New (Co)poly(hydroxyimide)s Based on 4,4′-Oxydiphthalic Anhydride—Effect of Composition on Properties, Including Gas Transport Ability
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Polyimides and (Co)poly(hydroxyimide)s
2.3. Membranes Formation
2.4. Characterization Methods
3. Result and Discussion
3.1. The (Co)polyimides Characterization
3.2. Thermal Properties
3.3. Optical and Mechanical Properties
3.4. Gas Transport Properties
4. Conclusions
- All of these (co)PIOHs revealed the hydrophobicity. The ODPA content in the molecule mainly influences the water contact angle measure.
- The influence of the stiffening of the polymer chain due to the hindered rotation of the benzene ring with the –CH3 groups ((co)PIOH-3) and reduced intermolecular attraction forces in hydrogen bond formation ((co)PIOH-1 and (co)PIOH-2) on the value of Tg was observed.
- In the Vis range, with the increase in the HAB contents in the polymer, the less incident radiation was transmitted by the obtained compound.
- The increase in HAB units in copolymer contributed to improving the mechanical properties of the tested materials.
- The gas permeation properties of the tested membranes mainly depend on the intersegment distance and the glass transition temperature of the investigated compounds; thus, the highest permeability coefficients were exhibited by the copolymer with the highest D ratio.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yi, C.; Li, W.; Shi, S.; He, K.; Ma, P.; Chen, M.; Yang, C. High-temperature-resistant and colorless polyimide: Preparations, properties, and applications. Sol. Energy 2020, 195, 340–354. [Google Scholar] [CrossRef]
- Bogert, M.T.; Renshaw, R.R. 4-Amino-0-phthalic acid and some of its derivatives. J. Am. Chem. Soc. 1908, 30, 1135–1144. [Google Scholar] [CrossRef]
- Xu, Z.; Croft, Z.L.; Guo, D.; Cao, K.; Liu, G. Recent development of polyimides: Synthesis, processing, and application in gas separation. J. Polym. Sci. 2021, 59, 943–962. [Google Scholar] [CrossRef]
- Megusar, J. Low temperature fast-neutron and gamma irradiation of Kapton® polyimide films. J. Nucl. Mater. 1997, 245, 185–190. [Google Scholar] [CrossRef]
- Hasegawa, M.; Horie, K. Photophysics, photochemistry, and optical properties of polyimides. Prog. Polym. Sci. 2001, 26, 259–335. [Google Scholar] [CrossRef]
- Narzary, B.B.; Baker, B.C.; Yadav, N.; D’Elia, V.; Faul, C.F. Crosslinked porous polyimides: Structure, properties and applications. Polym. Chem. 2021, 12, 6494–6514. [Google Scholar] [CrossRef]
- Benfridja, I.; Diaham, S.; Laffir, F.; Brennan, G.; Liu, N.; Kennedy, T. A universal study on the effect thermal imidization has on the physico-chemical, mechanical, thermal and electrical properties of polyimide for integrated electronics applications. Polymers 2022, 14, 1713. [Google Scholar] [CrossRef]
- Tharakan, S.A.; Muthusamy, S. The effects of long and bulky aromatic pendent groups with flexible linkages on the thermal, mechanical and electrical properties of the polyimides and their nanocomposites with functionalized silica. RSC Adv. 2021, 11, 16645–16660. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Yang, X.; Meng, Y.; Hu, P.; Huang, C.; Liu, F. From Molecular Design to Practical Applications: Strategies for Enhancing the Optical and Thermal Performance of Polyimide Films. Polymers 2024, 16, 2315. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Gao, X.; Cui, P.; Zou, T.; Hu, G.; Tao, L.; Zhai, L. Preparation and characterization of semi-alicyclic polyimides containing trifluoromethyl groups for optoelectronic application. Polymers 2020, 12, 1532. [Google Scholar] [CrossRef]
- Sawada, R.; Ando, S. Enhancing optical, dielectric, and thermal properties of bio-based polyimides incorporating isomannide with a bent and sterically constrained conformation. J. Mater. Chem. C 2023, 11, 15053–15064. [Google Scholar] [CrossRef]
- Castro-Blanco, R.A.; Rojas-Rodríguez, M.; Hernández, A.; Lozano, Á.E.; Alexandrova, L.; Aguilar-Lugo, C. Aromatic polyimides and copolyimides containing bulky t-butyltriphenylmethane units. Polym. Bull. 2020, 77, 5103–5125. [Google Scholar] [CrossRef]
- Sezer Hicyilmaz, A.; Celik Bedeloglu, A. Applications of polyimide coatings: A review. SN Appl. Sci. 2021, 3, 363. [Google Scholar] [CrossRef]
- Wu, Z.; He, J.; Yang, H.; Yang, S. Progress in aromatic polyimide films for electronic applications: Preparation, structure and properties. Polymers 2022, 14, 1269. [Google Scholar] [CrossRef]
- Ramgobin, A.; Fontaine, G.; Bourbigot, S. Investigation of the thermal stability and fire behavior of high performance polymer: A case study of polyimide. Fire Saf. J. 2021, 120, 103060. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, P.; Li, F.; Guo, H.; Kang, C.; Gao, L. Ultrahigh thermal-stability polyimides with low CTE and required flexibility by formation of hydrogen bonds between poly(amic acid)s. Eur. Polym. J. 2021, 148, 110369. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Feng, Q.; Wu, Y.; Liu, S.; Zhao, J. Improvement in Mechanical and Thermal Properties of Transparent Semi-Aromatic Polyimide by Crosslinking. Macromol. Chem. Phys. 2020, 221, 2000085. [Google Scholar] [CrossRef]
