Abstract
V0.5/Pt/TiO2 tandem catalysts exhibit both an outstanding low-temperature NH3 conversion rate and high N2 selectivity in NH3-SCO reactions, but the mechanism of high N2 selectivity remains unclear. In this work, Vx/Pt/TiO2 tandem catalysts were synthesized through a two-step impregnation–deposition method. The modulating effect of the V loading mount on NH3-SCO performance was evaluated, and the relevant reaction mechanism was explored systematically. The results demonstrated that the synergistic effect of tandem NH3 oxidation and NH3-SCR reactions could be regulated by changing the V loading amount, thereby modulating N2 selectivity. Compared with other Vx/Pt/TiO2 catalysts and previously reported SCO catalysts, the V0.5/Pt/TiO2 catalyst with a V loading amount of 0.5 wt.% exhibited outstanding NH3-SCO performance, which achieved complete NH3 conversion and >90% of N2 selectivity within a range of 250–450 °C. XPS, NH3-TPD, and O2-TPD results suggested that the increase in the V loading amount from 0.1 wt.% to 0.5 wt.% was conducive to increasing the relative contents of Pt0 and V5+ species, as well as the amount of acid sites, oxygen species, and oxygen vacancies. Consequently, the synergistic effect of tandem NH3 oxidation and NH3-SCR reactions was significantly enhanced, enabling the catalyst to exhibit excellent N2 selectivity. A further increase in the V loading amount from 0.5 wt.% to 0.9 wt.% would bring about the opposite effect to the above, resulting in a decline in catalytic performance. In situ DRIFTS results showed that a V loading amount of 0.5 wt.% was beneficial for -NH2 species to participate in NH3-SCO reactions, thereby boosting N2 selectivity.
1. Introduction
In response to increasingly stringent CO2 emission reduction regulations, the international shipping industry is exploring the application of NH3-fuel engines as the main engines for large merchant ships [1,2,3]. However, the serious environmental risks caused by NH3 emissions from NH3-fuel engines have become one of the main obstacles limiting the application of NH3-fuel engines on board [3,4,5,6,7,8]. It is urgent to explore practical and efficient technologies for removing NH3 from high-temperature flue gas to meet the needs of NH3-powered ships. At present, the selective catalytic oxidation (SCO) technique is considered more promising than other techniques in meeting the above needs [9,10,11]. This is because SCO technology can effectively convert NH3 into N2 through Equation (1) at relatively high temperatures and space velocities.
The NH3 conversion performance of SCO technology mainly depends on the design of the catalyst. During the past several decades, numerous types of NH3-SCO catalysts have been reported, which can be mainly classified into noble metal catalysts and transition metal oxide catalysts. Noble metal catalysts exhibit an outstanding NH3 conversion performance at low temperatures, which is attributed to their strong catalytic oxidation activity for NH3 [11,12,13,14,15]. But noble metal catalysts are prone to excessively oxidizing NH3 into NOx, resulting in a low N2 selectivity [13,14,15]. Transition metal oxide catalysts exhibit high N2 selectivity due to their moderate catalytic oxidation activity for NH3, but this makes it difficult to achieve a good NH3 conversion performance at low temperatures [15,16,17].
It is considered that the mainstream approach to designing SCO catalysts with excellent performance is to simultaneously pursue good NH3 conversion performance and high N2 selectivity over a broad temperature range. But this remains difficult to achieve at present. Aiming to synthesize SCO catalysts with excellent performance, the combination of transition metal oxides with a small amount of noble metal components was proposed as a strategy [18,19,20,21,22,23]. Its operation relies on an internal selective catalytic reduction (i-SCR) mechanism, which is mainly composed of the following two tandem reactions: Under catalysis of noble metal components, NH3 is oxidized to NO by O2 (Equation (2)). Subsequently, NO reacts with NH3 under the action of transition metal oxide components through SCR reaction (Equation (3)).
For the design of catalysts employing the above strategy, the selection of suitable noble metal and transition metal components is deemed to be of great importance. In recent years, Pt-V tandem catalysts have received extensive attention due to their high potential for achieving good NH3 conversion performance and high N2 selectivity simultaneously. Byun et al. introduced 0.3 wt.% Pt into the V3W2/TiO2 catalyst. The results showed that, within the temperature range of 330–380 °C, the Pt0.3V3W2/TiO2 catalyst could achieve complete conversion of NH3 and the corresponding N2 selectivity was higher than 90.0% [20]. The main reason for this was the synergistic effect between NH3-SCO and CO-SCR reactions. Kim et al. reported that the addition of 2 wt.% V into the Pt0.1/TiO2 catalyst could enhance the NH3-SCO performance. The Pt0.1/V2/TiO2 catalyst derived through optimization completely converted NH3 within the range of 250–350 °C, and the corresponding N2 selectivity was ~81% [12].
In our previous work, a Pt-V tandem catalyst was synthesized by directly mixing and grinding a certain mass of the VW/TiO2 catalyst with a small quantity of the Pt/Al2O3 catalyst [9]. Results of the experiments indicated that NH3-SCO and NH3-SCR reactions over the mixed catalysts displayed a synergistic effect, enabling them to exhibit excellent low-temperature redox performance. In situ DRIFTS results showed that NH3 conversion reactions on the surface of mixed catalysts followed both the i-SCR mechanism and hydrazine mechanism. Moreover, authors synthesized a series of Pt-modified V/TiO2 catalysts (0.04 wt.% Pt and 0.5 wt.% V) by using the impregnation–deposition method [22]. The effect of the V and Pt deposition sequence on NH3-SCO performance was explored. The results displayed that V0.5/Pt0.04/TiO2, Pt0.04/V0.5/TiO2, and Pt0.04V0.5/TiO2 catalysts all possessed outstanding NH3 conversion performance. They could achieve complete NH3 conversion at around 250 °C. But the V0.5/Pt0.04/TiO2 catalyst exhibited much better N2 selectivity than the other two catalysts. However, the mechanism by which the V0.5/Pt0.04/TiO2 tandem catalyst achieves high N2 selectivity remains unclear. According to the i-SCR mechanism, N2 selectivity is mainly determined by the V component. Thus, an exploration of the modulating effect of the V loading amount on NH3-SCO performance may be a promising approach to revealing the mechanism of the high N2 selectivity of the V0.5/Pt/TiO2 tandem catalyst. But similar studies have not been reported yet.
Herein, a series of Vx/Pt/TiO2 tandem catalysts were prepared via a two-step impregnation–deposition process. The influence of the V loading amount on the NH3-SCO performance of the Vx/Pt/TiO2 catalysts was systematically investigated. The results showed that, compared with other Vx/Pt/TiO2 catalysts, the V0.5/Pt/TiO2 catalyst exhibited outstanding comprehensive SCO performance. It could completely remove 3000 ppm of NH3 within the range of 250–450 °C and corresponding N2 selectivity was higher than 90%. Compared to previously reported NH3-SCO catalysts (Table S1), the V0.5/Pt/TiO2 catalyst also possessed a remarkable performance. The operating temperature window of the V0.5/Pt/TiO2 catalyst was well-matched with the range of exhaust temperatures in NH3-fuel engines (280–450 °C). In order to explore the mechanism underlying the excellent N2 selectivity of the V0.5/Pt/TiO2 catalyst, some characterization tests including N2 adsorption–desorption, X-ray Diffraction (XRD), X-ray Photoelectron Spectroscopy (XPS), a High-Resolution Transmission Electron Microscope (HRTEM), Energy Dispersive X-Ray Spectroscopy (EDX), O2 Temperature Programmed Desorption (O2-TPD), H2 Temperature-Programmed Reduction (H2-TPR), and NH3 Temperature-Programmed Desorption (NH3-TPD), and in situ Diffuse Reflection Infrared Fourier Transform Spectroscopy (in-situ DRIFTS) were carried out. The structure–efficiency relation and surface reaction pathways were discussed in depth based on characterization results. It is well known that for NH3-SCO catalysts, simultaneously achieving high NH3 conversion efficiency and N2 selectivity still remains a great challenge. This work is novel in uncovering the significant regulatory effect of V loading on N2 selectivity of Vx/Pt/TiO2 catalysts, elucidating the relevant regulatory mechanism, and revealing the mechanism by which the V0.5/Pt/TiO2 catalyst achieves a high NH3 conversion rate and N2 selectivity simultaneously.
2. Experimental Section
2.1. Catalyst Preparation
Based on our previous research [22], the Vx/Pt/TiO2 catalysts with 0.04 wt.% Pt and x wt.% V were prepared using a two-step impregnation–deposition method. Different mass fractions of V (x = 0.1, 0.3, 0.5, 0.7, 0.9 wt.%) were incorporated via impregnation in the second step. The detailed preparation processes of Vx/Pt/TiO2 catalysts were given in the Supplementary Materials. The Pt/TiO2 (0.04 wt.% Pt) and V/TiO2 (0.5 wt.% V) catalysts were synthesized by the impregnation method for better benchmarking. The specific synthesis procedures can be found in the Supplementary Materials.
2.2. Catalyst Characterization
Various techniques including XRD, N2 adsorption–desorption, XPS, HRTEM, EDX, NH3-TPD, O2-TPD, and H2-TPR were employed to characterize the prepared catalysts so as to reveal the physical and chemical properties. Additionally, in situ DRIFTS was used to investigate key intermediates in NH3-SCO reactions. Detailed information on catalyst characterization can be found in the Supplementary Materials.
