New Biobased Plasticizers for PVC Derived from Saturated Dimerized Fatty Acids
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
2.2. Test Methods
2.3. Synthesis of Plasticizers
2.4. Preparation of PVC Film Casting Method—The Compatibility Test
2.5. Preparation of PVC Blends
2.6. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Plasticizers and Their Compatibility Test with PVC
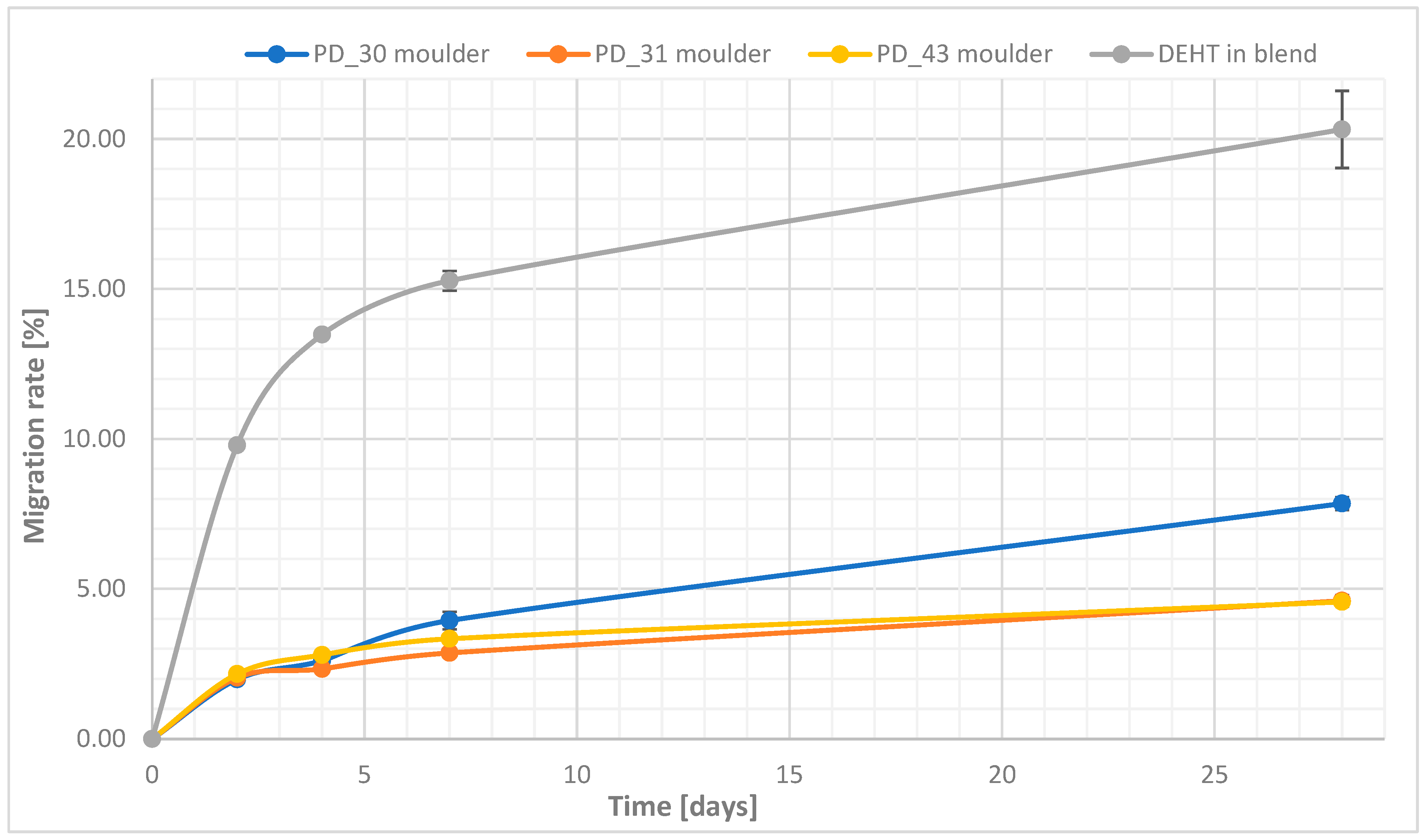
3.2. Characteristics of Plasticized PVC Blends
- (a)
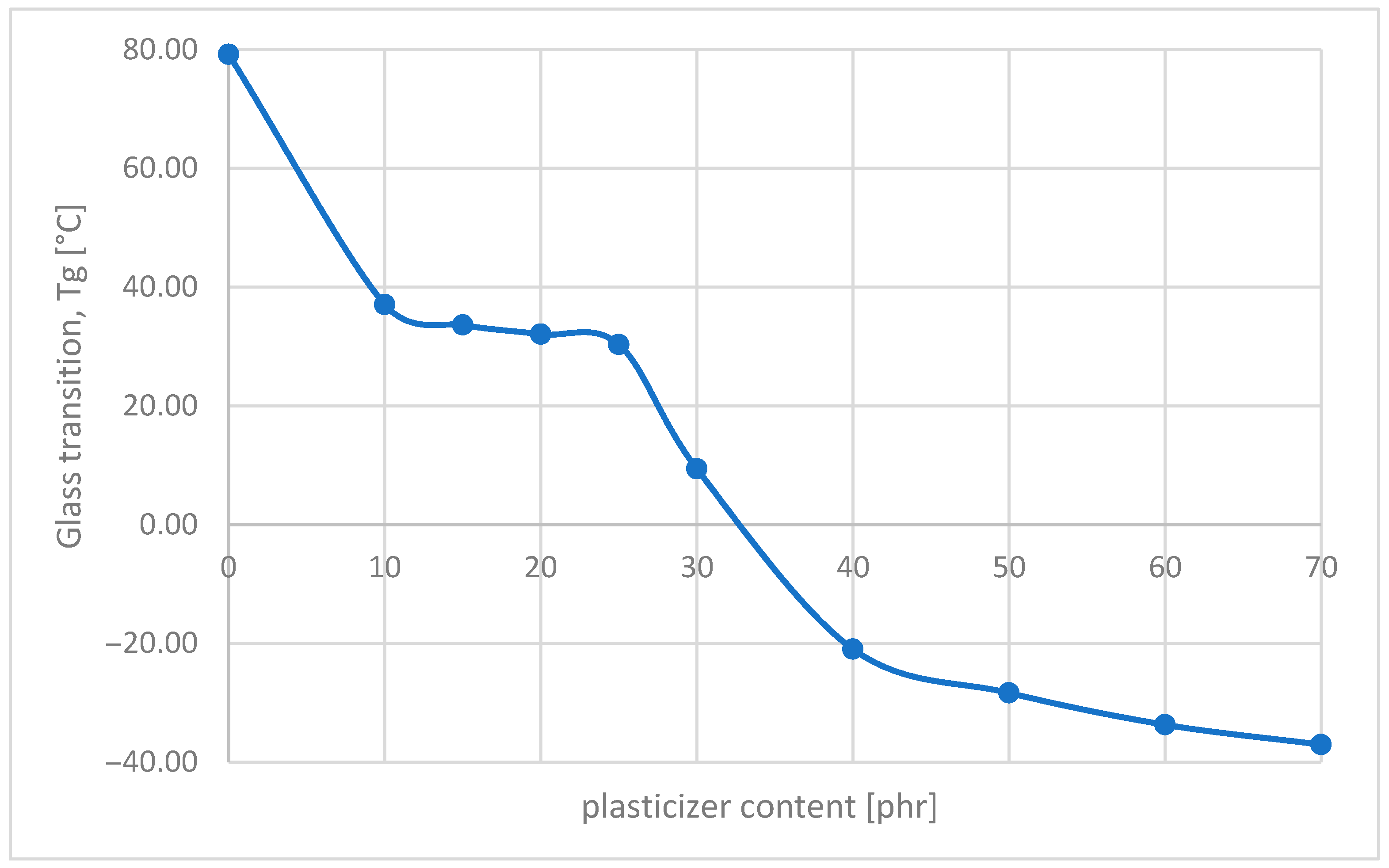
- Thermal properties
- (b)
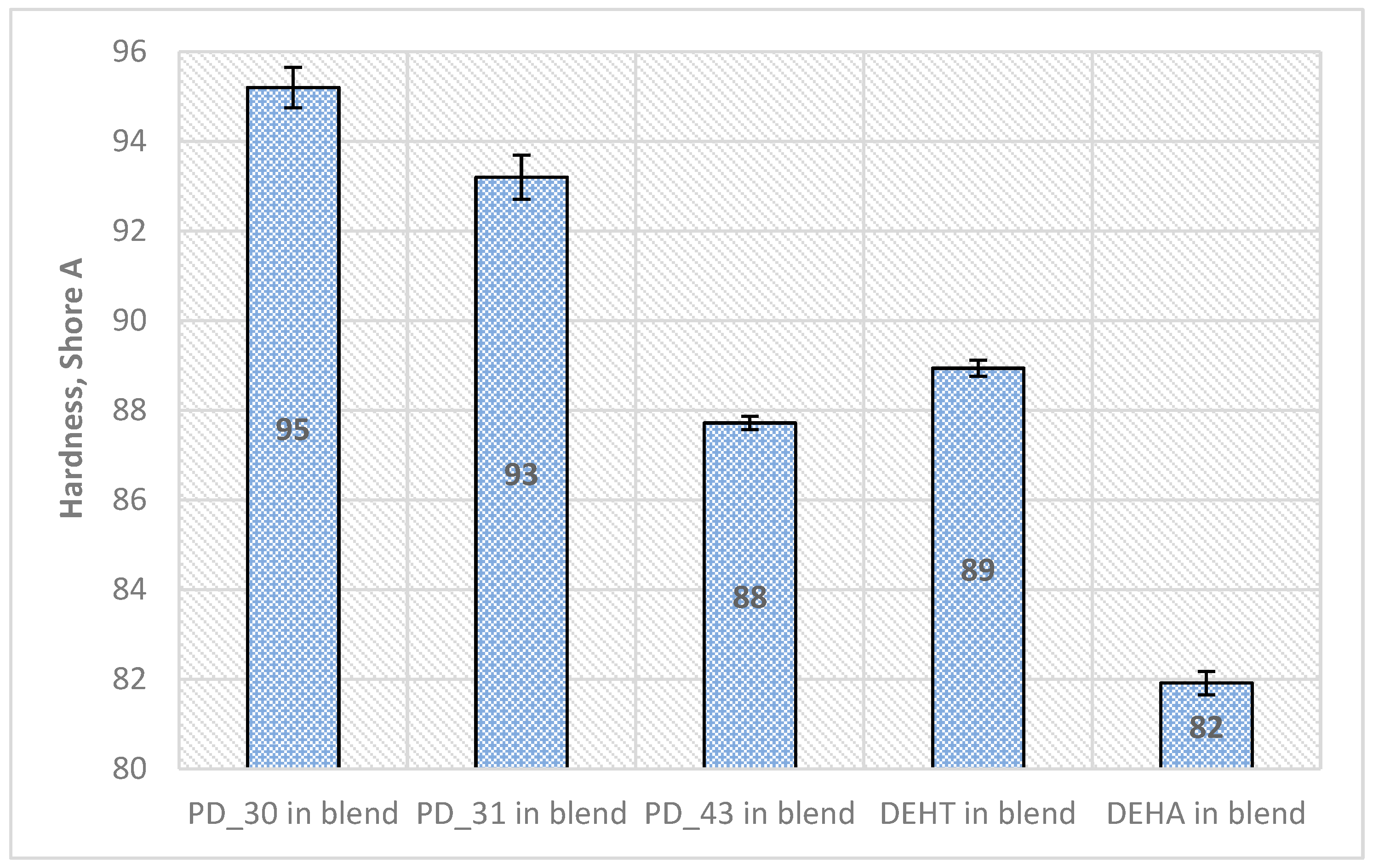
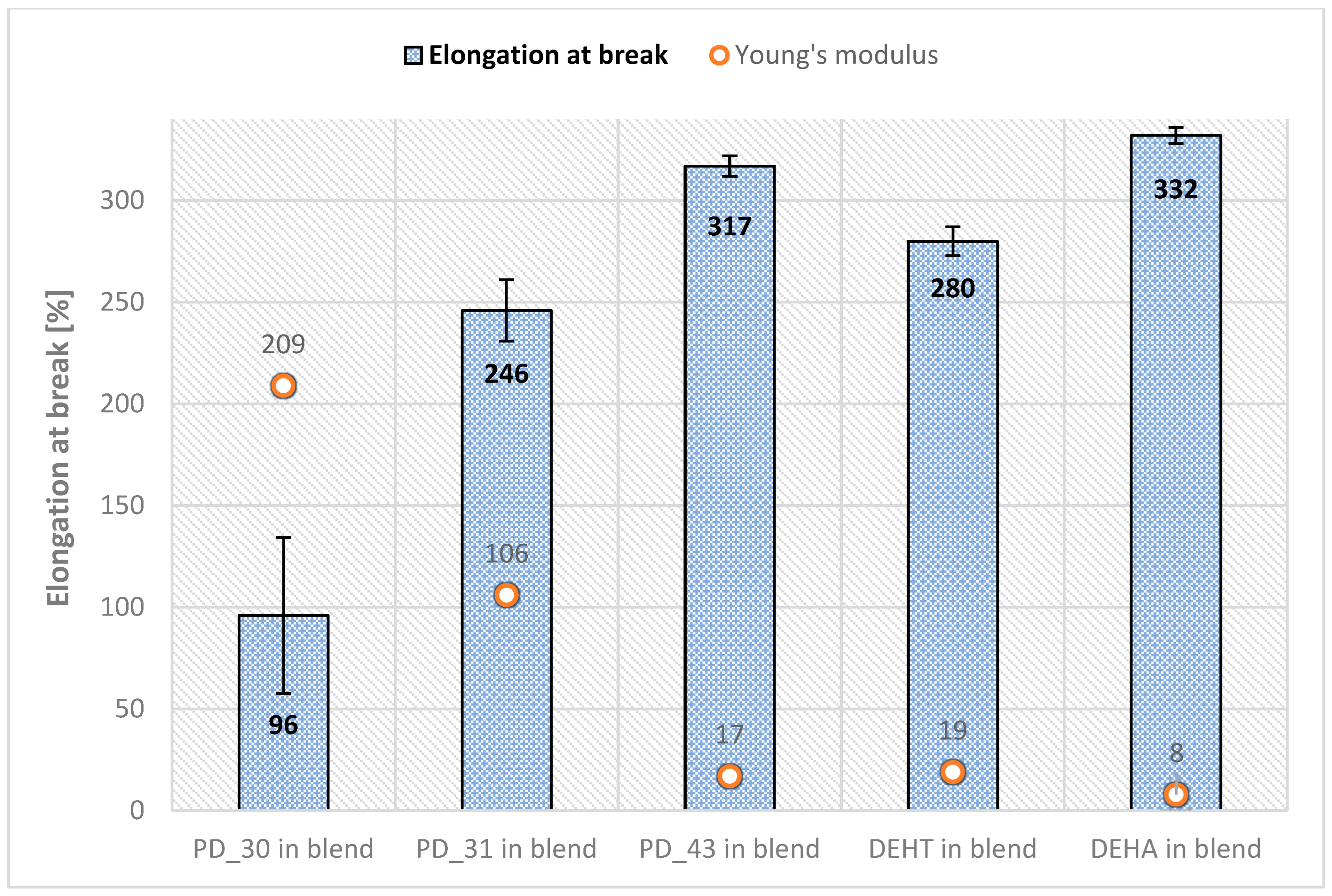
- Mechanical properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plastics Europe. Enabling a Sustainable Future: Plastics—The Fast Facts. 2024. Available online: https://plasticseurope.org/wp-content/uploads/2024/11/PE_TheFacts_24_digital-1pager.pdf (accessed on 31 January 2025).
- Wilkes, C.E.; Summers, J.W.; Daniels, C.A. PVC Handbook; Hanser-Verlag: Berlin, Germany, 2005. [Google Scholar]