- Lin, J.; Su, J.; Weng, M.; Xu, W.; Huang, J.; Fan, T.; Liu, Y.; Min, Y. Applications of flexible polyimide: Barrier material, sensor material, and functional material. Soft Sci. 2023, 3, 2–53. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Amooghin, A.E.; Bandehali, S.; Moghadassi, A.; Matsuura, T.; Van der Bruggen, B. Polyimides in membrane gas separation: Monomer’s molecular design and structural engineering. Prog. Polym. Sci. 2019, 91, 80–125. [Google Scholar] [CrossRef]
- Tan, X.; Rodrigue, D. A review on porous polymeric membrane preparation. Part II: Production techniques with polyethylene, polydimethylsiloxane, polypropylene, polyimide, and polytetrafluoroethylene. Polymers 2019, 11, 1310. [Google Scholar] [CrossRef]
- Ogbonna, V.E.; Popoola, A.P.I.; Popoola, O.M.; Adeosun, S.O. A review on polyimide reinforced nanocomposites for mechanical, thermal, and electrical insulation application: Challenges and recommendations for future improvement. Polym. Bull. 2020, 79, 663–695. [Google Scholar] [CrossRef]
- Liang, N.; Fujiwara, E.; Nara, M.; Ishige, R.; Ando, S. Colorless Copolyimide Films Exhibiting Large Stokes-Shifted Photoluminescence Applicable for Spectral Conversion. ACS Appl. Polym. Mater. 2021, 3, 3911–3921. [Google Scholar] [CrossRef]
- Sava, I.; Damaceanu, M.D.; Constantin, C.P.; Asandulesa, M.; Wolińska-Grabczyk, A.; Jankowski, A. Structure–promoted high performance properties of triphenylmethane-containing polyimides and copolyimides. Eur. Polym. J. 2018, 108, 554–569. [Google Scholar] [CrossRef]
- Sapegin, D.A.; Gubanova, G.N.; Kruchinina, E.V.; Volkov, A.Y.; Popova, E.N.; Vylegzhanina, M.E.; Setnickova, K.; Kononova, S.V. On the structure, morphology and transport through limitedly flexible chain sulfonated co-polyimide. Polymer 2021, 212, 123142. [Google Scholar] [CrossRef]
- Zhang, M.; Niu, H.; Wu, D. Polyimide fibers with high strength and high modulus: Preparation, structures, properties, and applications. Macromol. Rapid Commun. 2018, 39, 1800141. [Google Scholar] [CrossRef] [PubMed]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in polyimide-based materials for space applications. Adv. Mater. 2019, 31, 1807738. [Google Scholar] [CrossRef]
- Xiao, S.; Akinyi, C.; Longun, J.; Iroh, J.O. Polyimide Copolymers and Nanocomposites: A Review of the Synergistic Effects of the Constituents on the Fire-Retardancy Behavior. Energies 2022, 15, 4014. [Google Scholar] [CrossRef]
- Jeon, H.; Na, C.; Kwac, L.K.; Kim, H.G.; Chang, J.H. Effects of various types of organo-mica on the physical properties of polyimide nanocomposites. Sci. Rep. 2024, 14, 655. [Google Scholar] [CrossRef]
- Fang, Y.; He, X.; Kang, J.C.; Wang, L.; Ding, T.M.; Lu, X.; Zhang, S.Y.; Lu, Q. Colorless transparent and thermally stable terphenyl polyimides with various small side groups for substrate application. Eur. Polym. J. 2024, 202, 112640. [Google Scholar] [CrossRef]
- Weyhrich, C.W.; Will, J.W.; Nayyar, G.; Westover, C.C.; Patterson, S.; Arrington, C.B.; Williams, C.B.; Long, T.E. Temporally Stable Supramolecular Polymeric Salts Enabling High-Performance 3D All-Aromatic Polyimide Lattices. Small 2023, 19, 2303188. [Google Scholar] [CrossRef]
- Aristizábal, S.L.; Habboub, O.S.; Pulido, B.A.; Cetina-Mancilla, E.; Olvera, L.I.; Forster, M.; Nunes, S.P.; Scherf, U.; Zolotukhin, M.G. One-step, room temperature synthesis of well-defined, organo-soluble multifunctional aromatic polyimides. Macromolecules 2021, 54, 10870–10882. [Google Scholar] [CrossRef]
- Yu, S.; Zhou, J.; Xu, A.; Lao, J.; Luo, H.; Chen, S. The scalable and high performance polyimide dielectrics containing alicyclic structures for high-temperature capacitive energy storage. J. Chem. Eng. 2023, 469, 143803. [Google Scholar] [CrossRef]
- Toto, E.; Laurenzi, S.; Pellegrini, R.C.; Cavallini, E.; Santonicola, M.G. Eco-friendly synthesis of high-performance polyimide materials using bio-based greener solvents: Towards sustainable technologies in space environment. Mater. Today Sustain. 2024, 25, 100657. [Google Scholar] [CrossRef]
- Liu, L.; Duan, Y.; Yun, H.; Chen, X.; Liu, J.; Lv, S.; Zhang, Y. Progress on the research and development of the biomass-based polyimide. Ind. Crops Prod. 2024, 220, 119239. [Google Scholar] [CrossRef]
- Kumar, A.G.; Singh, A.; Komber, H.; Voit, B.; Tiwari, B.R.; Noori, M.T.; Ghangrekar, M.M.; Banerjee, S. Novel sulfonated Co-poly (ether imide) s containing trifluoromethyl, fluorenyl and hydroxyl groups for enhanced proton exchange membrane properties: Application in microbial fuel cell. ACS Appl. Mater. Interfaces 2018, 10, 14803–14817. [Google Scholar] [CrossRef]
- Liu, Q.; Paul, D.R.; Freeman, B.D. Gas permeation and mechanical properties of thermally rearranged (TR) copolyimides. Polymer 2016, 82, 378–391. [Google Scholar] [CrossRef]