2.3. Catalytic Performance Test
The NH3-SCO performance of the Vx/Pt/TiO2 catalysts was tested using a fixed-bed reactor (Ming Yang Company, Nanjing, China). A catalyst dosage of 0.2 mL was used in each test. Prior to testing, a N2 stream was used to purge the catalyst at 150 °C for 1 h, removing any residual contaminants. The catalyst was then exposed to a reactant gas flow of 200 mL/min, consisting of 3000 ppm NH3, 5 vol.% O2, and N2 as the balance gas. Then, reaction temperature was gradually increased from 200 to 450 °C at a rate of 2 °C/min. During this process, the concentrations of NH3, NO, NO2, and N2O were monitored by using a FT-IR spectrometer (IGS, ThermoFisher Scientific, Waltham, MA, USA). Based on Equations (4) and (5), the NH3 conversion efficiency and N2 selectivity could be calculated:
3. Results
3.1. NH3-SCO Performance
The NH3 conversion efficiencies of Vx/Pt/TiO2 catalysts are illustrated in Figure 1a. It can be seen that with the reaction temperature increased from 200 to 250 °C, the NH3 conversion efficiencies of the Vx/Pt/TiO2 catalysts increased significantly. When the reaction temperature was 250 °C, the NH3 conversion efficiencies of the V0.1/Pt/TiO2, V0.3/Pt/TiO2, and V0.3/Pt/TiO2 catalysts were 100%, while the NH3 conversion efficiencies of the V0.7/Pt/TiO2 and V0.9/Pt/TiO2 catalysts were 89.2% and 83.5%, respectively. When the reaction temperature was further increased from 250 to 300 °C, the NH3 conversion efficiencies of the V0.7/Pt/TiO2 and V0.9/Pt/TiO2 catalysts reached 100%. The above results indicate that, compared to V0.7/Pt/TiO2 and V0.9/Pt/TiO2 catalysts, the V0.1/Pt/TiO2, V0.3/Pt/TiO2, and V0.5/Pt/TiO2 catalysts exhibit better NH3 catalytic conversion performance.
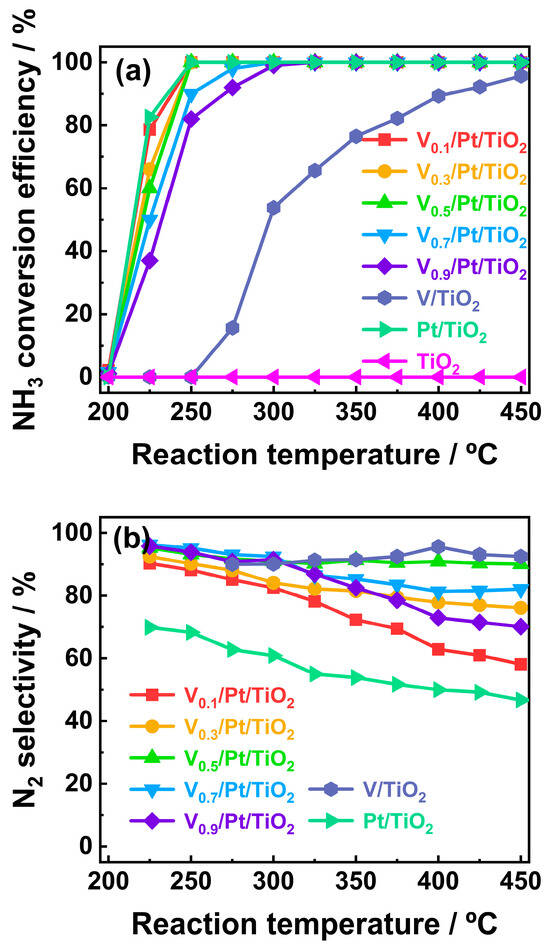
Figure 1.
(a) NH3 conversion efficiencies and (b) N2 selectivities of prepared catalysts. Reaction conditions: [NH3]in = 3000 ppm, [O2]in = 5 vol%, Flowrate = 200 mL/min, N2 balance gas.
The N2 selectivity of the Vx/Pt/TiO2 catalysts within the temperature range of 200–450 °C is shown in Figure 1b. It can be observed that when the loading amount of V was increased from 0.1 wt.% to 0.5 wt.%, the N2 selectivities of the Vx/Pt/TiO2 catalysts were distinctly enhanced. It is noteworthy that, compared with other Vx/Pt/TiO2 catalysts, the V0.5/Pt/TiO2 catalyst exhibited outstanding N2 selectivity. The N2 selectivity of the V0.5/Pt/TiO2 catalyst exceeded 90% across the entire temperature range. The above results imply that a moderate increase in the loading amount of V might be beneficial for enhancing NH3-SCR reactions over Vx/Pt/TiO2 catalysts, thereby promoting the generation of N2 via Equations (3), (6) and (7)
When the V loading amount was further increased from 0.5 wt.% to 0.9 wt.%, the N2 selectivity of the Vx/Pt/TiO2 catalysts gradually decreased. According to previous studies [22,23,24], this might be related to the excessive catalytic oxidation of NH3 by the V species.
The NH3-SCO performance of Pt/TiO2 (0.04 wt.% Pt) and V/TiO2 (0.5 wt.% V) catalysts and the bare TiO2 support were evaluated for benchmarking. The results are shown in Figure 1. It could be seen that the Pt/TiO2 catalyst exhibited excellent NH3 conversion performance. It could achieve complete conversion of 3000 ppm NH3 within the temperature range of 250–450 °C. Its NH3 conversion performance was close to that of the V0.1/Pt/TiO2, V0.3/Pt/TiO2, and V0.5/Pt/TiO2 catalysts, and was significantly better than that of the V0.7/Pt/TiO2 and V0.9/Pt/TiO2 catalysts. But the N2 selectivity of the Pt/TiO2 catalyst within the above temperature range was lower than 70%, which was significantly lower than that of the Vx/Pt/TiO2 catalysts. Compared with the Vx/Pt/TiO2 and Pt/TiO2 catalysts, the NH3 conversion performance of the V/TiO2 catalyst was relatively poor. It only shows the obvious NH3 conversion performance when the reaction temperature was higher than 250 °C. However, the V/TiO2 catalyst exhibited excellent N2 selectivity, which exceeded 90% within the temperature range of 275–450 °C. The bare TiO2 support did not show the catalytic activity within the whole temperature range.
In order to more intuitively display the influence of the V loading amount on the performance of the Vx/Pt/TiO2 catalyst, a summary schematic comparing the catalyst performance versus the V loading amount was drawn, which is shown in Figure 2. The reaction temperature corresponding to the schematic is 350 °C. As shown in Figure 2, with the V loading amount increased from 0.1 wt.% to 0.5 wt.%, the N2 selectivity of the Vx/Pt/TiO2 catalysts significantly improved from 72.3% to 91.3%. When the V loading amount further increased from 0.5 wt.% to 0.9 wt.%, the N2 selectivity of the Vx/Pt/TiO2 catalyst gradually decreased from 91.3% to 82.5%. During the process of increasing the V loading amount from 0.1 wt.% to 0.9 wt.%, the NH3 conversion efficiency of the Vx/Pt/TiO2 catalysts always remains at 100%. The above results indicated that, in this work, the V0.5/Pt/TiO2 catalyst with a V loading amount of 0.5 wt.% exhibited the best comprehensive SCO performance.
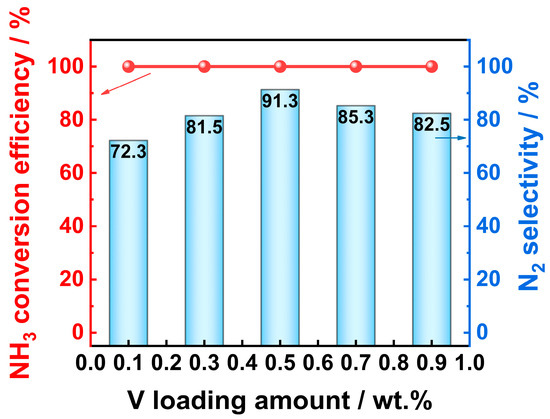
Figure 2.
Change in NH3-SCO performance of Vx/Pt/TiO2 catalysts with V loading amount. Reaction conditions: [NH3]in = 3000 ppm, [O2]in = 5 vol%, Flowrate = 200 mL/min, N2 balance gas.
The NH3-SCO experimental results demonstrated that, compared with other Vx/Pt/TiO2 catalysts, the V0.5/Pt/TiO2 catalyst exhibited excellent N2 selectivity. In comparison with the NH3-SCO catalysts reported previously (Table S1), the performance of the V0.5/Pt/TiO2 catalyst was also quite outstanding. Therefore, the V0.5/Pt/TiO2 catalyst was selected for further study.
