Ultra-Small Iron-Based Nanoparticles with Mild Photothermal-Enhanced Cascade Enzyme-Mimic Reactions for Tumor Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Materials and Reagents
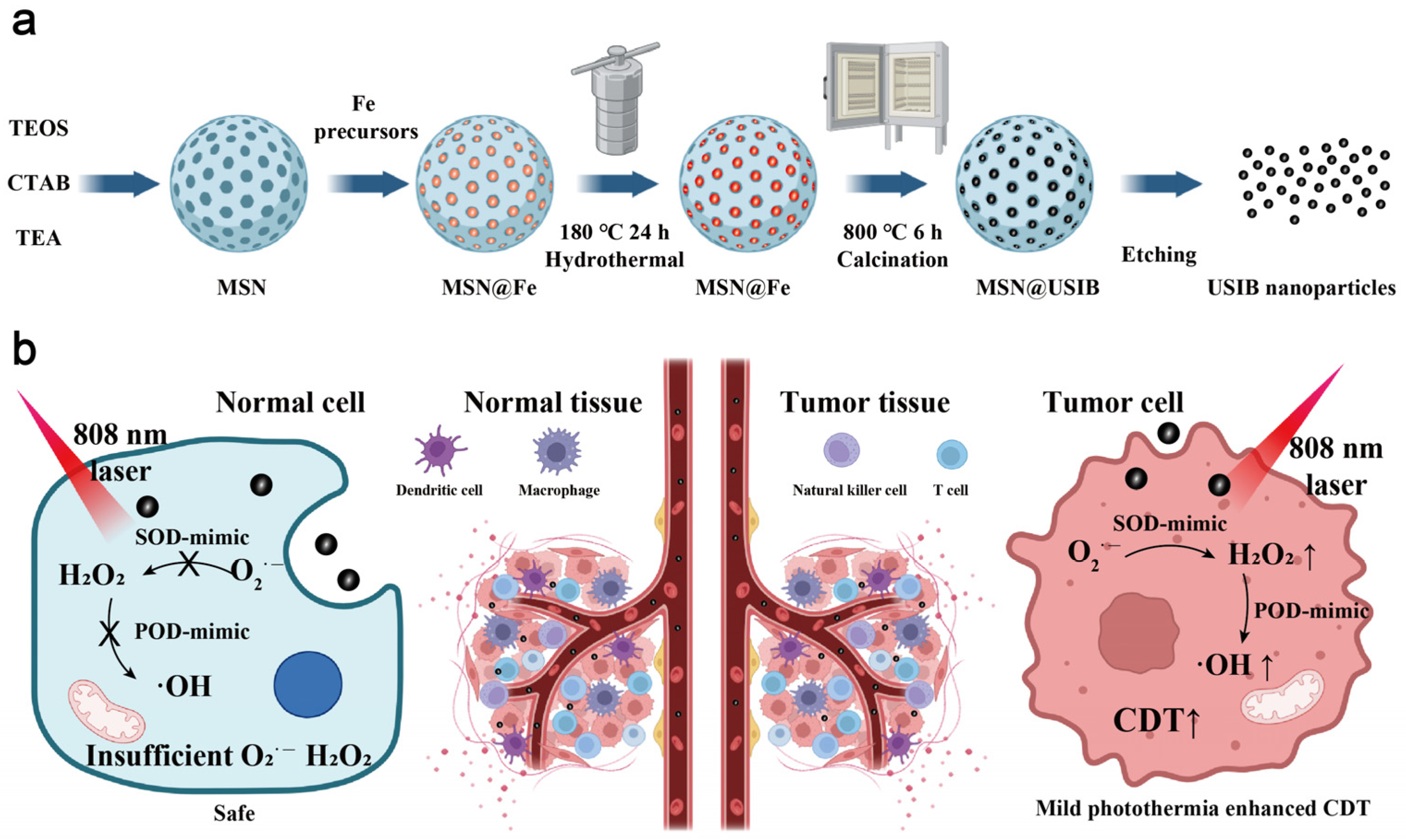
2.2. Synthesis of USIB Nanoparticles
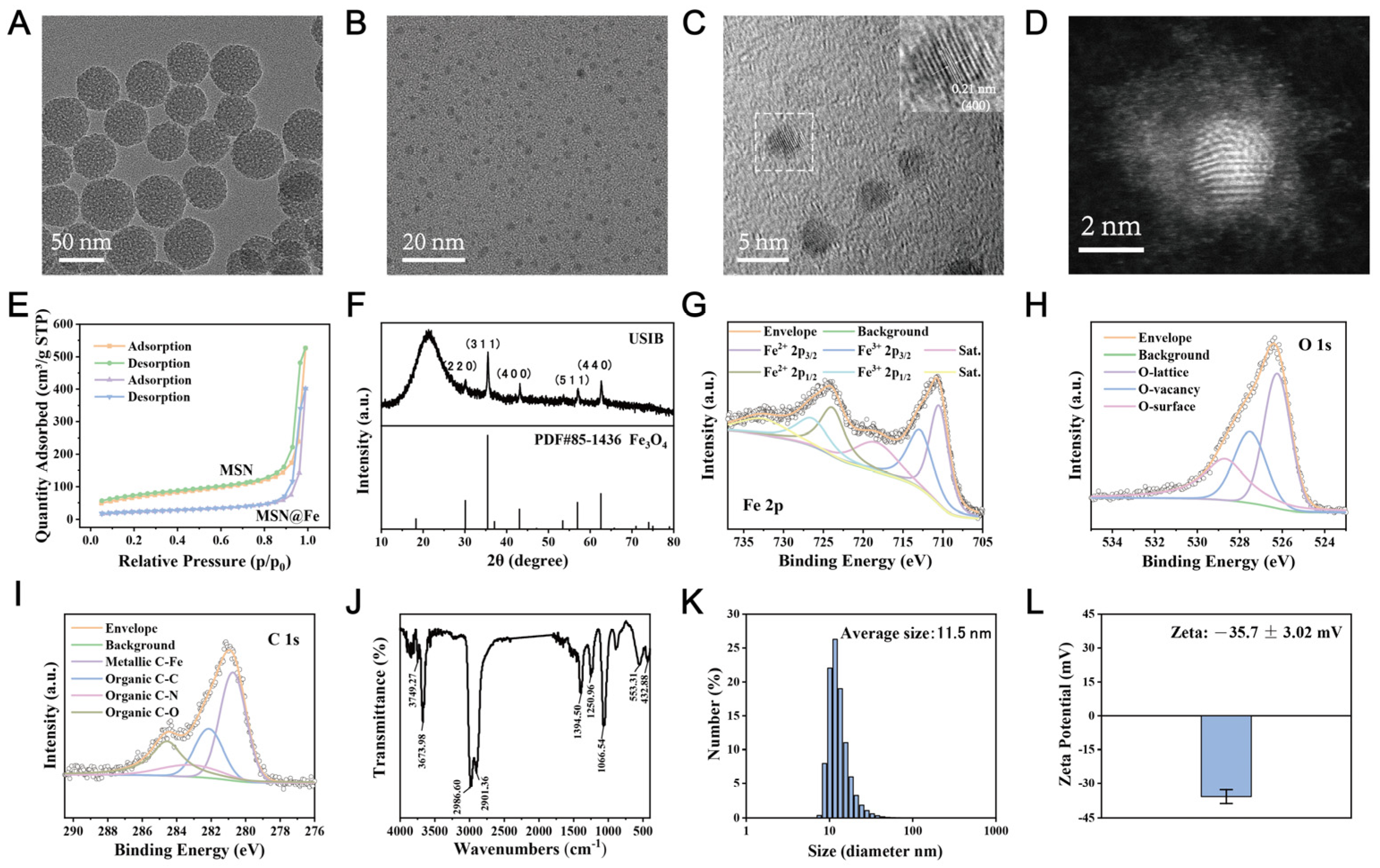
2.3. Characterization
2.4. Photothermal Performance of USIB Nanoparticles
2.5. Superoxide Dismutase Mimic Activity of USIB Nanoparticles
2.6. Peroxidase-Mimic Activity of USIB Nanoparticles
2.7. VSM and MRI Experiments
2.8. Cell Culture
2.9. Cellular Uptake Assay
2.10. MTT Assay
2.11. Cell Imaging
2.12. Flow Cytometry Analysis
2.13. Biosafety Assay
2.14. In Vivo MRI
2.15. In Vivo Therapeutic Evaluation of USIB Nanoparticles
3. Results and Discussion
3.1. Synthesis and Characterizations
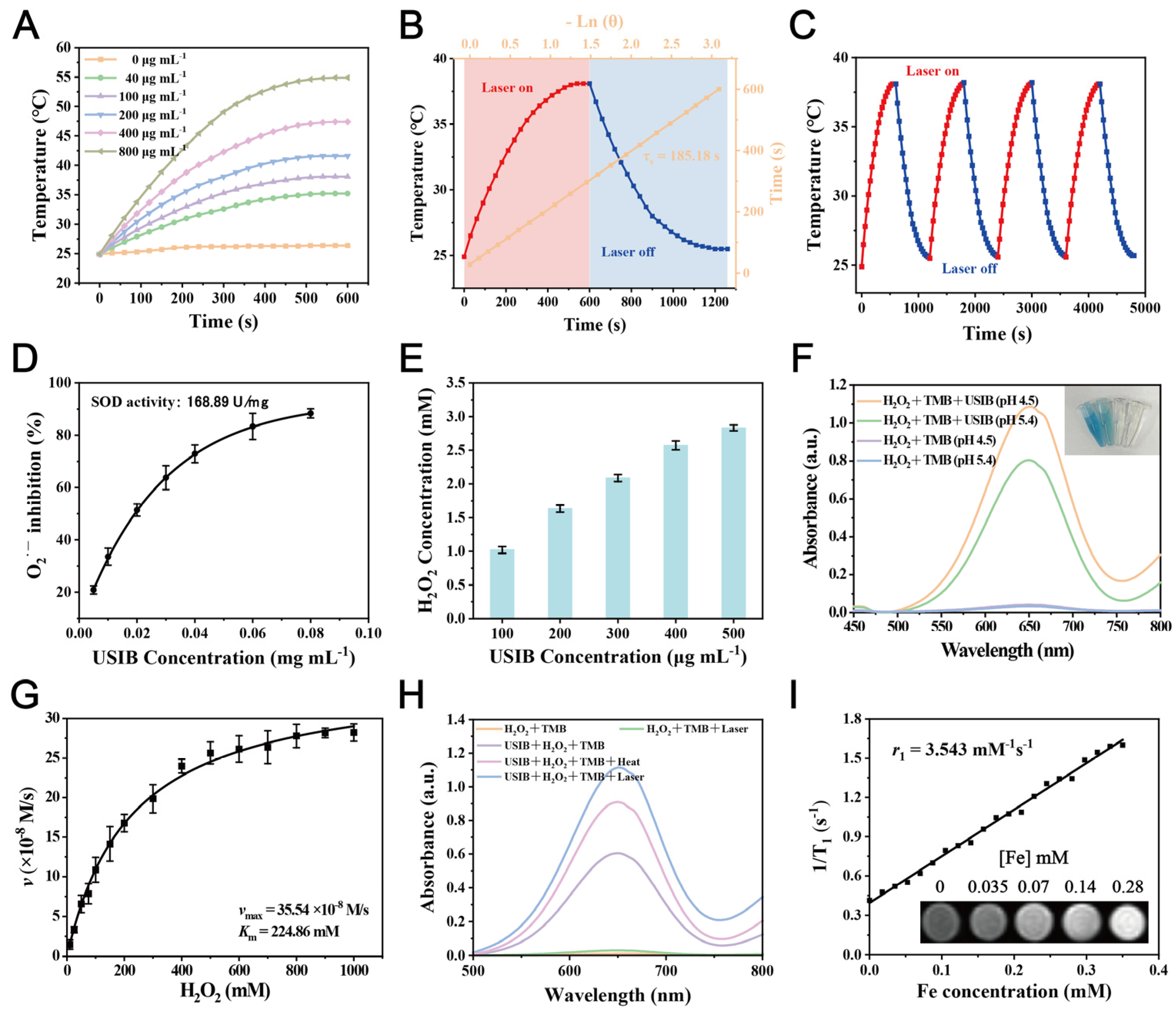
3.2. Photothermal Performance of USIB Nanoparticles
3.3. Enzyme-Mimic Property of USIB Nanoparticles
3.4. Magnetic Properties and Magnetic Resonance Image (MRI) of USIB Nanoparticles
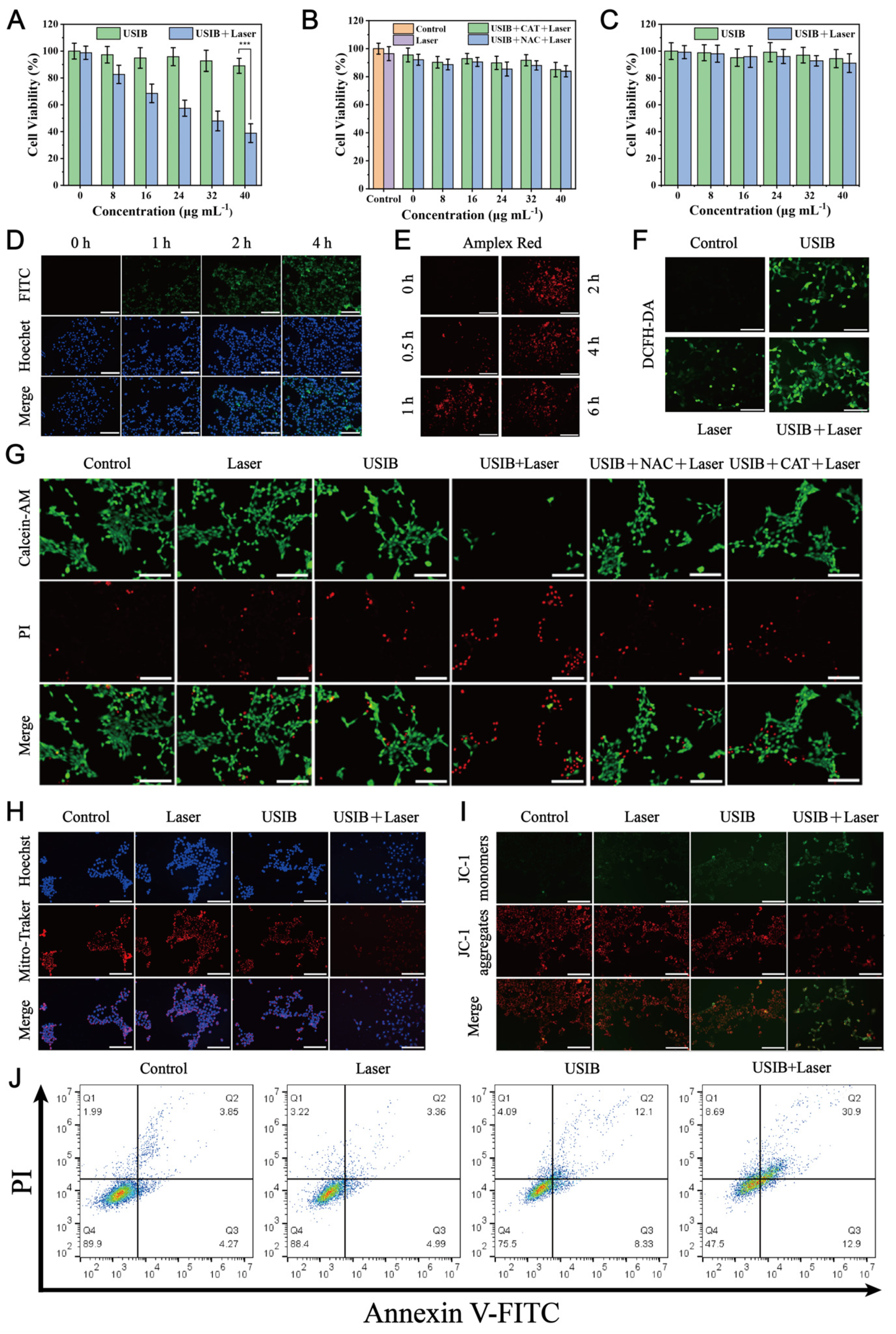
3.5. Laser-Enhanced Cytotoxicity of USIB Nanoparticles In Vitro
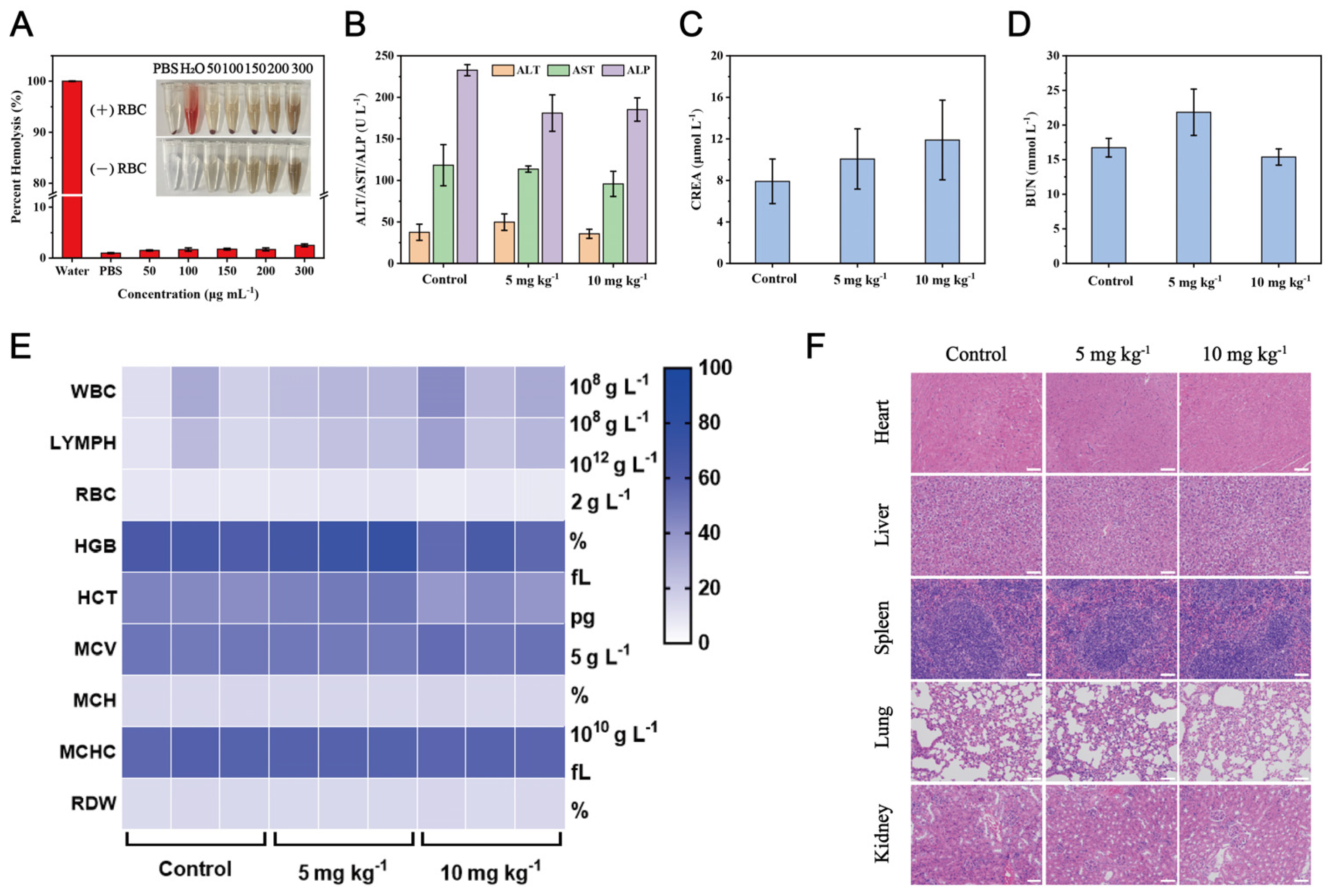
3.6. Biosafety Evaluation
3.7. Tumor Therapeutic Effect In Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CDT | Chemodynamic therapy |
| H2O2 | Hydrogen peroxide |
| USIB | Ultra-small iron-based |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| O2·− | Superoxide anions |
| ·OH | Hydroxyl radicals |
| TME | Tumor microenvironment |
| CAT | Catalase |
| GPx | Glutathione peroxidase |
| PTT | Photothermal therapy |
| TEOS | Tetraethyl orthosilicate |
| CTAB | Hexadecyl trimethyl ammonium bromide |
| TEA | Trolamine |
| NaOH | Sodium hydroxide |
| MB | Methylene blue |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
| NAC | N-acetyl cysteine |
| DMSO | Dimethyl sulfoxide |