- Velioğlu, S.; Tantekin-Ersolmaz, S.B.; Chew, J.W. Towards the generalization of membrane structure-property relationship of polyimides and copolyimides: A group contribution study. J. Membr. Sci. 2017, 543, 233–254. [Google Scholar] [CrossRef]
- Didenko, A.L.; Nesterova, A.S.; Anokhina, T.S.; Borisov, I.L.; Kudryavtsev, V.V. Poly(urethane-imides) and Poly(ester-imides) as Promising Materials for Gas Separation and Pervaporation Membranes. Membr. Membr. Technol. 2024, 6, 43–53. [Google Scholar] [CrossRef]
- Didenko, A.L.; Smirnova, V.E.; Popova, E.N.; Vaganov, G.V.; Kuznetсov, D.A.; Elokhovskii, V.Y.; Ivanov, A.G.; Svetlichnyi, V.M.; Yudin, V.E.; Kudryavtsev, V.V. Heat Resistance and Dynamic Mechanical and Rheological Properties of a Blend of Crystallizing Polymers, Polyimide and Copoly (urethane—Imide), at Identical Chemical Structure of the Imide Blocks in the Initial Polymers. Russ. J. Appl. Chem. 2020, 93, 45–56. [Google Scholar] [CrossRef]
- Shankar, M.R.; Smith, M.L.; Tondiglia, V.P.; Lee, K.M.; McConney, M.E.; Wang, D.H.; Tan, L.-S.; White, T.J. Contactless, photoinitiated snap-through in azobenzene-functionalized polymers. Proc. Natl. Acad. Sci. USA 2013, 110, 18792–18797. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Wojtowicz, M.; Sobolewska, A.; Noga, J.; Jarczyk-Jędryka, A.; Kozanecka-Szmigiel, A.; Schab-Balcerzak, E. Thermal, optical and photoinduced properties of a series of homo and co-polyimides with two kinds of covalently bonded azo-dyes and their supramolecular counterparts. Opt. Mater. 2015, 48, 139–149. [Google Scholar] [CrossRef]
- Konieczkowska, J.; Nocoń-Szmajda, K.; Ciemięga, A. Photomechanical effect in “side-chain” polyimides with low content of azopyridine chromophore. Express Polym. Lett. 2024, 18, 607–622. [Google Scholar] [CrossRef]
- Nocoń-Szmajda, K.; Jankowski, A.; Wolińska-Grabczyk, A.; Konieczkowska, J. Guest-host and functionalized side-chain azopolyimide membranes for controlled gas separation. Polymer 2021, 229, 124012. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Wei, X.; Jing, D.; Su, W.; Zhang, S. Hydroxyl-functionalized block co-polyimide enables simultaneously improved toughness and strength of tetrafunctional epoxy resin. Compos. Sci. Technol. 2022, 230, 109787. [Google Scholar] [CrossRef]
- Du, J.; Pu, C.; Sun, X.; Wang, Q.; Niu, H.; Wu, D. Preparation and interfacial properties of hydroxyl-containing polyimide fibers. Polymers 2023, 15, 1032. [Google Scholar] [CrossRef]
- Kononova, S.V.; Lebedeva, G.K.; Gubanova, G.N.; Kruchinina, E.V.; Vlasova, E.N.; Afanas’eva, N.V.; Popova, E.N.; Volkov, A.Y.; Bykova, E.N.; Zakharova, N.V. Effect of Hydroxyl-Containing Fragments on the Structure and Properties of Membrane-Forming Polyamide-Imides. Membranes 2013, 13, 716. [Google Scholar] [CrossRef]
- Lebedeva, G.; Kononova, S.; Kruchinina, E.; Vlasova, E.; Gofman, I.; Bol’shakov, M.; Romashkova, K. Novel hydroxyl-containing and thermo-dehydrocyclizable polycondensation polymers for multifunctional materials: Synthesis, properties, application. J. Appl. Polym. Sci. 2021, 139, 51978. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, H.; Cai, M.; Ma, X.; Wang, Z.; Yan, J. Systematic Investigation of Microstructures and Gas Separation Performance of Thermally Rearranged Polybenzoxazole Membranes Derived from 9,9-Bis(3,4-dicarboxyphenyl) fluorene Dianhydride. Macromolecules 2024, 57, 9877–9888. [Google Scholar] [CrossRef]
- Jankowski, A.; Grabiec, E.; Nocoń-Szmajda, K.; Marcinkowski, A.; Janeczek, H.; Wolińska-Grabczyk, A. Polyimide-based membrane materials for CO2 separation: A Comparison of segmented and aromatic (Co)polyimides. Membranes 2021, 11, 274. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, M.H.; Han, S.G.; Kim, H.Y.; Ahn, J.H.; Lee, E.M.; Won, Y.H. Synthesis and properties of nonlinear optical side chain soluble polyimides for photonics applications. J. Polym. Sci. Part A Polym. Chem. 1998, 36, 301–307. [Google Scholar] [CrossRef]
- Wu, Q.; Ma, X.; Zheng, F.; Lu, X.; Lu, Q. High performance transparent polyimides by controlling steric hindrance of methyl side groups. Eur. Polym. J. 2019, 120, 109235. [Google Scholar] [CrossRef]
- Park, J.Y.; Paul, D.R. Correlation and prediction of gas permeability in glassy polymer membrane materials via a modified free volume based group contribution method. J. Membr. Sci. 1997, 125, 23–39. [Google Scholar] [CrossRef]
- Huang, X.; Chen, B.; Mei, M.; Li, H.; Liu, C.; Wei, C. Synthesis and characterization of organosoluble, thermal stable and hydrophobic polyimides derived from 4-(4-(1-pyrrolidinyl)phenyl)-2,6-bis(4-(4-aminophenoxy) phenyl) pyridine. Polymers 2017, 910, 484. [Google Scholar] [CrossRef]
- Polymer Molecular Weight Distribution and Definitions of MW Averages. Available online: https://www.agilent.com/cs/library/technicaloverviews/public/5990-7890EN.pdf (accessed on 20 January 2025).