- ECHA. Appendices A and B to the Investigation Report on PVC and PVC Additives. 2023. Available online: https://echa.europa.eu/documents/10162/17233/rest_pvc_investigation_report_appendix_a_b_en.pdf/5a1e8057-b576-73fd-e163-4587874349d3?t=1701157496271 (accessed on 21 January 2025).
- Lewandowski, K.; Skórczewska, K. A brief review of poly(vinyl chloride) (PVC) recycling. Polymers 2022, 14, 3035. [Google Scholar] [CrossRef] [PubMed]
- Wypych, G. Principles of thermal degradation. In PVC Degradation and Stabilization, 3rd ed.; ChemTec Publishing: Toronto, ON, Canada, 2020; pp. 79–167. [Google Scholar]
- Wypych, G. Handbook of Plasticizers, 4th ed.; ChemTec Publishing: Toronto, ON, Canada, 2023. [Google Scholar]
- Basso, C.G.; de Araújo-Ramos, A.T.; Martino-Andrade, A.J. Exposure to phthalates and female reproductive health: A literature review. Reprod. Toxicol. 2022, 109, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Haggerty, D.K.; Rappolee, D.A.; Ruden, D.M. Phthalate exposure and long-term epigenomic consequences: A review. Front. Genet. 2020, 11, 405. [Google Scholar] [CrossRef]
- Albert, O.; Nardelli, T.; Lalancette, C.; Hales, B.; Robaire, B. Effects of in utero and lactational exposure to new generation green plasticizers on adult male rats: A comparative study with di(2-ethylhexyl) phthalate. Toxicol. Sci. 2018, 164, 129–141. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, H. Phthalates and their impacts on human health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef]
- Directive 2005/84/EC of the European Parliament and the Council of 14 December 2005. Amending for the 22nd time Council Directive 76/769/EEC on the approximation of the laws, regulations and administrative provisions of the Member States relating to restrictions on the marketing and use of certain dangerous substances and preparations (phthalates in toys and childcare articles). Off. J. Eur. Union 2005, L 344, 40–43.
