Effect of Fe Content on Phase Behavior of Sm–Co–Fe Alloys During Solidification and Aging
Abstract
1. Introduction
2. Materials and Methods
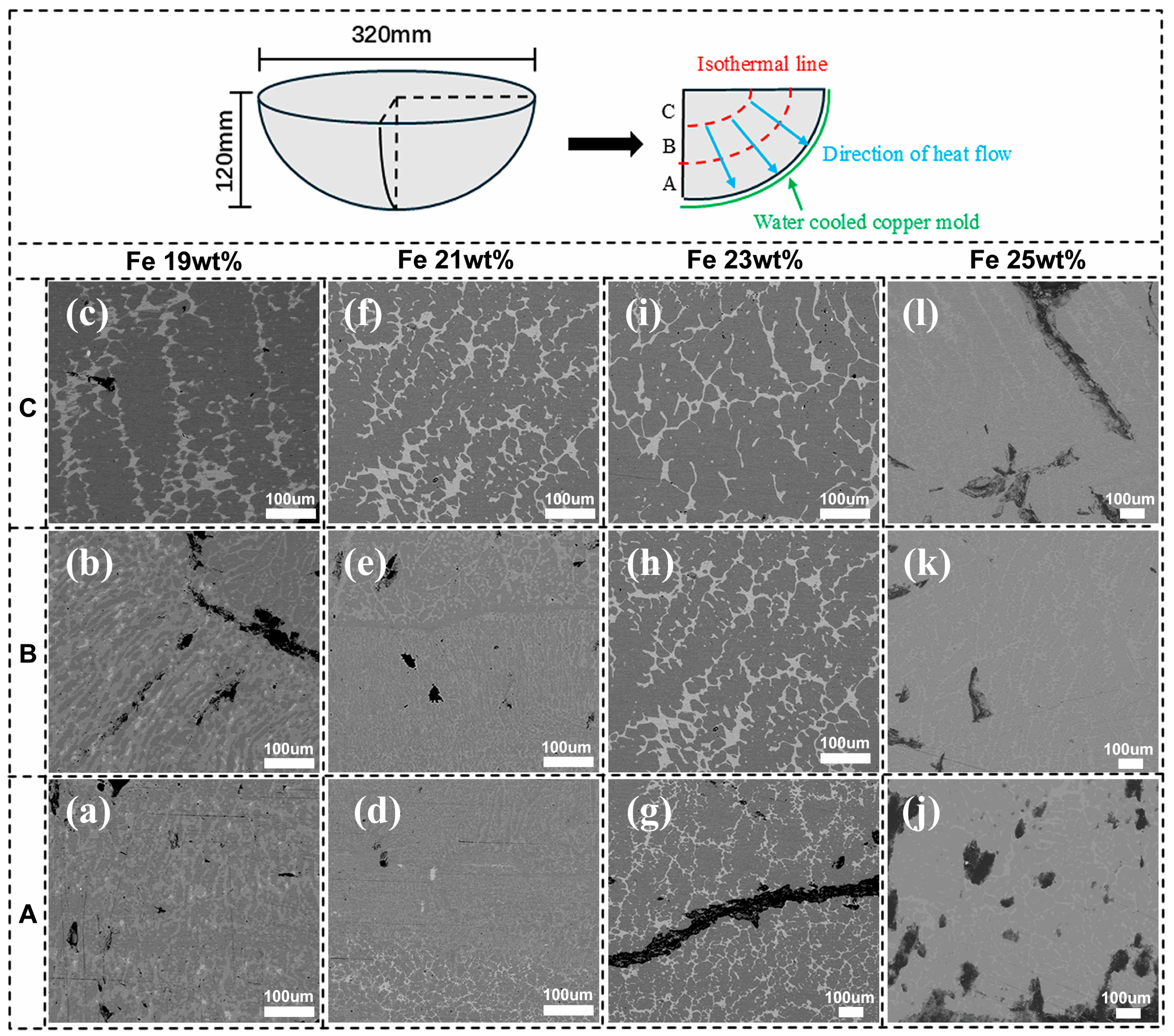
2.1. Experimental Procedure
2.2. Characterization Methods
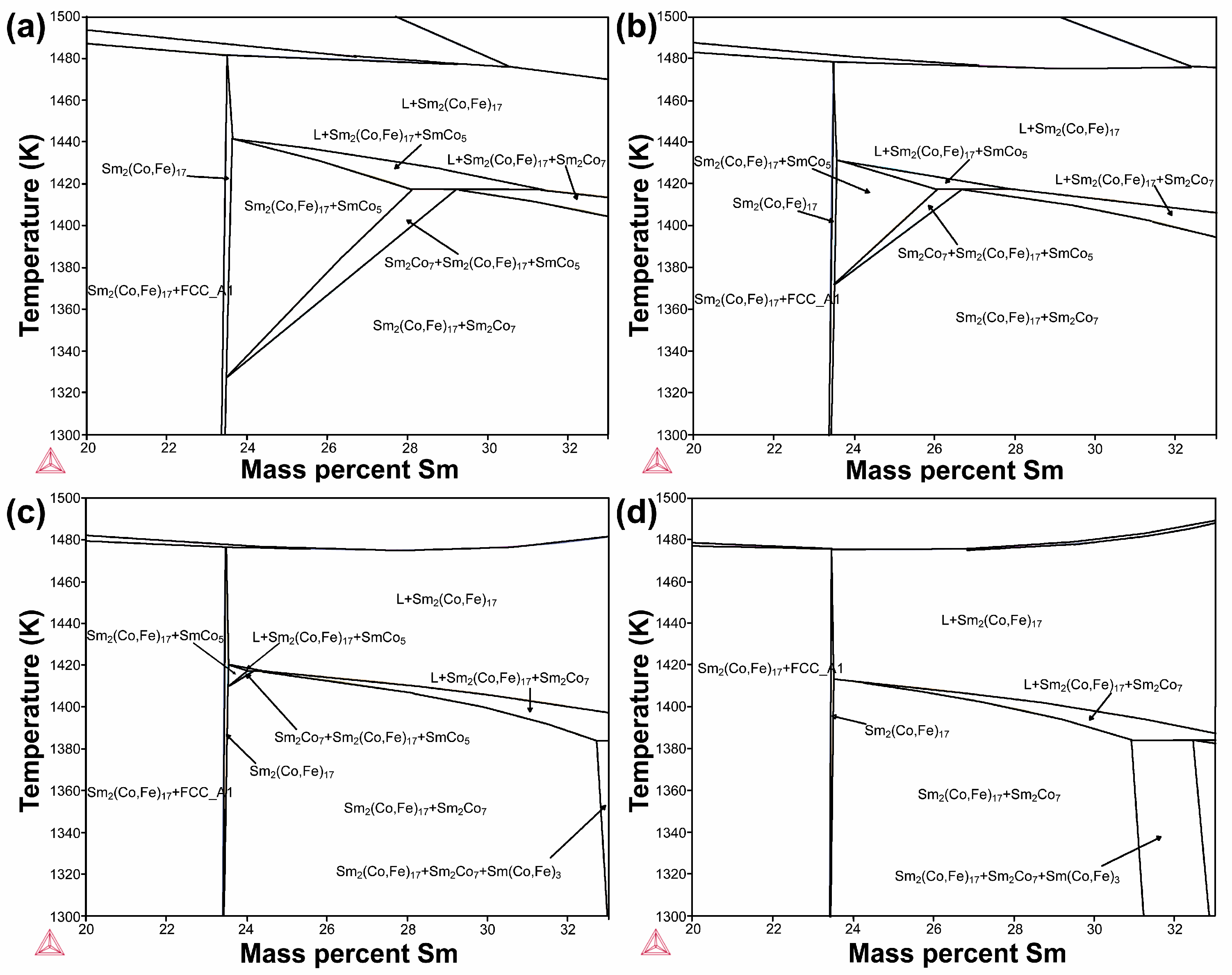
2.3. Thermodynamic Calculations
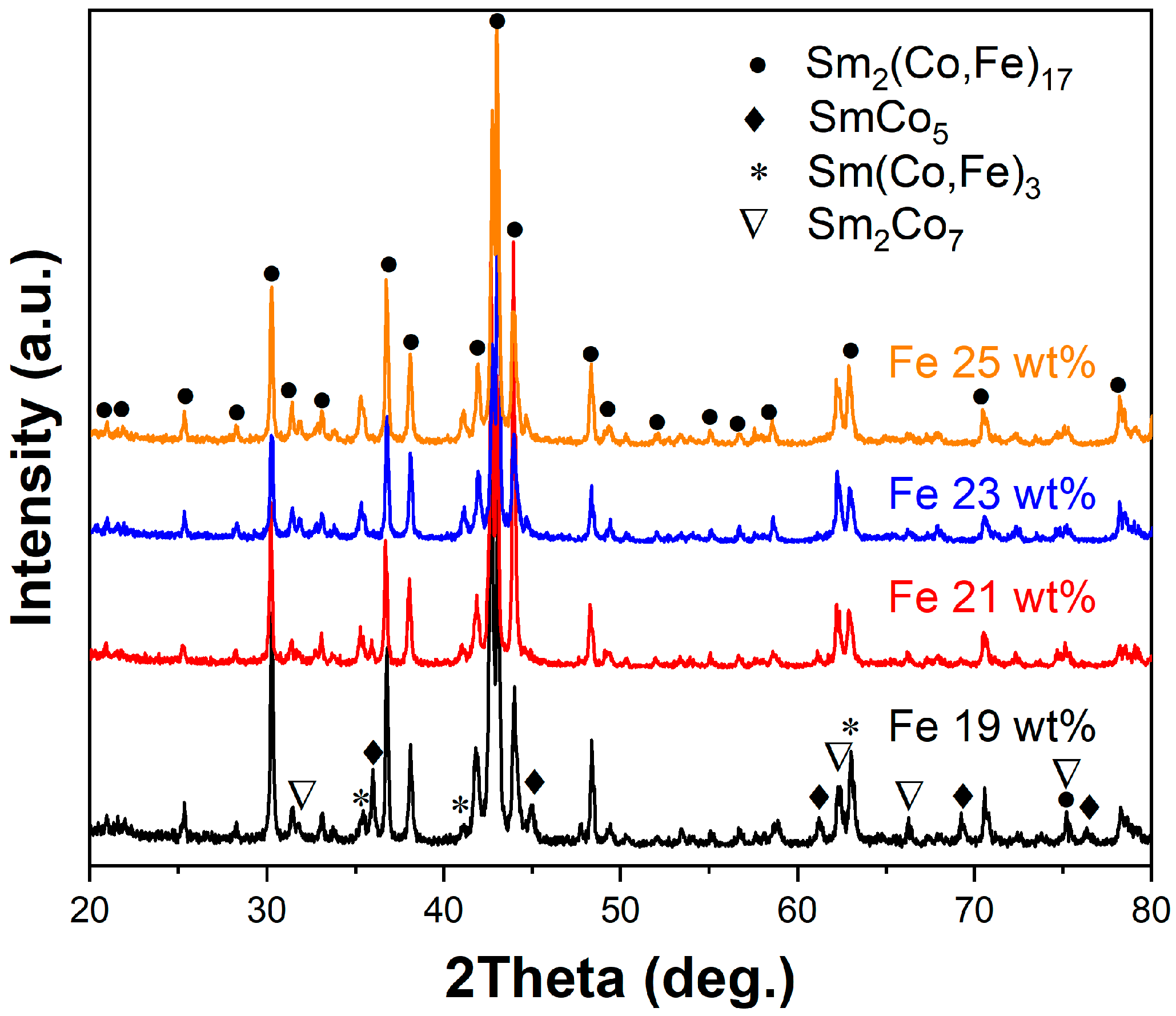
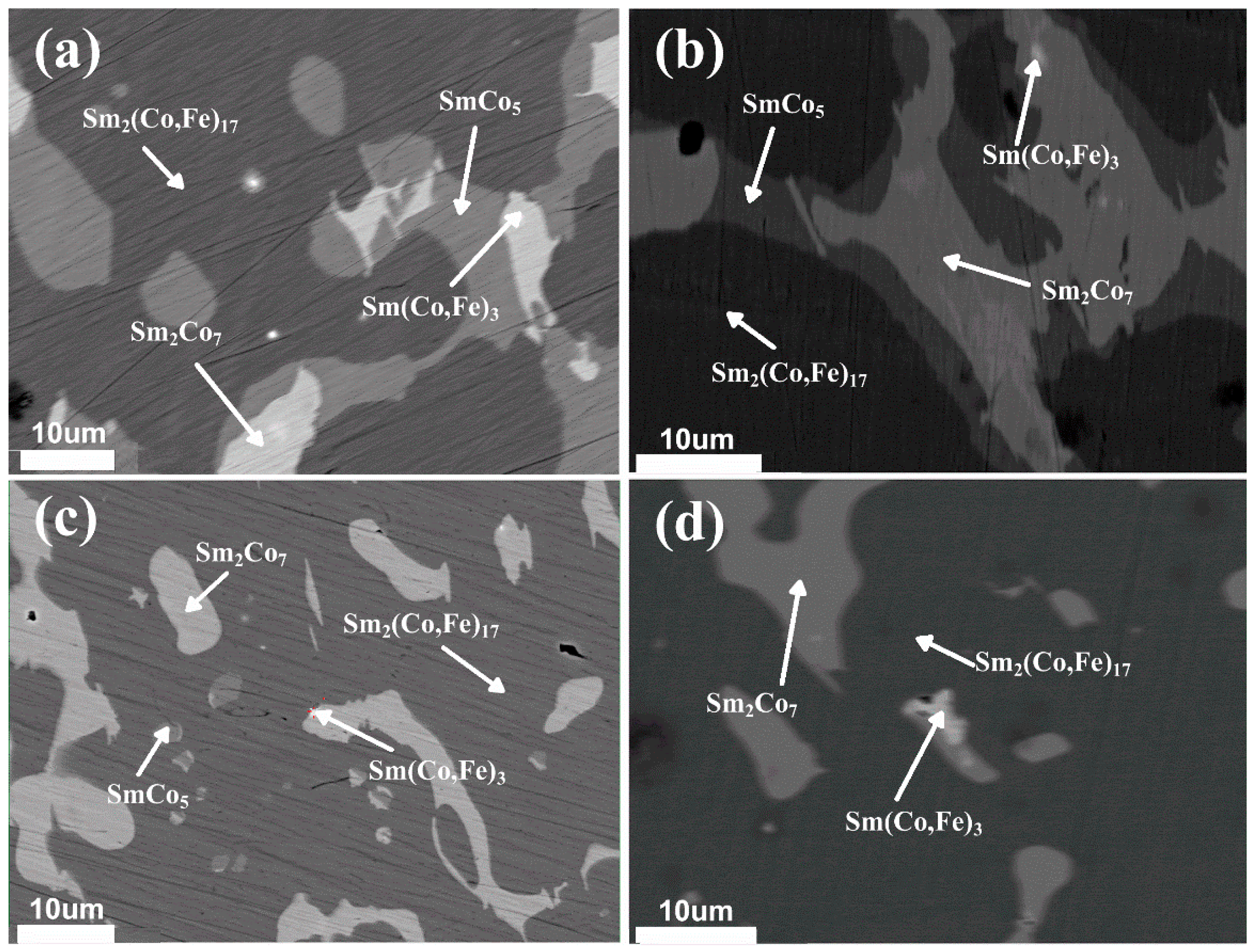
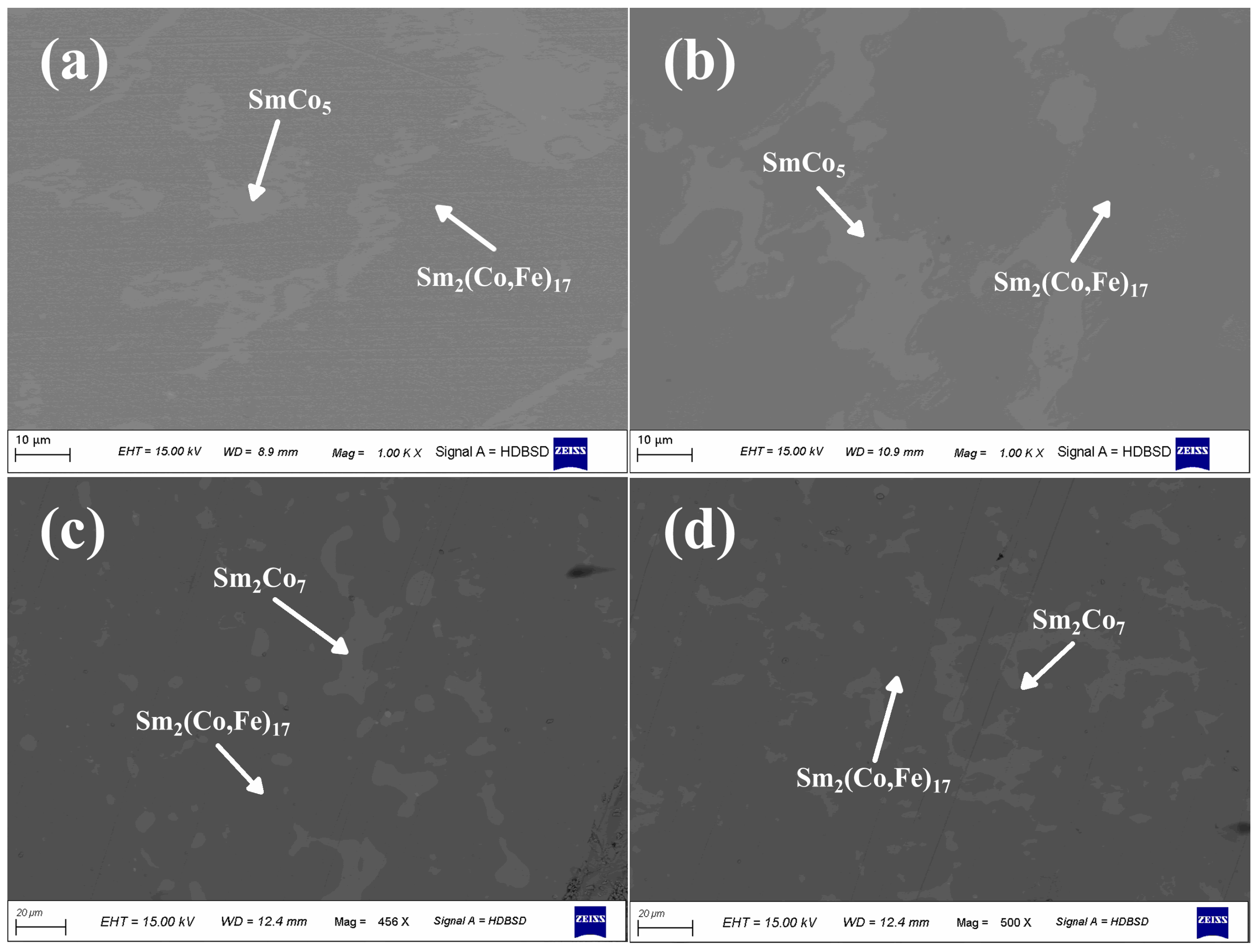
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, R.; Vero, K.; Borah, J.P. Historical overview and recent advances in permanent magnet materials. Mater. Today Commun. 2024, 41, 110538. [Google Scholar] [CrossRef]
- Song, X.; Liu, Y.; Yuan, T.; Wang, F.; Fan, J.P.; Ma, T.Y. Effect of post-solutionizing cooling rate on microstructure and magnetic properties of 2:17-type Sm-Co-Fe-Cu-Zr magnets. J. Alloys Compd. 2022, 896, 163080. [Google Scholar] [CrossRef]
- Guo, K.; Lu, H.; Xu, G.J.; Liu, D.; Wang, H.B.; Liu, X.M.; Song, X.Y. Recent progress in nanocrystalline Sm-Co based magnets. Mater. Today Chem. 2022, 25, 100983. [Google Scholar] [CrossRef]
