Abstract
The present paper deals with the synthesis, characterization, and properties of sol-gel-derived TiO2/TeO2/Nb2O5 nanopowders. The gels were prepared using a combination of organic [Ti (IV) n-butoxide, Nb (V) ethoxide (C10H25NbO5)] and inorganic [telluric acid (H6TeO6)] precursors. The aging of gels was performed in air for several days in order to enable further hydrolysis. The phase formation of the gels was investigated by XRD upon heating in the temperature range of 200–700 °C. It was established that the gels heat-treated up to 300 °C exhibited a predominantly amorphous phase in all binary and ternary compositions. The amount of amorphous phase gradually decreased with increasing temperature, and the first TiO2 (anatase) crystals were detected at about 400–500 °C. The average crystallite size of TiO2 (anatase) in the powdered samples heat-treated at 400 °C was about 10 nm. By DTA, it was established that the decomposition of organics is accompanied by strong weight loss occurring in the temperature range of 200–300 °C. The completeness of the hydrolysis-condensation reactions was verified by IR and UV–Vis analyses. The UV–Vis spectra of the as-prepared gels exhibited red shifting of the cut-off. Photoluminescence spectra exhibited a change in intensity with varying temperature and composition. The performed photocatalytic tests showed that all powders possess photocatalytic activity toward Malachite green organic dye. The obtained nanopowders exhibited good antibacterial properties against E. coli ATCC 25922. The obtained samples can be considered as prospective materials for use as environmental catalysts.
1. Introduction
The sol-gel synthesis of Nb2O5-containing TiO2 powders has attracted scientific interest in the last decade due to their remarkable physicochemical properties, such as electrochromic behavior, photoelectric and photocatalytic activity, excellent chemical stability, and corrosion resistance in both acidic and alkaline media [1,2]. Various thin TiO2/Nb2O5 films have been developed that possess various innovative applications in photonics for improving the optical performance of different devices (optical filters), waveguide-based optical circuits, and transparent conductive electrodes [3,4,5]. Numerous papers are mainly devoted to the preparation of high-quality Nb2O5 thin films using relatively simple and inexpensive techniques, such as sol-gel and spin or dip coating [3,5]. Obviously, the sol-gel method still attracts scientific attention because of its versatility, low cost, and low-temperature processing [6,7]. Additionally, it allows control of the microstructure of the coating and produces durable and chemically stable films. Moreover, the versatility of the sol-gel process in preparing porous film has an additional advantage to be exploited.
Recently, many studies have been conducted on the use of Nb2O5 nanoparticles for environmental remediation in water through photocatalytic processes. Regarding this, Nb2O5 showed great potential because of its stability in an aqueous medium, its surface acidity, and its redox and photocatalytic properties. Previous studies on Nb2O5-doped TiO2 powders also reported on dye degradation as well as the degradation of aromatic compounds [8,9]. Sedneva et al. [10] studied the effects of Nb amount and calcination temperature on photoactivity, phase composition, and crystallite size. Despite these papers on the Ti-Nb combination reported in the literature [11,12], investigations on the TiO2/N2O5 system have not been exhausted yet. There is still a lack of studies about the impact of the synthesis method on the properties of the obtained materials. It is also not clear what the effect of pH is on the photocatalytic activity of the oxides, as it is known that this factor directly affects the surface charges of the catalyst and the potential of the valence and conduction bands [13,14].
There are scarce data concerning the antibacterial activity of Nb2O5-containing sol-gel-derived powders. It was found that TiO2 + Nb2O5 films exhibited antibacterial potential against S. mutans [15]. It is also known that Nb2O5 is very important for the bioactivity of glasses. The antibacterial studies of CaF2–CaO–B2O3–P2O5–SrO glasses doped with different Nb2O5 concentrations found that they are effective toward Gram-negative Escherichia coli (E. coli) and Gram-positive Staphylococcus aureus (S. aureus) bacteria [16].
Our group has experience in the sol-gel synthesis of composite powders containing TiO2, and we have so far produced many binary, ternary, and multicomponent composites with strong photocatalytic and antibacterial qualities [17,18,19,20,21]. This work is an extension of our earlier studies on the sol-gel synthesis of powdered TiO2 nanocomposite. Since the emphasis is now on compositions with enhanced photocatalytic and antibacterial capabilities, the search for novel combinations of compositions that have not yet been studied has been expanded. To the best of our knowledge, there are no reported data on TiO2/TeO2/Nb2O5 sol-gel-derived powders and no studies of their antibacterial and photocatalytic applications, which underlines the novelty in the current work.
In the present paper, we study the synthesis and luminescent and antibacterial properties of TiO2/TeO2/Nb2O5 powders obtained by the sol-gel method. Their photocatalytic activity has also been verified.
2. Materials and Methods
2.1. Gelling and Drying
Based on our previous findings on gel formation in other ternary TiO2–TeO2–MnOm (B2O3, ZnO, SeO2) systems [19,20,21], compositions containing higher TiO2 content were selected—80TiO2/20Nb2O5, 80TiO2/10TeO2/10Nb2O5, and 50TiO2/25TeO2/25Nb2O5—denoted as samples A, B, and C, respectively. They were subjected to detailed investigations. The gels were prepared using a combination of Te(VI) acid (99.99%, Aldrich, St. Louis, MO, USA), along with Ti butoxide(IV) (≥99%, Fluka AG, Buchs, Switzerland) and niobium(V) ethoxide (C10H25NbO5) (Merck, Darmstadt, Germany) as precursors dissolved in ethylene glycol (C2H6O2) (99%, Aldrich) (Figure 1). Telluric acid (H6TeO6) was chosen to overcome the problem with the high hydrolysis rate of tellurium(VI) alkoxide, which has been analyzed in numerous papers [20,21]. The precursor solutions were subjected to 5–10 min of intensive stirring at room temperature to achieve complete dissolution. No direct addition of water was made to the precursor solutions. The sol-gel hydrolysis reaction was acquired from absorbed atmospheric moisture. The measured pH was 4–5, depending on composition. The gelation for the investigated compositions occurred immediately. To complete the hydrolysis, aging of the gels was performed in air for several days. The obtained gels were subjected to stepwise heating from 200 to 600 °C for one hour of exposure time in air. Aiming to verify the phase and structural transformations of the gels, heating in the range of 200–600 °C until powders were obtained was performed. The selection of the temperatures was made based on our previous investigations [20].