In order to evaluate the repeatability of the catalyst synthesis, three batches of the V0.5/Pt/TiO2 catalyst were, respectively, synthesized using the same process, and performance tests were carried out. The results are shown in Figure S1. The data of Test 1 in Figure S1 are the performance test data of the V0.5/Pt/TiO2 catalyst mentioned in the manuscript. The data of Test 2 and Test 3 are the performance test data of the other two batches of the V0.5/Pt/TiO2 catalyst synthesized under the same conditions. It can be seen from Figure S1 that the NH3 conversion performances of the three batches of the V0.5/Pt/TiO2 catalyst in the temperature range of 200–450 °C are quite similar, and so is the N2 selectivity. This indicated that the catalyst synthesis process has high reproducibility.
The common causes of catalyst aging or deactivation after long-term use include sintering of active components, changes in structure of active components, collapse of pore structure, and catalyst poisoning caused by external impurities [24,25,26]. The humid conditions may promote aging or deactivation of catalysts. This is because under certain conditions, H2O molecules may react with active components, thus resulting in a reduction in catalytic activity or even deactivation [25,27]. H2O molecules may also react with supports of the catalyst, resulting in collapse of the pore structure, thus leading to a decline in catalytic activity [28]. Since the combustion of NH3 in NH3-fuel engines would produce H2O vapor, it is advisable to conduct a preliminary test to obtain a brief understanding of the H2O resistance of the V0.5/Pt/TiO2 catalyst. The H2O resistance test was carried out under the following conditions: the concentration of the H2O vapor was 10 vol.%, the reaction temperature was set at 300 °C, and the total experimental duration was 8 h. The results are shown in Figure 3. It can be observed that the introduction of water vapor has no significant impact on the NH3 conversion rate and N2 selectivity of the V0.5/Pt/TiO2 catalyst, indicating that the catalyst possessed good water resistance.
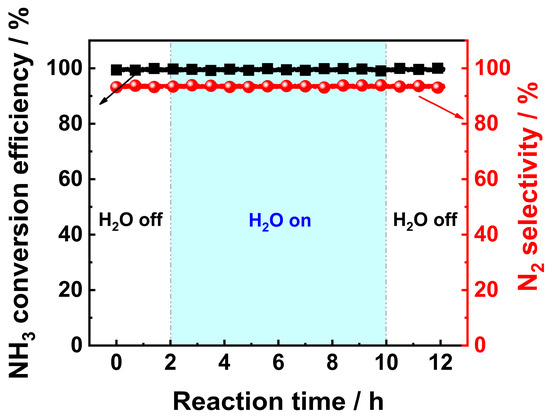
Figure 3.
H2O resistance test results of V0.5/Pt/TiO2 catalyst. Reaction conditions: [NH3]in = 3000 ppm, [O2]in = 5 vol%, [H2O]in = 10 vol.%, Flowrate = 200 mL/min, N2 balance gas.
For the purpose of investigating the mechanism for outstanding N2 selectivity of the V0.5/Pt/TiO2 catalyst, some characterization techniques have been employed to characterize physicochemical properties of Vx/Pt/TiO2 catalysts. The results of the catalyst characterization tests will be presented below, and they will be discussed in detail.
3.2. N2 Adsorption and Desorption
The specific surface area of a catalyst is usually closely related to the adsorption of reactants and the dispersion of active species on the catalyst surface, thereby having a significant impact on the performance of the catalyst [20,21]. Generally, a high specific surface area of the catalyst is conducive to adsorbing more NH3 and O2, which is beneficial for promoting NH3-SCO performance [22,23]. A high specific surface area of the catalyst is also conducive to active species being more evenly dispersed on the catalyst surface, thereby enriching active sites and improving the NH3-SCO performance [24]. To characterize the surface physical properties of the Vx/Pt/TiO2 catalysts, N2 adsorption and desorption experiments were performed, and the results are presented in Figure 4. Figure 4a shows the N2 adsorption–desorption isotherms of the Vx/Pt/TiO2 catalysts. According to previous studies, the isotherms could be categorized as type IV(a) with H3 hysteresis loops, which implied the existence of a large number of mesopores on the surface of the tested catalysts [22,23,24]. It can be seen from Figure 4b that the surface pore sizes of the Vx/Pt/TiO2 catalysts were predominantly around 46 nm, which is in agreement with the results shown in Figure 4a. It can also be observed from Figure 4b that, compared with other Vx/Pt/TiO2 catalysts, the V0.5/Pt/TiO2 catalyst possessed more abundant 45 nm pores on the surface. As shown in Table S2, the specific surface area of the V0.5/Pt/TiO2 catalyst was higher than those of other Vx/Pt/TiO2 catalysts. But there was little difference in surface pore volume and pore diameter between the V0.5/Pt/TiO2 catalyst and each of the other Vx/Pt/TiO2 catalysts. The results shown in Figure 4b and Table S2 indicate that 0.5 wt.% of the V loading amount might be conducive to the formation of mesopores on the surface of the V0.5/Pt/TiO2 catalyst, thus resulting in a relatively higher specific surface area of the V0.5/Pt/TiO2 catalyst. Generally, a higher specific surface area is conducive to the dispersion of active metal components and the adsorption of reactants on the catalyst surface, thus improving the catalytic activity [29]. Thus, the relatively larger specific surface area of the V0.5/Pt/TiO2 catalyst contributed to its excellent NH3-SCO performance.
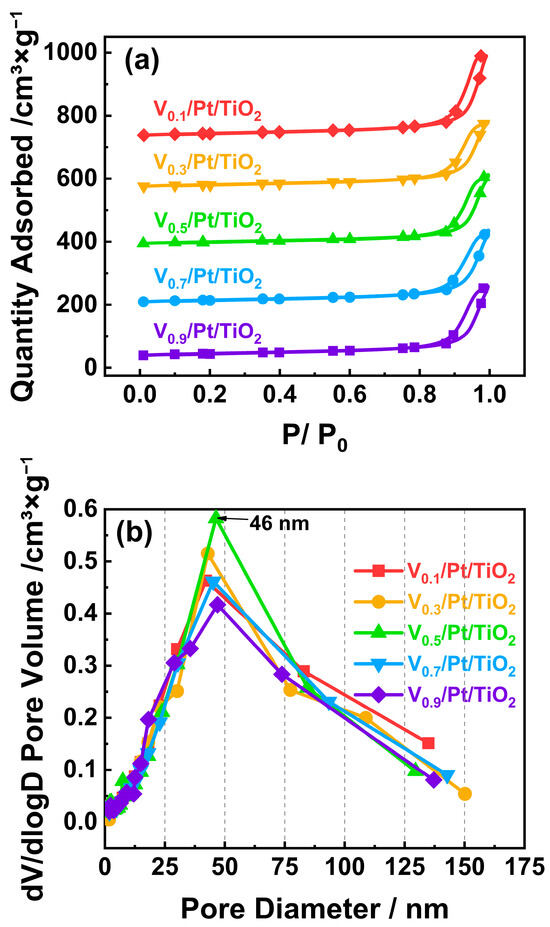
Figure 4.
(a) N2 adsorption–desorption isotherms and (b) pore size distribution curves of Vx/Pt/TiO2 catalysts.
3.3. XRD
The crystal structures of active species and support are closely related to the performance of the catalyst. The XRD technique is considered a common technique for obtaining the above information. XRD tests were conducted on the Vx/Pt/TiO2 catalyst, and the results are presented in Figure 5. Several obvious diffraction peaks can be observed in the XRD spectra of the Vx/Pt/TiO2 catalysts. Diffraction peaks centered at 25.0°, 36.6°, 38.0°, 38.8°, 48.1°, 54.3°, 55.2°, 62.8°, 68.9°, 70.6°, and 75.3° could be attributed to anatase TiO2 phases (ICDD #21-1276) [9,22,23]. The diffraction peak centered at 27.5° could be attributed to rutile TiO2 phases (ICDD #21-1272) [24]. It can be seen from Figure 5 that there were no peaks attributed to the VOx phase in the XRD spectra of the V0.1/Pt/TiO2, V0.3/Pt/TiO2, and V0.5/Pt/TiO2 catalysts. This suggested that the VOx species over the above three catalysts were in a highly dispersed state or an amorphous state. As shown in Figure 5, a small diffraction peak (56.8°) emerged in each of the XRD spectra of the V0.7/Pt/TiO2 and V0.9/Pt/TiO2 catalysts. These peaks were ascribed to the VOx species (ICDD #19-1401), indicating that there were tiny VOx crystals over the V0.7/Pt/TiO2 and V0.9/Pt/TiO2 catalysts [22,23,24]. The above results show that when the V loading amounts were 0.1 wt.%, 0.3 wt.%, and 0.5 wt.%, the VOx species could be well dispersed on the catalyst surface. When the V loading amounts were 0.7 and 0.9 wt.%, the VOx species on the surface of the catalyst agglomerated slightly. It can be seen from Figure 5 that no diffraction peaks that could be attributed to the Pt species appeared in the XRD spectra of the Vx/Pt/TiO2 catalysts. This phenomenon could be primarily ascribed to the extremely low Pt contents in the Vx/Pt/TiO2 catalysts.