- Wolińska-Grabczyk, A.; Wójtowicz, M.; Jankowski, A.; Grabiec, E.; Kubica, P.; Musioł, M.; Sobota, M. Synthesis, characterization, and gas permeation properties of thermally rearranged poly(hydroxyimide)s filled with mesoporous MCM-41 silica. Polymer 2018, 158, 32–45. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Xiao, G.; Li, L.; Hou, M.; Hu, J.; Wang, T. Synthesis and gas permeation properties of thermally rearranged poly(ether-benzoxazole)s with low rearrangement temperatures. RSC Adv. 2020, 10, 17461–17472. [Google Scholar] [CrossRef]
- Nocoń-Szmajda, K.; Wolińska-Grabczyk, A.; Jankowski, A.; Dryzek, J.; Dryzek, E.; Janeczek, H.; Grabiec, E.; Musioł, M. Effects of ionic liquid doping on gas transport properties of thermally rearranged poly(hydroxyimide)s. Sep. Purif. Technol. 2021, 254, 117664. [Google Scholar] [CrossRef]
- Nocoń-Szmajda, K.; Wolińska-Grabczyk, A.; Jankowski, A.; Szeluga, U.; Wójtowicz, M.; Konieczkowska, J.; Hercog, A. Gas transport properties of mixed matrix membranes based on thermally rearranged poly(hydroxyimide)s filled with inorganic porous particles. Sep. Purif. Technol. 2020, 242, 116778. [Google Scholar] [CrossRef]