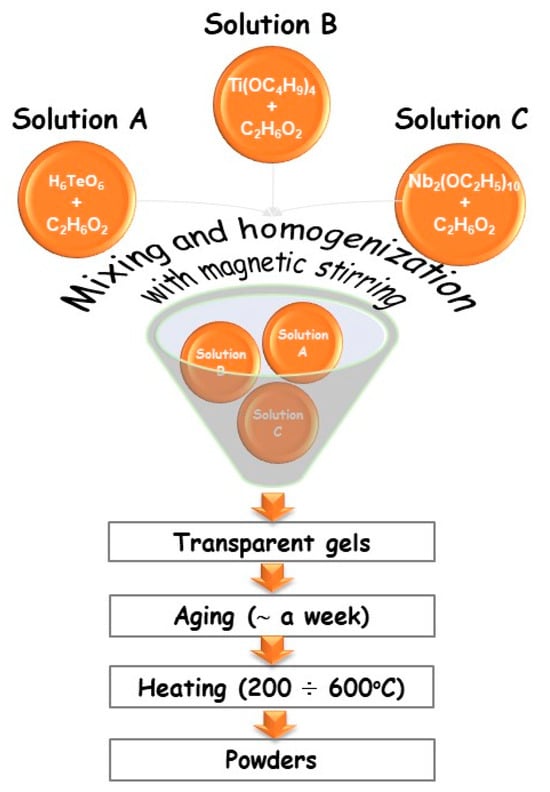
Figure 1.
Scheme for the sol-gel synthesis of the samples.
2.2. Sample Characterization
Powder XRD patterns were registered at room temperature with a Bruker D8 Advance (Berlin, Germany) X-ray powder diffractometer with a Cu Ka radiation (k = 1.54056 Å) with a LynxEye solid position sensitive detector and X-ray tube operated at 40 kV and 40 mA. X-ray diffraction patterns were recorded in the range of 5.3–80° for 2 h with a step of 0.02° 2 h. Differential thermal analysis (LABSYSTM EVO apparatus, Setaram, Lyon, France) with a Pt-Pt/Rh thermocouple at a heating rate of 10 K/min in air flow and with Al2O3 as a reference material was used to study the decomposition process of the gels. The accuracy of the temperature was ±5 °C, and the heating of the samples was limited up to 700 °C. Gases evolved (EGA) during the thermal treatments were analyzed by mass spectrometry (MS) with a Pfeiffer OmniStarTM mass spectrometer (Pfeiffer Vacuum Technology AG, Wetzlar, Germany). Mass spectra recorded for the investigated samples show the m/z = 14, 18, and 44 signals, being ascribed to CH2, H2O, and CO2, respectively. The infrared spectra were registered in the range of 1600–400 cm−1 using the KBr pellet technique on a Nicolet-320 FTIR spectrometer (Madison, WI, USA) with 64 scans and a resolution of ±1 cm−1. A UV–Vis diffused reflectance spectrophotometer Evolution 300 (Thermo Electron Corporation, Madison, WI, USA) with a magnesium oxide reflectance standard as the baseline was used for recording the optical absorption spectra of the powdered samples in the wavelength range of 200–800 nm. Planck’s equation was applied for the determination of the absorption edge and optical bandgap (Eg) [22,23,24]. Photoluminescence spectra of the powders were measured at an excitation wavelength of 325 nm in the range of 340–640 nm using a Fluorolog-3 spectrofluorometer (Horiba/Jobin-Yvon, Longjumeau, France). The specific surface areas (BETs) were determined by low-temperature (77.4 K) nitrogen adsorption in a NOVA 1200e surface area and pore analyzer (Quantachrome, Boynton Beach, FL, USA) at relative pressures p/p0 = 0.1–0.3 using the BET equation.
2.2.1. Photocatalysis
The photocatalytic performance of the synthesized TiO2-containing samples was evaluated by using Malachite green (MG) as a representative dye pollutant. All photodegradation experiments were carried out with a 5 ppm aqueous solution of MG at room temperature. In each run, 100 mg of TiO2-based sample was suspended in 150 mL of a solution of MG and sonicated for 10 min. Prior to irradiation, the suspensions were magnetically swirled in the dark for 30 min to ensure the establishment of an adsorption–desorption equilibrium at the sample surface. Then, the reaction mixtures were illuminated with a UV lamp (Sylvania BLB 50 Hz 8 W T5, emitting primarily at 365 nm) fixed at a distance of 10 cm above the solution’s surface. During illumination, the dispersions were continuously stirred in order to ensure satisfactory mixing of the reacting particles in the mixtures. Before and at regular intervals during the irradiation, aliquots of 3 mL of the suspensions were withdrawn and centrifuged (5000 rpm) for 10 min. The residual concentration of MG dye in the supernatants was determined by measuring the absorbance using a UV–Vis spectrophotometer (Genesys 10 S) at λmax = 618 nm. The percentage of residual MG dye is expressed as C/C0 × 100%, where C0 is the concentration of MG at zero time, and C is its concentration at time t of illumination.
2.2.2. Test of Antibacterial Activity
All investigated samples heat-treated at 400 and 600 °C were verified for antibacterial activity against E. coli. The antimicrobial effect was tested on the Gram-negative bacteria Escherichia coli ATCC 25922, supplied by the National Bank of Industrial microorganisms and cell cultures (NBIMCC), Bulgaria. A spot test assay was performed at the 3rd and 24th hours to assess the antimicrobial activity of the nanopowder dispersions. The broth microdilution method was used to determine the minimal bactericidal (MBC) and minimal inhibitory concentration (MIC).
The bacterial suspension was adjusted to a turbidity of 0.5 McFarland (108 colony-forming units per milliliter) using a densitometer. To determine the MIC and MBC, a 96-well plate was used. Each well was dripped with 100 µL of bacterial suspension and 100 µL of nanopowders dispersed in deionized water at different concentrations (35, 25, 17.5, 15, 12.5, 10, 6, 3, and 1.5 mg/mL) after sonication for 15 min in an ultrasonic bath by Sonorex, Bandelin (Germany). For every tested sample, a separate control was prepared that only consisted of bacterial suspension. Then, the 96-well plate was incubated overnight (20–24 h) at a temperature of 35 ± 2 °C.
At the 3rd and 24th hour from the incubation of the well plate, a spot test was prepared: 5 μL samples from each well were plated onto solid media and incubated at the same temperature. Bacterial growth in each spot was evaluated on the following day, indicating the inhibitory or bactericidal effect of the samples used. To quantify viable bacteria, tenfold serial dilutions were prepared based on the growth results from the 3rd-hour spot test and inoculated onto Mueller–Hinton agar plates.
