Studies on the Interaction Between the Functional Monomer 4-Methacryloxyethyl Trimellitic Anhydride and Hydroxyapatite and Stability of the Obtained Hybrids
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Hydroxyapatite
2.2. Interaction of the Functional Acidic Monomer 4-META with Hydroxyapatite
2.3. Stability of the Hybrids in Environments with Different pH
2.4. Characterization
2.4.1. Chemical Analysis
2.4.2. Powder X-Ray Diffraction (XRD) Analysis
2.4.3. Low-Temperature Absorption for Specific Surface Area Determination
2.4.4. Solid-State Nuclear Magnetic Resonance (NMR) Analysis
3. Results
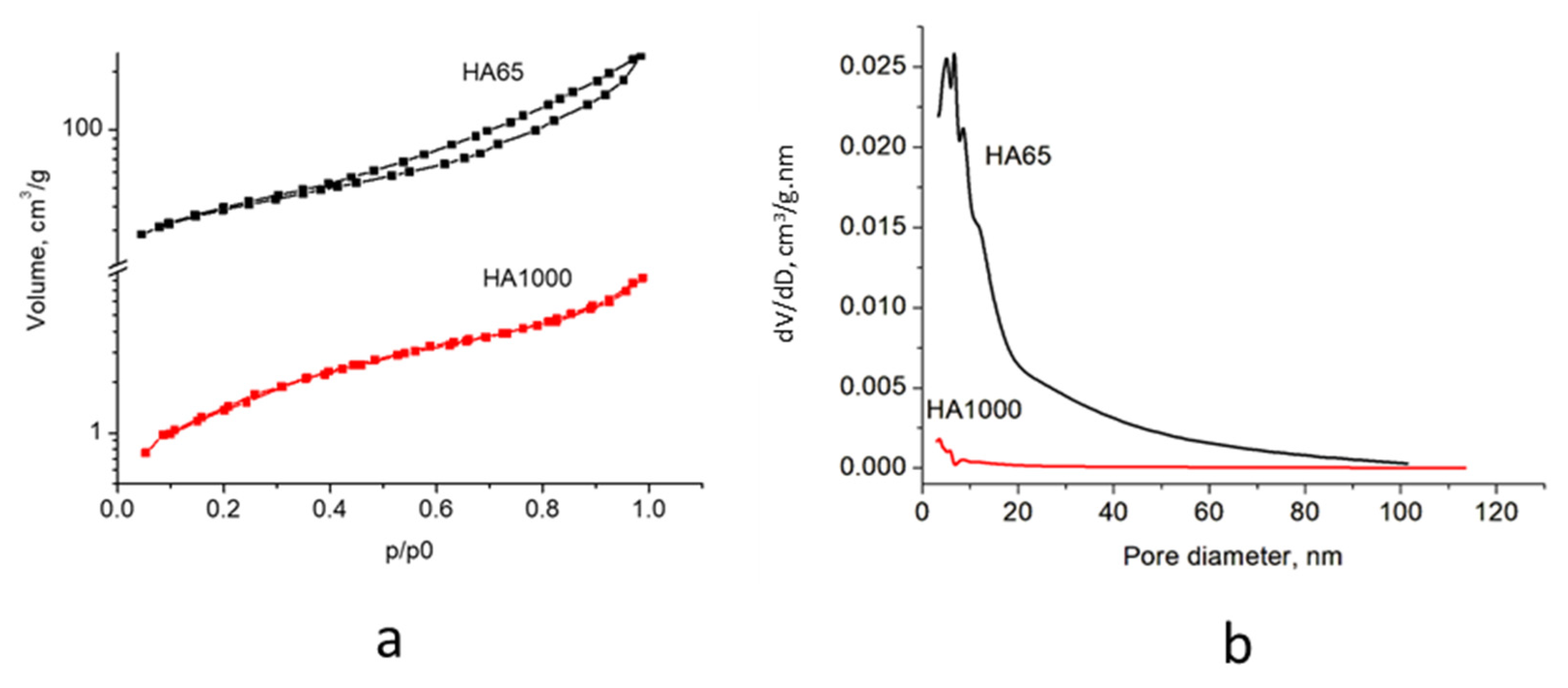
3.1. Synthesis of Hydroxyapatite
| Sample | Crystallite Size (Bragg Peaks (002)) | Literature |
|---|---|---|
| HA65 | 20.2 | This study |
| HA1000 | 141 | This study |
| Enamel | 89.7 | [22] |
| 100 | [23] | |
| Dentin | 30.9 | [22] |
| 35 | [23] |
| Sample | SBET, m2/g | Vt, cm3/g | Dav nm |
|---|---|---|---|
| HA65 | 138 | 0.38 | 11 |
| HA1000 | 6 | 0.01 | 8 |
| Enamel [25] | 8.7 | ||
| Dentin [25] | 150 |
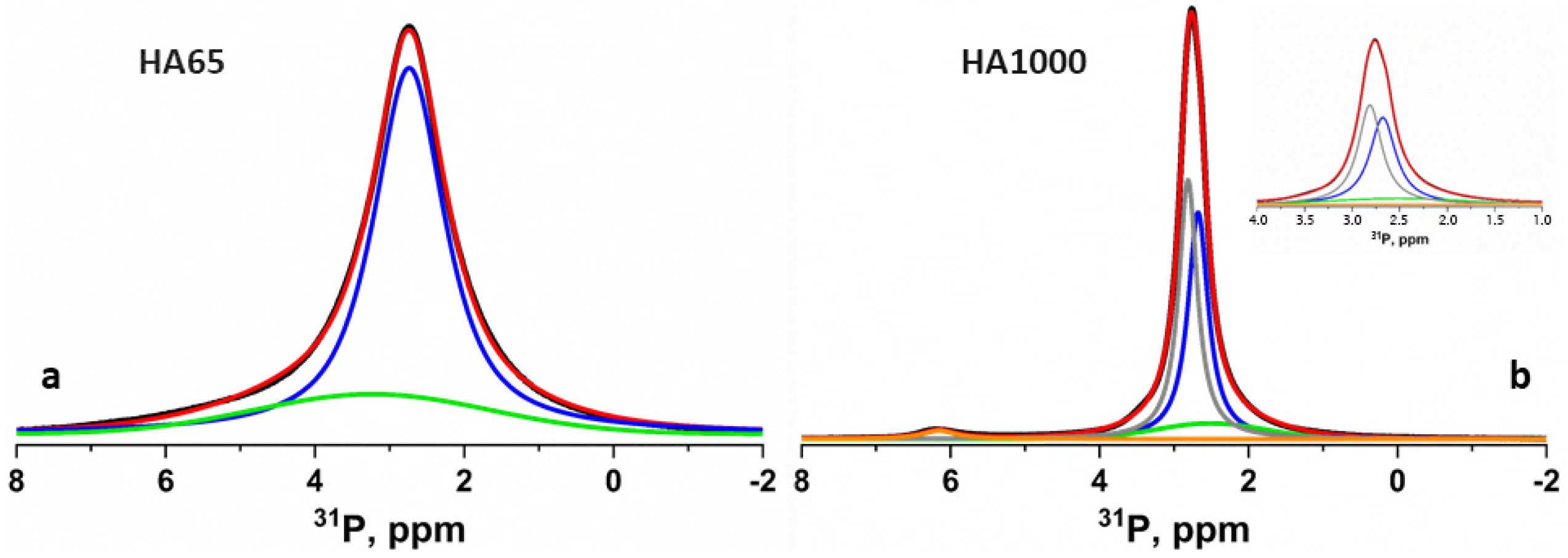
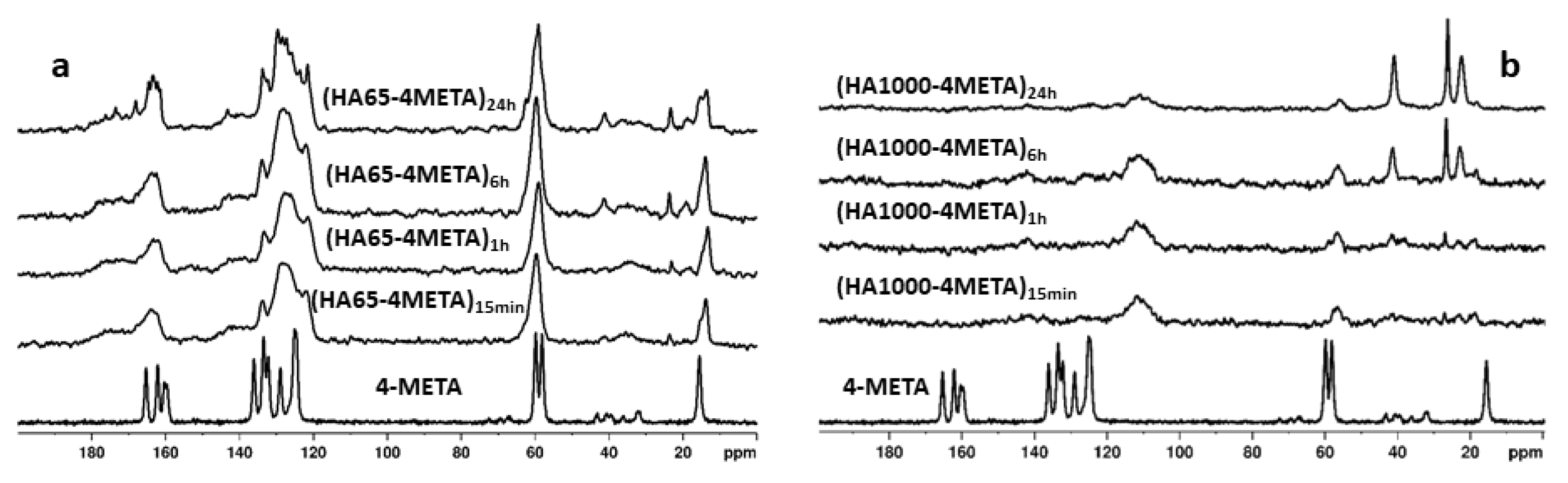
3.2. Interaction of the Functional Acidic Monomer 4-META with Hydroxyapatite
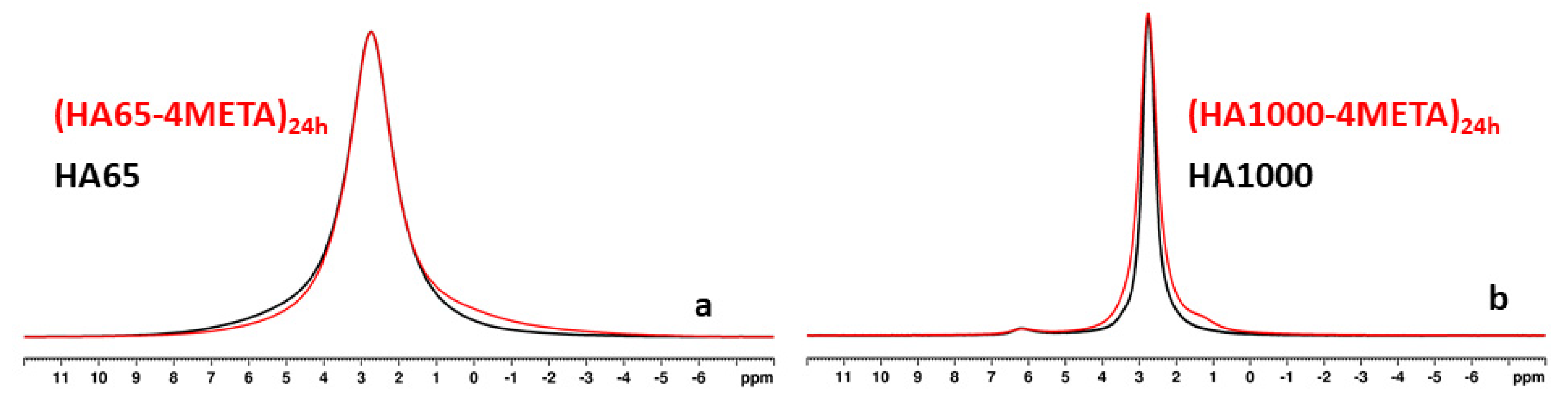
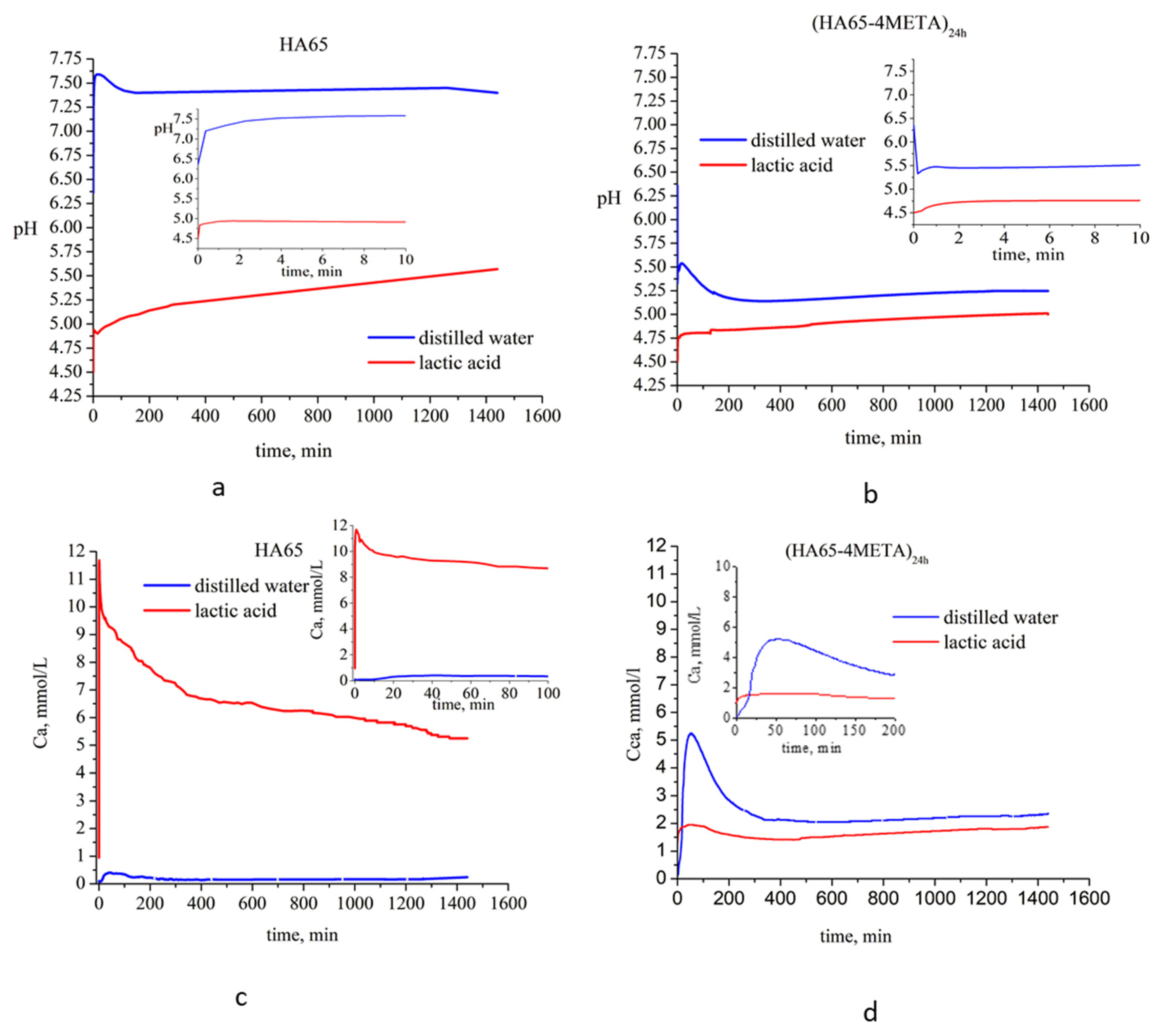
3.3. Stability of the Hybrids in Environments with Different pH
| Solution | Free Ca2+ Ions | Total |
|---|---|---|
| HA65 | ||
