Evaluation of Patellar Groove Prostheses in Veterinary Medicine: Review of Technological Advances, Technical Aspects, and Quality Standards
Abstract
1. Introduction
1.1. 3D-Printed Patient-Specific Implants in Veterinary Medicine
1.2. Patellar Groove Implants
- Explain the biomechanical and anatomical factors and pathologies that necessitate patellar groove replacement, thereby enhancing the understanding of the conditions that warrant surgical intervention;
- Review quality requirements and standards for the human medicine equivalent to veterinary patellar groove replacement, product—knee implants;
- Review the materials used to fabricate knee and patellar groove implants, concentrating on their mechanical properties, biocompatibility, surface modifications, and clinical performance, to inform future material selection;
- Compare the advantages and disadvantages of patellar groove implants available on the market;
- Highlight technological advancements, particularly in designing and manufacturing custom implants and surgical guides.
1.3. Literature Selection Methodology
2. Anatomy and Physiology
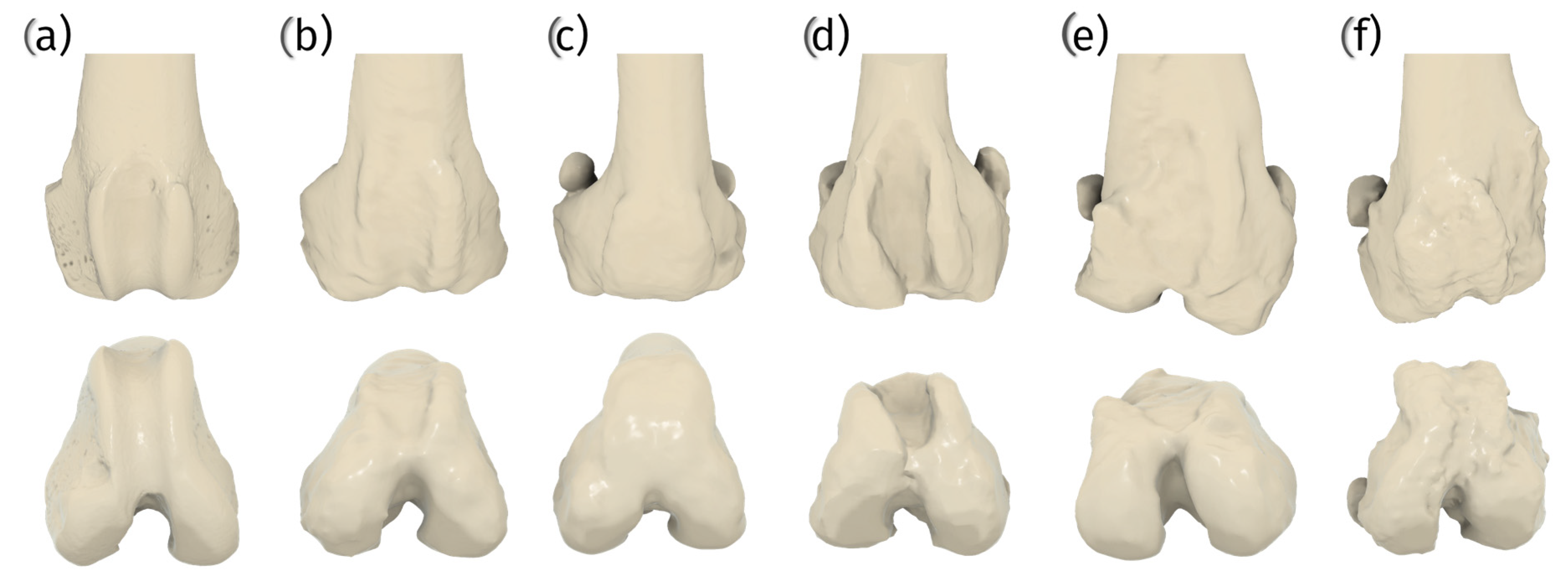
2.1. The Main Causes of Patellar Dislocation and Indications for Patellar Groove Arthroplasty
2.1.1. Role of Angular Deformities in Patellar Luxation
- Lateral Bowing of the Distal Femur (Varus Deformity)
- Hypoplasia of the Medial Condyle
- Torsion of the Tibial Tuberosity
- Medial Bowing of the Proximal Tibia
2.1.2. Secondary Changes in the Trochlea
2.1.3. Indications for Patellar Groove Arthroplasty
3. Standards and Requirements for Knee Implants in Human Medicine: A Reference for Veterinary Applications
3.1. ISO Standards for Orthopedic Implants
3.2. Comparing Human and Veterinary Knee Biomechanics: Implications for Implant Design
- The peak joint forces in the canine stifle reach 3.5–4.5 times the animal’s body weight during high-impact activities such as running or jumping. In comparison, human knee prostheses are designed to withstand 5–7 times the human body weight, particularly during stair climbing or squatting;
- In dogs, the tibiofemoral contact areas are smaller relative to their body weight, resulting in higher localized pressures on articulating surfaces;
- Human knees achieve full extension, while canine and feline stifles remain in a mild flexion, influencing implant kinematics and stress distribution [46]
3.3. Critical Aspects of Joint Prosthesis
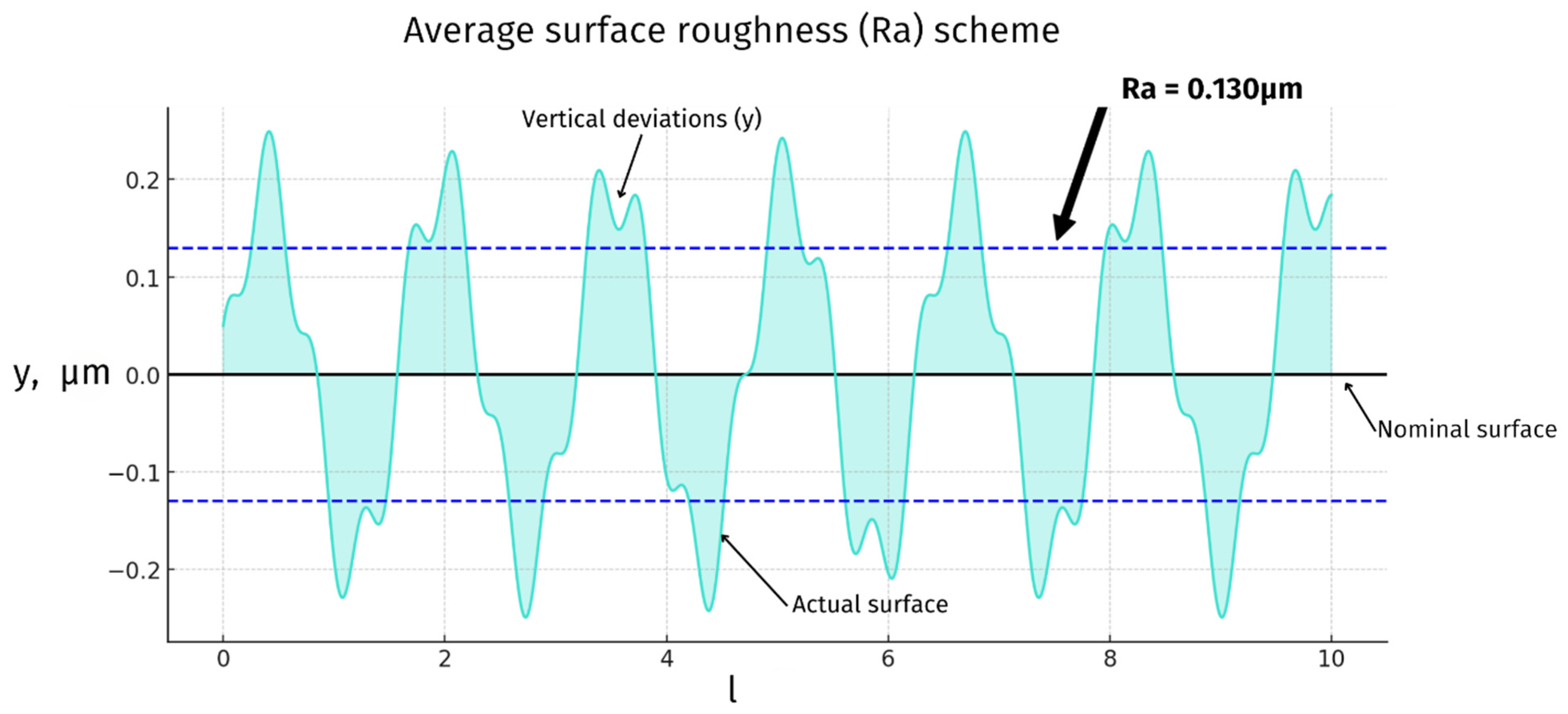
3.4. Joint Articulating Surfaces—Roughness
3.5. Materials Biocompatibility
3.6. Cleanliness
3.7. Toward Veterinary-Specific Implant Regulations: Challenges and Future Directions
- Material and biomechanical standards: Establishing species-specific guidelines for material properties, mechanical strength, surface roughness, and biocompatibility. Adaptations of ISO 5832 (metallic materials), ISO 10993 (biological evaluation), and ISO 7207 (knee prosthesis components) can serve as a foundation but must be refined to suit veterinary needs [31,32,33,34,35,36,37,38,39,40];
- Size-specific testing and classification: Given the extreme range of patient sizes involved in veterinary medicine, regulatory bodies should introduce size-based mechanical testing categories, ensuring that implants perform reliably across different weight classes. Load-bearing requirements should reflect the quadrupedal gait of animals, which differs from human bipedal locomotion;
- Quality control and post-market surveillance: A standardized certification process for veterinary implant manufacturers should be developed, requiring pre-clinical testing, long-term monitoring, and complication reporting. A collaborative effort between regulatory agencies, veterinary orthopedic societies, and manufacturers would be necessary to implement these measures effectively.