The MIC was defined as the lowest concentration of the sample that inhibited the growth of Escherichia coli, while the MBC was determined as the lowest concentration of the sample that achieved a ≥99.9% reduction in colony-forming units per milliliter (CFU/mL). The concentration of surviving treated bacteria is calculated using the following formula: CFU/mL = (number of colonies × dilution factor)/volume of the inoculum sample.
3. Results and Discussion
3.1. XRD and DTA Analyses
The crystallinity and phase formation of the investigated samples calcined at different temperatures (200–600 °C) were analyzed by XRD. The XRD patterns of the samples are shown in Figure 2. As seen in the figure, the samples containing 80 mol% TiO2 preserved the amorphous state up to 300 °C, while in the third sample (sample C, 50 mol% TiO2), first TiO2 (anatase, JCPDS 78-2486) along with TiTe3O8 (JCPDS 50-0250) crystals are already detected at 200 °C and are present up to 300 °C. The main peculiarity in the XRD pattern of sample C is that the amount of crystalline TiTe3O8 phase increased with increasing temperature. In the other two samples, A and B, the first anatase crystals appeared at 400 °C, and this remained the dominant phase up to 600 °C in both samples. Similar to the ternary sample C, the XRD pattern of sample B exhibited the presence of TiTe3O8 at 600 °C, but that phase appeared only at the highest temperature, while in sample C, the same phase coexisted with the TiO2 (anatase) in the temperature range of 400–600 °C. The average crystallite size of the TiO2 (anatase) particles, calculated from the broadening of the diffraction line using Sherrer’s equation, is in the range of 10–30 nm, as the XRD pattern of sample B at 400 °C exhibited a broader peak than the other samples. The obtained XRD data are very similar to those reported in other papers [25,26].
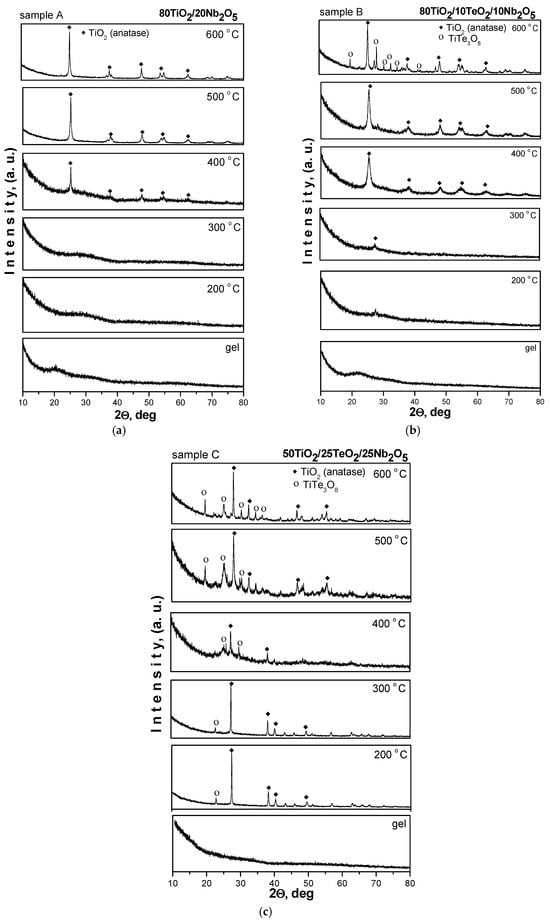
Figure 2.
XRD patterns of investigated samples (a) 80TiO2/20Nb2O5. (b) 80TiO2/10TeO2/10Nb2O5. (c) 50TiO2/25TeO2/25Nb2O5.
3.2. DTA Results
The thermal decomposition data of investigated gels in air are shown in Figure 3. As seen, all gels are characterized by endothermic peaks in the range of 100–200 °C, with weight loss between 8 and 18% depending on composition. The DTA curves of binary gel A differ from those of the ternary ones (B and C). Generally, the evaporation of organic solvent and the local removal of water that has formed a connection with the material’s surface are the primary causes of mass loss. Upon examining the DTA/TG curves, several exothermic peaks may be seen throughout the 220–440 °C range. The first one is observed in the 250–290 °C range for all investigated gels, which is related to the decomposition of Ti (IV) butoxide and niobium (V) ethoxide. It has to be noted that this peak is stronger for binary sample A, and its low intensity in the ternary samples could be attributed to the presence of TeO2, which delays the decomposition of the metal alkoxides. The observed weight loss exhibited the highest value of about 20% in sample A, which, according to the TG curve, finished at nearly 420 °C. The second exothermic peak differs for the binary and ternary samples—for sample A, it is about 430 °C, while for the ternary ones, it is near 320 °C (Figure 3). This peak could be attributed to the crystallization of TiO2 (anatase), and it is accompanied by a strong weight loss for the ternary samples (12 and 25%). The last exothermic peak is at about 570 °C for sample A and ~530–540 °C for the ternary samples (B and C), and the calculated weight loss is very low, ~5% in all samples. The calculated total weight loss for the investigated samples is below 50%. For comparison, other authors have stated similar temperatures for DTA investigations [10].
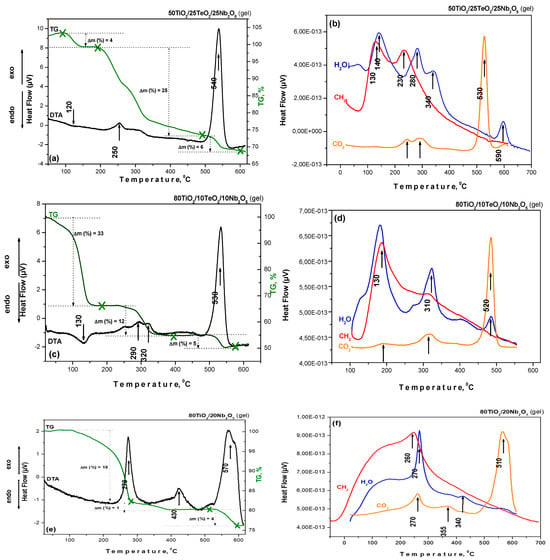
Figure 3.
DTA/TG curves (a,c,e) and mass spectra (b,d,f) of the investigated compositions.