- Godwin, A.D. Plasticizer. In Applied Plastics Engineering Handbook: Processing, Materials, and Applications, 2nd ed.; Kutz, M., Ed.; Plastics Design Library (PDL); Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Marcilla, A.; García, S.; García-Quesada, J.C. Study of the migration of PVC plasticizers. J. Anal. Appl. Pyrol. 2004, 71, 457–463. [Google Scholar] [CrossRef]
- Yin, B.; Hakkarainen, M. Oligomeric isosorbide esters as alternative renewable resource plasticizers for PVC. J. Appl. Polym. Sci. 2011, 119, 2400–2407. [Google Scholar] [CrossRef]
- Zhou, J.; Ritter, H. Copolyesters as non-toxic plasticizers. Polym. Int. 2011, 60, 1158–1161. [Google Scholar] [CrossRef]
- Saurabh, T.; Patnaik, M.; Bhagt, S.L.; Renge, V.C. Epoxidation of vegetable oils: Review. Int. J. Eng. Adv. Technol. 2011, II, 491–501. [Google Scholar]
- Sun, B.; Chaudhary, B.I.; Shen, C.-Y.; Mao, D.; Yuan, D.-M.; Dai, G.-C.; Li, B.; Cogen, J.M. Thermal Stability of epoxidized soybean oil and its absorption and migration in poly(vinyl chloride). Polym. Eng. Sci. 2013, 53, 1645–1656. [Google Scholar] [CrossRef]
- Akter, S.; Rahman, M.M.; Auerbach, M.; Langer, B. Effect of Bio-Based Plasticizers from Modified Vegetable Oils in a New Formulation of PVC Materials. J. Appl. Polym. Sci. 2024, 142, e56527. [Google Scholar] [CrossRef]
- Qadeer, A.; Kirsten, K.L.; Ajmal, Z.; Jiang, X.; Zhao, X. Alternative Plasticizers as Emerging Global Environmental and Health Threat: Another Regrettable Substitution? Environ. Sci. Technol. 2022, 56, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Z.; Wang, K.; Huang, J.; Li, K.; Nie, X.; Jiang, J. Epoxidized castor oil-based diglycidyl-phthalate plasticizer: Synthesis and thermal stabilizing effects on poly(vinyl chloride). J. Appl. Polym. Sci. 2019, 136, 47142. [Google Scholar] [CrossRef]
- Bajetto, G.; Scutera, S.; Menotti, F.; Banche, G.; Chiaradia, G.; Turesso, C.; De Andrea, M.; Vallino, M.; Van Es, D.S.; Biolatti, M.; et al. Antimicrobial Efficacy of a Vegetable Oil Plasticizer in PVC Matrices. Polymers 2024, 16, 1046. [Google Scholar] [CrossRef]
- Ja, P.; Bo, C.; Zhang, M.; Hu, L.; Zhou, Y. Synthesis of soybean oil based polyester plasticizer and its property mixing with polyvinyl chloride. Nongye Gongcheng Xuebao/Trans. Chin. Soc. Agric. Eng. 2014, 30, 314–320. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, E.; Wang, H.; Huang, D.; Ding, G.; Zhao, Z.; Wei, Z. Synthesis and evaluation of oleic acid-derived hyperbranched polyester as an efficient plasticizer for PVC. J. Appl. Polym. Sci. 2024, 141, e55847. [Google Scholar] [CrossRef]
- Waskiewicz, S.; Langer, E.; Tannenberg, M.; Dziendzioł, P.; Jurczyk, S. Synthesis and study of properties of new oligoesters based on soybean oil as potential poly(vinyl chloride) plasticizers. J. Appl. Polym. Sci. 2023, 141, e54865. [Google Scholar] [CrossRef]
- Gartili, A.; Caillol, S.; Briou, B.; Lapinte, V. One step beyond for CNSL-based plasticizers for PVC: Use of cardol. Eur. J. Lipid Sci. Technol. 2024, 126, 2400086. [Google Scholar] [CrossRef]
- Greco, A.; Brunetti, D.; Renna, G.; Mele, G.; Maffezzoli, A. Plasticizer for poly(vinyl chloride) from cardanol as a renewable resource material. Polym. Degrad. Stab. 2010, 95, 2169–2174. [Google Scholar] [CrossRef]
- EN ISO 660; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. ISO: Geneva, Switzerland, 2020.
- ISO 255; Belt Drives—Pulleys for V-belts (System Based on Datum Width)—Geometrical Inspection of Grooves. ISO: Geneva, Switzerland, 2023.
- EN ISO 527-2; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. ISO: Geneva, Switzerland, 2012.
- PN-EN ISO 868; Plastics and Ebonite—Determination of Indentation Hardness by Means of a Durometer (Shore Hardness). ISO: Geneva, Switzerland, 2003.
- EN ISO 177; Plastics—Determination of Migration of Plasticizers. ISO: Geneva, Switzerland, 2016.