- Yang, J.J.; Zhang, D.T.; Zhang, H.G.; Li, Y.Q.; Meng, C.Z.; Teng, Y.; Liu, W.Q.; Yue, M. Combination strategy for high-performance Sm(CoFeCuZr)z sintered permanent magnet: Synergistic improvement of the preparation process. Acta Mater. 2023, 251, 11890. [Google Scholar] [CrossRef]
- Shang, Z.F.; Yue, M.; Li, Y.Q.; Zhang, D.T.; Xie, Z.H.; Wang, Y.Q. The effect of multi-scale Cu distribution regulation on magnetic properties of Sm(CoFeCuZr)z magnets. J. Magn. Magn. Mater. 2020, 502, 166484. [Google Scholar] [CrossRef]
- Li, Z.Z.; Meng, Q.; Zhang, L.L.; Oana, R.L. How do rare earth prices respond to economic and geopolitical factors? Resour. Policy 2023, 85, 103853. [Google Scholar] [CrossRef]
- Gjoka, M.; Sarafidis, C.; Giaremis, S. Towards Production of Cost-Effective Modification of SmCo5-Type Alloys Suitable for Permanent Magnets. Materials 2024, 17, 808. [Google Scholar] [CrossRef]
- Yuan, T.; Song, X.; Zhou, X.L.; Jia, W.T.; Musa, M.; Wang, J.D.; Ma, T.Y. Role of primary Zr-rich particles on microstructure and magnetic properties of 2:17-type Sm-Co-Fe-Cu-Zr permanent magnets. J. Mater. Sci. Technol. 2020, 53, 73–81. [Google Scholar] [CrossRef]
