in Tailings Sand Using a Stabilizer Synthesized from Granite Sawdust for Mine Remediation. Materials. 2026; 19(1):199. https://doi.org/10.3390/ma19010199
Chicago/Turabian StyleShi, Yanping, Mengjia Liang, Man Xue, Zhi Li, Xianyu Yang, Chuyuan Ma, Longchen Duan, and Jihua Cai. 2026. "Immobilization of Lead and Zinc in Tailings Sand Using a Stabilizer Synthesized from Granite Sawdust for Mine Remediation" Materials 19, no. 1: 199. https://doi.org/10.3390/ma19010199
APA StyleShi, Y., Liang, M., Xue, M., Li, Z., Yang, X., Ma, C., Duan, L., & Cai, J. (2026). Immobilization of Lead and Zinc in Tailings Sand Using a Stabilizer Synthesized from Granite Sawdust for Mine Remediation. Materials, 19(1), 199. https://doi.org/10.3390/ma19010199