| MTT | Methylthiazolyldiphenyl-tetrazolium bromide |
| MSN | Mesoporous silica nanoparticle |
| TEM | Transmission electron microscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| BET | Brunauer–Emmett–Teller |
| FT-IR | Fourier transform infrared |
| UV-Vis | Ultraviolet-visible |
| NIR | Near-infrared |
| ITI | Infrared thermal imaging |
| 4T1 | Mouse breast cancer |
| L929 | Mouse fibroblast |
| ROS | Reactive oxygen species |
| MRI | Magnetic Resonance Image |
| H&E | Hematoxylin and eosin |
| TUNEL | Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling |
References
- Zhou, Y.; Fan, S.; Feng, L.; Huang, X.; Chen, X. Manipulating Intratumoral Fenton Chemistry for Enhanced Chemodynamic and Chemodynamic-Synergized Multimodal Therapy. Adv. Mater. 2021, 33, e2104223. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, Y.; He, M.; Bu, W. Chemodynamic Therapy: Tumour Microenvironment-Mediated Fenton and Fenton-like Reactions. Angew. Chem. Int. Ed. 2019, 58, 946–956. [Google Scholar] [CrossRef]
- Li, S.L.; Jiang, P.; Jiang, F.L.; Liu, Y. Recent Advances in Nanomaterial-Based Nanoplatforms for Chemodynamic Cancer Therapy. Adv. Funct. Mater. 2021, 31, 2100243. [Google Scholar] [CrossRef]
- Chen, W.; Yang, M.; Li, J.; Chen, Z.; Hu, L.; Zhang, J.; Cai, L.; Qiu, L.; Chen, J. GSH-Activatable Metal-Phenolic Networks for Photothermal-Enhanced Chemotherapy and Chemodynamic Therapy. J. Funct. Biomater. 2023, 14, 436. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 902. [Google Scholar] [CrossRef]
- Huo, M.; Wang, L.; Chen, Y.; Shi, J. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat. Commun. 2017, 8, 357. [Google Scholar] [CrossRef]
- Di, X.; Pei, Z.; Pei, Y.; James, T.D. Tumor microenvironment-oriented MOFs for chemodynamic therapy. Coord. Chem. Rev. 2023, 484, 215098. [Google Scholar] [CrossRef]