- da Silva, M.A.; Vieira, M.G.A.; Maçumoto, A.C.G.; Beppu, M.M. Polyvinylchloride (PVC) and natural rubber films plasticized with a natural polymeric plasticizer obtained through polyesterification of rice fatty acid. Polym. Test. 2011, 30, 478–484. [Google Scholar] [CrossRef]
- Lindström, A.; Hakkarainen, M. Environmentally Friendly Plasticizers for Poly(vinyl chloride)—Improved Mechanical Properties and Compatibility by Using Branched Poly(butylene adipate) as a Polymeric Plasticizer. J. Appl. Polym. Sci. 2006, 100, 2180–2188. [Google Scholar] [CrossRef]
- Hattimattur, V.J.; Sangale, V.R.; Zade, P.S.; Mandake, M.B.; Walke, S. Review: Epoxidation of Vegetable oils. Int. J. Trend Res. Dev. 2018, 5, 542–548. [Google Scholar]
- Zhang, Z.; Jiang, P.P.; Liu, D.; Feng, S.; Zhang, P.; Wang, Y.; Fu, J.; Agus, H. Research progress of novel bio-based plasticizers and their applications in poly(vinyl chloride). J. Mater. Sci. 2021, 56, 10155–10182. [Google Scholar] [CrossRef]
- Bocque, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.J. Petro-Based and Bio-Based Plasticizers: Chemical Structures to Plasticizing Properties. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 11–33. [Google Scholar] [CrossRef]
- Bae, W.S.; Tabor, R.; Rogers, K.A.; Mukerjee, S.L. Polymeric Plasticizer Compositions. U.S. Patent US2017/0121457A1, 2017. [Google Scholar]
- Ziska, J.J.; Barlow, J.W.; Paul, D.R. Miscibility in PVC-polyester blends. Polymer 1981, 22, 918–923. [Google Scholar] [CrossRef]
- Langer, E.; Bortel, K.; Lenartowicz-Klik, M.; Waśkiewicz, S. Plasticizers derived from post-consumer PET. In Research Trends and Potential Applications; Plastics Design Library; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Rijavec, T.; Strlič, M.; Kralj Cigić, I. Plastics in heritage collections: Poly(vinyl chloride) degradation and characterization. Acta Chim. Slov. 2020, 67, 993–1013. [Google Scholar] [CrossRef]