- Huang, J.Y.; Zuo, S.L.; Zhang, Q.; Dai, H.Y. 2:17-type SmCo permanent magnets with controllable remanence temperature coefficients via substitution of heavy rare earth elements. Rare Met. 2024, 43, 3990–3996. [Google Scholar] [CrossRef]
- Gong, Y.S.; Qiu, Z.G.; Liang, S.Z.; Zheng, X.R. Strategy of preparing SmCo based films with high coercivity and remanence ratio achieved by temperature and chemical optimization. J. Rare Earths 2024, 42, 1289–1297. [Google Scholar] [CrossRef]
- Li, Q.F.; Wang, C.; Chen, H.S. Influence of 1:7 H phase stability on the evolution of cellular microstructure in Fe-rich Sm-Co-Fe-Cu-Zr magnets. J. Alloys Compd. 2023, 959, 170604. [Google Scholar]
- Zhou, X.L.; Liu, Y.; Song, X.; Jia, W.T.; Xiao, A.D.; Yuan, T.; Liu, F.; Wang, F.; Ma, T.Y. Enhanced magnetic properties of Fe-rich Sm-Co-Fe-Cu-Zr magnets by compressive stress-aging. Materialia 2021, 20, 101230. [Google Scholar]
- Li, Q.F.; Wang, C.; Lin, Z.C.; Wang, L.; Xiao, Y.F.; Zhang, P.; Cao, X.Z.; Fang, Y.K.; Liao, Q.L.; Yang, J.B.; et al. Phase separation and chemistry evolution in Fe-rich Sm(CobalFe0.31Cu0.07Zr0.025)7.7 magnets: The effect of initial defect. Acta Mater. 2023, 245, 118664. [Google Scholar]