3.3. Spectroscopic Characterization
IR Investigations
The IR spectra of the samples heated at different temperatures are shown in Figure 4. The vibration band assignments were made based on spectral data discussed in our previous investigations on sol-gel-derived binary and ternary composite powders containing TiO2 [27,28]. As seen in the figure, the IR spectra of the binary sample 80TiO2/20Nb2O5 differ from those of the ternary ones. Generally, two main regions could be distinguished in the IR spectra of the gels: 1500–1030 cm−1 and 900–400 cm−1. The similarity with our previous works is expressed by the presence of bands at 1120, 1090–1080, and 1040–1030 cm−1 that could be assigned to the Ti-O-C stretching vibrations contributing to the formation of a mixed organic–inorganic amorphous structure. The position of these bands provides additional information for the determination of the degree of hydrolysis [29,30]. On the other hand, the bending vibrations of CH3 and CH2 groups are also situated in this region [31,32]. As seen in the figure, the low intensity of absorption bands in the 1120–1040 cm−1 region is an indication of more completed hydrolysis-condensation processes. Additionally, the obtained results show that the intensity of these bands decreased in the following order: sample C > sample B > sample A. The lowest intensity is observed in the spectrum of 80TiO2/20BNb2O5 (sample A) as compared to the ternary compositions, which indicates that niobate units are incorporated in the titanate network and lead to a greater degree of hydrolysis in the gel and the sample heated at 200 °C. Obviously, the decrease in the TiO2 content and addition of TeO2 retard the hydrolysis-condensation processes, as was observed in the spectrum of sample C in comparison to the other two samples.
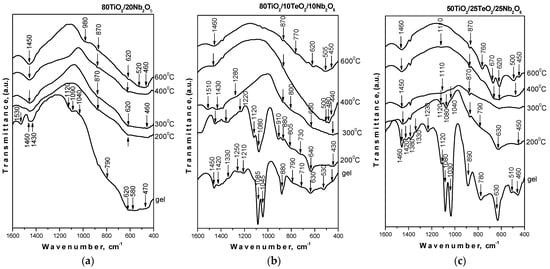
Figure 4.
IR spectra of investigated samples heat-treated at different temperatures: (a) 80TiO2/20Nb2O5, (b) 80TiO2/10TeO2/10Nb2O5, and (c) 50TiO2/25TeO2/25Nb2O5.
The spectral behavior of the samples in the range of (300–600 °C) is similar, and only vibrations of inorganic structural units could be seen (Figure 4). These bands are low-intensity and broadened, which is typical for disordered systems. In all compositions, the observed bands in the region of 700–400 cm−1 are related to the vibrations of a Ti-O-Ti network [33,34,35]. On the other hand, the ternary compositions exhibited bands at 670 and 620 cm−1, which are assigned to the vibrations of TeO4 trigonal bipyramids (tbps) [34,35]. At higher temperatures (600 °C), these bands become stronger when the Nb2O5 content is 25 mol%, as seen in the IR spectrum of sample C, which has the highest TeO2 content (25 mol%). This phenomenon was found by Kabalci et al. [35], who investigated the structure of TeO2/Nb2O5/TiO2 glasses. Obviously, there are strong overlaps in the 750–400 region, which hinders the more precise assignments of the inorganic bands. The infrared band at 870 cm−1 can be ascribed to the ν1 stretching vibration of short Nb–O bonds in isolated NbO6 octahedra [27,36]. The IR analysis demonstrated that the organic–inorganic amorphous phase obtained by the sol-gel method entirely changed into an inorganic one above 400 °C. The results obtained correlate well with the XRD data already discussed above, as well as with our preceding investigations on obtaining TiO2-containing compositions by the sol-gel method.
3.4. Diffuse Reflectance Spectroscopy (DRS) in the UV–Visible Region
The optical absorption spectra of investigated gels were compared to those of TiO2 obtained by Ti(IV) butoxide gel (Figure 5). The absorption edge and optical band gap values of the samples are summarized in Table 1. All gels possess good absorption in the UV region, where the ternary sample 80TiO2/10TeO2/10Nb2O5 exhibited the highest absorption, while sample C—which had the lowest TiO2 content—showed a decrease in the absorption. This observation might be associated with interface charge transfer between TiO2 and Nb2O5 [37,38].
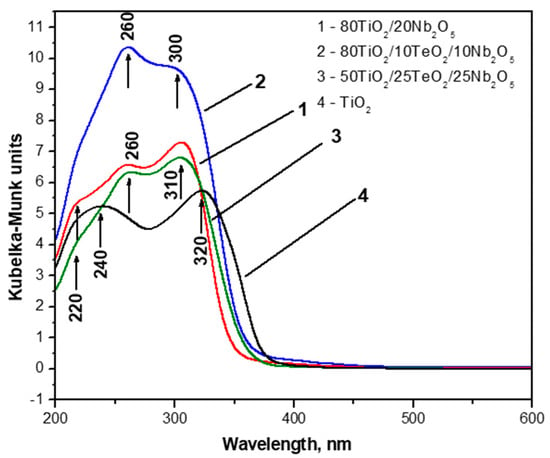
Figure 5.
UV–Vis spectra of investigated samples compared with Ti(IV) butoxide gel and pure TiO2.

Table 1.
Cut-off and optical band gap values (Eg) of the investigated gel compositions.
The UV–Vis spectra exhibited two maxima at 230–260 nm and 300–325 nm, which could be ascribed to the isolated TiO4 and TiO6 units, respectively [39]. It is seen that the TiO2 gel showed comparable intensity of UV peaks at 240 nm and 320 nm, which is an indication of the commensurate amount of TiO4 and TiO6 polyhedra in the gel network. The intensities of these bands in the other gels vary depending on composition. The UV–Vis spectra were also used to determine the optical band gap (Eopt) of the investigated samples (Table 1). In all absorption spectra, the absorption edge values of the Nb2O5-containing gels were calculated between 348.89 and 363.2 nm. This shows a blue shift for these powders in comparison to the pure TiO2 gel. The estimated band gaps of the investigated gels are in the range of 3.41 to 3.55 eV, which is similar to that reported in the literature [37,40,41]. According to the literature, the band gap of pure Nb2O5 is 3.4 eV as a typical n-type wide-bandgap semiconductor, and the obtained values are very close to that [42]. Some authors have stated that the wavelength and intensity of Nb2O5 absorption spectra depend on the size, crystalline type, morphology, and method of synthesis. The smaller size of Nb2O5 particles leads to blue shifting in the absorption spectra [40,43].