| H2O | 0.18 ± 0.01 | 0.29 ± 0.01 |
| 1 mmol/L lactic acid | 5.24 ± 0.02 | 7.25 ± 0.01 |
| (HA65-4-META)24h | ||
| H2O | 2.40 ± 0.01 | 2.45 ± 0.01 |
| 1 mmol/L lactic acid | 1.50 ± 0.01 | 2.50 ± 0.01 |
4. Discussion
4.1. Interaction of the Functional Acidic Monomer 4-META with Hydroxyapatite
4.2. Stability of the Hybrids in Environments with Different pH
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gauthier, R.; Aboulleil, H.; Chenal, J.M.; Chevalier, J.; Colon, P.; Grosgogeat, B. Consideration of Dental Tissues and Composite Mechanical Properties in Secondary Caries Development: A Critical Review. J. Adhes. Dent. 2021, 23, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Jokstad, A. Secondary caries and microleakage. Dent. Mater. 2016, 32, 11–25. [Google Scholar] [CrossRef]
- Signori, C.; CaCIA collaborative group; Uehara, J.L.S.; Romero, V.H.D.; Moro, B.L.P.; Braga, M.M.; Mendes, F.M.; Cenci, M.S. Comparison of two clinical approaches based on visual criteria for secondary caries assessments and treatment decisions in permanent posterior teeth. BMC Oral Health 2022, 22, 77. [Google Scholar] [CrossRef] [PubMed]
- Nedeljkovic, I.; De Munck, J.; Vanloy, A.; Declerck, D.; Lambrechts, P.; Peumans, M.; Teughels, W.; Van Meerbeek, B.; Van Landuyt, K.L. Secondary caries: Prevalence, characteristics, and approach. Clin. Oral Investig. 2020, 24, 683–691. [Google Scholar] [CrossRef]