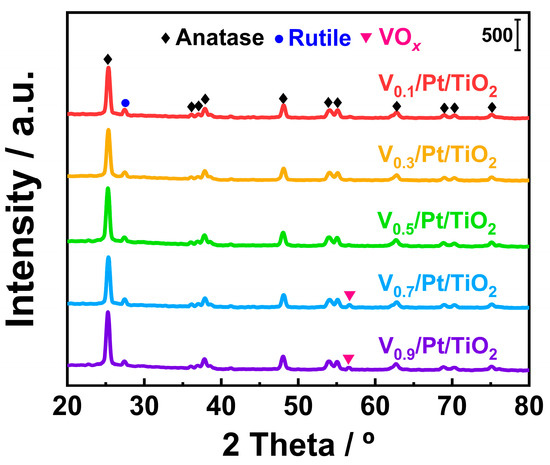
Figure 5.
XRD spectra of Vx/Pt/TiO2 catalysts.
3.4. XPS
The NH3-SCO performance of the catalyst is greatly influenced by the valence states and relative contents of the elements on the surface. XPS experiments were performed to analyze the valence states and relative contents of the main elements over the Vx/Pt/TiO2 catalysts. The test results are presented in Figure 6 and Table S3. As shown in Figure 6a, the V 2p spectrum of each Vx/Pt/TiO2 catalyst could be split into three sub-peaks. Sub-peaks centered at 515.6, 516.5, and 517.2 eV were attributable to the V3+, V4+, and V5+ species, respectively [22,23,24]. As shown in Table S3, with the V loading amount increasing from 0.1 wt.% to 0.5 wt.%, the V5+/(V3++V4++V5+) ratios of the Vx/Pt/TiO2 catalysts increased from 28.20% to 32.65%. When the V loading amount was further increased from 0.5 wt.% to 0.9 wt.%, the V5+/(V3++V4++V5+) ratios of the Vx/Pt/TiO2 catalysts gradually decreased from 32.65% to 25.22%. It has been reported that the V5+ species possesses good NH3-SCR catalytic activity [24]. Thus, the relatively high V5+/(V3++V4++V5+) ratio of the V0.5/Pt/TiO2 catalyst was conducive to promoting the generation of N2, enabling the V0.5/Pt/TiO2 catalyst to exhibit excellent N2 selectivity.
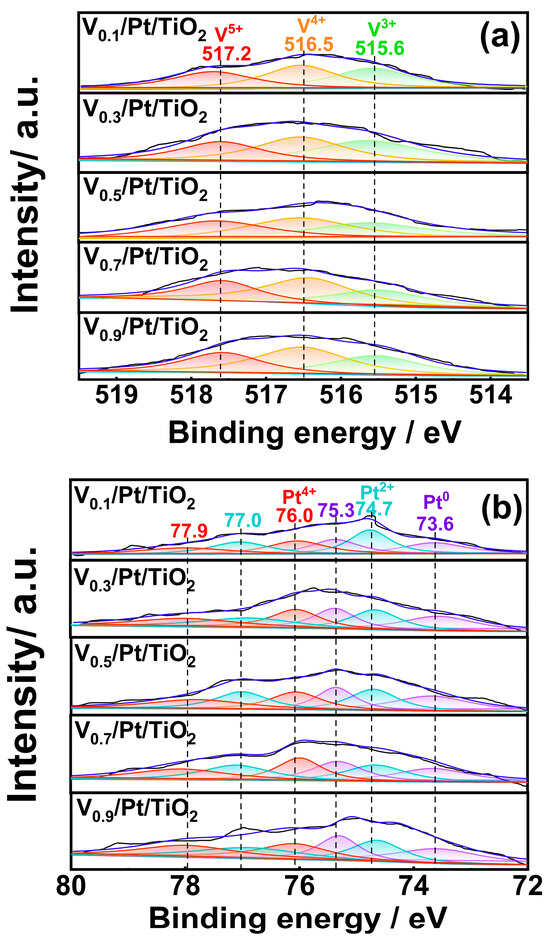
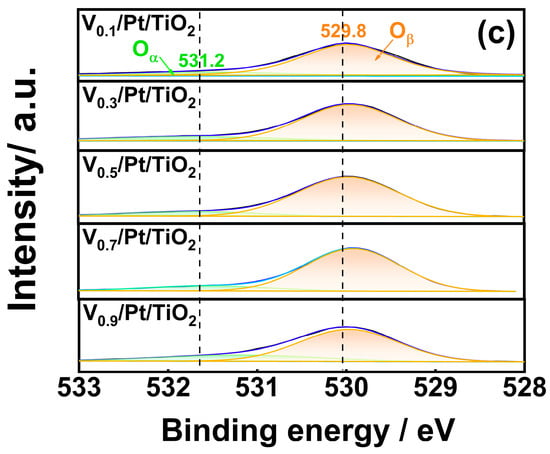
Figure 6.
(a) V 2p, (b) Pt 4f, (c) O 1s XPS spectra of Vx/Pt/TiO2 catalysts.
The Pt 4f spectra of the Vx/Pt/TiO2 catalyst are presented in Figure 6b. It can be seen that each of spectra could be resolved into four sub-peaks. As marked in Figure 6b, sub-peaks in the range of 73.6 to 77.9 eV could be ascribed to Pt0, Pt²⁺, and Pt⁴⁺ species [27,28]. As shown in Table S3, when the V loading amount was increased from 0.1 wt.% to 0.5 wt.%, the Pt0/(Pt0+Pt2++Pt4+) ratio of the Vx/Pt/TiO2 catalyst increased from 35.56% to 37.66%. When the V loading amount was further increased from 0.5 wt.% to 0.9 wt.%, the Pt0/(Pt0+Pt2++Pt4+) ratio of the Vx/Pt/TiO2 catalyst decreased from 37.66% to 35.23%. This suggested that adopting a V loading amount of 0.5 wt.% was conducive to the generation of the Pt0 species. Compared to the Pt2+ and Pt4+ species, the Pt0 species possess much higher catalytic oxidation activity, which is mainly attributed to its high activity in O2 dissociation [22,23]. Therefore, the existence of a relatively larger amount of Pt0 species was beneficial for the V0.5/Pt/TiO2 catalyst to oxidize more NH3 into NOx, providing more reactants for the NH3-SCR reaction, thus promoting the generation of N2.
Figure 6c displays the O 1s spectra of the Vx/Pt/TiO2 catalysts. It can be seen that the spectrum of each Vx/Pt/TiO2 catalyst could be resolved into two sub-peaks. Sub-peaks centered at 531.2 eV and 529.8 eV could be ascribed to chemisorbed oxygen species (Oα) and lattice oxygen species (Oβ), respectively. Table S3 shows that the Oα/(Oα+Oβ) ratio of the V0.5/Pt/TiO2 catalyst is 15.23%, which is higher than those of other Vx/Pt/TiO2 catalysts. Compared with Oβ species, the Oα species possess higher activity in the NH3 oxidation reaction [29,30,31,32]. Thus, a relatively larger amount of Oα species was conducive to oxidation of more NH3 into NOx and N2. Subsequently, NOx could be converted into N2 through NH3-SCR reactions, enabling the V0.5/Pt/TiO2 catalyst to exhibit a good N2 selectivity.
3.5. O2-TPD
The surface oxygen species can participate in NH3 catalytic oxidation reactions, thereby influencing the SCO performance of the catalysts. For the purpose of characterizing the active oxygen species over the Vx/Pt/TiO2 catalysts, O2-TPD experiments were performed, and the results are shown in Figure 7. Previous studies pointed out that O2 desorption below 250 °C mainly resulted from the desorption of physically adsorbed oxygen [9,22,23]. O2 desorption between 250 °C and 600 °C was mainly caused by the desorption of chemically adsorbed oxygen from oxygen vacancies. Desorption of O2 between 600 °C and 900 °C was mainly related to the desorption of surface lattice oxygen.
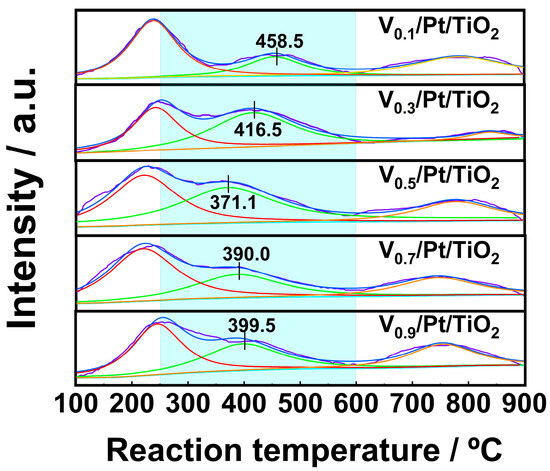
Figure 7.
O2-TPD profiles of Vx/Pt/TiO2 catalysts.
It can be seen from Figure 7 that for the O2-TPD profile of each Vx/Pt/TiO2 catalyst, there is a single peak within the range of 100–250 °C, which implies that a great amount of physically adsorbed oxygen desorbed from the surface of the Vx/Pt/TiO2 catalyst in this temperature range. As shown in Figure 7, each of the O2-TPD profiles of the Vx/Pt/TiO2 catalysts had one peak in the range of 250 to 600 °C. The peak of the V0.5/Pt/TiO2 catalyst within the above temperature range was centered at 371.1 °C, which was significantly lower than the peak temperatures of the other Vx/Pt/TiO2 catalysts. This indicates that, compared with other Vx/Pt/TiO2 catalysts, the oxygen vacancies on the surface of the V0.5/Pt/TiO2 catalyst exhibit higher activity within the range of 250 to 600 °C [33]. This was beneficial for the V0.5/Pt/TiO2 catalyst to adsorb and activate O2, enabling it to exhibit excellent NH3-SCO performance.