3.5. Luminescent Properties
Room-temperature emission PL spectra of the investigated samples heated at 400 and 600 °C are shown in Figure 6. Two obvious signals at 400 and 430 nm are observed, originating from the irradiative recombination of free electrons in shallow traps and sub-bands below the conductive band and free holes at the valance band edge [44,45,46]. The shape of the spectra is similar for all samples, indicating that the addition of different TeO2 and Nb2O5 amounts to TiO2 does not induce new emission phenomena. It is obvious that the samples heat-treated at a higher temperature (600 °C) exhibited stronger PL signals. As mentioned above, the intensity of the spectra results from both the recombination of electron–hole pairs (the lower the intensity, the lower the recombination) and the presence of lattice defects or oxygen vacancies (the higher the intensity, the greater their number) [47,48,49]. Generally, the intensities of the two PL signals are reduced in samples containing lower Nb2O5 amounts, except those in sample A, which did not contain TeO2. Perhaps its presence decreases the recombination rate of photogenerated electron–hole pairs. Therefore, the binary 80TiO2/20Nb2O5 is expected to exhibit promoted activity in photocatalytic tests. It is also noticed that samples containing 20 and 25 mol% Nb2O5 showed stronger signals at 400 nm, which is probably due to the presence of oxygen vacancies [50]. For all samples, the main emission band is around 400 nm, and according to Tam et al. [51], this can be attributed to the indirect band-to-band transition of electrons in TiO2. On the other hand, this short-wavelength PL could be ascribed to the near-band gap emission of Nb2O5 [1,50]. The other PL emission band (430 nm) could be ascribed to the structure defects [1,40].
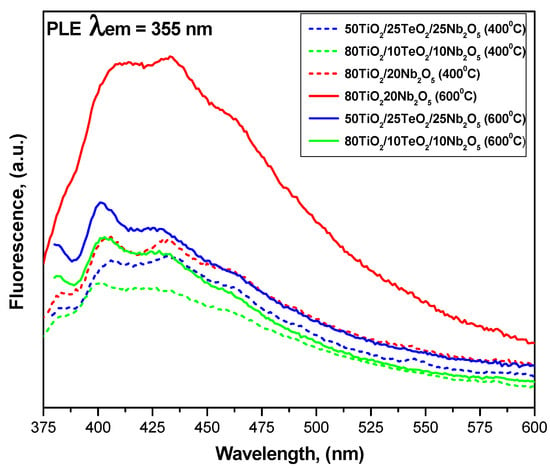
Figure 6.
Room-temperature emission PL spectra of investigated samples heated at 400 and 600 °C.
3.6. Photocatalytic Properties
The photocatalytic activity of the as-synthesized TiO2 samples was tested by the degradation of MG under UV irradiation. Figure 6 presents the results of photocatalytic experiments with the investigated samples compared with those of pure TiO2 synthesized from Ti(IV) butoxide. As can be seen, the photocatalytic performance of the sample 80TiO2/20Nb2O5 was more efficient than the other assayed samples toward MG degradation (Figure 7). After 120 min of UV irradiation, the MG dye was completely decolorized by sample A (80TiO2/20Nb2O5), while for samples B, C, and pure TiO2, the percentages of residual MG were about 75%, 88%, and 50%, respectively. The BET measurements provide additional confirmation of the photocatalytic results of the species. The specific surface area (SSA) of the ternary samples is about 35–45 m2/g, while the binary one exhibited a value of 70 m2/g.
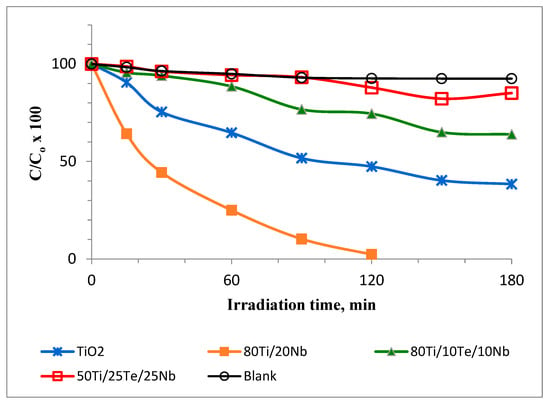
Figure 7.
Photocatalytic properties of investigated samples.
A literature survey [51] summarized that the probable mechanism for the discoloration of MG dye could be explained as follows: excited surface electrons interact with the dissolved oxygen molecule to form superoxide radicals, and holes oxidize water to produce highly active hydroxyl radicals while allowing nanoparticles to interact with the ionic dye. Regarding the fundamental constituents, the chromophores responsible for characteristic colors in MG were broken down; thus, the dye degraded in the presence of powders under UV light illumination.
3.7. Antibacterial Properties
Aiming to obtain more complete information concerning the antimicrobial activity of the prepared samples, two kinds of tests—quantitative (microdilution method) and qualitative (spot test) were performed. The performed spot test was used to predict the antibacterial activity of the as-obtained samples. Bearing in mind the obtained data, only results from the quantitative analysis have been presented. Moreover, it should be noted that the antibacterial activity of all samples was studied without UV light, as in the Refs. [51,52].
As shown in Figure 8, both ternary samples heated at 400 °C demonstrated antibacterial properties, and their MBC and MIC were determined. The MBC for 80TiO2/10TeO2/10Nb2O5 (sample B) is 12.5 mg/mL, and the MIC is 10 mg/mL. For sample C (50TiO2/25TeO2/25Nb2O5), the MBC stands at 4.5 mg/mL.
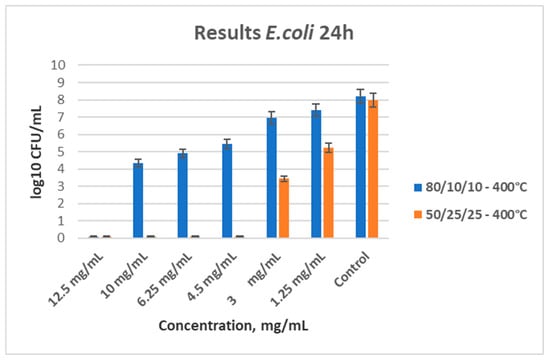
Figure 8.
Antibacterial effect of both ternary samples heat-treated at 400 °C, with different concentrations influencing E. coli.
Figure 9 shows the results from the tested antibacterial properties of the two ternary samples heated at 600 °C. Again, their MBC and MIC were determined with the broth microdilution method. Sample B shows a good antimicrobial effect, and the minimal bactericidal concentration is determined to be 3.0 mg/mL. For sample C, the MBC value was found to be 4.5 mg/mL. Obviously, there is an increase in the minimal bactericidal concentration for sample C, which is probably due to the decreased content of titania.