- Perdigão, J. New developments in dental adhesion. Dent. Clin. N. Am. 2007, 51, 333–357. [Google Scholar] [CrossRef]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1. [Google Scholar] [CrossRef]
- Kalchinov, V.; Yantcheva, S.; Vasileva, R.; Sezanova, K.; Rabajieva, D.; Shestakova, P. Chemical aspects of the dental adhesives. A literature review. Medinform 2024, 11, 1925–1935. [Google Scholar] [CrossRef]
- Nakabayashi, N.; Kojima, K.; Masuhara, E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J. Biomed. Mater. Res. 1982, 16, 265–273. [Google Scholar] [CrossRef]
- Yoshida, Y.; Nagakane, K.; Fukuda, R.; Nakayama, Y.; Okazaki, M.; Shintani, H.; Inoue, S.; Tagawa, Y.; Suzuki, K.; De Munck, J.; et al. Comparative study on adhesive performance of functional monomers. J. Dent. Res. 2004, 83, 454–458. [Google Scholar] [CrossRef]
- Demirci, M.; Sancakli, H.S.; Uysal, O. Clinical evaluation of a polyacid-modified resin composite (Dyract) in class V carious lesions: 5-year results. Clin. Oral Investig. 2008, 12, 157–163. [Google Scholar] [CrossRef]
- Hanabusa, M.; Yoshihara, K.; Yoshida, Y.; Okihara, T.; Yamamoto, T.; Momoi, Y.; Van Meerbeek, B. Interference of functional monomers with polymerization efficiency of adhesives. Eur. J. Oral Sci. 2016, 124, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Hiraishi, N.; Tochio, N.; Kigawa, T.; Otsuki, M.; Tagami, J. Monomer-collagen interactions studied by saturation transfer difference NMR. J. Dent. Res. 2013, 92, 284. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.J.; Tang, J.; Saito, T. A novel bio-active adhesive monomer induces odontoblast differentiation: A comparative study. Int. Endod. J. 2020, 53, 1413–1429. [Google Scholar] [CrossRef]
- Fujisawa, S.; Ito, S. 1H-NMR studies of the interaction of dental adhesive monomer, 4-META with calcium. Dent. Mater. J. 1999, 18, 54–62. [Google Scholar] [CrossRef]
- Ohno, H.; Hashimoto, M.; Araki, Y.; Nezu, T.; Endo, K. Chemical interaction of 4-META with enamel in resin-enamel bonds. Dent. Mater. J. 2021, 40, 683–688. [Google Scholar] [CrossRef]
- Nagakane, K.; Yoshida, Y.; Hirata, I.; Fukuda, R.; Nakayama, Y.; Shirai, K.; Ogawa, T.; Suzuki, K.; Van Meerbeek, B.; Okazaki, M. Analysis of chemical interaction of 4-MET with hydroxyapatite using XPS. Dent. Mater. J. 2006, 25, 645–649. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Rabadjieva, D.; Gergulova, R.; Ruseva, K.; Bonchev, A.; Shestakova, P.; Simeonov, M.; Vasileva, R.; Tatchev, D.; Titorenkova, R.; Vassileva, E. Polycarboxy/Sulfo Betaine—Calcium Phosphate Hybrid Materials with a Remineralization Potential. Materials 2023, 16, 6640. [Google Scholar] [CrossRef]
- Disha, S.A.; Hossain, S.; Habib, L.; Ahmed, S. Calculation of crystallite sizes of pure and metals doped hydroxyapatite engaging Scherrer method, Halder-Wagner method, Williamson-Hall model, and size-strain plot. Results Mater. 2024, 21, 100496. [Google Scholar] [CrossRef]