4. Materials for Implants
4.1. Metallic Alloys
4.2. Ceramic Materials
4.3. Polymeric Materials
4.4. Biomechanical Considerations
4.5. Material Performance of Custom vs. Off-the-Shelf Implants
4.6. Implants Materials Summary
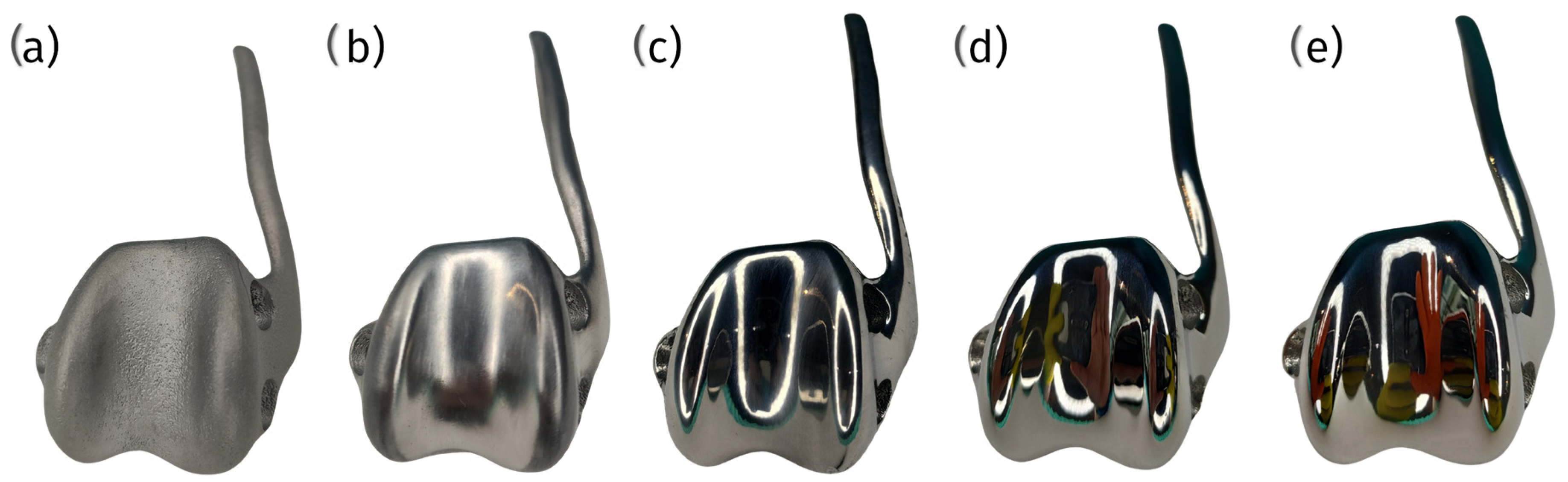
5. Surface Modifications—Articulating Joint Surface
5.1. Diamond-like Carbon
5.2. Titanium Nitride
6. Design and Surface Modifications for Osteointegration and Bone Ingrowth
6.1. Pore Size
6.2. Porous Coatings
Types of Porous Coatings
- 1.
- Plasma-sprayed coatings
- (a)
- Titanium and titanium alloy coatings—plasma spraying is used to apply a layer of titanium or titanium alloy onto the surface of implants. These coatings provide a rough, porous surface, promoting bone cell attachment and growth (Figure 7);
- (b)