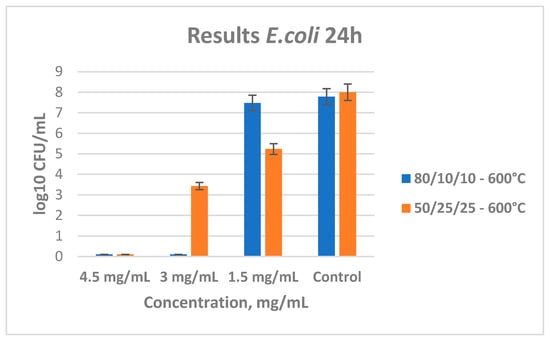
Figure 9.
Antibacterial effect of ternary samples (B and C) heat-treated at 600 °C with different concentrations influencing E. coli.
The tested binary composition did not show a bactericidal effect with the following concentrations: 35, 25, 17.5, 12.5, and 6.25 mg/mL. As shown in Figure 10, a slightly bacteriostatic effect for the sample heated at 600 °C at a concentration of 35 mg/mL was observed. The absence of bactericidal activity in the binary sample further supports the significant role of tellurium in antimicrobial action. Obviously, the observed antibacterial activity of our powders can be attributed to the synergetic effect of anatase, TeO2, and Nb2O5. The plate count method was used to determine the quantity of treated bacteria. The bacterial count in the control sample was measured at 108 CFU, while the treated sample showed a reduction in bacterial count to 106 CFU. This demonstrated a 100-fold decrease in colony count in the treated sample compared to the control.
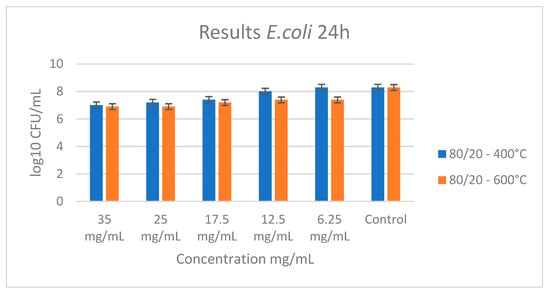
Figure 10.
Antibacterial effect of sample A (80TiO2/20Nb2O5) heat-treated at 400 °C and 600 °C with different concentrations influencing E. coli.
It is well known that antibacterial activity can be explained by the nature of titanium dioxide, and one of the main mechanisms of its action is through the generation of reactive oxygen species (ROS) on its surface. This process can occur even in the absence of photoactivation [51]. It has been reported that an electromagnetic attraction between microorganisms and the TiO2 NPs is due to the positive charge carried by the TiO2 and the negative charges carried by the microorganism’s surface, leading to oxidation reactions. Generally, in this way, TiO2 deactivates cellular enzymes and DNA by coordinating with electron-donating groups, such as thiols, carbohydrates, amides, indoles, and hydroxyls. As a result, the bacterial cell walls lead to increased permeability and cell death [52].
4. Conclusions
The sol-gel method was used for the preparation of binary and ternary TiO2/TeO2/Nb2O5 gels. By using XRD and IR analysis, it was determined that niobium oxide retained the organic components up to 300 °C and that above 400 °C, the organic–inorganic amorphous phase entirely changed into an inorganic one. Applying UV–Vis spectroscopy, two maxima relating to the isolated TiO4 units and condensed TiO6 groups were observed at about 230–260 nm and 305–325 nm, respectively. The photoluminescence spectra exhibited a change in intensity with varying temperatures and TeO2 amounts. The photocatalytic tests showed that the binary sample possesses superior photocatalytic activity toward Malachite green organic dye. The two ternary samples exhibited good antibacterial properties against E. coli ATCC 25922, but the best one was 80TiO2/10TeO2/10Nb2O5 heat-treated at 600 °C. The obtained results reveal that the latter could be used as an antibacterial agent.
Author Contributions
Conceptualization, A.B.-N. and R.I.; methodology, A.B.-N., R.I., A.S. and I.I.; investigation, A.B.-N. and A.S.; writing—original draft preparation, A.B.-N. and K.I.; writing—review and editing, R.I. and I.I.; luminescent properties, P.P.; antibacterial properties, K.I. and L.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The author A. Bachvarova-Nedelcheva is thankful for the support from the European Regional Development Fund under the Research Innovation and Digitization for Smart Transformation program 2021–2027 under the Project BG16RFPR002-1.014-0006 National Centre of Excellence Mechatronics and Clean Technologies. Research equipment from the distributed research infrastructure INFRAMAT, supported by the Bulgarian Ministry of Education and Science, was used.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Georgiev, R.; Georgieva, B.; Vasileva, M.; Ivanov, P.; Babeva, T. Optical Properties of Sol-Gel Nb2O5 Films with Tunable Porosity for Sensing Applications. Adv. Condens. Matter Phys. 2015, 2015, 403196. [Google Scholar] [CrossRef]
- Raba, A.M.; Bautista-Ruíz, J.; Joya, M.R. Synthesis and Structural Properties of Niobium Pentoxide Powders: A Comparative Study of the Growth Process. Mater. Res. 2016, 19, 1381–1387. [Google Scholar] [CrossRef]
- Lee, C.-C.; Tien, C.-L.; Hsu, J.-C. Internal stress and optical properties of Nb2O5 thin films deposited by ion-beam sputtering. Appl. Opt. 2002, 41, 2043–2047. [Google Scholar] [CrossRef] [PubMed]
- Mizuuchi, K.; Ohta, H.; Yamamoto, K.; Kato, M. Second harmonic generation with a high-index-clad waveguide. Opt. Lett. 1997, 22, 1217–1219. [Google Scholar] [CrossRef]
- Dhar, A.; Alford, T.L. Optimization of Nb2O5/Ag/Nb2O5 multilayers as transparent composite electrode on flexible substrate with high figure of merit. J. Appl. Phys. 2012, 112, 103113. [Google Scholar] [CrossRef]
- Akpan, U.G.; Hameed, B.H. Parameters Affecting the Photocatalytic Degradation of Dyes Using TiO2-Based Photocatalysts: A Review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic Activity Improvement and Application of UV-TiO2 Photocatalysis in Textile Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Sacco, O.; Murcia, J.J.; Lara, A.E.; Hernández-Laverde, M.; Rojas, H.; Navío, J.A.; Hidalgo, M.C.; Vaiano, V. Pt–TiO2–Nb2O5, Heterojunction as Effective Photocatalyst for the Degradation of Diclofenac and Ketoprofen. Mater. Sci. Semicond. Process. 2020, 107, 104839. [Google Scholar] [CrossRef]