- Neimark, A.V.; Ravikovitch, P.I. Capillary Condensation in MMS and Pore Structure Characterization. Micro. Mesopor. Mater. 2001, 44, 697–707. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; Le Celve, S.; Alonson, B.; Durand, J.O.; Bujoli, B.; Gan, Z.H.; Hoatson, G. Modelling one- and two-dimensional solid-state NMR spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Hanlie, H.; Liyun, T.; Tao, J. The crystal characteristics of enamel and dentin by XRD method. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2006, 21, 9–12. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Nanosized and nanocrystalline calcium orthophosphates. Acta Biomater. 2010, 6, 715–734. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar]
- Dibdin, G.H.; Poole, D.F. Surface area and pore size analysis for human enamel and dentine by water vapour sorption. Arch. Oral Biol. 1982, 27, 235–241. [Google Scholar] [CrossRef]
- Jager, C.; Welzel, T.; Meyer-Zaika, W.; Epple, M. A solid-state NMR investigation of the structure of nanocrystalline hydroxyapatite. Magn. Reson. Chem. 2006, 44, 573–580. [Google Scholar] [CrossRef]
- Fujita, K.; Ma, S.; Li, R.; Li, J.; Ikemi, T.; Nishiyama, N. Effect of Solvent Type on the Degradation of 4-MET. Dent. Mater. J. 2007, 26, 792–799. [Google Scholar] [CrossRef]