3.6. Redox Performance
The NH3-SCO performance of the catalysts is strongly associated with their redox performance. In order to investigate the redox performance of the Vx/Pt/TiO2 catalysts, H2-TPR tests were carried out over a temperature range of 100 to 800 °C, and the results are shown in Figure 8. The H2-TPR profiles of each Vx/Pt/TiO2 catalyst contained three reduction peaks. The reduction peaks at 268.7, 230.0, 188.7, 226.9, and 262.7 °C were attributed to the reduction processes of the Pt2+ and V5+ species. During these processes, Pt2+ was reduced to Pt0 and V5+ was reduced to V4+ [31,32,33,34]. The reduction processes of the V4+ species to the V3+ species and subsequent reduction in the V3+ species accounted for reduction peaks at 505.9, 416.0, 375.0, 479.8, and 430.1 °C [31,32,33,34,35]. As TiO2 was difficult to react with H2 under 600 °C, reduction peaks at 746.9, 728.9, 688.3, 742.1, and 727.8 °C were attributed to the reduction in the TiO2 species [34].
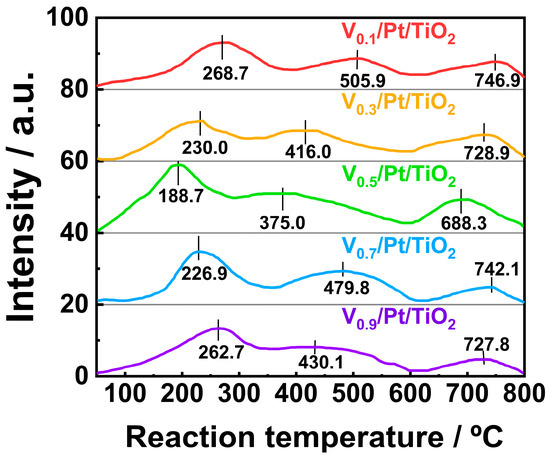
Figure 8.
H2-TPR profiles of Vx/Pt/TiO2 catalysts.
It is worth noting that the temperatures corresponding to three reduction peaks in the H2-TPR profile of the V0.5/Pt/TiO2 catalyst were lower than those of the other Vx/Pt/TiO2 catalysts. This might be ascribed to the strong interaction between the active components and TiO2 support [36]. The above results indicate that throughout the entire temperature, the V0.5/Pt/TiO2 catalyst exhibited better redox performance compared to other Vx/Pt/TiO2 catalysts. In addition, as shown in Table S4, the H2 consumption value (0.75 mmol/g) of the V0.5/Pt/TiO2 catalyst was higher than those of other Vx/Pt/TiO2 catalysts. This indicated that compared with other Vx/Pt/TiO2 catalysts, there were more abundant reducible species on the surface of the V0.5/Pt/TiO2 catalyst. Generally, outstanding redox performance and abundant reducible species are beneficial for the catalyst to exhibit excellent activity in the NH3-SCR reaction. Thus, the V0.5/Pt/TiO2 catalyst could achieve higher N2 selectivity via the i-SCR mechanism.
3.7. NH3-TPD
Surface acidity played a pivotal role in facilitating adsorption and activation of NH3 on the catalyst surface, which was critical for the NH3-SCO reaction. The surface acidity of the Vx/Pt/TiO2 catalysts was tested through NH3-TPD experiments, and the results are displayed in Figure 9. Figure 9a displays the NH3-TPD profiles of the Vx/Pt/TiO2 catalysts. Based on previous studies, the NH3-TPD profiles in the temperature range of 50 to 200 °C were ascribed to NH3 desorption from weak acid sites. NH3-TPD profiles between 200 and 350 °C were attributed to the desorption of NH3 from medium acid sites [36,37,38]. The NH3-TPD profiles in the temperature range of 350 to 500 °C were ascribed to the NH3 desorption from strong acid sites [39,40,41]. The quantities of acid sites on the surface of Vx/Pt/TiO2 catalysts were calculated by integrating the areas of sub-peaks in NH3-TPD profiles, and the results are shown in Figure 9b. It can be seen that with the V loading amount increasing from 0.1 wt.% to 0.5 wt.%, the total acid quantities of Vx/Pt/TiO2 catalysts gradually increased from 4.2 to 5.2 mmol/g. When the V loading amount was further increased from 0.5 wt.% to 0.9 wt.%, the total acid quantities of Vx/Pt/TiO2 catalysts decreased from 5.2 to 3.2 mmol/g. This suggested that compared with other Vx/Pt/TiO2 catalysts, the V0.5/Pt/TiO2 catalyst had more abundant acid sites on its surface. It can be seen from Figure 9b that the quantities of the weak and medium acid sites on the surface of the V0.5/Pt/TiO2 catalyst were higher than those of other Vx/Pt/TiO2 catalysts. This was conducive to the adsorption and activation of NH3 on the surface of the V0.5/Pt/TiO2 catalyst [34,35,36,37,38,39,40,41,42,43,44,45,46], enabling the V0.5/Pt/TiO2 catalyst to exhibit excellent N2 selectivity.
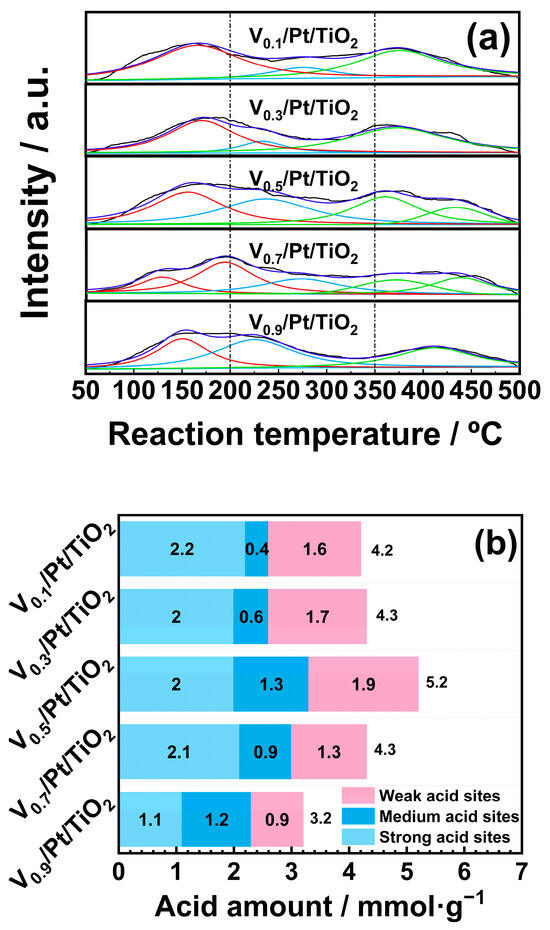
Figure 9.
(a) NH3-TPD profiles and (b) acid sites quantities of Vx/Pt/TiO2 catalysts.
3.8. HRTEM and EDX
HRTEM tests were conducted on Vₓ/Pt/TiO2 catalysts, and EDX tests were carried out on the V0.5/Pt/TiO2 catalyst. The results are shown in Figure 10 and Figure 11. It can be seen from Figure 10 that the Vx/Pt/TiO2 catalysts all exhibited a granular microscopic morphology, with a particle size of approximately 20–50 nm. This was mainly because in this work, P25 TiO2 nanoparticles were used as the catalyst support. It could be observed that distinct lattice fringes existed in the highly magnified images. As marked in Figure 10, these fringes could be attributed to anatase TiO2 species (ICDD 21-1276), rutile TiO2 species (ICPD 21-1272), the Pt species (ICDD 43-1100), and the V2O5 species (ICDD 19-1401) [27,46]. This indicated that there were nanocrystals of the above-mentioned species on the surface of the catalysts.
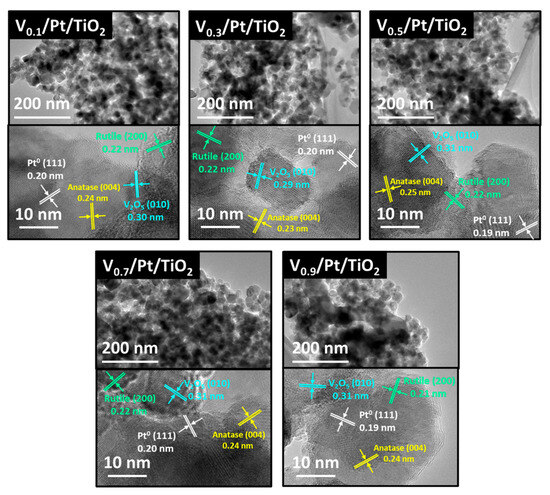
Figure 10.
HRTEM images of Vx/Pt/TiO2 catalysts.