- Hydroxyapatite (HA) coatings—hydroxyapatite, a naturally occurring mineral form of calcium apatite, is plasma-sprayed onto implants to create a bioactive surface that encourages direct bone bonding (Figure 7);
- 2.
- Metal bead coatings:
- 3.
- Metallic fiber mesh coatings:
- 4.
- Porous tantalum trabecular metal:
- 5.
- Bioactive glass coatings:
- 6.
- Tricalcium phosphate (TCP) coatings:
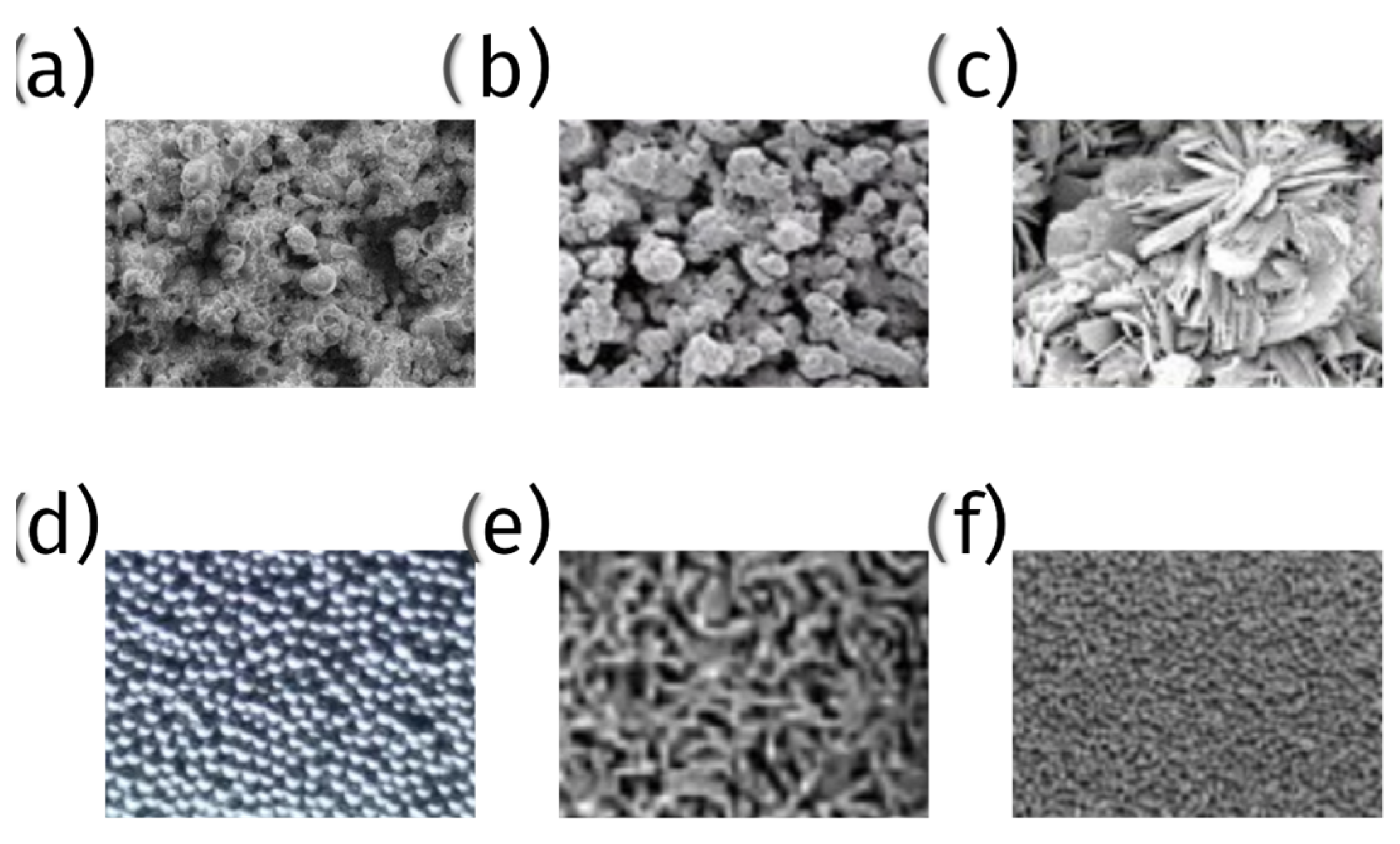
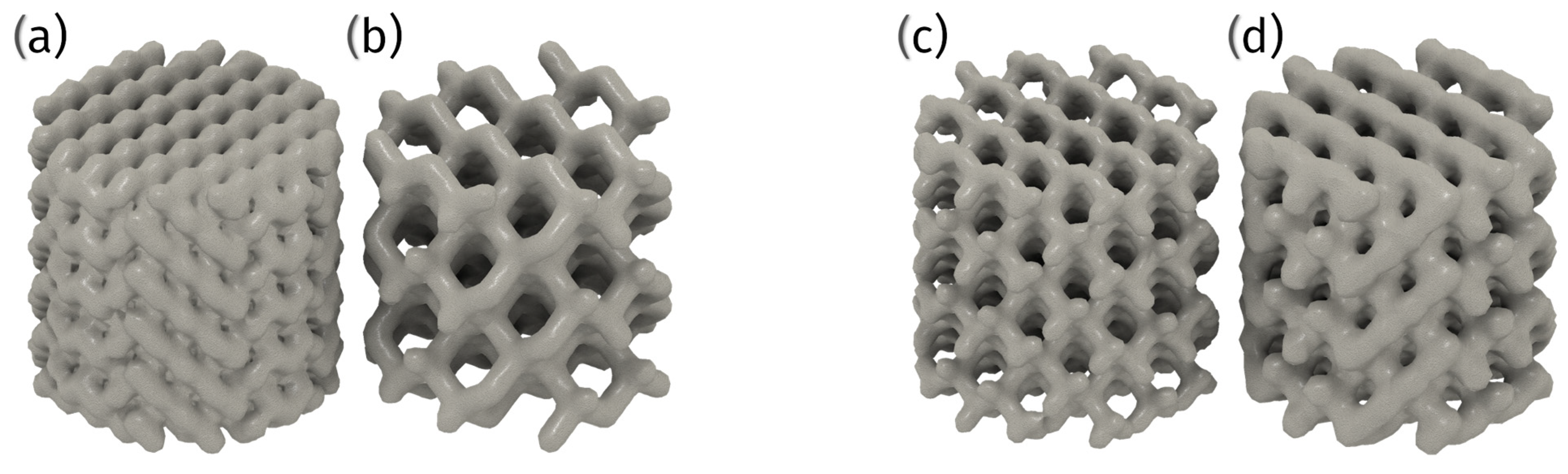
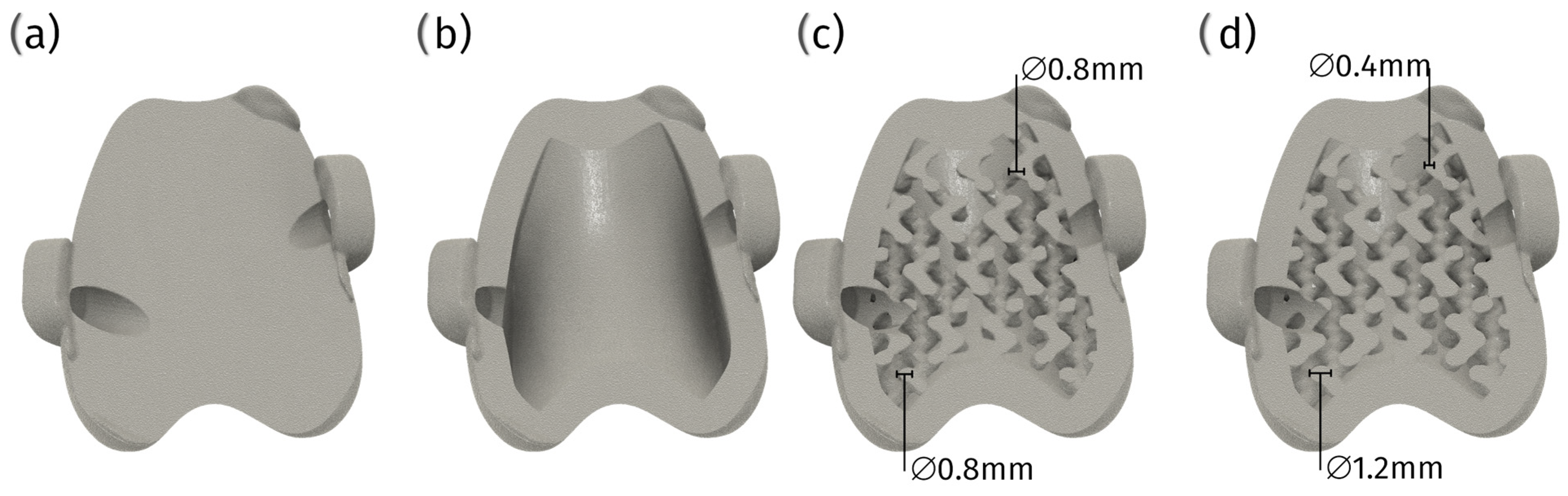
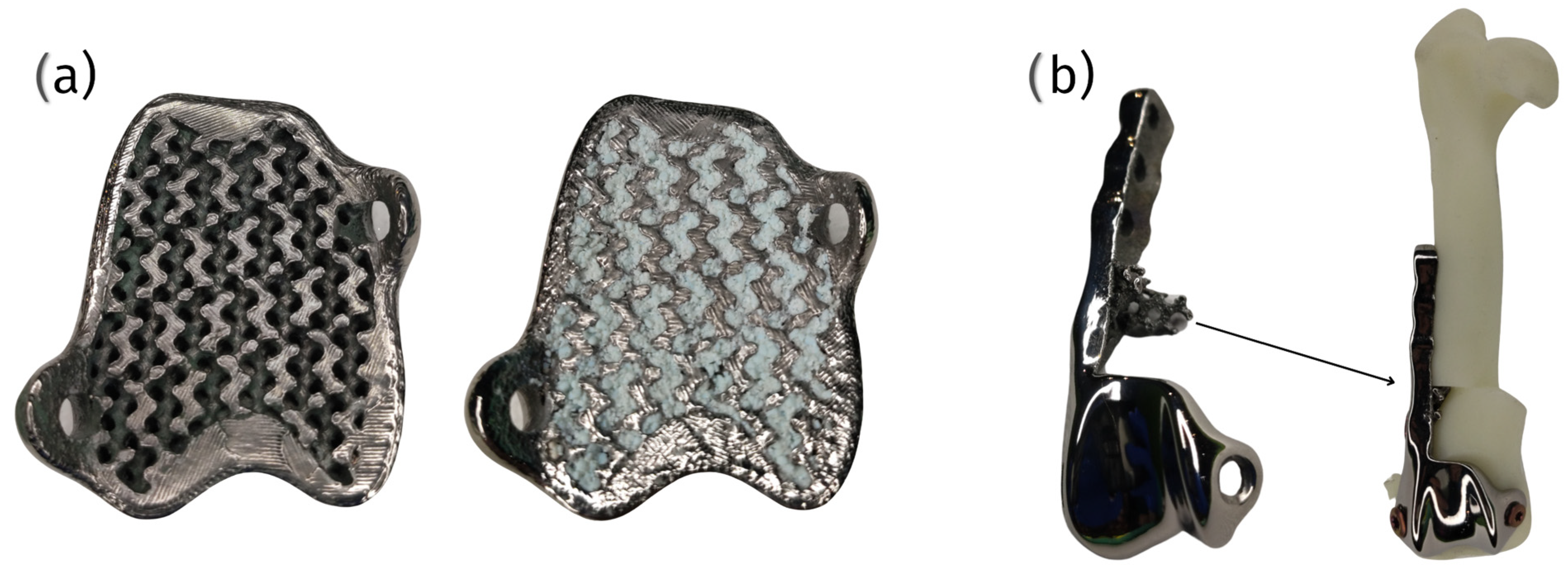
6.3. 3D Printed Porous Structures
6.3.1. Techniques and Materials
6.3.2. Advantages and Challenges
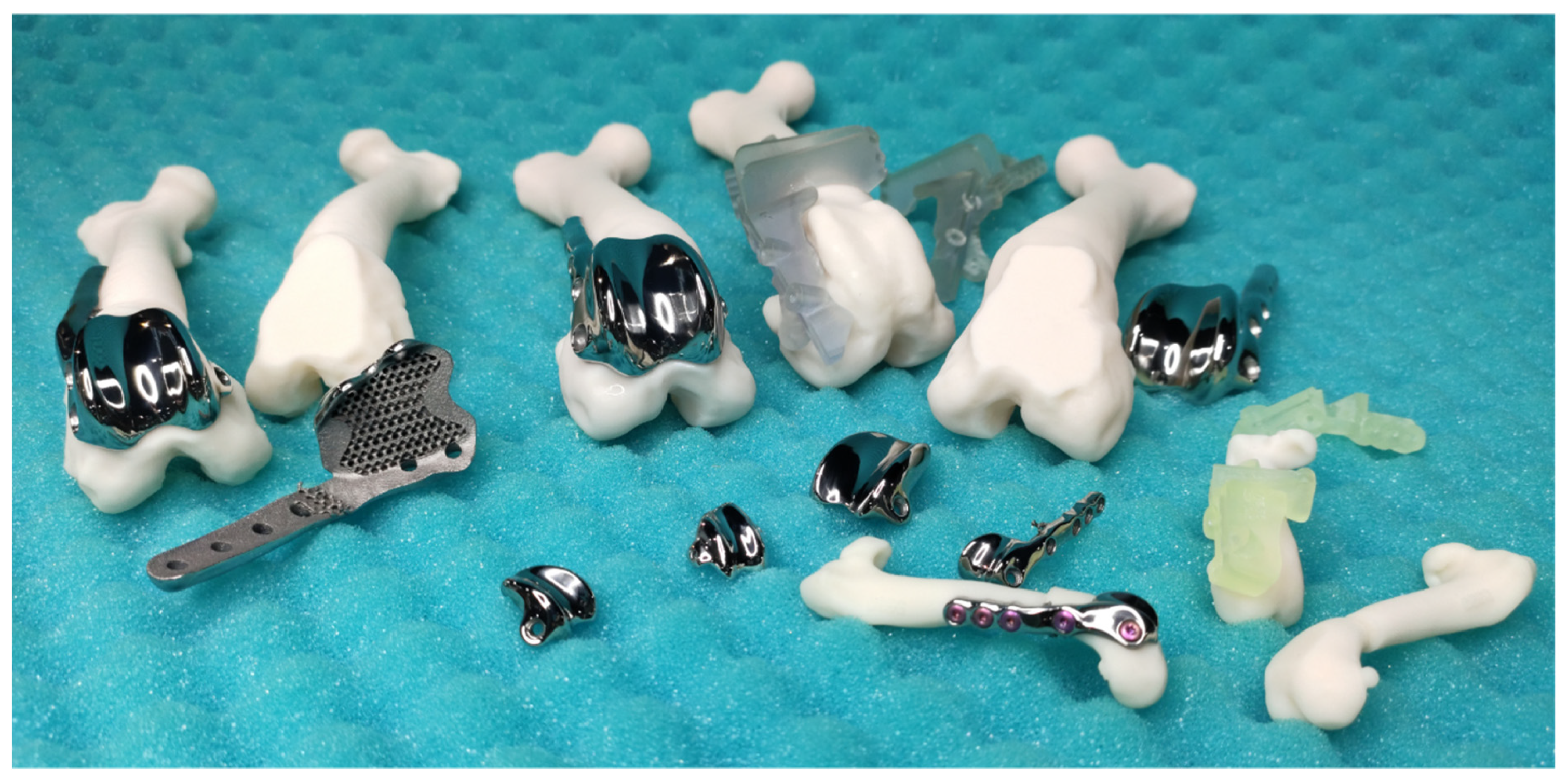
7. Current Market Review
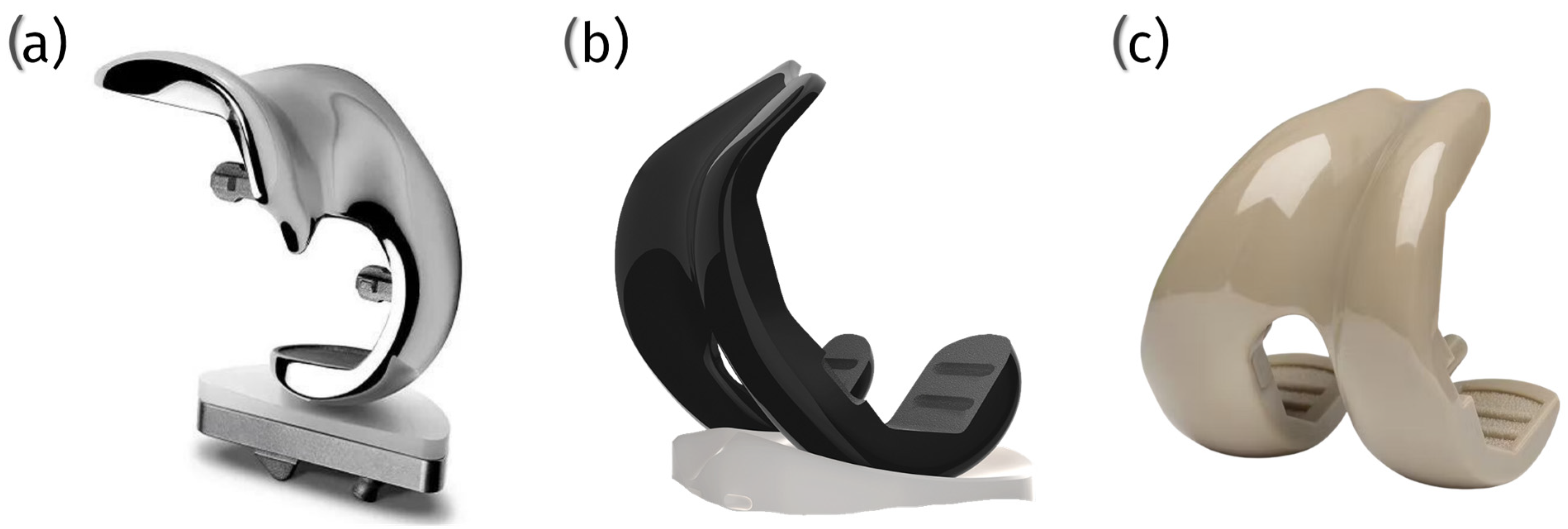
7.1. KYON Patellar Groove Replacement (PGR)
- Groove component: This element is fabricated from a titanium alloy and coated with amorphous diamond-like carbon (ADLC). The ADLC coating imparts a smooth, complex, and scratch-resistant surface with a low coefficient of friction, thereby minimizing the heat generated by interaction with the surface;
- Base plate: constructed from commercially pure titanium and coated with calcium phosphate, this component is designed to facilitate integration with the bone.
- -
- Immediate stability: the implant provides immediate stability for the patella within the artificial groove, reducing the risk of luxation;
- -
- Low friction: the ADLC coating on the groove component ensures smooth patellar movement, minimizing friction and wear;
- -
- Rapid bone ingrowth: the BioCer® surface treatment on the base plate promotes rapid bone ingrowth, enhancing implant stability;
- -
- Size options: the KYON PGR is available in over 10 sizes, allowing surgeons to select the most appropriate fit for various dog breeds.