- McNeill, I.C.; Memetea, L.; Cole, W.J. A study of the products of PVC thermal degradation. Polym. Degrad. Stab. 1995, 49, 181–191. [Google Scholar] [CrossRef]
- Coltro, L.; Pitta, J.B.; Madaleno, E. Performance evaluation of new plasticizers for stretch PVC films. Polym. Test. 2013, 32, 272–278. [Google Scholar] [CrossRef]
- van Krevelen, D.W.; te Nijenhuis, K. Properties of Polymers, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Venkatram, S.; Kim, C.; Chandrasekaran, A.; Ramprasad, R. Critical Assessment of the Hildebrand and Hansen Solubility Parameters for Polymers. J. Chem. Inf. Model. 2019, 59, 4188–4194. [Google Scholar] [CrossRef]
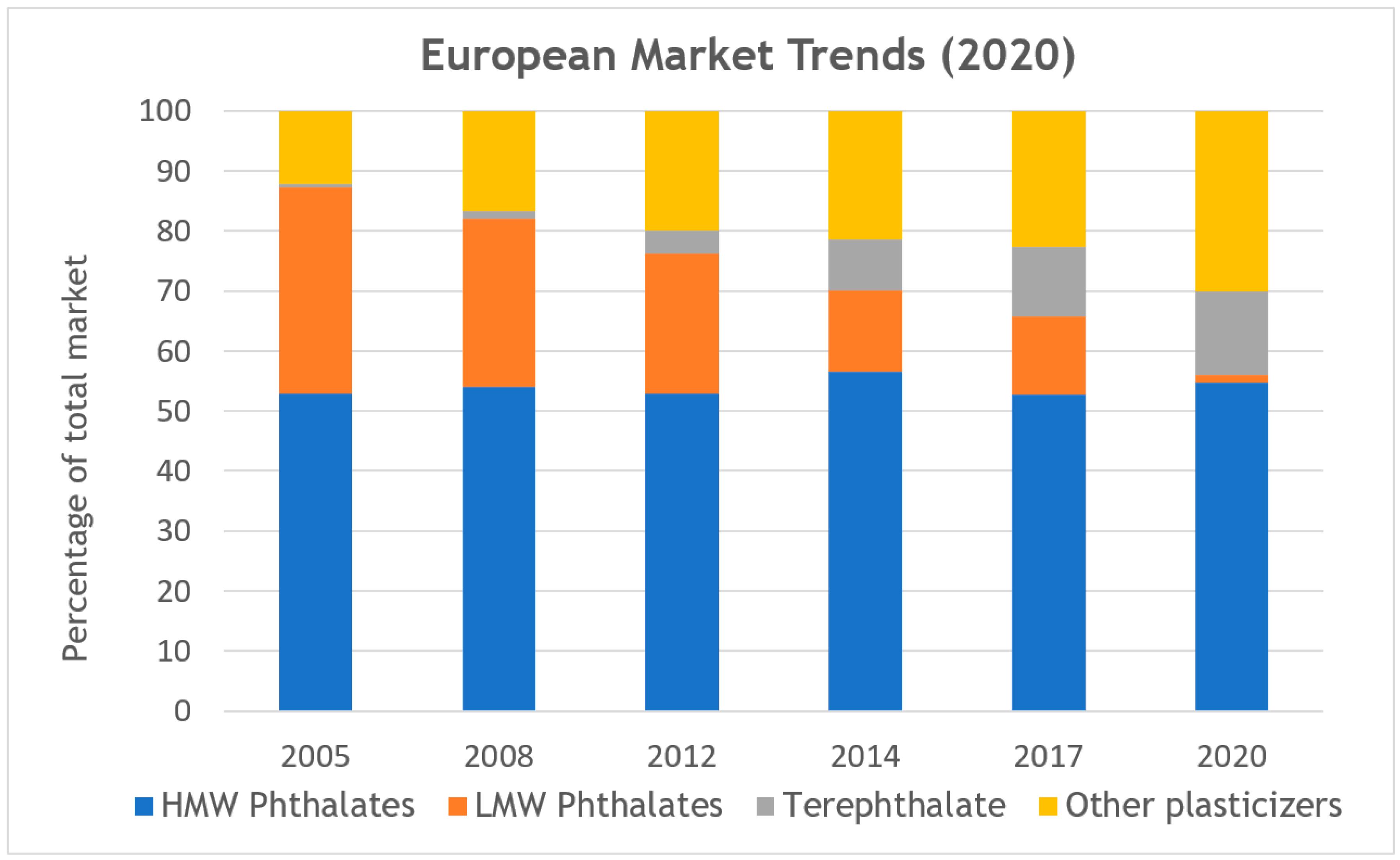

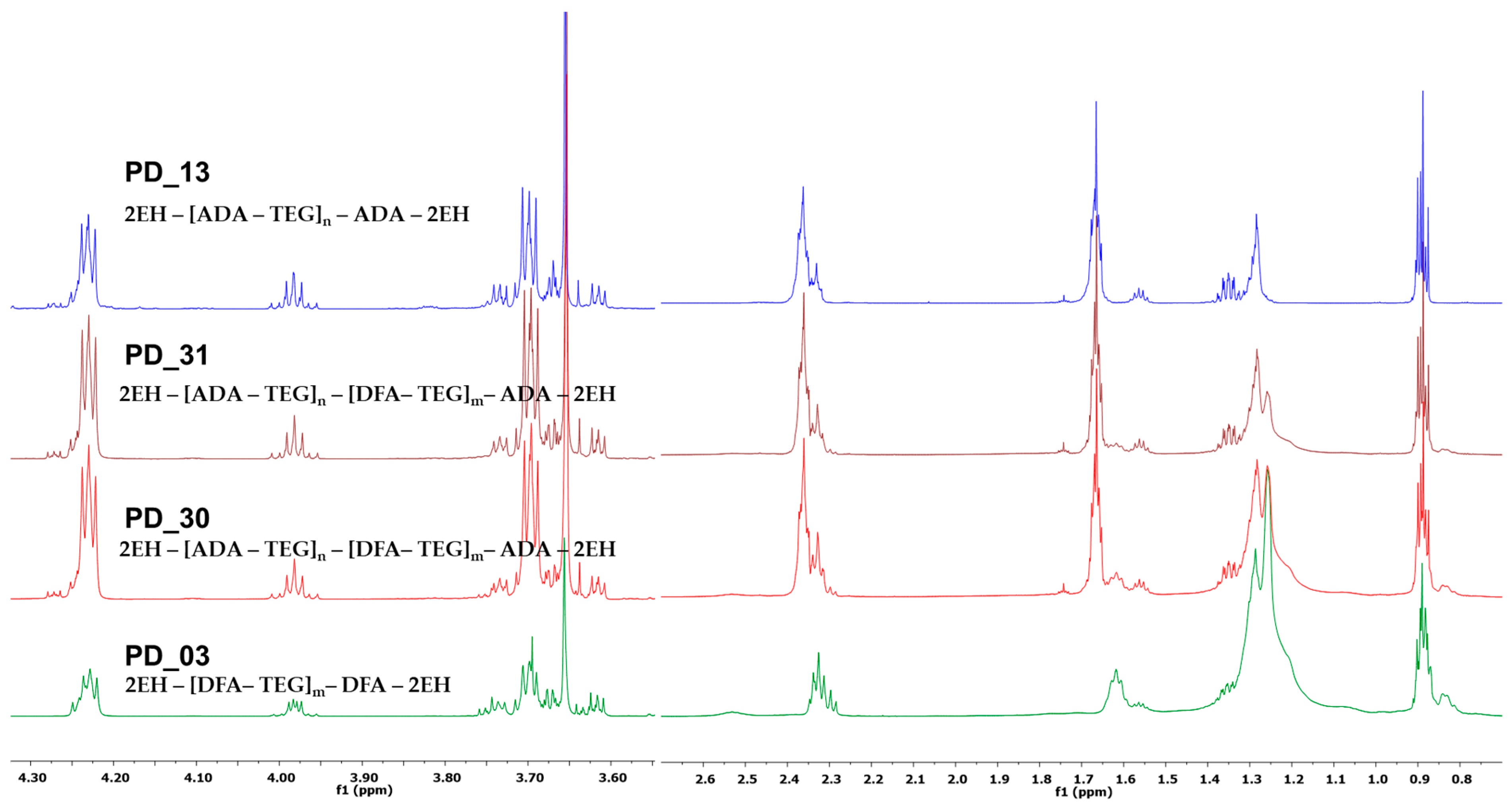
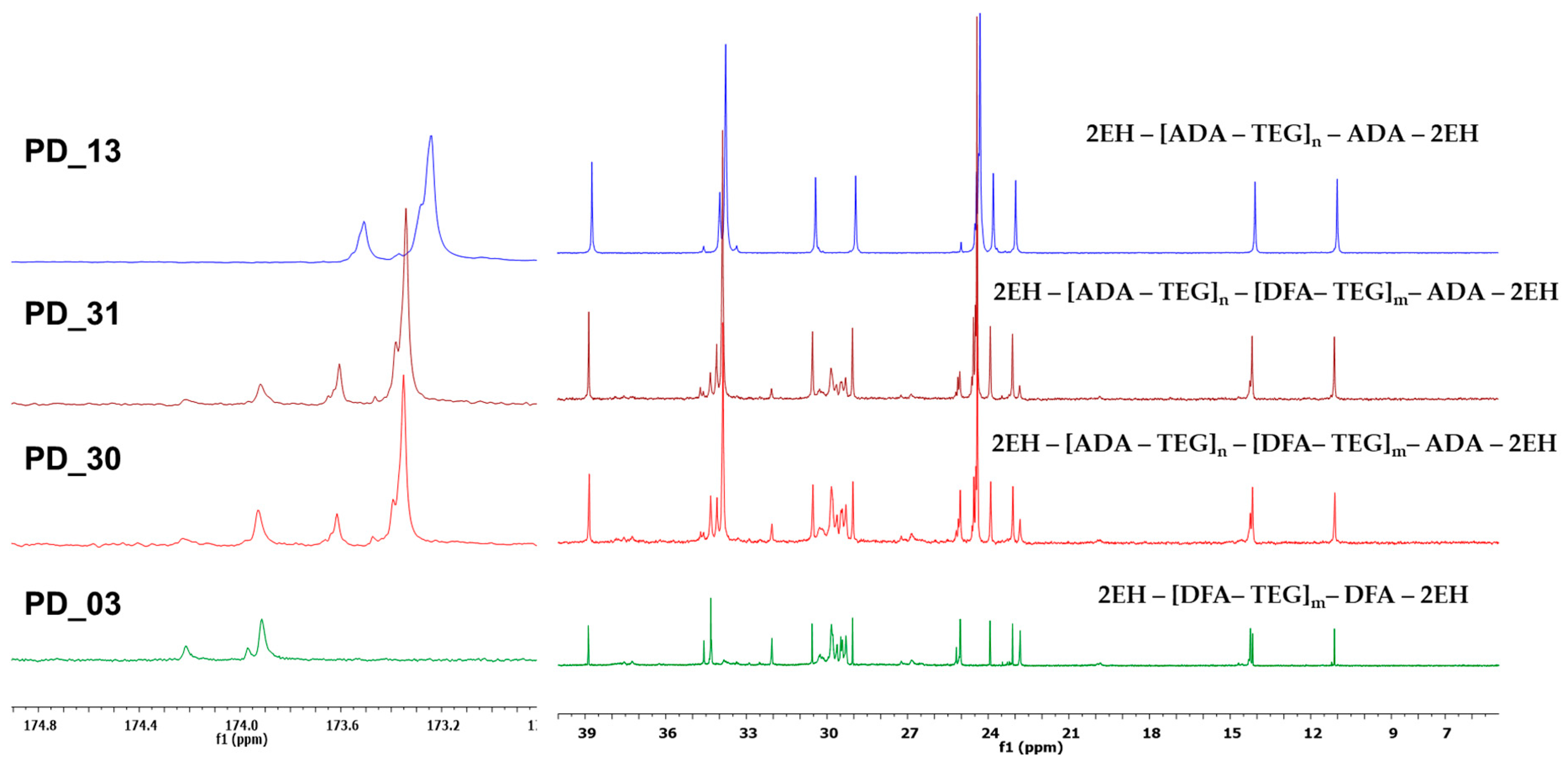

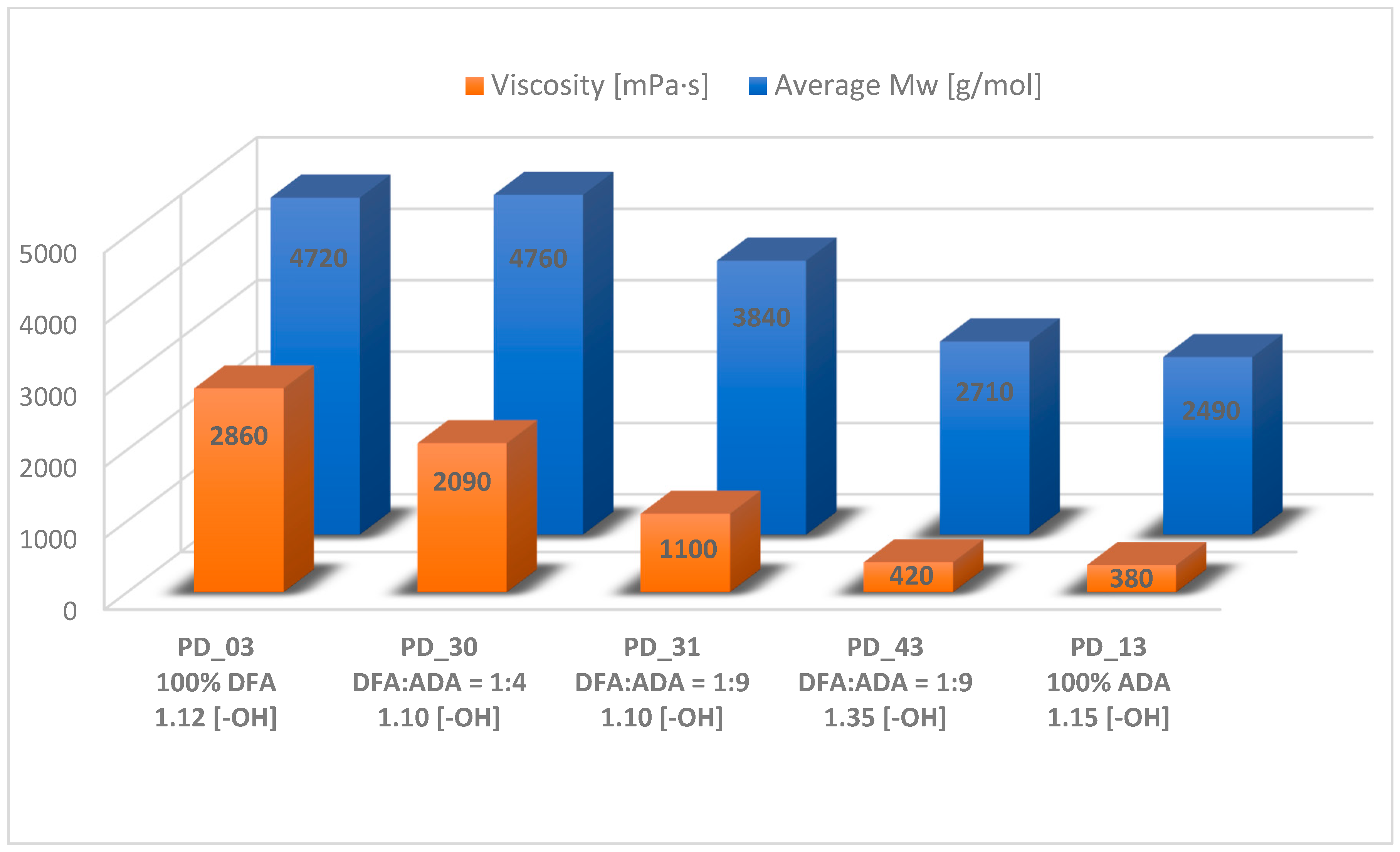

| Sample Code | Molar Ratios of Acids DFA:ADA | Summary Based on Equation (2) | Reagents [mol] | Cat. Fascat 4100 [wt%] | AV on the End of Step [mg KOH/g] | Time of Step [h] | Other Information |
|---|---|---|---|---|---|---|---|
| PD_03 | 100% DFA | 1.12 | DFA 0.25 TEG 0.24 2-EH 0.09 | 0.20 | 0.8 | 9 | synthesis in 500 mL flask; clear product |
| PD_13 | 100% ADA | 1.15 | I ADA 0.44 TEG 0.42 | I 0.20 | I 39.0 II 1.6 | I 3 II 6 | precipitate after 2 years |
| II 2EH 0.17 | |||||||
| PD_14 | 1:9 | 1.15 | I ADA 0.42 TEG 0.44 | I 0.15 II 0.05 | I 62.6 II 30.9 III 1.8 | I 1 II 7 III 7 | slightly cloudy product; precipitate after 2–3 months |
| II DFA 0.05 | |||||||
| III 2-EH 0.19 | |||||||
| PD_17 | 1:9 | 1.35 | I ADA 0.42 TEG 0.44 DFA 0.05 | I 0.20 | I 30.6 II 1.1 | I 5 II 5 | excess 2EH was distilled |
| II 2EH 0.37 | |||||||
| PD_30 | 1:4 | 1.10 | I ADA 1.25 TEG 1.47 DFA 0.31 | I 0.15 II 0.05 | I 31.6 II 0.9 | I 7 II 10 | synth. in 1000 mL flask; larger scale = longer synth. time |
| II 2EH 0.49 | |||||||