- Rafael, R.A.; Noronha, F.B.; Gaspar, A.B. Synthesis and Characterization of Ti-Nb2O5 Catalysts for Discoloration Reaction of Bromophenol Blue and Indigo Carmine Dyes. Top. Catal. 2020, 63, 1066–1076. [Google Scholar] [CrossRef]
- Sedneva, T.A.; Lokshin, E.P.; Belikov, M.L.; Belyaevskii, A.T. TiO2- and Nb2O5-Based Photocatalytic Composites. Inorg. Mater. 2013, 49, 382–389. [Google Scholar] [CrossRef]
- Cui, H.; Dwight, K.; Soled, S.; Wold, A. Surface Acidity and Photocatalytic Activity of Nb2O5/TiO2 Photocatalysts. J. Solid State Chem. 1995, 115, 187–191. [Google Scholar] [CrossRef]
- Fuziki, M.E.K.; Ribas, L.S.; Abreu, E.; Fernandes, L.; dos Santos, O.A.A.; Brackmann, R.; de Tuesta, J.L.D.; Tusset, A.M.; Lenzi, G.G. N-Doped TiO2-Nb2O5 Sol–Gel Catalysts: Synthesis, Characterization, Adsorption Capacity, Photocatalytic and Antioxidant Activity. Catalysts 2023, 13, 1233. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The Absolute Energy Positions of Conduction and Valence Bands of Selected Semiconducting Minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Gondal, M.A.; Sayeed, M.N.; Seddigi, Z. Laser Enhanced Photocatalytic Removal of Phenol from Water Using p-Type NiO Semiconductor Catalyst. J. Hazard. Mater. 2008, 155, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Silva Souza, L.V.; Pavanello, L.; Picolo, M.Z.D.; Kury, M.; Matos, I.C.R.T.; Cogo-Müller, K.; Florez, F.L.E.; Cavalli, V. Mechanical and antibacterial properties of an experimental flowable composite containing Nb2O5 and NF_TiO2 nanoparticles. J. Mech. Behav. Biomed. Mater. 2023, 143, 105919. [Google Scholar]
- Madhavi, B.; Siva Sesha Reddy, A.; Syam Prasad, P.; Prasad, A.; Pavani, P.; Devi, K.; Kumar, V.R.; Veeraiah, N. The impact of Nb2O5 on in-vitro bioactivity and antibacterial activity of CaF2–CaO–B2O3–P2O5–SrO glass system. Ceram. Int. 2021, 47, 28328–28337. [Google Scholar] [CrossRef]
- Stoyanova, A.; Bachvarova-Nedelcheva, A.; Iordanova, R.; Ivanova, N.; Hitkova, H.; Sredkova, M. Synthesis, photocatalytic and antibacterial properties of nanosized ZnTiO3 powders obtained by different sol-gel methods. Dig. J. Nanomater. Biostr. 2012, 7, 777–784. [Google Scholar]
- Shalaby, A.; Bachvarova-Nedelcheva, A.; Iordanova, R.; Dimitriev, Y.; Stoyanova, A.; Hitkova, H.; Ivanova, N. Sol-gel synthesis and properties of nanocomposites in the Ag/TiO2/ZnO system. J. Optoel. Adv. Mater. 2015, 17, 248–256. [Google Scholar]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Stoyanova, A.; Georgieva, N.; Angelova, T. Sol-gel synthesis of Se and Te containing TiO2 nanocomposites with photocatalytic and antibacterial properties. J. Optoel. Adv. Mater. 2016, 18, 5–9. [Google Scholar]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Stoyanova, A.; Georgieva, N.; Nemska, V.; Foteva, T. Effect of B2O3 on the Structure, Properties and Antibacterial Abilities of Sol-Gel-Derived TiO2/TeO2/B2O3 Powders. Materials 2023, 16, 6400. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, A.; Hitkova, H.; Bachvarova Nedelcheva, A.; Iordanova, R.; Ivanova, N.; Sredkova, M. Synthesis and antibacterial activity of TiO2/ZnO nanocomposites prepared via nonhydrolytic route. J. Chem. Techn. Metall. 2013, 48, 154–161. [Google Scholar]
- Lecomte, A.; Bamiere, F.; Coste, S.; Thomas, P.; Champarnaud-Mesjard, J.C. Sol–gel processing of TeO2 thin films from citric acid stabilised tellurium isopropoxide precursor. J. Europ. Cer. Soc. 2007, 27, 1151–1158. [Google Scholar] [CrossRef]
- Weng, L.; Hodgson, S. Sol–gel processing of tellurite materials from tellurium ethoxide precursor. Mater. Sci. Eng. 2001, 87, 77–82. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Naydenov, A.; Stoyanova, A.; Georgieva, N.; Nemska, V.; Foteva, T. Sol–gel obtaining of TiO2/TeO2 nanopowders with biocidal and environmental applications. Catalysts 2023, 13, 257. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Georgieva, N.; Nemska, V.; Stoyanova, A. Photocatalytic and antibacterial assessment of Sol–gel derived TiO2/TeO2/ZnO powders. J. Chem. Technol. Metall. 2022, 57, 589–597. [Google Scholar]
- Da Silva, A.L.; Muche, D.N.F.; Dey, S.; Hotza, D.; Castro, R.H.R. Photocatalytic Nb2O5-doped TiO2 nanoparticles for glazed ceramic tiles. Ceram. Intern. 2016, 42, 5113–5122. [Google Scholar] [CrossRef]
- Ücker, C.L.; Riemke, F.; Goetzke, V.; Moreira, M.L.; Raubach, C.W.; Longo, E.; Cava, S. Facile preparation of Nb2O5/TiO2 heterostructures for photocatalytic application. Chem. Phys. Impact 2022, 4, 100079–100086. [Google Scholar] [CrossRef]
- Iordanova, R.; Bachvarova-Nedelcheva, A.; Gegova, R.; Dimitriev, Y. Sol–gel synthesis of composite powders in the TiO2-TeO2-SeO2 system. J. Sol. Gel Sci. Technol. 2016, 79, 12–28. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Gegova, R.; Dimitriev, Y. Crystallization of gels in the binary TiO2-MnOm (MnOm =TeO2, SeO2, B2O3, ZnO) systems. Bulg. Chem. Commun. 2017, 49, 110–118. [Google Scholar]