7.2. Innoplant TRA
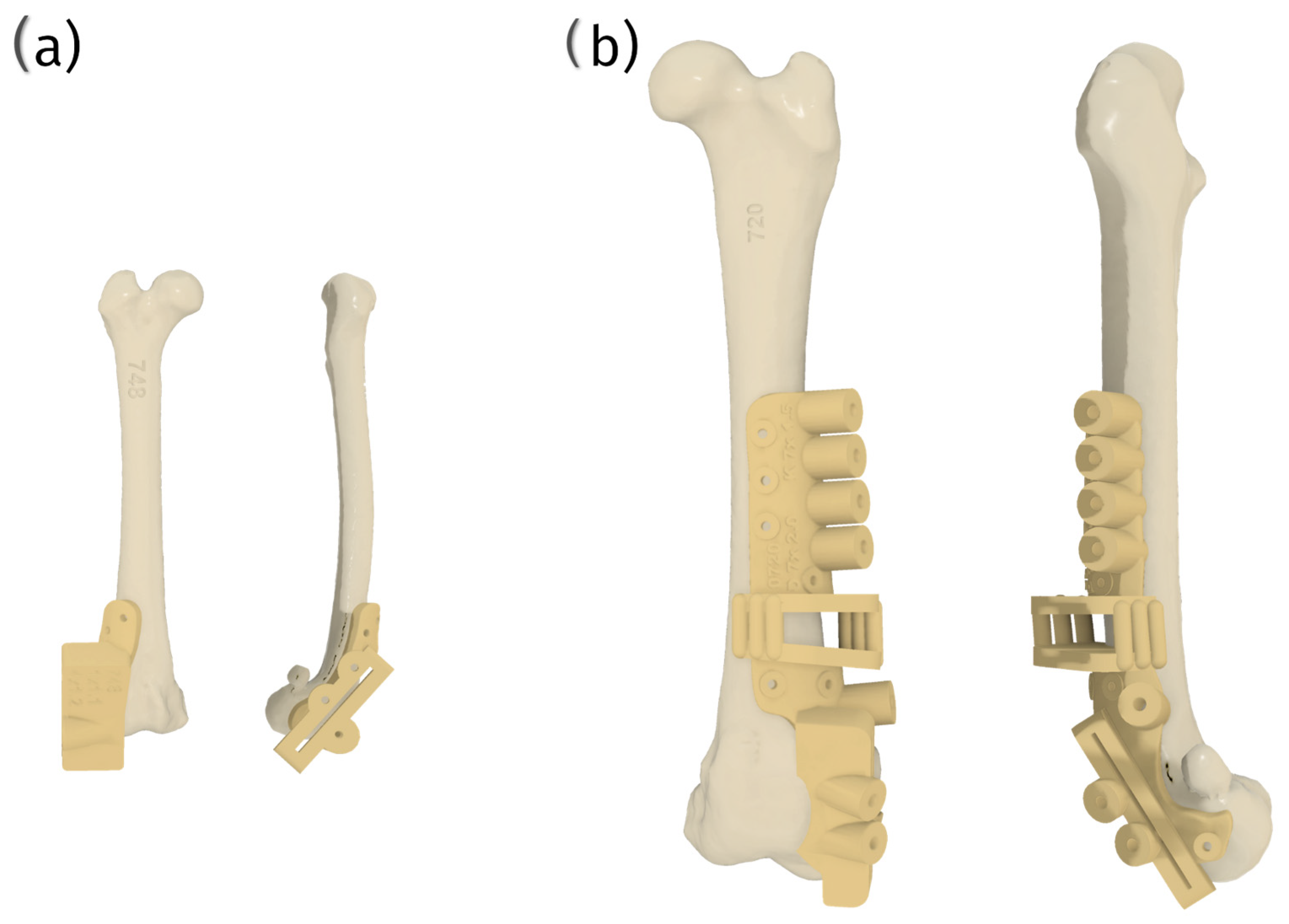
7.3. Surgical Procedures
7.4. Current Off-Shelf Products’ Disadvantages and Limitations
7.5. Challenges in Maintaining Anatomical Surface Levels
7.6. Implications for Patella Wear
7.7. Implant Placement Challenges and Biomechanical Considerations
8. Advances in Technology
8.1. Advantages and Potential of Custom Patellar Groove Implants
8.2. Technological Implementation and Future Directions
8.3. Challenges and Considerations
Risks of Combined Prosthesis/Plate Implants
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MPL | medial patellar luxation |
| PGR | patellar groove replacement |
| CT | computed tomography |
| MRI | magnetic resonance imaging |
| PEEK | polyetheretherketone |
| DLC | diamond-like carbon |
| TiN | titanium nitride |
| PIRAC | powder immersion reaction assisted coating |
| PVD | physical vapor deposition |
| SLM | selective laser melting |
| HA | hydroxyapatite |
| TCP | tricalcium phosphate |
| EBM | electron beam melting ebm |
| ADLC | amorphous diamond-like carbon |
| TRA | trochlear ridge arthroplasty |
| cPGI | custom patellar groove implants |
| ccPGI | corrective custom patellar groove implants |
References
- Carwardine, D.R.; Gosling, M.J.; Burton, N.J.; O’Malley, F.L.; Parsons, K.J. Three-Dimensional-Printed Patient-Specific Osteotomy Guides, Repositioning Guides and Titanium Plates for Acute Correction of Antebrachial Limb Deformities in Dogs. Vet. Comp.Orthop. Traumatol. 2021, 34, 43–52. [Google Scholar] [CrossRef]
- Liu, W.-C.; Robu, I.S.; Patel, R.; Leu, M.C.; Velez, M.; Chu, T.-M.G. The effects of 3D bioactive glass scaffolds and BMP-2 on bone formation in rat femoral critical size defects and adjacent bones. Biomed. Mater. 2014, 9, 045013. [Google Scholar] [CrossRef]
- Fitzpatrick, N.; Guthrie, J.W. Hemipelvic and proximal femoral limb salvage endoprosthesis with tendon ongrowth in a dog. Veter. Surg. 2018, 47, 963–969. [Google Scholar] [CrossRef]
- Castelli, E.; Schmierer, P.A.; Pozzi, A. Custom acetabular prosthesis for total hip replacement: A case report in a dog with acetabular bone loss after femoral head and neck ostectomy. Veter. Surg. 2019, 48, 1520–1529. [Google Scholar] [CrossRef]
- Bray, J.P.; Kersley, A.; Downing, W.; Crosse, K.R.; Worth, A.J.; House, A.K.; Yates, G.; Coomer, A.R.; Brown, I.W.M. Clinical outcomes of patient-specific porous titanium endoprostheses in dogs with tumors of the mandible, radius, or tibia: 12 cases (2013–2016). J. Am. Veter. Med Assoc. 2017, 251, 566–579. [Google Scholar] [CrossRef]
- Kim, Y.; Park, Y.; Park, J.; Jeong, S.M.; Lee, H. Bilateral Patellar Groove Replacement in a Dog with Iatrogenic Trochlear Groove Damage. J. Vet. Clin. 2016, 33, 295–299. [Google Scholar] [CrossRef]
- Hespel, A.; Wilhite, R.; Hudson, J. Invited Review-Applications For 3d Printers in Veterinary Medicine. Vet. Radiol. Ultrasound 2014, 55, 347–358. [Google Scholar] [CrossRef]
- Oană, L.A. A surgical technique of medial patellar luxation in dogs. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Vet. Med. 2010, 67, 6031. [Google Scholar]
- Pinna, S.; Venturini, A.; Tribuiani, A.M. Rotation of the femoral trochlea for treatment of medial patellar luxation in a dog. J. Small Anim. Pr. 2008, 49, 163–166. [Google Scholar] [CrossRef]
- Longo, F.; Memarian, P.; Knell, S.C.; Contiero, B.; Pozzi, A. Computed tomographic measurements of the femoral trochlea in dogs with and without medial patellar luxation. Vet. Surg. 2022, 52, 395–406. [Google Scholar] [CrossRef]
- De Giacinto, E.; Troiano, D.; Denti, F.; Buiatti, M.; Petazzoni, M. Computed Tomographic Trochlear Depth Measurement in Normal Dogs. Vet. Comp. Orthop. Traumatol. 2018, 31, 431–437. [Google Scholar] [CrossRef]
- Ericksen, T.D.; Stobie, D.; Culbert, L.; Valenzano, D.; Bogart, D.F. Dome trochleoplasty for correction of patella alta and patella luxation in dogs > 20 kg. J. Am. Vet. Med Assoc. 2023, 261, 0307. [Google Scholar] [CrossRef]
- Goring, R.L.; de Haan, J.J.; Talcott, K.W. Rectangular Recession Trochleoplasty for Treatment of Patellar Luxation in Dogs and Cats. Vet. Comp. Orthop. Traumatol. 2000, 13, 39–43. [Google Scholar] [CrossRef]
- Johnson, A.L.; Probst, C.W.; Decamp, C.E.; Rosenstein, D.S.; Hauptman, J.G.; Weaver, B.T.; Kern, T.L. Comparison of Trochlear Block Recession and Trochlear Wedge Recession for Canine Patellar Luxation Using a Cadaver Model. Vet. Surg. 2001, 30, 140–150. [Google Scholar] [CrossRef]
- Sasaki, A.; Hidaka, Y.; Mochizuki, M.; Honnami, M. Computed Tomographic Measurements of the Sulcus Angle of the Femoral Trochlea in Small-Breed Dogs with and without Medial Patellar Luxation. Vet. Comp. Orthop. Traumatol. 2022, 35, 314–320. [Google Scholar] [CrossRef]
- Nicetto, T.; Longo, F.; Contiero, B.; Isola, M.; Petazzoni, M. Computed tomographic localization of the deepest portion of the femoral trochlear groove in healthy dogs. Vet. Surg. 2020, 49, 1246–1254. [Google Scholar] [CrossRef]
- Dokic, Z.; Lorinson, D.; Weigel, J.P.; Vezzoni, A. Patellar groove replacement in patellar luxation with severe femoro-patellar osteoarthritis. Vet. Comp. Orthop. Traumatol. 2015, 28, 124–130. [Google Scholar] [CrossRef]
- Panichi, E.; Sassaroli, S.; Ciccarese, G.M.; Riccio, V.; Balestriere, C.; Barbaccia, M.; Cappellari, F.; Burkhan, E.; Piccionello, A.P. Use of a Custom-Made Patellar Groove Replacement in an American Staffordshire Terrier Puppy with a Severe Bone Defect in the Femoral Trochlea Caused by Hematogenous Osteomyelitis. Animals 2024, 14, 909. [Google Scholar] [CrossRef]
- Jaworski, J.; Krukowski, M.; Gosling, M.; Burton, N. Patellar Groove Replacement in a Cat. VCOT Open 2022, 05, e71–e77. [Google Scholar] [CrossRef]
- Kowaleski, M.; Boudrieau, R.; Pozzi, A. Stifle joint. In Veterinary Surgery: Small Animal; Tobias, K.M., Johnston, S., Eds.; Elsevier: St. Louis, LA, USA, 2017; pp. 1071–1167. [Google Scholar]
- Di Dona, F.; Della Valle, G.; Fatone, G. Patellar luxation in dogs. Vet. Med. Res. Rep. 2018, 9, 23–32. [Google Scholar] [CrossRef]
- Rezende, C.M.; Torres, R.C.S.; Nepomuceno, A.C.; Lara, J.S.; Varón, J.A.C. Patellar Luxation in Small Animals. In Canine Medicine–Recent Topics and Advanced Research; InTech: London, UK, 2016. [Google Scholar] [CrossRef]
- Arthurs, G.I.; Langley-Hobbs, S.J. Complications Associated with Corrective Surgery for Patellar Luxation in 109 Dogs. Vet. Surg. 2006, 35, 559–566. [Google Scholar] [CrossRef] [PubMed]
- de la Oliva Cases, P.L.; Grierson, J. Patellar luxation in dogs. Companion Anim. 2019, 24, 293–298. [Google Scholar] [CrossRef]
- Janssens, L.; Béosier, Y.; Daems, R. Grossly apparent cartilage erosion of the patellar articular surface in dogs with congenital medial patellar luxation. Vet. Comp. Orthop. Traumatol. 2009, 22, 222–224. [Google Scholar] [CrossRef] [PubMed]