- Fan, H.; Guo, Z. Tumor microenvironment-responsive manganese-based nanomaterials for cancer treatment. Coord. Chem. Rev. 2023, 480, 215027. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Nanocatalytic Medicine. Adv. Mater. 2019, 31, 1901778–1901823. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, X.; Liu, Z.; Cheng, L. Recent progress of chemodynamic therapy-induced combination cancer therapy. Nano Today 2020, 35, 100946. [Google Scholar] [CrossRef]
- Gao, H.; Cao, Z.; Liu, H.; Chen, L.; Bai, Y.; Wu, Q.; Yu, X.; Wei, W.; Wang, M. Multifunctional nanomedicines-enabled chemodynamic-synergized multimodal tumor therapy via Fenton and Fenton-like reactions. Theranostics 2023, 13, 1974–2014. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Jiang, G.; Wan, Y.; Liu, J.; Pi, F. Nanomaterials-modulated Fenton reactions: Strategies, chemodynamic therapy and future trends. Chem. Eng. J. 2023, 466, 142960. [Google Scholar] [CrossRef]
- Liu, J.; Dong, S.; Gai, S.; Dong, Y.; Liu, B.; Zhao, Z.; Xie, Y.; Feng, L.; Yang, P.; Lin, J. Design and Mechanism Insight of Monodispersed AuCuPt Alloy Nanozyme with Antitumor Activity. ACS Nano 2023, 17, 20402–20423. [Google Scholar] [CrossRef]
- Yan, R.; Ren, J.; Wen, J.; Cao, Z.; Wu, D.; Qin, M.; Xu, D.; Castillo, R.; Li, F.; Wang, F.; et al. Enzyme Therapeutic for Ischemia and Reperfusion Injury in Organ Transplantation. Adv. Mater. 2022, 34, e2105670. [Google Scholar] [CrossRef]
- Wu, S.; Nan, F.; Zhang, K.; Hao, W.; Shi, D.; Li, Y.; Deng, W.; Jarhen, N.; Li, K.; Xiao, Y.; et al. A novel metal-organic framework encapsulated iridium oxide nanozyme enhanced antisense oligonucleotide combo for osteoarthritis synergistic therapy. Aggregate 2024, 5, e635. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, M.; Wang, S.; Jiang, W.; Yang, F.; Shen, X.; Zhang, L.; Yan, X.; Jiang, B.; Fan, K. A Self-Catalytic NO/O2 Gas-Releasing Nanozyme for Radiotherapy Sensitization through Vascular Normalization and Hypoxia Relief. Adv. Mater. 2024, 36, e2403921. [Google Scholar] [CrossRef]