- Velasco, M.J.; Rubio, F.; Rubio, J.; Oteo, J.L. FT-IR Study of the Hydrolysis and Polymerization of Tetraethyl Orthosilicate and Polydimethyl Siloxane in the Presence of Tetrabutyl Orthotitanate. Spectr. Lett. 1999, 32, 289–304. [Google Scholar] [CrossRef]
- Dubey, R.S.; Krishnamurthy, K.V.; Singh, S. Experimental studies of TiO2 nanoparticles synthesized by Sol–gel and solvothermal routes for DSSCs application. Results Phys. 2019, 14, 102390. [Google Scholar] [CrossRef]
- Doeuff, S.; Henry, M.; Sanchez, C.; Livage, J. Hydrolysis of titanium alkoxides: Modification of the molecular precursor by acetic acid. J. Non-Cryst. Solids 1987, 89, 206–216. [Google Scholar] [CrossRef]
- Uzunova-Bujnova, M.; Dimitrov, D.; Radev, D.; Bojinova, A.; Todorovsky, D. Effect of the mechanoactivation on the structure, sorption and photocatalytic properties of titanium dioxide. Mater. Chem. Phys. 2008, 110, 291–298. [Google Scholar] [CrossRef]
- El-Malawany, R. Tellurite Glasses, Physical Properties and Data; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Kabalci, I.; Gökçe, H. Investigation of Infrared and Raman Spectra of TeO2-Nb2O5-TiO2 Glasses. Acta Phys. Pol. A 2014, 125, 877–882. [Google Scholar] [CrossRef]
- Aleksandrov, L.; Milanova, M.; Yordanova, A.; Iordanova, R.; Nedyalkov, N.; Petrova, P.; Tagiara, N.S.; Palles, D.; Kamitsos, E.I. Synthesis, structure and luminescence properties of Eu3+-doped 50ZnO.40B2O3.5WO3.5Nb2O5 glass. Phys. Chem. Glas. Eur. J. Glass Sci. Technol. B 2023, 64, 101–109. [Google Scholar]
- Yan, J.; Wu, G.; Guan, N.; Li, L. Nb2O5/TiO2 heterojunctions: Synthesis strategy and photocatalytic activity. Appl. Catal. B Environ. 2014, 152–153, 280–288. [Google Scholar] [CrossRef]
- Prashant, V. Kamat, Manipulation of Charge Transfer Across Semiconductor Interface. A Criterion That Cannot Be Ignored in Photocatalyst Design. J. Phys. Chem. Lett. 2012, 3, 663–672. [Google Scholar]
- Barlier, V.; Bounor-Legare, V.; Boiteux, G.; Davenas, J. Hydrolysis–condensation reactions of titanium alkoxides in thin films: A study of the steric hindrance effect by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2008, 254, 5408–5412. [Google Scholar] [CrossRef]
- Yan, C.; Xue, D. Formation of Nb2O5 nanotube arrays through phase transformation. Adv. Mater. 2008, 20, 1055. [Google Scholar] [CrossRef]
- Orel, B.; Maček, M.; Grdadolnik, J.; Meden, A. In situ UV-Vis and ex situ IR spectroelectrochemical investigations of amorphous and crystalline electrochromic Nb2O5 films in charged/discharged states. J. Solid State Electrochem. 1998, 2, 221–236. [Google Scholar] [CrossRef]
- Mohite, N.; Shinde, M.; Kumar Gupta, A.; Waghadkar, Y.; Gosavi, S.W.; Mohite, K.C.; Chauhan, R.; Rane, S. Facile synthesis of hollow urchin-like Nb2O5 nanostructures and their performance in dye-sensitized solar cells. J. Solid State Electrochem. 2020, 24, 6346–6352. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Zhao, Q.; He, X.; Feng, J. Effect of heat treatment on the UV–vis–NIR and PL spectra of TiO2 films. J. Electron Spectrosc. Relat. Phenom. 2005, 148, 158–163. [Google Scholar] [CrossRef]
- Rex, R.E.; Knorr, F.J.; McHale, J.L. Characterization of Oxygen Vacancy Associates within Hydrogenated TiO2: A Positron Annihilation Study. J. Phys. Chem. C 2013, 117, 7949–7951. [Google Scholar] [CrossRef]
- Feng, W.; Wu, G.J.; Li, L.D.; Guan, N.J. Solvent-free selective photocatalytic oxidation of benzyl alcohol over modified TiO2. Green Chem. 2011, 13, 3265–3272. [Google Scholar] [CrossRef]
- Di Paola, A.; Bellardita, M.; Marc, G.; Palmisano, L.; Parrino, F.; Amadelli, R. Preparation of Sm-loaded brookite TiO2 photocatalysts. Catal. Today 2011, 161, 35–40. [Google Scholar] [CrossRef]
- Liqiang, J.; Xiaojun, S.; Baifu, X.; Baiqi, W.; Weimin, C.; Honggang, F. The preparation and characterization of La doped TiO2 nanoparticles and their photocatalytic activity. J. Solid State Chem. 2004, 177, 3375–3382. [Google Scholar] [CrossRef]
- Umair, M.; Palmisano, G.; Sakkaf, R.A.; Jitan, S.A.; Pintar, A.; Zerjav, G.; Palmisano, L.; Loddo, V.; Bellardita, M. Pt-Nb2O5-TiO2 based semiconductors for photo-reforming of glucose and fructose aqueous solutions. Appl. Surf. Sci. 2024, 648, 159030. [Google Scholar] [CrossRef]
- Jaybhaye, S.; Shinde, N.; Jaybhaye, S.; Narayan, H. Photocatalytic Degradation of Organic Dyes Using Titanium Dioxide (TiO2) and Mg-TiO2 Nanoparticles. J. Nanotechnol. Nanomater. 2022, 3, 67–76. [Google Scholar]
- Thakur, N.; Thakur, N.; Kumar, A.; Thakur, V.K.; Kalia, S.; Arya, V.; Kumar, A.; Kumar, S.; Kyzas, G.Z. A critical review on the recent trends of photocatalytic, antibacterial, antioxidant and nanohybrid applications of anatase and rutile TiO2 nanoparticles. Sci. Total Environ. 2024, 914, 169815. [Google Scholar] [CrossRef]
- Tam, K.H.; Djurišić, A.B.; Chan, C.M.N.; Xi, Y.Y.; Tse, C.W.; Leung, Y.H.; Chan, W.K.; Leung, F.C.C.; Au, D.W.T. Antibacterial activity of ZnO nanorods prepared by a hydrothermal method. Thin Solid Film. 2008, 516, 6167–6174. [Google Scholar] [CrossRef]
- Nakano, R.; Hara, M.; Ishiguro, H.; Yao, Y.; Ochiai, T.; Nakata, K.; Murakami, T.; Kajioka, J.; Sunada, K.; Hashimoto, K.; et al. Broad spectrum microbicidal activity of photocatalysis by TiO2. Catalysts 2013, 3, 310–323. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).