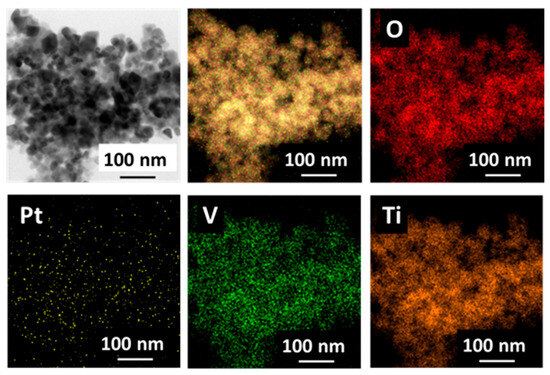
Figure 11.
EDX mappings of V0.5/Pt/TiO2 catalyst.
The EDX test was employed to characterize the dispersion state of O, Pt, V, and Ti elements on the surface of the V0.5/Pt/TiO2 catalyst, and the results are shown in Figure 11. The results suggested that O, Pt, V, and Ti elements were uniformly distributed on the surface of the V0.5/Pt/TiO2 catalyst. Pt and V elements were in intimate contact with one another, which contributed to facilitating the interaction between the Pt and V species.
3.9. In Situ DRIFTS
The surface reaction pathways of NH3-SCO catalysts are closely related to their N2 selectivity. With the aim of exploring reaction pathways over Vx/Pt/TiO2 catalysts, in situ DRIFTS experiments were carried out for the reaction between NH3 and O2 at 350 °C, and the results are shown in Figure 12. The results of in situ DRIFTS experiments have been discussed as follows. Detailed information about in situ DRIFTS experiments is presented in the Supplementary Materials.
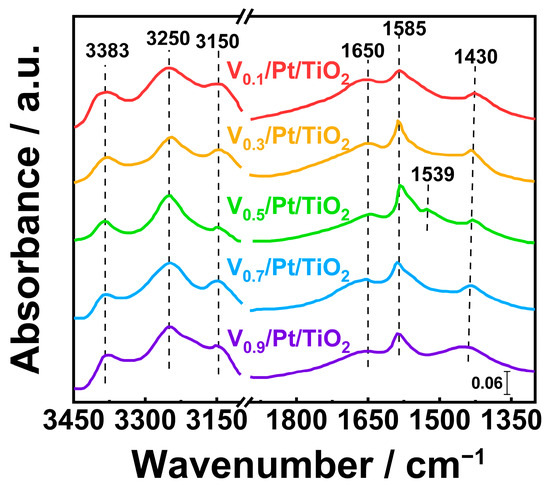
Figure 12.
In situ DRIFT spectra of NH3 and O2 co-adsorbed over Vx/Pt/TiO2 catalysts at 350 °C for 30 min.
As shown in Figure 12, the NH3 species (3383, 3150 cm−1), NH3 species coordinated at Lewis acid sites (3250 cm−1), NO2 species (1650 cm−1), bidentate nitrate species (1585 cm−1), and NH4+ species coordinated at Brønsted acid sites (1436 cm−1) on the surface of Vx/Pt/TiO2 catalysts were capable of taking part in the NH3-SCO reaction [47,48,49,50,51,52,53]. It can be seen from Figure 12 that when both NH3 and O2 were introduced into reaction cell, a distinct signal of -NH2 species (1539 cm−1) appeared in the in situ DRIFTS spectrum of the V0.5/Pt/TiO2 catalyst [35]. But during the same reaction process, the signal of the -NH2 species did not appear in the in situ DRIFTS spectra of other Vx/Pt/TiO2 catalysts. This implied that the -NH2 species could be involved in the NH3-SCO reaction over the V0.5/Pt/TiO2 catalyst. Since the -NH2 species was a key intermediate in the i-SCR mechanism [53,54], the appearance of the -NH2 species over the V0.5/Pt/TiO2 catalyst might be associated with the promotion of the i-SCR reaction. This facilitated the formation of N2, consequently enhancing the N2 selectivity.
4. Discussion
HRTEM, EDX, XRD, XPS, and H2-TPR results indicated that there were Pt0 and V2O5 nanocrystals on the surface of the V0.5/Pt/TiO2 catalyst. The Pt0 species had a strong ability for catalytic oxidation of NH3. V2O5 species possessed remarkable catalytic performance for NH3-SCR reactions. Thus, both NH3-SCO and NH3-SCR reactions existed on the surface of the Vₓ/Pt/TiO2 catalyst, as shown in Figure 13. This enabled NH3 to be converted into N2 through the i-SCR mechanism. XPS and H2-TPR results implied that the valence states of the V and Pt species could be regulated by varying the V loading amount, thereby regulating the synergistic effect between NH3-SCO and NH3-SCR reactions. When the V loading amount was 0.5 wt.%, there were abundant Pt⁰ and V⁵⁺ species on the surface of the V0.5/Pt/TiO2 catalyst. This was beneficial for making the synergistic effect between NH3-SCO and NH3-SCR reactions more efficient, thus enabling the V0.5/Pt/TiO2 catalyst to exhibit excellent N2 selectivity. N2 adsorption–desorption, NH3-TPD, O2-TPD, and in situ DRIFTS results suggested that the V0.5/Pt/TiO2 catalyst possessed a higher specific surface area, abundant surface acid sites, and oxygen vacancies, as well as better reaction pathways. This also contributed to enabling the V0.5/Pt/TiO2 catalyst to exhibit excellent N2 selectivity.
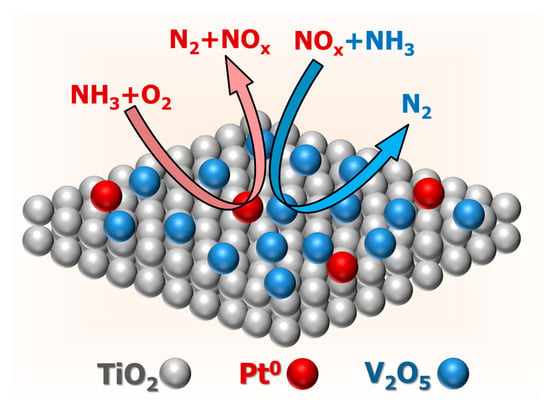
Figure 13.
Schematic diagram of reaction pathways on the surface of Vx/Pt/TiO2 catalysts.
Due to the limitation of the article length, a systematic exploration of hydrothermal aging characteristics of the V0.5/Pt/TiO2 catalyst has not been carried out in this work. In future work, the NH3-SCO performance of the V0.5/Pt/TiO2 catalyst under different hydrothermal aging conditions will be systematically explored. The influence mechanism of the hydrothermal aging process on the catalytic performance will be analyzed, and corresponding strategies for improving the catalytic performance will be proposed.
5. Conclusions
In this work, the Vx/Pt/TiO2 tandem catalysts were synthesized for SCO of NH3 by the two-step impregnation–deposition method. The synergistic effect of NH3-SCO and NH3-SCR reactions over Vx/Pt/TiO2 tandem catalysts could be regulated by changing the V loading amount, thereby modulating the N2 selectivity. When the Pt loading was 0.5 wt.%, there were relatively abundant Pt0 species and V5+ species on the surface of the V0.5/Pt/TiO2 catalyst. These two species, respectively, possessed excellent NH3 catalytic oxidation performance and NH3-SCR performance, thus enabling NH3-SCO and NH3-SCR reactions to achieve highly efficient synergy. In addition, the V0.5/Pt/TiO2 catalyst had a relatively high specific surface area, abundant surface acid sites, good redox properties, and reaction pathways that are more favorable for the generation of N2, which are also the reasons for its excellent N2 selectivity.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ma18081782/s1, Figure S1: (a) NH3 conversion efficiencies and (b) N2 selectivities of V0.5/Pt/TiO2 catalyst in the repeatability test.; Table S1: NH3-SCO performance comparison for V0.5/Pt/TiO2 catalyst and other catalysts in previous work; Table S2: Specific surface areas and average pore sizes of Vx/Pt/TiO2 catalysts; Table S3: XPS results of Vx/Pt/TiO2 catalysts; Table S4: H2 consumption values of Vx/Pt/TiO2 catalysts. References [55,56,57,58,59,60,61,62] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, Y.G.; methodology, Y.G. and W.M.; investigation, Y.G. and W.M.; writing—original draft preparation, Y.G.; writing—review and editing, Y.G. and P.L.; funding acquisition, P.L. and W.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was founded by the “National Key Research and Development Program of China”, grant number 2021YFB2601601 and the “Fundamental Research Funds for China Waterborne Transport Research Institute”, grant number 72404.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lagouvardou, S.; Psaraftis, H.N.; Zis, T. Impacts of a bunker levy on decarbonizing shipping: A tanker case study. Transp. Res. Part D Transp. Environ. 2022, 106, 103257. [Google Scholar] [CrossRef]
- Liu, J.; Cavagnaro, R.J.; Deng, Z.D.; Shao, Y.; Kuo, L.J.; Nguyen, M.T.; Glezakou, V. Renewable ammonia as an energy fuel for ocean exploration and transportation. Mar. Technol. Soc. J. 2020, 54, 126–136. [Google Scholar] [CrossRef]
- Kanberoğlu, B.; Kökkülünk, G. Assessment of CO2 emissions for a bulk carrier fleet. J. Clean. Prod. 2021, 283, 124590. [Google Scholar] [CrossRef]
- Dimitriou, P.; Javaid, R. A review of ammonia as a compression ignition engine fuel. Int. J. Hydrogen Energy 2020, 45, 7098–7118. [Google Scholar] [CrossRef]