- Salmoral, A.; Clarke, S.; Smith, K. Femur fracture associated with canine patellar groove replacement. Vet. Rec. Case Rep. 2023, 11, e699. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Griffon, D.J.; Thomas, M.W.; Constable, P.D. Proximodistal Alignment of the Canine Patella: Radiographic Evaluation and Association with Medial and Lateral Patellar Luxation. Vet. Surg. 2008, 37, 201–211. [Google Scholar] [CrossRef]
- Sasaki, A.; Hidaka, Y.; Mochizuki, M.; Honnami, M. Measurement of Femoral Trochlear Morphology in Dogs Using Ultrasonography. Vet. Comp. Orthop. Traumatol. 2023, 36, 294–301. [Google Scholar] [CrossRef]
- Irvine, W.O. Concepts of etiologies and effects of normal human knee pressure variations. Anat. Physiol. 2015, 5, 172. [Google Scholar] [CrossRef]
- Murakami, T. Biomechanics of human and artificial joints. In Biotribology of Natural and Artificial Joints; Elsevier: Amsterdam, Netherlands, 2023; pp. 29–79. ISBN 9780128236697. [Google Scholar]
- ISO 5832-1; Implants for Surgery–Metallic Materials–Part 1: Wrought Stainless Steel. ISO: Geneva, Switzerland, 2024.
- ISO 5832-2; Implants for Surgery–Metallic Materials–Part 2: Unalloyed Titanium. ISO: Geneva, Switzerland, 2018.
- ISO 5832-3; Implants for Surgery–Metallic Materials–Part 3: Wrought Titanium 6-Aluminium 4-Vanadium Alloy. ISO: Geneva, Switzerland, 2021.
- ISO 5832-4; Implants for Surgery–Metallic Materials–Part 4: Cobalt-Chromium-Molybdenum Casting Alloy. ISO: Geneva, Switzerland, 2024.
- ISO 5832-12; Implants for Surgery–Metallic Materials–Part 12: Wrought Cobalt-Chromium-Molybdenum Alloy. ISO: Geneva, Switzerland, 2019.
- ISO 10993-1; Biological Evaluation of Medical Devices–Part 1: Evaluation and Testing within a Risk Management Process. ISO: Geneva, Switzerland, 2018.
- ISO 10993-5; Biological Evaluation of Medical Devices–Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- ISO 10993-11; Biological Evaluation of Medical Devices–Part 11: Tests for Systemic Toxicity. ISO: Geneva, Switzerland, 2017.
- ISO 7207-1; Implants for Surgery–Components for Partial and Total Knee Joint Prostheses–Part 1: Classification, Definitions, and Designation of Dimensions. ISO: Geneva, Switzerland, 2007.
- ISO 7207-2; Implants for Surgery–Components for Partial and Total Knee Joint Prostheses–Part 2: Articulating Surfaces Made of Metal, Ceramic, and Plastic Materials. ISO: Geneva, Switzerland, 2011.
- ISO 13485; Medical Devices—Quality Management Systems—Requirements for Regulatory Purposes. ISO: Geneva, Switzerland, 2016.
- ISO 14243-1; Implants for Surgery–Wear of Total Knee-Joint Prostheses–Part 1: Loading and Displacement Parameters for Wear-Testing Machines with Load Control and Corresponding Environmental Conditions for the Test. ISO: Geneva, Switzerland, 2009.
- ISO 14243-2; Implants for Surgery–Wear of Total Knee-Joint Prostheses–Part 2: Methods of Measurement. ISO: Geneva, Switzerland, 2016.
- ISO 21534; Implants for Surgery–Joint Replacement Implants–Particular Requirements. ISO: Geneva, Switzerland, 2007.
- ISO 21535; Non-Active Surgical Implants–Joint Replacement Implants–Specific Requirements for Hip-Joint Replacement Implants. ISO: Geneva, Switzerland, 2023.
- Proffen, B.L.; McElfresh, M.; Fleming, B.C.; Murray, M.M. A comparative anatomical study of the human knee and six animal species. Knee 2012, 19, 493–499. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ajwani, S.H.; Sutton, P.; Charalambous, C.P. Engineering advances in knee arthroplasty. In Advances in Medical and Surgical Engineering; Waqar, A., Phoenix, D., Jackson, M., Charalambous, P., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 55–70. ISBN 9780128197127. [Google Scholar] [CrossRef]
- Murr, L.; Amato, K.; Li, S.; Tian, Y.; Cheng, X.; Gaytan, S.; Martinez, E.; Shindo, P.; Medina, F.; Wicker, R. Microstructure and mechanical properties of open-cellular biomaterials prototypes for total knee replacement implants fabricated by electron beam melting. J. Mech. Behav. Biomed. Mater. 2011, 4, 1396–1411. [Google Scholar] [CrossRef]
- Bal, B.S.; Oonishi, H. Ceramic femoral components in total knee replacement. In Bioceramics in Joint Arthroplasty. Ceramics in Orthopaedics; Steinkopff: Berlin/Heidelberg, Germany, 2003; pp. 135–136. ISBN 9783798514171. [Google Scholar]
- Bergschmidt, P.; Bader, R.; Kluess, D.; Zietz, C.; Mittelmeier, W. The All-Ceramic Knee Endoprosthesis—The Gap Between Expectation and Experience with Ceramic Implants. Semin. Arthroplast. JSES 2012, 23, 262–267. [Google Scholar] [CrossRef]
- de Ruiter, L.; Janssen, D.; Briscoe, A.; Verdonschot, N. A preclinical numerical assessment of a polyetheretherketone femoral component in total knee arthroplasty during gait. J. Exp. Orthop. 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-P.; Guo, D.; Wang, S.-H.; Yang, Y.-Q.; Li, G. Structural stability of a polyetheretherketone femoral component—A 3D finite element simulation. Clin. Biomech. 2019, 70, 153–157. [Google Scholar] [CrossRef]
- Cowie, R.M.; Briscoe, A.; Fisher, J.; Jennings, L.M. PEEK-OPTIMA™ as an alternative to cobalt chrome in the femoral component of total knee replacement: A preliminary study. Proc. Inst. Mech. Eng. Part H. J. Eng. Med. 2016, 230, 1008–1015. [Google Scholar] [CrossRef]
- Galas, A.; Banci, L.; Innocenti, B. The Effects of Different Femoral Component Materials on Bone and Implant Response in Total Knee Arthroplasty: A Finite Element Analysis. Materials 2023, 16, 5605. [Google Scholar] [CrossRef] [PubMed]
- Boyle, A.J.; Landsman, T.L.; Wierzbicki, M.A.; Nash, L.D.; Hwang, W.; Miller, M.W.; Tuzun, E.; Hasan, S.M.; Maitland, D.J. In vitro and in vivo evaluation of a shape memory polymer foam-over-wire embolization device delivered in saccular aneurysm models. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1407–1415. [Google Scholar] [CrossRef]
- Memarian, P.; Pishavar, E.; Zanotti, F.; Trentini, M.; Camponogara, F.; Soliani, E.; Gargiulo, P.; Isola, M.; Zavan, B. Active Materials for 3D Printing in Small Animals: Current Modalities and Future Directions for Orthopedic Applications. Int. J. Mol. Sci. 2022, 23, 1045. [Google Scholar] [CrossRef]
- Choi, S.; Oh, Y.-I.; Park, K.-H.; Lee, J.-S.; Shim, J.-H.; Kang, B.-J. New clinical application of three-dimensional-printed polycaprolactone/β-tricalcium phosphate scaffold as an alternative to allograft bone for limb-sparing surgery in a dog with distal radial osteosarcoma. J. Vet. Med. Sci. 2019, 81, 434–442. [Google Scholar] [CrossRef]
- McKee, W.M.; May, C.; Macias, C.; Lapish, J.P. Pantarsal arthrodesis with a customised medial or lateral bone plate in 13 dogs. Veter. Rec. 2004, 154, 165–170. [Google Scholar] [CrossRef]
- Dorea, H.C.; McLaughlin, R.M.; Cantwell, H.D.; Read, R.; Armbrust, L.; Pool, R.; Roush, J.K.; Boyle, C. Evaluation of healing in feline femoral defects filled with cancellous autograft, cancellous allograft or Bioglass. Vet. Comp. Orthop. Traumatol. 2005, 18, 157–168. [Google Scholar] [CrossRef]