| PD_31 | 1:9 | 1.10 | I ADA 2.50 TEG 2.62 DFA 0.28 | I 0.15 II 0.05 | I 42.0 II 1.0 | I 9 II 17 | synth. in 2000 mL flask; larger scale required for dry-blend production |
| II 2-EH 0.87 | |||||||
| PD_43 | 1:9 | 1.35 | I ADA 2.08 TEG 2.18 DFA 0.23 | I 0.10 II 0.10 | I 41.4 II 0.7 | I 7.5 II 4.5 | the same as PD_17 but on a larger scale—2000 mL; excess 2EH was distilled |
| II 2EH 1.88 |
| Sample Code | Molar Ratios of Acids DFA:ADA | Product Properties | ||||||
|---|---|---|---|---|---|---|---|---|
| Molecular Weight (SEC) | Viscosity 25 °C [mPa·s] | Density 25 °C [g/cm3] | Color Scales | |||||
| [g/mol] | [g/mol] | Ð | Hazen | Iodine | ||||
| PD_03 | 100% DFA | 2780 | 4720 | 1.7 | 2860 | 0.96 | 448 | 2.9 |
| PD_13 | 100% ADA | 1460 | 2490 | 1.7 | 380 | 1.10 | 146 | 0.7 |
| PD_14 | 1:9 | 1430 | 2580 | 1.8 | 570 | 1.06 | 795 (precipitate) | 5.3 |
| PD_17 | 1:9 | 1020 | 1840 | 1.8 | 260 | 1.04 | 401 | 2.4 |
| PD_30 | 1:4 | 2640 | 4760 | 1.8 | 2090 | 1.05 | 340 | 3.0 |
| PD_31 | 1:9 | 2130 | 3840 | 1.8 | 1100 | 1.08 | 742 | 5.4 |
| PD_43 | 1:9 | 1590 | 2710 | 1.7 | 420 | 1.06 | 232 | 1.8 |
| PVC Film Code | Transparency | Flexibility (Can the PVC Film Be Easily Bent?) | Is the Surface of the Film Dry? | Tg [°C] | Qualificate to PVC Blends? |
|---|---|---|---|---|---|
| PVC_film without plasticizer | − white color | − rigid | + | 80 | comparison sample |
| DEHT_film | + | + | + | −14 | comparison sample |
| PD_03_film | − | − rigid | − greasy surface | 79 | − opaque film with white discoloration |
| PD_13_film | +/− partly opaque | +/− partly rigid | − greasy after a few days | −25 | − loses its positive properties after time |
| PD_14_film | + | + | + | −30 | − precipitate in plasticizers |
| PD_17_film | + | + | + | −29 | + but there is too much 2EH in plasticizer = low Mw |
| PD_30_film | + | + | + | −28 | + slight shrinkage of the film after drying |
| PD_31_film | + | + | + | −27 | + slight shrinkage of the film after drying |
| PD_43_film | + | + | + | −28 | + the same as PD_17_film |