- Torres, A.; Soto, C.; Carmona, J.; Comesaña-Gandara, B.; de la Viuda, M.; Palacio, L.; Prádanos, P.; Simorte, M.T.; Sanz, I.; Muñoz, R.; et al. Gas Permeability through Polyimides: Unraveling the Influence of Free Volume, Intersegmental Distance and Glass Transition Temperature. Polymers 2023, 16, 13. [Google Scholar] [CrossRef]
- Grabiec, E.; Schab-Balcerzak, E.; Wolińska-Grabczyk, A.; Jankowski, A.; Jarząbek, B.; Kożuch-Krawczyk, J.; Kurcok, M. Physical, optical and gas transport properties of new processable polyimides and poly(amideimide)s obtained from 4,4′-[oxybis(4,1-phenylenethio)] dianiline and aromatic dianhydrides. Polym. J. 2011, 43, 621–629. [Google Scholar] [CrossRef]
- Kubica, P.; Wolińska-Grabczyk, A. Correlation between Cohesive Energy Density, Fractional Free Volume, and Gas Transport Properties of Poly(ethylene-co-vinyl acetate) Materials. Int. J. Polym. Sci. 2015, 2015, 861979. [Google Scholar] [CrossRef]
- Mi, Y.; Stern, S.A.; Trohalaki, S. Dependence of the gas permeability of some polyimide isomers on their intrasegmental mobility. J. Membr. Sci. 1993, 77, 41–48. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves: I. Preparation and experimental results. J. Membr. Sci. 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Kim, T.H.; Koros, W.J.; Husk, G.R.; O’brien, K.C. Relationship between gas separation properties and chemical structure in a series of aromatic polyimides. J. Membr. Sci. 1988, 37, 45–62. [Google Scholar] [CrossRef]