- Reiter, A.J.; Kong, S.C. Combustion and emissions characteristics of compression-ignition engine using dual ammonia-diesel fuel. Fuel 2011, 90, 87–97. [Google Scholar] [CrossRef]
- Niki, Y. Reductions in unburned ammonia and nitrous oxide emissions from an ammonia-assisted diesel engine with early timing diesel pilot injection. J. Eng. Gas Turbines Power 2021, 143, 091014. [Google Scholar] [CrossRef]
- Niki, Y.; Nitta, Y.; Sekiguchi, H.; Hirata, K. Diesel fuel multiple injection effects on emission characteristics of diesel engine mixed ammonia gas into intake air. J. Eng. Gas Turbines Power 2019, 141, 061020. [Google Scholar] [CrossRef]
- Cui, J.; Chen, W.; Wang, B.; Fan, Y.; Tian, H.; Long, W.; Liu, X. Effects of relative position of injectors on the performance of ammonia/diesel two-stroke engines. Energy 2024, 309, 133085. [Google Scholar] [CrossRef]
- Gao, Y.; Han, Z.; Cheng, S.; Xu, D.; Pan, X. A mixed catalyst prepared by mechanically milling VW/TiO2 and low content of Pt/Al2O3 for SCO of high-concentration NH3. New J. Chem. 2022, 46, 20108–20119. [Google Scholar] [CrossRef]
- Gao, F.; Liu, Y.; Sani, Z.; Tang, X.; Yi, H.; Zhao, S.; Yu, Q.; Zhou, Y. Advances in selective catalytic oxidation of ammonia (NH3-SCO) to dinitrogen in excess oxygen: A review on typical catalysts, catalytic performances and reaction mechanisms. J. Environ. Chem. Eng. 2021, 9, 104575. [Google Scholar] [CrossRef]
- Lan, T.; Zhao, Y.; Deng, J.; Zhang, J.; Shi, L.; Zhang, D. Selective catalytic oxidation of NH3 over noble metal-based catalysts: State of the art and future prospects. Catal. Sci. Technol. 2020, 10, 5792–5810. [Google Scholar] [CrossRef]
- Kim, G.J.; Kwon, D.W.; Shin, J.H.; Kim, K.W.; Hong, S.C. Influence of the addition of vanadium to Pt/TiO2 catalyst on the selective catalytic oxidation of NH3 to N2. Environ. Technol. 2019, 40, 2588–2600. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, J.; He, G.; Chen, M.; Zhang, C.; He, H. Nanosize effect of Al2O3 in Ag/Al2O3 catalyst for the selective catalytic oxidation of ammonia. ACS Catal. 2018, 8, 2670–2682. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, H.; Wang, S.; Cheng, H.; Qin, Y.; Wang, Z. Role of the support on the behavior of Ag-based catalysts for NH3 selective catalytic oxidation (NH3-SCO). Appl. Surf. Sci. 2014, 316, 373–379. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.H.; Kwon, D.W.; Lee, K.Y.; Ha, H.P. Unveiling the traits of rare earth metal (RM)-substituted bimetallic Ce0.5RM0.5V1O4 phases to activate selective NH3 oxidation and NOx reduction. Appl. Surf. Sci. 2020, 518, 146238. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Wang, Y.; Wang, W.; Zhou, S.; Zhang, S.; Li, W.; Li, S. Revealing the promotional effect of Ce doping on the low-temperature activity and SO2 tolerance of Ce/FeVO4 catalysts in NH3-SCR. J. Environ. Chem. Eng. 2022, 10, 107588. [Google Scholar] [CrossRef]
- Liang, C.; Li, X.; Qu, Z.; Tade, M.; Liu, S. The role of copper species on Cu/γ-Al2O3 catalysts for NH3-SCO reaction. Appl. Surf. Sci. 2012, 258, 3738–3743. [Google Scholar] [CrossRef]
- Wang, F.; Zhu, Y.; Li, Z.; Shan, Y.; Shan, W.; Shi, X.; Yu, Y.B.; Zhang, C.B.; Li, K.; Ning, P.; et al. Promoting effect of acid sites on NH3-SCO activity with water vapor participation for Pt-Fe/ZSM-5 catalyst. Catal. Today 2021, 376, 311–317. [Google Scholar] [CrossRef]
- Chang, S.; Harle, G.; Ma, J.; Yi, J. The effect of textural properties of CeO2-SiO2 mixed oxides on NH3-SCO activity of Pt/CeO2-SiO2 catalyst. Appl. Catal. A-Gen. 2020, 604, 117775. [Google Scholar] [CrossRef]
- Byun, S.W.; Lee, S.J.; Kim, M.; Bae, W.B.; Shin, H.; Hazlett, M.J.; Kang, D.; Tesfaye, B.; Park, P.W.; Kang, S.B. High N2 selectivity of Pt-VW/TiO2 oxidation catalyst for simultaneous control of NH3 and CO emissions. Chem. Eng. J. 2022, 444, 136517. [Google Scholar] [CrossRef]
- Liu, W.; Long, Y.; Zhou, Y.; Liu, S.; Tong, X.; Yin, Y.; Li, X.; Hu, K.; Hu, J. Excellent low temperature NH3-SCR and NH3-SCO performance over Ag-Mn/Ce-Ti catalyst: Evaluation and characterization. Mol. Catal. 2022, 528, 112510. [Google Scholar] [CrossRef]
- Gao, Y.; Han, Z.; Lu, S.; Pan, X. Influence of deposition order of dual active components on the NH3-SCO performance of the bimetallic Pt-V system supported on TiO2. New J. Chem. 2023, 47, 11143–11155. [Google Scholar] [CrossRef]
- Kwon, D.W.; Lee, S.M.; Hong, S.C. Influence of attrition milling on V/Ti catalysts for the selective oxidation of ammonia. Appl. Catal. A-Gen. 2015, 505, 557–565. [Google Scholar] [CrossRef]
- Shen, M.; Li, C.; Wang, J.; Xu, L.; Wang, W.; Wang, J. New insight into the promotion effect of Cu doped V2O5/WO3-TiO2 for low temperature NH3-SCR performance. RSC Adv. 2015, 5, 35155–35165. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, L.; Lin, C.; Shan, W.; He, H. Hydrothermal aging treatment activates V2O5/TiO2 catalysts for NOx abatement. Environ. Sci. Technol. 2022, 56, 9744–9750. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, G.; Wang, C.; Gao, F.; Wang, J.; Shen, M. Mechanistic insights into the deactivation of Pd/BEA methane combustion catalysts by hydrothermal aging. Mol. Catal. 2024, 558, 114027. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, K.; Yang, X.; Chen, X.; Shen, X.; Li, Y.; Fang, Y.; Liu, Y.; Zhao, J.; Yang, X.; et al. In-situ formed stable Pt nanoclusters on ceria-zirconia solid solutions induced by hydrothermal aging for efficient low-temperature CO oxidation. Chem. Eng. J. 2024, 498, 155427. [Google Scholar] [CrossRef]
- Liu, S.R.; Luo, S.T.; Wu, X.D.; Wang, T.J.; Ran, R.; Weng, D.; Si, Z.; Liu, S. Application of silica-alumina as hydrothermally stable supports for Pt catalysts for acid-assisted soot oxidation. Rare Met. 2023, 42, 1614–1623. [Google Scholar] [CrossRef]
- Song, D.; Shao, X.; Yuan, M.; Wang, L.; Zhan, W.; Guo, Y.; Lu, G. Selective catalytic oxidation of ammonia over MnOx-TiO2 mixed oxides. RSC Adv. 2016, 6, 88117–88125. [Google Scholar] [CrossRef]
- Guo, J.; Yang, W.; Zhang, Y.; Gan, L.; Fan, C.; Chen, J.; Peng, Y.; Li, J. A multiple-active-site Cu/SSZ-13 for NH3-SCO: Influence of Si/Al ratio on the catalytic performance. Catal. Commun. 2020, 135, 105751. [Google Scholar] [CrossRef]
- Sun, M.; Liu, J.; Song, C.; Ogata, Y.; Rao, H.; Zhao, X.; Xu, H.D.; Chen, Y. Different reaction mechanisms of ammonia oxidation reaction on Pt/Al2O3 and Pt/CeZrO2 with various Pt states. ACS Appl. Mater. Interfaces 2019, 11, 23102–23111. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.M.; Liu, Y.R.; Zhao, P.P.; Cen, B.H.; Tang, C.; Jia, A.P.; Lu, J.; Luo, M.F. Total oxidation of propane over Pt-V/SiO2 catalysts: Remarkable enhancement of activity by vanadium promotion. Appl. Catal. A-Gen. 2020, 590, 117337. [Google Scholar] [CrossRef]
- Tian, Y.; Han, Z.; Zhou, Z.; Zhao, H.; Zeng, Q.; Li, Y.; Ma, D. Synthesis of Cu/CeTiOx tandem catalyst with dual-function sites for selective catalytic oxidation of ammonia. Chem. Eng. J. 2025, 503, 158212. [Google Scholar] [CrossRef]
- Jabłońska, M. TPR study and catalytic performance of noble metals modified Al2O3, TiO2 and ZrO2 for low-temperature NH3-SCO. Catal. Commun. 2015, 70, 66–71. [Google Scholar] [CrossRef]