- Fujita-Nakajima, K.; Aoki-Tabei, N.; Arita, A.; Nishiyama, N. NMR study on the demineralization mechanism of the enamel and dentin surfaces in MDP-based all-in-one adhesive. Dent. Mater. J. 2018, 37, 693–701. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalchinov, V.; Sezanova, K.; Shestakova, P.; Yantcheva, S.; Vasileva, R.; Rabadjieva, D. Studies on the Interaction Between the Functional Monomer 4-Methacryloxyethyl Trimellitic Anhydride and Hydroxyapatite and Stability of the Obtained Hybrids. Materials 2025, 18, 1689. https://doi.org/10.3390/ma18081689
Kalchinov V, Sezanova K, Shestakova P, Yantcheva S, Vasileva R, Rabadjieva D. Studies on the Interaction Between the Functional Monomer 4-Methacryloxyethyl Trimellitic Anhydride and Hydroxyapatite and Stability of the Obtained Hybrids. Materials. 2025; 18(8):1689. https://doi.org/10.3390/ma18081689
Chicago/Turabian StyleKalchinov, Vasil, Kostadinka Sezanova, Pavletta Shestakova, Sevda Yantcheva, Radosveta Vasileva, and Diana Rabadjieva. 2025. "Studies on the Interaction Between the Functional Monomer 4-Methacryloxyethyl Trimellitic Anhydride and Hydroxyapatite and Stability of the Obtained Hybrids" Materials 18, no. 8: 1689. https://doi.org/10.3390/ma18081689
APA StyleKalchinov, V., Sezanova, K., Shestakova, P., Yantcheva, S., Vasileva, R., & Rabadjieva, D. (2025). Studies on the Interaction Between the Functional Monomer 4-Methacryloxyethyl Trimellitic Anhydride and Hydroxyapatite and Stability of the Obtained Hybrids. Materials, 18(8), 1689. https://doi.org/10.3390/ma18081689