- Yu, B.; Wang, W.; Sun, W.; Jiang, C.; Lu, L. Defect Engineering Enables Synergistic Action of Enzyme-Mimicking Active Centers for High-Efficiency Tumor Therapy. J. Am. Chem. Soc. 2021, 143, 8855–8865. [Google Scholar] [CrossRef]
- Yang, H.; Lin, P.; Zhang, B.; Li, F.; Ling, D. A Nucleophilicity-Engineered DNA Ligation Blockade Nanoradiosensitizer Induces Irreversible DNA Damage to Overcome Cancer Radioresistance. Adv. Mater. 2024, 36, e2410031. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, L.; Liu, X.; Ye, C.; Zhu, P.; Tan, T.; Wang, D.; Wang, Y. Tuning oxidant and antioxidant activities of ceria by anchoring copper single-site for antibacterial application. Nat. Commun. 2024, 15, 1010. [Google Scholar] [CrossRef]
- Li, Z.; Ding, B.; Li, J.; Chen, H.; Zhang, J.; Tan, J.; Ma, X.; Han, D.; Ma, P.; Lin, J. Multi-Enzyme Mimetic MoCu Dual-Atom Nanozyme Triggering Oxidative Stress Cascade Amplification for High-Efficiency Synergistic Cancer Therapy. Angew. Chem. Int. Ed. 2024, 64, e202413661. [Google Scholar] [CrossRef]
- Demkiv, O.; Nogala, W.; Stasyuk, N.; Grynchyshyn, N.; Vus, B.; Gonchar, M. The Peroxidase-like Nanocomposites as Hydrogen Peroxide-Sensitive Elements in Cholesterol Oxidase-Based Biosensors for Cholesterol Assay. J. Funct. Biomater. 2023, 14, 315. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, B.; Li, J.; Han, D.; Chen, H.; Tan, J.; Meng, Q.; Zheng, P.; Ma, P.A.; Lin, J. Valence-tailored copper-based nanoparticles for enhanced chemodynamic therapy through prolonged ROS generation and potentiated GSH depletion. Nano Res. 2024, 17, 6342–6352. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, D.; Tian, G.; He, Q.; Zhang, X.; Liao, J.; Mei, L.; Chen, L.; Gao, L.; Zhao, L.; et al. Coordination-Driven Self-Assembly Strategy-Activated Cu Single-Atom Nanozymes for Catalytic Tumor-Specific Therapy. J. Am. Chem. Soc. 2023, 145, 4279–4293. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Cao, F.; Li, W.; Zhang, L.; You, Y.; Deng, Q.; Dong, K.; Ren, J.; Qu, X. Bioinspired Construction of a Nanozyme-Based H2O2 Homeostasis Disruptor for Intensive Chemodynamic Therapy. J. Am. Chem. Soc. 2020, 142, 5177–5183. [Google Scholar] [CrossRef]
- Shen, H.; Fu, Y.; Liu, F.; Zhang, W.; Yuan, Y.; Yang, G.; Yang, M.; Li, L. AuCePt porous hollow cascade nanozymes targeted delivery of disulfiram for alleviating hepatic insulin resistance. J. Nanobiotechnol. 2024, 22, 660. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Shi, P.; Hu, X.; Fan, X.; Wu, Y.; Pan, J.; Bai, Q.; Li, Q. Single-atom nanozyme liposome-integrated microneedles for in situ drug delivery and anti-inflammatory therapy in Parkinson’s disease. J. Nanobiotechnol. 2024, 22, 643. [Google Scholar] [CrossRef]
- Gao, F.; Liang, W.; Chen, Q.; Chen, B.; Liu, Y.; Liu, Z.; Xu, X.; Zhu, R.; Cheng, L. A Curcumin-Decorated Nanozyme with ROS Scavenging and Anti-Inflammatory Properties for Neuroprotection. Nanomaterials 2024, 14, 389. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Zhang, M.; Yu, Z.; Zhang, M. Oral Administration of Platinum Nanoparticles with SOD/CAT Cascade Catalytic Activity to Alleviate Ulcerative Colitis. J. Funct. Biomater. 2023, 14, 548. [Google Scholar] [CrossRef]
- Sheng, J.; Wu, Y.; Ding, H.; Feng, K.; Shen, Y.; Zhang, Y.; Gu, N. Multienzyme-Like Nanozymes: Regulation, Rational Design, and Application. Adv. Mater. 2024, 36, e2211210. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Liu, H.; Qin, S.; Li, M.; Shi, L.; Zhou, C.; Liao, T.; Li, C.; Lv, Q.; et al. Nickel-Based Metal-Organic Frameworks Promote Diabetic Wound Healing via Scavenging Reactive Oxygen Species and Enhancing Angiogenesis. Small 2024, 20, e2305076. [Google Scholar] [CrossRef]