- Hirayama, Y.; Yoshinaga, T.; Kusuki, Y.; Ninomiya, K.; Sakakibara, T.; Tamari, T. Relation of gas permeability with structure of aromatic polyimides I. J. Membr. Sci. 1996, 111, 169–182. [Google Scholar] [CrossRef]
- Stern, S.A.; Mi, Y.; Yamamoto, H.; Clair, A.K.S. Structure/permeability relationships of polyimide membranes. Applications to the separation of gas mixtures. J. Polym. Sci. Part B Polym. Phys. 1989, 27, 1887–1909. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
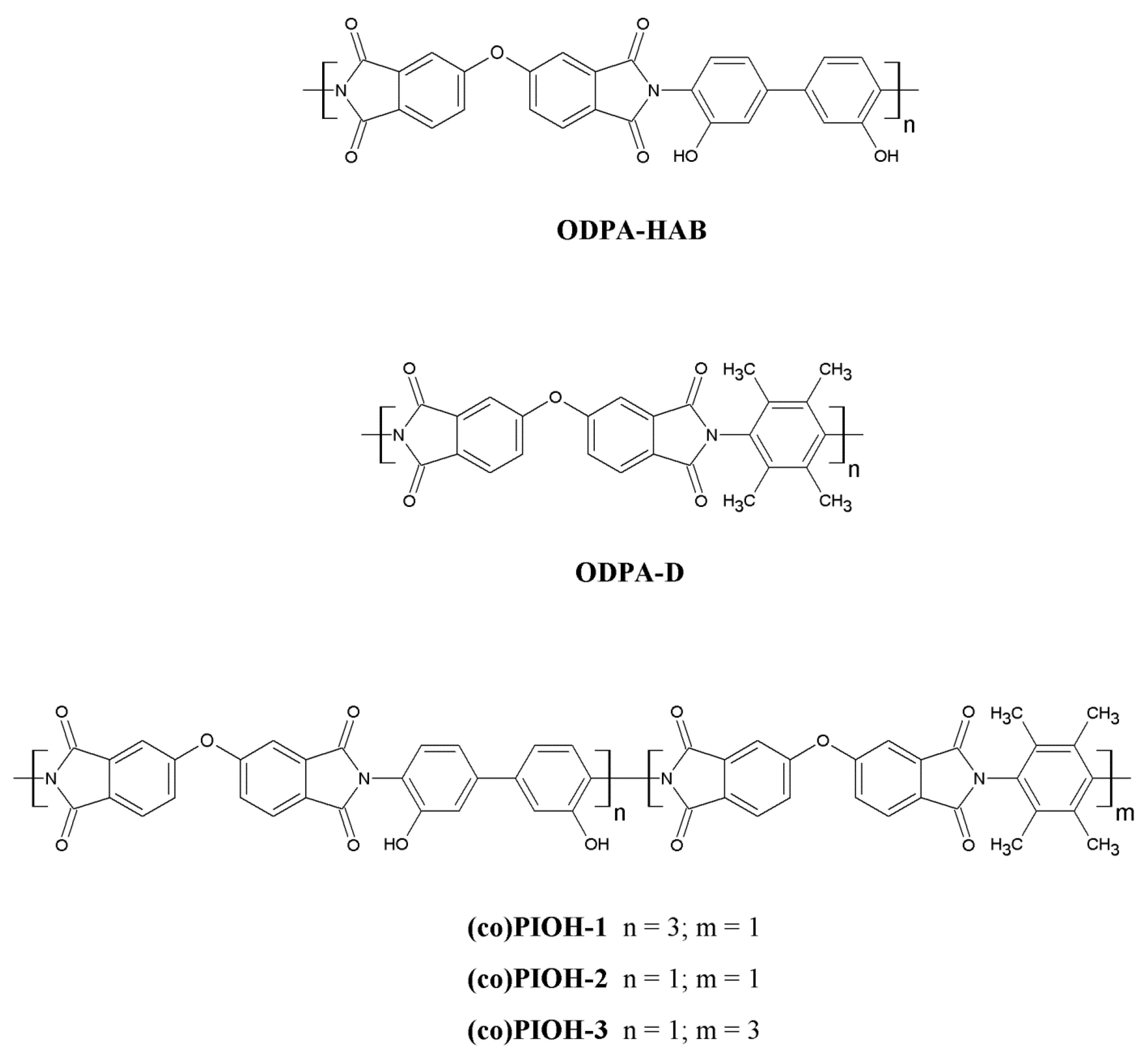
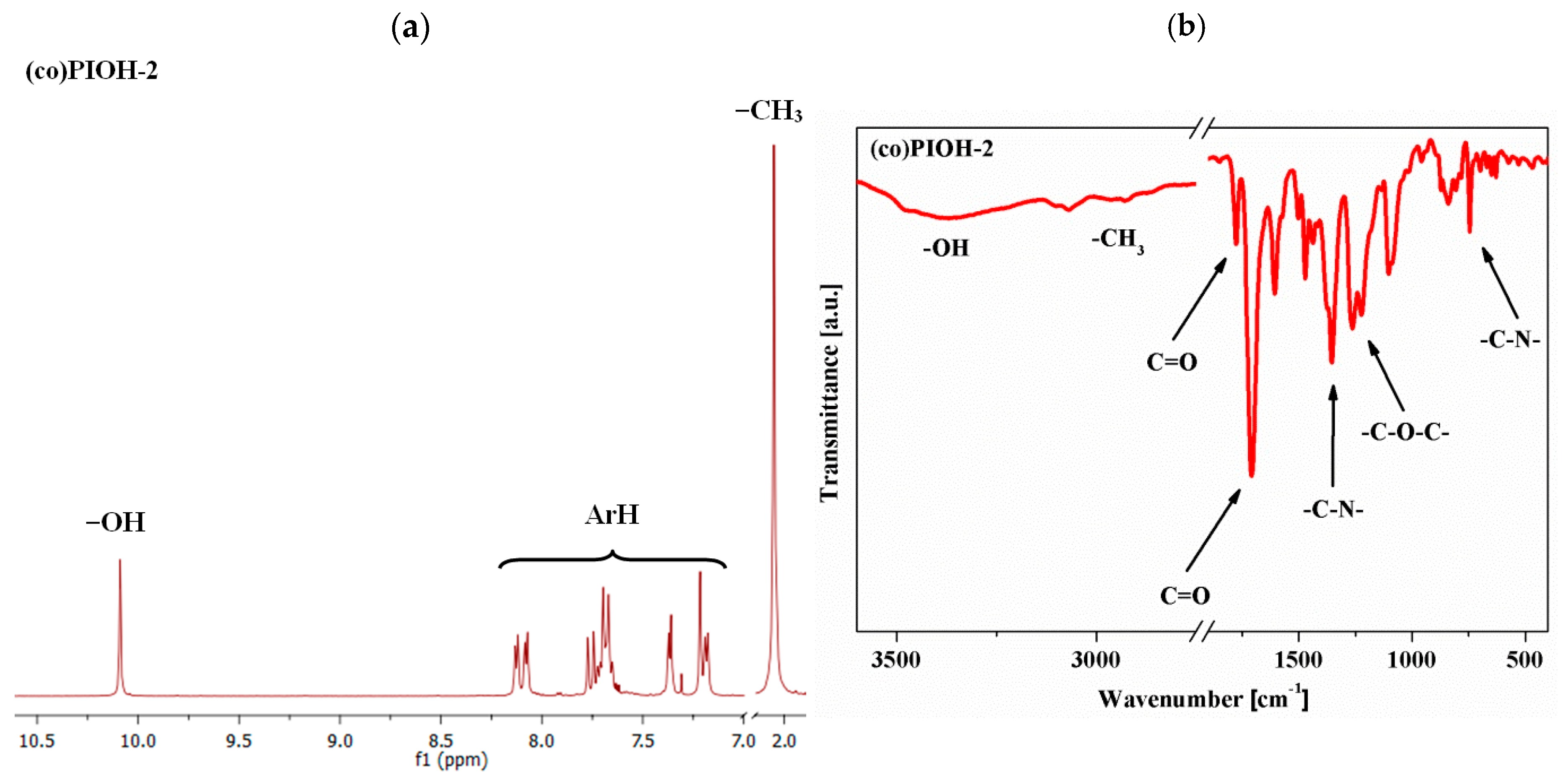
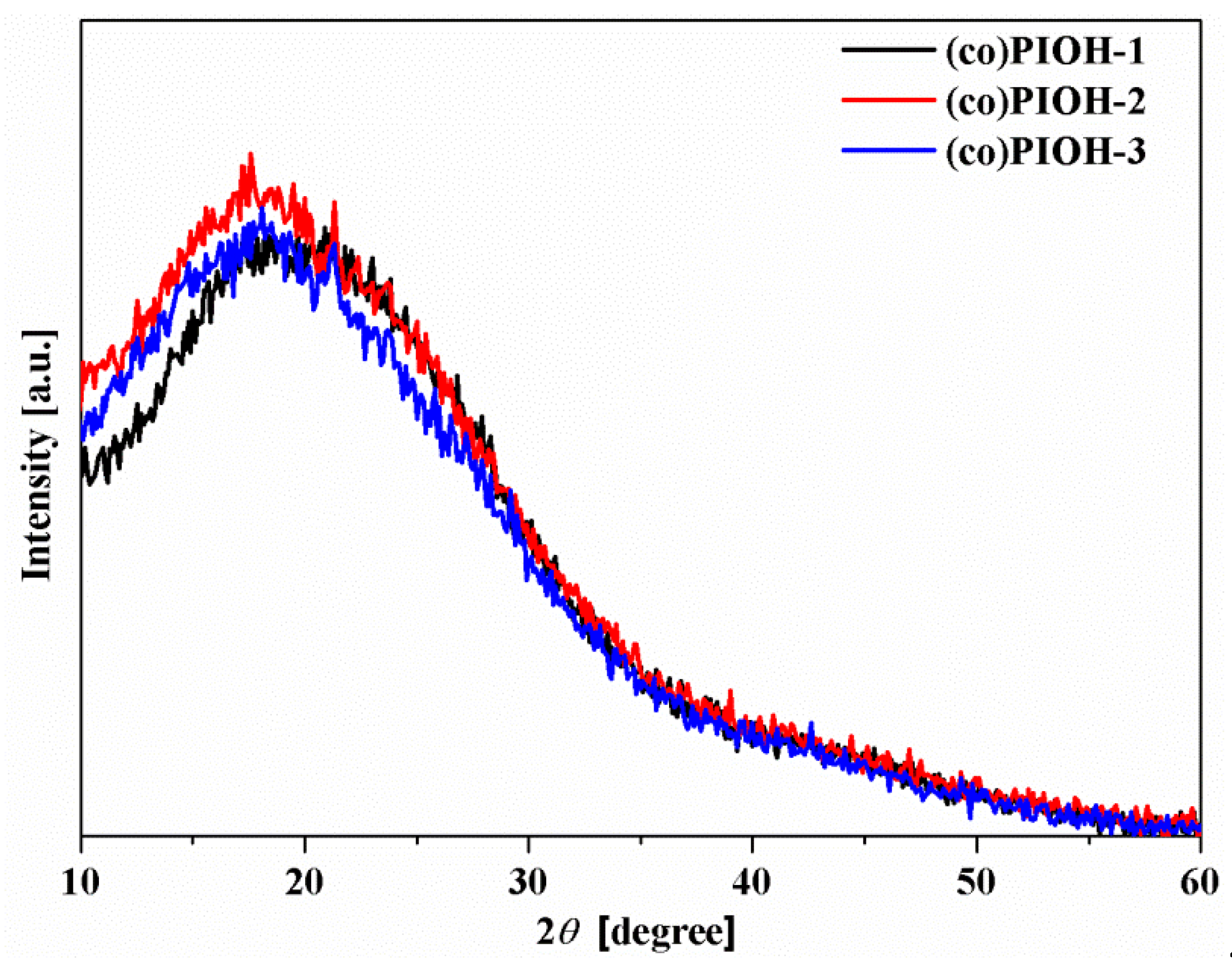


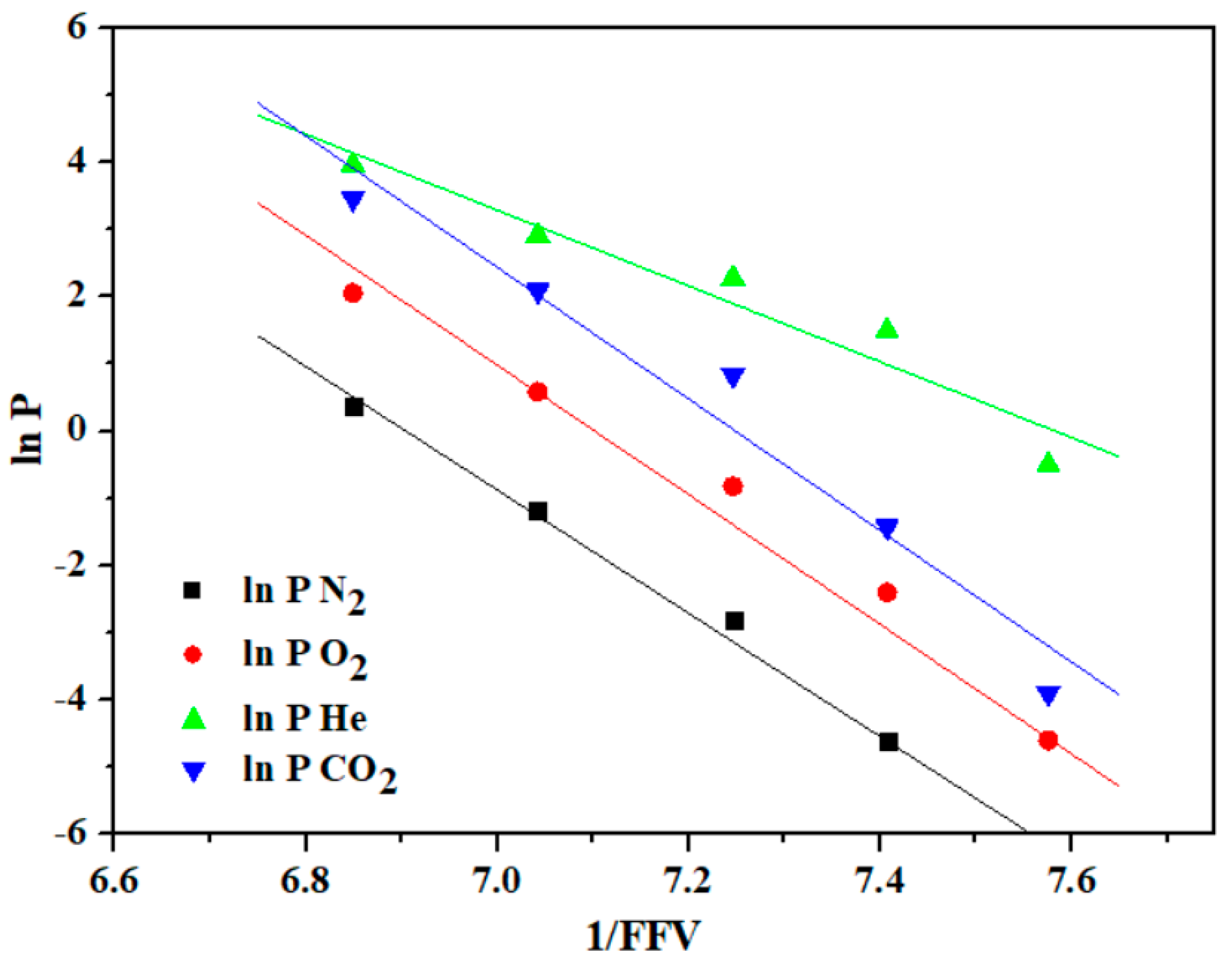
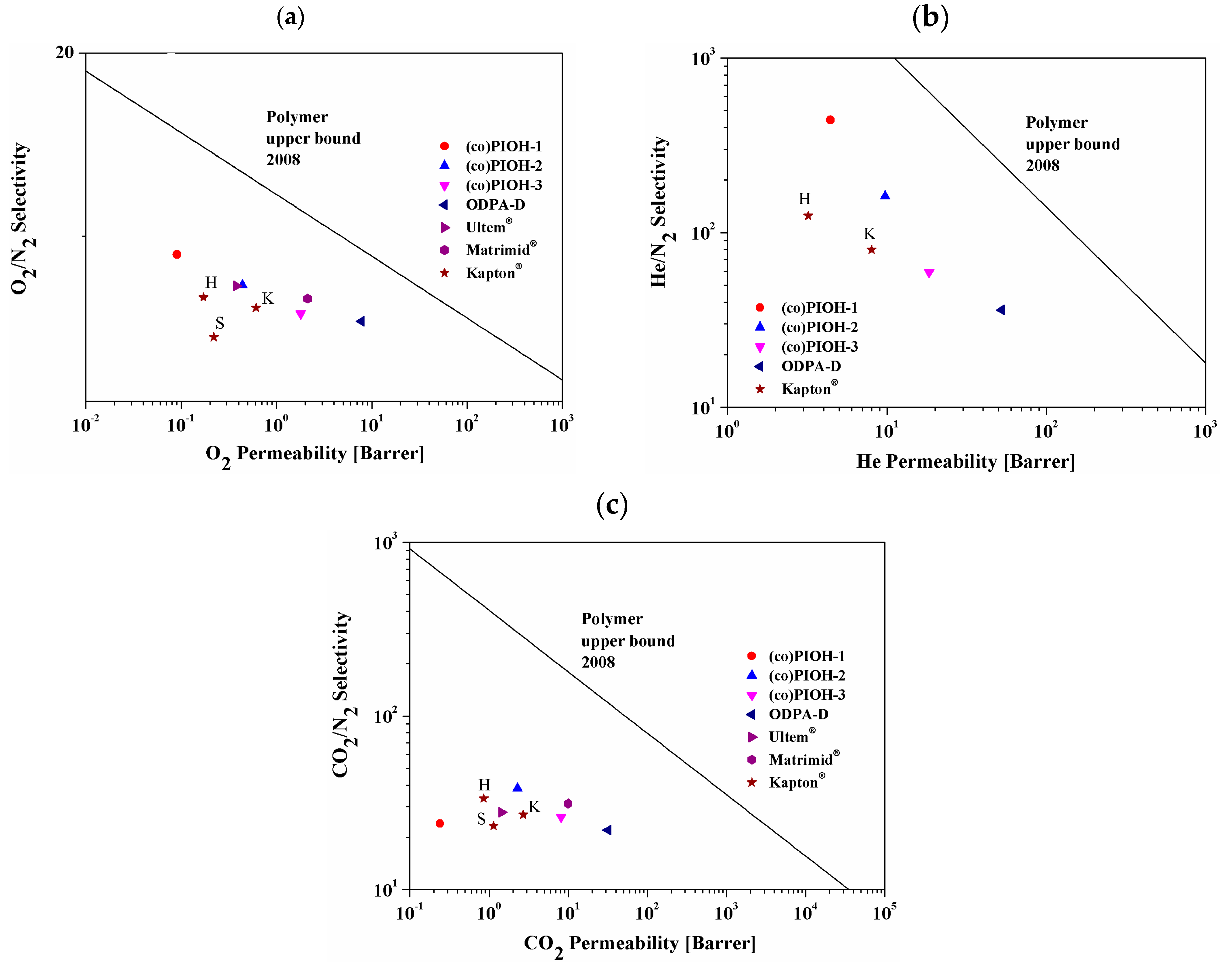
| Compound Code | d-Spacing (Å) | Density (g/cm3) | FFV a | Mn b (g/mol) | Mw c (g/mol) | Mw/Mn d |
|---|---|---|---|---|---|---|
| (co)PIOH-1 | 5.25 | 1.3262 | 0.135 | 50,000 | 129,000 | 2.6 |
| (co)PIOH-2 | 5.84 | 1.3115 | 0.138 | 34,000 | 80,000 | 2.4 |
| (co)PIOH-3 | 6.43 | 1.2694 | 0.142 | 89,000 | 209,000 | 2.3 |
| ODPA-HAB | 4.59 | 1.3657 | 0.132 | 65,000 | 172,000 | 2.6 |
| ODPA-D | 5.33 [49] | 1.2531 | 0.146 | 23,000 | 70,000 | 3.0 |
| Molecule Code | DSC | TGA | ||