| Tg [°C] | Temperature [°C] | |||
|---|---|---|---|---|
| Mass Loss of 5% | 1st Stage Decomposition | 2nd Stage Decomposition | ||
| PD_30 in blend | nd * | 281 | 300 | 466 |
| PD_31 in blend | −25 | 270 | 300 | 463 |
| PD_43 in blend | −27 | 261 | 300 | 460 |
| DEHT in blend | −16 | 265 | 286 | 475 |
| DEHA in blend | −35 | 238 | 283 | 469 |
| Plasticizer | The General Formula of Oligoester | Molar Ratio of DFA:ADA | δ, (MJ/m3)1/2 | δpolymer–δplasticizer | ||
|---|---|---|---|---|---|---|
| SEC | cal. | |||||
| DEHT | 390 | - | - | 17.93 | 1.77 | |
| PD_30 | 4760 | 4850 | 2-EH-(ADA-TEG)13-DFA-TEG-DFA-2-EH | 1:6.5 | 18.02 | 1.68 |
| 4750 | 2-EH-(ADA-TEG)10-(DFA-TEG)2-DFA-2-EH | 1:3.3 | 17.78 | 2.02 | ||
| PD_31 | 3840 | 3810 | 2-EH-(ADA-TEG)9-DFA-TEG-DFA-2-EH | 1:4.5 | 17.87 | 1.83 |
| 3910 | 2-EH-(ADA-TEG)12-DFA-2-EH | 1:12 | 18.13 | 1.57 | ||
| PD_43 | 2700 | 2610 | 2-EH-(ADA-TEG)7-DFA-2-EH | 1:7 | 17.97 | 1.73 |
| 2870 | 2-EH-(ADA-TEG)8-DFA-2-EH | 1:8 | 18.02 | 1.68 | ||
| 3130 | 2-EH-(ADA-TEG)9-DFA-2-EH | 1:9 | 18.07 | 1.63 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziendzioł, P.; Waśkiewicz, S.; Jaszcz, K. New Biobased Plasticizers for PVC Derived from Saturated Dimerized Fatty Acids. Materials 2025, 18, 2155. https://doi.org/10.3390/ma18092155
Dziendzioł P, Waśkiewicz S, Jaszcz K. New Biobased Plasticizers for PVC Derived from Saturated Dimerized Fatty Acids. Materials. 2025; 18(9):2155. https://doi.org/10.3390/ma18092155
Chicago/Turabian StyleDziendzioł, Patryk, Sylwia Waśkiewicz, and Katarzyna Jaszcz. 2025. "New Biobased Plasticizers for PVC Derived from Saturated Dimerized Fatty Acids" Materials 18, no. 9: 2155. https://doi.org/10.3390/ma18092155
APA StyleDziendzioł, P., Waśkiewicz, S., & Jaszcz, K. (2025). New Biobased Plasticizers for PVC Derived from Saturated Dimerized Fatty Acids. Materials, 18(9), 2155. https://doi.org/10.3390/ma18092155