- Fartushna, I.; Bajenova, I.; Shakirova, J.; Khvan, A.; Cheverikin, V.; Zanaeva, E.; Kondratiev, A. Experimental investigation of the phase equilibria in Co–Fe-Sm system with special attention to the effect of Fe substitution in structure and magnetic properties of intermetallic phases. Intermetallics 2022, 143, 107502. [Google Scholar]
- Musa, M.; Song, X.; Zhou, X.L.; Jia, W.T.; Yuan, T.; Ma, T.Y.; Ren, X.B. Grain boundary effect on the microstructure of solution-treated Fe-rich Sm-Co-Fe-Cu-Zr alloys. J. Alloys Compd. 2021, 853, 156974. [Google Scholar]
- Cao, J.; Zhang, T.L.; Xu, H.; Liu, J.H.; Hu, M.Y.; Xi, L.L.; Wang, H.; Jiang, C.B. Exploring microstructural origin of squareness-coercivity trade-off in iron-rich Sm-Co-Fe-Cu-Zr magnets. Scr. Mater. 2022, 209, 114418. [Google Scholar] [CrossRef]
- Yu, N.J.; Gao, W.Y.; Pan, M.X.; Yang, H.F.; Wu, Q.; Zhang, P.Y.; Ge, H.L. Influence mechanism of Fe content on the magnetic properties of Sm2Co17-type sintered magnets: Microstructure and Microchemistry. J. Alloys Compd. 2020, 818, 152908. [Google Scholar]