|---|---|---|---|---|
| Tg a (°C) | T5% b (°C) | Tmax c (°C) | Residual Weight d (%) | |
| (co)PIOH-1 | 265 | 375 | 407, 521 | 58 |
| (co)PIOH-2 | 257 | 386 | 410, 520 | 60 |
| (co)PIOH-3 | 281 | 376 | 432, 508 | 60 |
| ODPA-HAB | 246 | 405 | 448, 630 | 56 |
| ODPA-D | 307 [49] | 484 [49] | 520 [49] | 58 [49] |
| Compound Code | E a (GPa) | Rm b (MPa) | A c (%) |
|---|---|---|---|
| (co)PIOH-1 | 3.38 ± 0.14 | 184.1 ± 11.6 | 2.7 ± 0.2 |
| (co)PIOH-2 | 2.76 ± 0.60 | 167.7 ± 18.0 | 2.5 ± 0.1 |
| (co)PIOH-3 | 2.37 ± 0.69 | 101.1 ± 8.0 | 2.3 ± 0.1 |
| Molecule Code | Permeability (Barrer) | Ideal Selectivity | ||||
|---|---|---|---|---|---|---|
| N2 | O2 | He | CO2 | α O2/N2 | α CO2/N2 | |
| (co)PIOH-1 | 0.01 | 0.09 | 4.42 | 0.24 | 9.00 | 24.00 |
| (co)PIOH-2 | 0.06 | 0.44 | 9.74 | 2.29 | 7.33 | 38.17 |
| (co)PIOH-3 | 0.31 | 1.79 | 18.37 | 8.12 | 5.77 | 26.19 |
| ODPA-HAB | - | 0.01 | 0.60 | 0.02 | - | - |
| ODPA-D | 1.45 [49] | 7.79 [49] | 52.40 [49] | 31.90 [49] | 5.37 [49] | 22.00 [49] |
| Molecule Code | Permeability (Barrer) | Ideal Selectivity | ||||
|---|---|---|---|---|---|---|
| N2 | O2 | He | CO2 | α O2/N2 | α CO2/N2 | |
| Matrimid®_K a | 0.32 | 2.12 | - | 10.0 | 6.62 | 31.25 |
| Ultem®_K a | 0.052 | 0.38 | - | 1.45 | 7.31 | 27.88 |