- Shi, T.; Cui, Y.; Yuan, H.; Qi, R.; Yu, Y. Burgeoning Single-Atom Nanozymes for Efficient Bacterial Elimination. Nanomaterials 2023, 13, 2760. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; An, X.; Liu, L.; Cao, X.; Wu, Z.; Jia, W.; Sun, J.; Wang, H.; Huo, J.; Sun, Z.; et al. Self-Cascade Redox Modulator Trilogically Renovates Intestinal Microenvironment for Mitigating Endotoxemia. ACS Nano 2024, 18, 2131–2148. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, L.; Zhang, Y.; Liu, X.; Song, A.; Ren, J.; Qu, X. A Self-Adaptive Pyroptosis Inducer: Optimizing the Catalytic Microenvironment of Nanozymes by Membrane-Adhered Microbe Enables Potent Cancer Immunotherapy. Adv. Mater. 2024, 36, e2310063. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Kim, T.; Choi, I.; Soh, M.; Kim, D.; Kim, Y.; Jang, H.; Yang, H.; Kim, J.Y.; Park, H.; et al. Ceria Nanoparticles that can Protect against Ischemic Stroke. Angew. Chem. Int. Ed. 2012, 51, 11039–11043. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, L.; Qiu, K.; Liao, X.; Rees, T.W.; Zhao, Z.; Ji, L.; Chao, H. An Ultrasmall RuO2 Nanozyme Exhibiting Multienzyme-like Activity for the Prevention of Acute Kidney Injury. ACS Appl. Mater. Inter. 2020, 12, 31205–31216. [Google Scholar] [CrossRef]
- Liu, T.; Xiao, B.; Xiang, F.; Tan, J.; Chen, Z.; Zhang, X.; Wu, C.; Mao, Z.; Luo, G.; Chen, X.; et al. Ultrasmall copper-based nanoparticles for reactive oxygen species scavenging and alleviation of inflammation related diseases. Nat. Commun. 2020, 11, 2716–2788. [Google Scholar] [CrossRef]