- Schneider, G.; Henig, E.-T.H.; Lukas, H.L.; Petzow, G. Phase relations in the samarium-poor Sm-Co-Fe system. J. Less-Common. Met. 1985, 110, 159–170. [Google Scholar]
- Liu, P.P.; Dai, F.L.; Luo, L.; Chen, D.K.; Yao, Q.R.; Wang, J.; Rao, G.H.; Zhou, H.Y. Experimental study and thermodynamic calculation of the Sm-Co-Fe system. Calphad 2022, 78, 102447. [Google Scholar]
- Liu, X.B.; Altounian, Z. Magnetic moments and exchange interaction in Sm(Co, Fe)5 from first-principles. Comp. Mater. Sci. 2011, 50, 841–846. [Google Scholar]
- Li, C.; Yuan, Y.; Li, F.; Wei, Q.; Huang, Y. Modification and verification of Miedema model for predicating thermodynamic properties of binary precipitates in multi-element alloys. Phys. B Condens. Matter 2022, 627, 413540. [Google Scholar]
- Zhang, L.L.; Zheng, Q.J.; Jiang, H.X.; He, J.; Zhao, J.Z. Effect of La addition on microstructure evolution of hypoeutectic Al–6Si alloys. J. Mater. Sci. 2020, 55, 7546–7554. [Google Scholar]
- Dong, G.Y.; You, X.G.; Xu, Z.H.; Wang, Y.N.; Tan, Y. A new model for studing the evaporation behavior of alloy elements in DD98M alloy during electron beam smelting. Vacuum 2022, 195, 110641. [Google Scholar]