| Kapton®_K b,c | 0.10 b | 0.61 c | 8.0 b | 2.7 b | 6.1 | 27.0 |
| Kapton®_S d | 0.049 | 0.22 | - | 1.14 | 4.5 | 23.27 |
| Kapton®_H e | 0.0256 | 0.171 | 3.20 | 0.858 | 6.68 | 33.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pająk, A.K.; Jankowski, A.; Schab-Balcerzak, E. New (Co)poly(hydroxyimide)s Based on 4,4′-Oxydiphthalic Anhydride—Effect of Composition on Properties, Including Gas Transport Ability. Materials 2025, 18, 2193. https://doi.org/10.3390/ma18102193
Pająk AK, Jankowski A, Schab-Balcerzak E. New (Co)poly(hydroxyimide)s Based on 4,4′-Oxydiphthalic Anhydride—Effect of Composition on Properties, Including Gas Transport Ability. Materials. 2025; 18(10):2193. https://doi.org/10.3390/ma18102193
Chicago/Turabian StylePająk, Agnieszka Katarzyna, Andrzej Jankowski, and Ewa Schab-Balcerzak. 2025. "New (Co)poly(hydroxyimide)s Based on 4,4′-Oxydiphthalic Anhydride—Effect of Composition on Properties, Including Gas Transport Ability" Materials 18, no. 10: 2193. https://doi.org/10.3390/ma18102193
APA StylePająk, A. K., Jankowski, A., & Schab-Balcerzak, E. (2025). New (Co)poly(hydroxyimide)s Based on 4,4′-Oxydiphthalic Anhydride—Effect of Composition on Properties, Including Gas Transport Ability. Materials, 18(10), 2193. https://doi.org/10.3390/ma18102193