- DFranch, J.; Barba, A.; Rappe, K.; Maazouz, Y.; Ginebra, M. Use of three-dimensionally printed β-tricalcium phosphate synthetic bone graft combined with recombinant human bone morphogenic protein-2 to treat a severe radial atrophic nonunion in a Yorkshire terrier. Veter. Surg. 2020, 49, 1626–1631. [Google Scholar] [CrossRef]
- Goczkowski, M.; Gobin, M.; Hindié, M.; Agniel, R.; Larreta-Garde, V. Properties of interpenetrating polymer networks associating fibrin and silk fibroin networks obtained by a double enzymatic method. Mater. Sci. Eng. C 2019, 104, 109931. [Google Scholar] [CrossRef]
- Timercan, A.; Brailovski, V.; Petit, Y.; Lussier, B.; Séguin, B. Design, 3D printing, modeling, and in vitro testing of patient-specific endo-prostheses for limb-sparing surgery in dogs. Med Eng. Phys. 2019, 71, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Fedel, M. Blood compatibility of diamond-like carbon (DLC) coatings. In Diamond-Based Materials for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2013; pp. 71–102. ISBN 9780857093400. [Google Scholar]
- Love, C.; Cook, R.; Harvey, T.; Dearnley, P.; Wood, R. Diamond like carbon coatings for potential application in biological implants—A review. Tribol. Int. 2013, 63, 141–150. [Google Scholar] [CrossRef]
- Singh, A.; Ehteshami, G.; Massia, S.; He, J.; Storer, R.G.; Raupp, G. Glial cell and fibroblast cytotoxicity study on plasma-deposited diamond-like carbon coatings. Biomaterials 2003, 24, 5083–5089. [Google Scholar] [CrossRef]
- Choudhury, D.; Lackner, J.; Fleming, R.A.; Goss, J.; Chen, J.; Zou, M. Diamond-like carbon coatings with zirconium-containing interlayers for orthopedic implants. J. Mech. Behav. Biomed. Mater. 2017, 68, 51–61. [Google Scholar] [CrossRef]
- Alakoski, E.; Tiainen, V.-M.; Soininen, A.; Konttinen, Y.T. Load-Bearing Biomedical Applications of Diamond-Like Carbon Coatings–Current Status. Open Orthop. J. 2008, 2, 43–50. [Google Scholar] [CrossRef]
- Makarov, V.; Strel’nitskij, V.; Dedukh, N.; Nikolchenko, O. Diamond-like carbon coatings in endoprosthetics (literature review). Orthop. Traumatol. Prosthet. 2019, 2, 102–111. [Google Scholar] [CrossRef]
- Hauert, R. A review of modified DLC coatings for biological applications. Diam. Relat. Mater. 2003, 12, 583–589. [Google Scholar] [CrossRef]
- AbuAlia, M.; Fullam, S.; Cinotti, F.; Manninen, N.; Wimmer, M.A. Titanium Nitride Coatings on CoCrMo and Ti6Al4V Alloys: Effects on Wear and Ion Release. Lubricants 2024, 12, 96. [Google Scholar] [CrossRef]
- Orthopedics, N.A.J.; Alexander, J.S.; Hurst, J.M.; Morris, M.J.; Lombardi, A.V.; Berend, K.R.; Crawford, D.A. Use of Ion Beam Enhanced Deposition (IBED) Titanium Nitride for Knee Arthroplasty Implants. Surg. Technol. Online 2023, 42, 239–243. [Google Scholar] [CrossRef]
- Shenhar, A.; Gotman, I.; Radin, S.; Ducheyne, P.; Gutmanas, E. Titanium nitride coatings on surgical titanium alloys produced by a powder immersion reaction assisted coating method: Residual stresses and fretting behavior. Surf. Coat. Technol. 2000, 126, 210–218. [Google Scholar] [CrossRef]
- Grądzka-Dahlke, M. Comparison of Functional Properties of Thin Layers on Titanium and Cobalt Implant Alloys. Defect Diffus. Forum 2010, 297–301, 1053–1058. [Google Scholar] [CrossRef]
- Yuan, Z.; He, Y.; Lin, C.; Liu, P.; Cai, K. Antibacterial surface design of biomedical titanium materials for orthopedic applications. J. Mater. Sci. Technol. 2021, 78, 51–67. [Google Scholar] [CrossRef]
- Sidun, J.; Dąbrowski, J.R. Bone Ingrowth Processes on Porous Metalic Implants. Solid State Phenom. 2009, 147–149, 776–781. [Google Scholar] [CrossRef]
- Sidun, J.; Dabrowski, J. Investigation of Osseointegration of Porous Materials for Orthopedic Implants. Acta Mech. Slovaca 2010, 14, 78–85. [Google Scholar] [CrossRef]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsushita, T.; Kokubo, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef]
- Kapat, K.; Srivas, P.K.; Rameshbabu, A.P.; Maity, P.P.; Jana, S.; Dutta, J.; Majumdar, P.; Chakrabarti, D.; Dhara, S. Influence of Porosity and Pore-Size Distribution in Ti6Al4 V Foam on Physicomechanical Properties, Osteogenesis, and Quantitative Validation of Bone Ingrowth by Micro-Computed Tomography. ACS Appl. Mater. Interfaces 2017, 9, 39235–39248. [Google Scholar] [CrossRef]
- Lopez-Heredia, M.A.; Goyenvalle, E.; Aguado, E.; Pilet, P.; Leroux, C.; Dorget, M.; Weiss, P.; Layrolle, P. Bone growth in rapid prototyped porous titanium implants. J. Biomed. Mater. Res. Part A 2007, 85A, 664–673. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Peng, H.; Yang, J.; Li, Y.; Xu, J. Optimize the pore size-pore distribution-pore geometry-porosity of 3D-printed porous tantalum to obtain optimal critical bone defect repair capability. Mater. Sci. Eng. C 2023, 154, 213638. [Google Scholar] [CrossRef]
- Dall’ava, L.; Hothi, H.; Henckel, J.; Di Laura, A.; Tirabosco, R.; Eskelinen, A.; Skinner, J.; Hart, A. Osseointegration of retrieved 3D-printed, off-the-shelf acetabular implants. Bone Jt. Res. 2021, 10, 388–400. [Google Scholar] [CrossRef]
- Zheng, J.-P.; Chen, L.-J.; Chen, D.-Y.; Shao, C.-S.; Yi, M.-F.; Zhang, B. Effects of pore size and porosity of surface-modified porous titanium implants on bone tissue ingrowth. Trans. Nonferrous Met. Soc. China 2019, 29, 2534–2545. [Google Scholar] [CrossRef]
- Pilliar, R.M. Powder Metal-Made Orthopedic Implants with Porous Surface for Fixation by Tissue Ingrowth. Clin. Orthop. Relat. Res. 1983, 176, 42–51. [Google Scholar] [CrossRef]
- Iqbal, M.; Kumar, A.; Shabadi, R.; Singh, S. Fabrication and Surface Modification of Biomaterials for Orthopedic Implant: A Review. Surf. Rev. Lett. 2021, 30, 2141008. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, D.; Zan, X.; Ye, Q.; Sheng, S. Engineering the surfaces of orthopedic implants with osteogenesis and antioxidants to enhance bone formation in vitro and in vivo. Colloids Surfaces B. Biointerfaces 2022, 212, 112319. [Google Scholar] [CrossRef]
- Alontseva, D.; Azamatov, B.; Yantsen, Y.S.; Voinarovych, S.; Nazenova, G. A Brief Review of Current Trends in the Additive Manufacturing of Orthopedic Implants with Thermal Plasma-Sprayed Coatings to Improve the Implant Surface Biocompatibility. Coatings 2023, 13, 1175. [Google Scholar] [CrossRef]
- Cheong, V.S.; Fromme, P.; Mumith, A.; Coathup, M.J.; Blunn, G.W. Novel adaptive finite element algorithms to predict bone ingrowth in additive manufactured porous implants. J. Mech. Behav. Biomed. Mater. 2018, 87, 230–239. [Google Scholar] [CrossRef]
- Koju, N.; Niraula, S.; Fotovvati, B. Additively Manufactured Porous Ti6Al4V for Bone Implants: A Review. Metals 2022, 12, 687. [Google Scholar] [CrossRef]
- ASTM F1472; Standard Specification for Wrought Titanium-6Aluminum-4Vanadium Alloy for Surgical Implant Applications (UNS R56400). ASTM: West Conshohocken, PA, USA, 2023.