- Kawano, H. Effective Work Functions of the Elements Database, Most probable value, Previously recommended value, Polycrystalline thermionic contrast, Change at critical temperature, Anisotropic dependence sequence, Particle size dependence. Prog. Surf. Sci. 2022, 97, 100583. [Google Scholar]

| Element | /d.u. | φ/V | μ | r/p | Tm/K | |
|---|---|---|---|---|---|---|
| Sm | 1.21 | 3.2 | 7.37 | 0.7 | 0.7 | 1345 |
| Co | 1.75 | 5.1 | 3.55 | 0.04 | 1 | 1768 |
| Fe | 1.77 | 4.97 | 3.69 | 0.04 | 1 | 1811 |
| Nominal Composition (wt.%) | Measured Composition by EPMA(at.%) | Identified Phases by EPMA | |||
|---|---|---|---|---|---|
| Sm | Co | Fe | |||
| a | Sm25.5Co55.5Fe19 | 9.6 | 64.4 | 26 | Sm2(Co,Fe)17 |
| 14.8 | 64.5 | 20.7 | SmCo5 | ||
| 19.3 | 63.2 | 17.5 | Sm2Co7 | ||
| 23.96 | 56.44 | 19.6 | Sm(Co,Fe)3 | ||
| b | Sm25.5Co53.5Fe21 | 9.4 | 60.27 | 30.33 | Sm2(Co,Fe)17 |
| 13.67 | 61.2 | 25.13 | SmCo5 | ||
| 20.6 | 60.1 | 19.3 | Sm2Co7 | ||
| 24.2 | 57.79 | 18.01 | Sm(Co,Fe)3 | ||
| c | Sm25.5Co51.5Fe23 | 9.44 | 61.37 | 29.19 | Sm2(Co,Fe)17 |
| 13.38 | 63.6 | 23.02 | SmCo5 | ||
| 19.57 | 60.56 | 19.87 | Sm2Co7 | ||