- Cui, X.; Ruan, Q.; Zhuo, X.; Xia, X.; Hu, J.; Fu, R.; Li, Y.; Wang, J.; Xu, H. Photothermal Nanomaterials: A Powerful Light-to-Heat Converter. Chem. Rev. 2023, 123, 6891–6952. [Google Scholar] [CrossRef]
- Fan, W.; Yung, B.; Huang, P.; Chen, X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem. Rev. 2017, 117, 13566–13638. [Google Scholar] [CrossRef]
- Jana, D.; He, B.; Chen, Y.; Liu, J.; Zhao, Y. A Defect-Engineered Nanozyme for Targeted NIR-II Photothermal Immunotherapy of Cancer. Adv. Mater. 2022, 36, e2206401. [Google Scholar] [CrossRef]
- Xue, C.; Li, M.; Liu, C.; Li, Y.; Fei, Y.; Hu, Y.; Cai, K.; Zhao, Y.; Luo, Z. NIR-Actuated Remote Activation of Ferroptosis in Target Tumor Cells through a Photothermally Responsive Iron-Chelated Biopolymer Nanoplatform. Angew. Chem. Int. Ed. 2021, 60, 8938–8947. [Google Scholar] [CrossRef]
- Niu, X.; Zhu, Y.; Ding, C.; Ma, J.; Wei, P.; Lin, Y.; Fang, W.; He, Q.; Li, C.; Cheng, J.; et al. Dual-Active Center AgFeCu Nanocatalyst for Tumor Destruction via Self-Catalytically Enhanced Mild Photothermal Therapy. Adv. Funct. Mater. 2023, 33, e2306778. [Google Scholar] [CrossRef]
- Dong, S.; Dong, Y.; Zhao, Z.; Liu, J.; Liu, S.; Feng, L.; He, F.; Gai, S.; Xie, Y.; Yang, P. “Electron Transport Chain Interference” Strategy of Amplified Mild-Photothermal Therapy and Defect-Engineered Multi-Enzymatic Activities for Synergistic Tumor-Personalized Suppression. J. Am. Chem. Soc. 2023, 145, 9488–9507. [Google Scholar] [CrossRef] [PubMed]
- Tao, N.; Jiao, L.; Li, H.; Deng, L.; Wang, W.; Zhao, S.; Chen, W.; Chen, L.; Zhu, C.; Liu, Y. A Mild Hyperthermia Hollow Carbon Nanozyme as Pyroptosis Inducer for Boosted Antitumor Immunity. ACS Nano 2023, 17, 22844–22858. [Google Scholar] [CrossRef] [PubMed]
- GB/T 37966-2019; Nanotechnologies-Measurementmethod for Peroxidase-like Activity of Iron Oxide Nanoparticles. China National Standards: Beijing, China, 2019.