- Yu, Z.; Cai, H.; Liu, Z. Factors that impact the patellofemoral contact stress in the TKA: A review. Arthroplasty 2023, 5, 44. [Google Scholar] [CrossRef]
- Adam, R.; Moldovan, C.; Tudorache, S.; Hârșovescu, T.; Orban, C.; Pogărășteanu, M.; Rusu, E. Patellar Resurfacing in Total Knee Arthroplasty, a Never-Ending Controversy; Case Report and Literature Review. Diagnostics 2023, 13, 383. [Google Scholar] [CrossRef]
- Trębacz, P.; Frymus, J.; Barteczko, A.; Pawlik, M.; Kurkowska, A.; Czopowicz, M. A 3D Printed Anatomically Pre-Contoured Plate for the Treatment of Y-T Humeral Condylar Fractures: A Feline Cadaveric Study. Animals 2024, 14, 537. [Google Scholar] [CrossRef]



| Standard Series | Standard |
|---|---|
| ISO 5832 This series specifies the requirements for metallic materials used to manufacture surgical implants. | ISO 5832-1: Implants for surgery—Metallic materials—Part 1: Wrought stainless steel. |
| ISO 5832-2: Implants for surgery—Metallic materials—Part 2: Unalloyed titanium. | |
| ISO 5832-3: Implants for surgery—Metallic materials—Part 3: Wrought titanium 6-aluminium 4-vanadium alloy. | |
| ISO 5832-4: Implants for surgery—Metallic materials—Part 4: Cobalt-chromium-molybdenum casting alloy. | |
| ISO 5832-12: Implants for surgery—Metallic materials—Part 12: Wrought cobalt-chromium-molybdenum alloy. | |
| ISO 1099 This series provides guidelines for the biological evaluation of medical devices, including orthopedic implants. | ISO 10993-1: Biological evaluation of medical devices—Part 1: Evaluation and testing within a risk management process. |
| ISO 10993-5: Biological evaluation of medical devices—Part 5: Tests for in vitro cytotoxicity. | |
| ISO 10993-11: Biological evaluation of medical devices—Part 11: Tests for systemic toxicity. | |
| ISO 7207 This series pertains to the components used in knee joint prostheses. | ISO 7207-1: Implants for surgery—Components for partial and total knee joint prostheses—Part 1: Classification, definitions, and designation of dimensions. |
| ISO 7207-2: Implants for surgery—Components for partial and total knee joint prostheses—Part 2: Articulating surfaces made of metal, ceramic, and plastic materials. This part specifies surface finish requirements for the articulating surfaces of total and partial knee joint prostheses classified in ISO 7207-1. | |
| ISO 13485: Medical devices—Quality management systems—Requirements for regulatory purposes | |
| ISO 14243 This series outlines the methods for testing the wear of knee joint prostheses. | ISO 14243-1: Implants for surgery—Wear of total knee-joint prostheses—Part 1: Loading and displacement parameters for wear-testing machines with load control and corresponding environmental conditions for the test. |
| ISO 14243-2: Implants for surgery—Wear of total knee-joint prostheses—Part 2: Methods of measurement | |
| ISO 21534: Implants for surgery—Joint replacement implants—Particular requirements. | |
| ISO 21535: Non-active surgical implants—Joint replacement implants—Specific requirements for hip-joint replacement implants. While specific to hip joint replacement, these principles and testing methods are also relevant to knee joint prostheses. | |
| Material | Density | Biocompatibility | Wear Resistance | Bone Integration | Brittleness | Manufacturability/Price |
|---|---|---|---|---|---|---|
| Cobalt Alloys | ~8.3 g/cm3 | Good | Very High | Good | Low | Difficult/High |
| Titanium Alloys | ~4.4 g/cm3 | Good | Moderate | Very good | Low | Moderate/Moderate |
| Bioceramics (Zirconia) | ~5.7–6.1 g/cm3 | Very good | High | Very Good | High | Difficult/High |
| Polymers (PEEK) | ~1.3 g/cm3 | Good | Moderate | Poor | Low | Moderate/High |
| Selective Laser Melting (SLM) | Electron Beam Melting (EBM) | |
|---|---|---|
| Process | Utilizes a high-power laser to melt and fuse metal powder layer by layer selectively. | Employs an electron beam to melt metal powder in a vacuum environment, layer by layer. |
| Materials | Commonly includes titanium alloys (e.g., Ti-6Al-4V), cobalt-chromium alloys, and stainless steel. | Titanium alloys were chosen primarily for their biocompatibility and mechanical properties. |
| Advantages | Facilitates high precision and the creation of intricate lattice structures with controlled porosity, promoting enhanced mechanical properties and bone ingrowth. | Produces parts with excellent mechanical properties, suitable for high-performance implants with complex geometries. |
| Implant Measured Dimensions, mm | ||||
|---|---|---|---|---|
| Implant Size | Width | Length | Height (From Base to Groove Condyles Peak Surface) | Thickness (From Base to the Bottom of the Groove) |
| #1 | 6.5 | 11.5 | 5.5 | 3.7 |
| #1.5 | 7.5 | 13.0 | 6.0 | 3.9 |
| #2 | 8.5 | 14.5 | 6.5 | 4.1 |
| #2.5 | 9.5 | 16.5 | 7.0 | 4.3 |
| #3 | 10.5 | 18.0 | 7.5 | 4.6 |
| #4 | 12.5 | 21.5 | 8.5 | 5.0 |
| #5 | 14.5 | 25.0 | 9.5 | 5.5 |
| #6 | 16.5 | 28.0 | 10.5 | 6.0 |
| #7 | 18.5 | 32.0 | 11.5 | 6.5 |
| #8 | 21.0 | 36.0 | 13.0 | 7.0 |
| #9 | 23.5 | 40.5 | 14.5 | 7.5 |
| #10 | 26.0 | 45.0 | 16.0 | 8.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, M.; Trębacz, P.; Barteczko, A.; Kurkowska, A.; Piątek, A.; Paszenda, Z.; Basiaga, M. Evaluation of Patellar Groove Prostheses in Veterinary Medicine: Review of Technological Advances, Technical Aspects, and Quality Standards. Materials 2025, 18, 1652. https://doi.org/10.3390/ma18071652
Pawlik M, Trębacz P, Barteczko A, Kurkowska A, Piątek A, Paszenda Z, Basiaga M. Evaluation of Patellar Groove Prostheses in Veterinary Medicine: Review of Technological Advances, Technical Aspects, and Quality Standards. Materials. 2025; 18(7):1652. https://doi.org/10.3390/ma18071652
Chicago/Turabian StylePawlik, Mateusz, Piotr Trębacz, Anna Barteczko, Aleksandra Kurkowska, Agata Piątek, Zbigniew Paszenda, and Marcin Basiaga. 2025. "Evaluation of Patellar Groove Prostheses in Veterinary Medicine: Review of Technological Advances, Technical Aspects, and Quality Standards" Materials 18, no. 7: 1652. https://doi.org/10.3390/ma18071652
APA StylePawlik, M., Trębacz, P., Barteczko, A., Kurkowska, A., Piątek, A., Paszenda, Z., & Basiaga, M. (2025). Evaluation of Patellar Groove Prostheses in Veterinary Medicine: Review of Technological Advances, Technical Aspects, and Quality Standards. Materials, 18(7), 1652. https://doi.org/10.3390/ma18071652