- Góra-Marek, K.; Brylewska, K.; Tarach, K.A.; Rutkowska, M.; Jabłońska, M.; Choi, M.; Chmielarz, L. IR studies of Fe modified ZSM-5 zeolites of diverse mesopore topologies in the terms of their catalytic performance in NH3-SCR and NH3-SCO processes. Appl. Catal. B-Environ. 2015, 179, 589–598. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, Q.; Wang, D.; Hong, Z.; Qu, Z.; Li, X. Hollow ZSM-5 zeolite encapsulated Ag nanoparticles for SO2-resistant selective catalytic oxidation of ammonia to nitrogen. Sep. Purif. Technol. 2019, 209, 1016–1026. [Google Scholar] [CrossRef]
- Hua, Z.; Xin, Q.; Ren, W.; Zheng, Z.; Zhou, F.; Liu, S.; Yang, Y.; Gao, X. Enhanced performance of Nb2O5 decorated RuO2/Sn0.2Ti0.8O2 for selective catalytic oxidation of ammonia. Process Saf. Environ. Prot. 2022, 160, 948–957. [Google Scholar] [CrossRef]
- Macina, D.; Opioła, A.; Rutkowska, M.; Basąg, S.; Piwowarska, Z.; Michalik, M.; Chmielarz, L. Mesoporous silica materials modified with aggregated transition metal species (Cr, Fe and Cr-Fe) in the role of catalysts for selective catalytic oxidation of ammonia to dinitrogen. Mater. Chem. Phys. 2017, 187, 60–71. [Google Scholar] [CrossRef]
- Lin, M.; An, B.; Takei, T.; Shishido, T.; Ishida, T.; Haruta, M.; Murayama, T. Features of Nb2O5 as a metal oxide support of Pt and Pd catalysts for selective catalytic oxidation of NH3 with high N2 selectivity. J. Catal. 2020, 389, 366–374. [Google Scholar] [CrossRef]
- Im, H.G.; Lee, M.J.; Kim, W.G.; Kim, S.J.; Jeong, B.; Ye, B.; Lee, H.; Kim, H.D. High-Dispersed V2O5-CuOx Nanoparticles on h-BN in NH3-SCR and NH3-SCO Performance. Nanomaterials 2022, 12, 2329. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Q.; Ran, G.; Kong, M.; Ren, S.; Yang, J.; Li, J. V2O5-modified Mn-Ce/AC catalyst with high SO2 tolerance for low-temperature NH3-SCR of NO. Chem. Eng. J. 2019, 370, 810–821. [Google Scholar] [CrossRef]
- Li, X.; Han, Z.; Wang, X.; Yang, S.; Liu, G.; Gao, Y.; Li, C. Acid treatment of ZrO2-supported CeO2 catalysts for NH3-SCR of NO: Influence on surface acidity and reaction mechanism. J. Taiwan Inst. Chem. Eng. 2022, 132, 104144. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Yang, X.; Chen, Y.; Hu, X.; Wu, X. CeMn/TiO2 catalysts prepared by different methods for enhanced low-temperature NH3-SCR catalytic performance. Chem. Eng. Sci. 2021, 238, 116588. [Google Scholar] [CrossRef]
- Yu, Y.; Wei, D.; Tong, Z.; Wang, J.; Chen, J.; He, C. Rationally engineered ReOx-CuSO4/TiO2 catalyst with superior NH3-SCO efficiency and remarkably boosted SO2 tolerance: Synergy of acid sites and surface adsorbed oxygen. Chem. Eng. J. 2022, 442, 136356. [Google Scholar] [CrossRef]
- Zhai, G.; Han, Z.; Wu, X.; Du, H.; Gao, Y.; Yang, S.; Song, L.; Dong, J.; Pan, X. Pr-modified MnOx catalysts for selective reduction of NO with NH3 at low temperature. J. Taiwan Inst. Chem. Eng. 2021, 125, 132–140. [Google Scholar] [CrossRef]
- Gao, Y.; Han, Z.T.; Cheng, S.S.; Lu, S.J.; Pan, X.X. The enhancement effect of Cu modification on N2 selectivity of V0.5/Pt0.04/TiO2 catalyst for selective catalytic oxidation of NH3. J. Taiwan Inst. Chem. Eng. 2023, 149, 104947. [Google Scholar] [CrossRef]
- Song, K.; Zhao, S.; Li, Z.; Li, K.; Xu, Y.; Zhang, Y.; Cheng, Y.; Shi, J.W. Zinc and phosphorus poisoning tolerance of Cu-SSZ-13 and Ce-Cu-SSZ-13 in the catalytic reduction of nitrogen oxides. J. Colloid Interface Sci. 2023, 629, 243–255. [Google Scholar] [CrossRef]
- Han, Z.; Du, H.; Xu, D.; Gao, Y.; Yang, S.; Song, L.; Dong, J.; Pan, X. Fe and Mn mixed oxide catalysts supported on Sn-modified TiO2 for the selective catalytic reduction of NO with NH3 at low temperature. New J. Chem. 2022, 46, 1621–1636. [Google Scholar] [CrossRef]
- Wang, F.; Ma, J.; He, G.; Chen, M.; Wang, S.; Zhang, C.; He, H. Synergistic effect of TiO2-SiO2 in Ag/Si-Ti catalyst for the selective catalytic oxidation of ammonia. Ind. Eng. Chem. Res. 2018, 57, 11903–11910. [Google Scholar] [CrossRef]
- Gao, Y.; Han, Z.; Zhai, G.; Song, L.; Dong, J.; Yang, S.; Pan, X. Mechanistic insight into the promoting effect of partial substitution of Mn by Ce on N2 selectivity of MnTiOx catalyst for NH3-SCR of NO. J. Taiwan Inst. Chem. Eng. 2022, 133, 104269. [Google Scholar] [CrossRef]
- Jabłońska, M.; Wolkenar, B.; Beale, A.M.; Pischinger, S.; Palkovits, R. Comparison of Cu-Mg-Al-Ox and Cu/Al2O3 in selective catalytic oxidation of ammonia (NH3-SCO). Catal. Commun. 2018, 110, 5–9. [Google Scholar] [CrossRef]
- Peng, Y.; Si, W.; Li, X.; Luo, J.; Li, J.; Crittenden, J.; Hao, J. Comparison of MoO3 and WO3 on arsenic poisoning V2O5/TiO2 catalyst: DRIFTS and DFT study. Appl. Catal. B-Environ. 2016, 181, 692–698. [Google Scholar] [CrossRef]
- Yao, H.; Chen, Y.; Wei, Y.; Zhao, Z.; Liu, Z.; Xu, C. A periodic DFT study of ammonia adsorption on the V2O5 (001), V2O5 (010) and V2O5 (100) surfaces: Lewis versus Brönsted acid sites. Surf. Sci. 2012, 606, 1739–1748. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Jiang, X.; Sun, H.; Qu, Z. Study of synergistic effect between CuO and CeO2 over CuO@CeO2 core-shell nanocomposites for NH3-SCO. Catal. Sci. Technol. 2019, 9, 2968–2981. [Google Scholar] [CrossRef]
- Shrestha, S.; Harold, M.P.; Kamasamudram, K.; Yezerets, A. Selective oxidation of ammonia on mixed and dual-layer Fe-ZSM-5+Pt/Al2O3 monolithic catalysts. Catal. Today 2014, 231, 105–115. [Google Scholar] [CrossRef]
- Sun, M.; Wang, S.; Li, Y.; Wang, Q.; Xu, H.; Chen, Y. Promotion of catalytic performance by adding Cu into Pt/ZSM-5 catalyst for selective catalytic oxidation of ammonia. J. Taiwan Inst. Chem. E. 2017, 78, 401–408. [Google Scholar] [CrossRef]
- Sun, M.; Wang, S.; Li, Y.; Xu, H.; Chen, Y. Promotion of catalytic performance by adding W into Pt/ZrO2 catalyst for selective catalytic oxidation of ammonia. Appl. Surf. Sci. 2017, 402, 323–329. [Google Scholar] [CrossRef]
- Wang, F.; He, G.; Zhang, B.; Chen, M.; Chen, X.; Zhang, C.; He, H. Insights into the activation effect of H2 pretreatment on Ag/Al2O3 catalyst for the selective oxidation of ammonia. ACS Catal. 2019, 9, 1437–1445. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Ning, P.; Song, Z.; Liu, X.; Duan, Y. In situ DRIFTS studies on CuO-Fe2O3 catalysts for low temperature selective catalytic oxidation of ammonia to nitrogen. Appl. Surf. Sci. 2017, 419, 733–743. [Google Scholar] [CrossRef]
- Gang, L.; Van Grondelle, J.; Anderson, B.G.; Van Santen, R.A. Selective low temperature NH3 oxidation to N2 on copper-based catalysts. J. Catal. 1999, 186, 100–109. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T. Selective catalytic oxidation of ammonia to nitrogen over Fe2O3-TiO2 prepared with a sol-gel method. J. Catal. 2002, 207, 158–165. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, S.B.; Hong, S.C. Characterization and reactivity of natural manganese ore catalysts in the selective catalytic oxidation of ammonia to nitrogen. Chemosphere 2003, 50, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).