| 23.39 | 58.59 | 18.02 | Sm(Co,Fe)3 | ||
| d | Sm25.5Co49.5Fe25 | 9.47 | 56.9 | 33.63 | Sm2(Co,Fe)17 |
| 14.5 | 57.47 | 28.03 | Sm2Co7 | ||
| 24.62 | 55.08 | 20.3 | Sm(Co,Fe)3 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Fang, Y.; Wu, W.; Zhao, B.; Zheng, M.; Lei, M.; Zhang, J. Effect of Fe Content on Phase Behavior of Sm–Co–Fe Alloys During Solidification and Aging. Materials 2025, 18, 1854. https://doi.org/10.3390/ma18081854
Zhu Z, Fang Y, Wu W, Zhao B, Zheng M, Lei M, Zhang J. Effect of Fe Content on Phase Behavior of Sm–Co–Fe Alloys During Solidification and Aging. Materials. 2025; 18(8):1854. https://doi.org/10.3390/ma18081854
Chicago/Turabian StyleZhu, Zhi, Yikun Fang, Wei Wu, Bo Zhao, Meng Zheng, Ming Lei, and Jiashuo Zhang. 2025. "Effect of Fe Content on Phase Behavior of Sm–Co–Fe Alloys During Solidification and Aging" Materials 18, no. 8: 1854. https://doi.org/10.3390/ma18081854
APA StyleZhu, Z., Fang, Y., Wu, W., Zhao, B., Zheng, M., Lei, M., & Zhang, J. (2025). Effect of Fe Content on Phase Behavior of Sm–Co–Fe Alloys During Solidification and Aging. Materials, 18(8), 1854. https://doi.org/10.3390/ma18081854