- Yang, L.; Tian, B.; Xie, Y.; Dong, S.; Yang, M.; Gai, S.; Lin, J. Oxygen-Vacancy-Rich Piezoelectric BiO2−x Nanosheets for Augmented Piezocatalytic, Sonothermal, and Enzymatic Therapies. Adv. Mater. 2023, 35, e2300648. [Google Scholar] [CrossRef]
- Shi, X.; Liu, Q.; Gong, Z.; Jin, Z.; Guo, H.; Wen, X.; Li, C. Vacancy-rich Fe-Ce mixed oxides as nanoenzymes for efficient antioxidant activity in vitro. Appl. Surf. Sci. 2024, 664, 160256. [Google Scholar] [CrossRef]
- Hu, L.; Sun, W.; Tang, Y.; Li, S.; Zhang, B.; Sun, X.; Ji, W.; Ma, L.; Deng, H.; Han, S.; et al. Photothermal effect enhancing graphene quantum dots/semiconducting polymer/nanozyme-mediated cancer catalytic therapy. Carbon 2021, 176, 148–156. [Google Scholar] [CrossRef]
- Vyas, A.; Petrášek, Z.; Nidetzky, B. Limits of Non-invasive Enzymatic Activation by Local Temperature Control. Small 2024, 20, e2312220. [Google Scholar] [CrossRef]
- Chen, Q.; He, S.; Zhang, F.; Cui, F.; Liu, J.; Wang, M.; Wang, D.; Jin, Z.; Li, C. A versatile Pt-Ce6 nanoplatform as catalase nanozyme and NIR-II photothermal agent for enhanced PDT/PTT tumor therapy. Sci. China Mater. 2021, 64, 510–530. [Google Scholar] [CrossRef]
- Pan, M.M.; Li, P.; Yu, Y.P.; Jiang, M.; Yang, X.; Zhang, P.; Nie, J.; Hu, J.; Yu, X.; Xu, L. Bimetallic Ions Functionalized Metal–Organic-Framework Nanozyme for Tumor Microenvironment Regulating and Enhanced Photodynamic Therapy for Hypoxic Tumor. Adv. Healthc. Mater. 2023, 12, e2300821. [Google Scholar] [CrossRef]
- Dai, R.; Liu, Q.; Zhang, B.; Zhang, X.; Gao, M.; Li, D.; Kang, W.; Chen, L.; Zhao, M.; Zheng, Z.; et al. A Single NIR-II Laser-Triggered Self-Enhancing Photo/Enzyme-coupled Three-in-One Nanosystems for Breast Cancer Phototheranostics. Adv. Healthc. Mater. 2024, 13, e2302783. [Google Scholar] [CrossRef]
- Kim, B.H.; Lee, N.; Kim, H.; An, K.; Park, Y.I.; Choi, Y.; Shin, K.; Lee, Y.; Kwon, S.G.; Na, H.B.; et al. Large-Scale Synthesis of Uniform and Extremely Small-Sized Iron Oxide Nanoparticles for High-Resolution T1 Magnetic Resonance Imaging Contrast Agents. J. Am. Chem. Soc. 2011, 133, 12624–12631. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Kim, S.; Yoo, D.; Shin, T.H.; Kim, H.; Gomes, M.D.; Kim, S.H.; Pines, A.; Cheon, J. Distance-dependent magnetic resonance tuning as a versatile MRI sensing platform for biological targets. Nat. Mater. 2017, 16, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Zhao, F.; Gao, W.; Yang, X.; Ju, Y.; Zhao, L.; Guo, W.; Xie, J.; Liang, X.; Tao, X.; et al. Magnetic Reactive Oxygen Species Nanoreactor for Switchable Magnetic Resonance Imaging Guided Cancer Therapy Based on pH-Sensitive Fe5C2@Fe3O4 Nanoparticles. ACS Nano 2019, 13, 10002–10014. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, J.; Li, S.; Zhu, X.; Yu, H.; Gao, H.; Qi, J.; Ying, Y.; Qiao, L.; Zheng, J.; Li, J.; et al. Ultra-Small Iron-Based Nanoparticles with Mild Photothermal-Enhanced Cascade Enzyme-Mimic Reactions for Tumor Therapy. Materials 2025, 18, 1649. https://doi.org/10.3390/ma18071649
Yu J, Li S, Zhu X, Yu H, Gao H, Qi J, Ying Y, Qiao L, Zheng J, Li J, et al. Ultra-Small Iron-Based Nanoparticles with Mild Photothermal-Enhanced Cascade Enzyme-Mimic Reactions for Tumor Therapy. Materials. 2025; 18(7):1649. https://doi.org/10.3390/ma18071649
Chicago/Turabian StyleYu, Jing, Shuangshan Li, Xun Zhu, Hongyan Yu, Hao Gao, Jiarui Qi, Yao Ying, Liang Qiao, Jingwu Zheng, Juan Li, and et al. 2025. "Ultra-Small Iron-Based Nanoparticles with Mild Photothermal-Enhanced Cascade Enzyme-Mimic Reactions for Tumor Therapy" Materials 18, no. 7: 1649. https://doi.org/10.3390/ma18071649
APA StyleYu, J., Li, S., Zhu, X., Yu, H., Gao, H., Qi, J., Ying, Y., Qiao, L., Zheng, J., Li, J., & Che, S. (2025). Ultra-Small Iron-Based Nanoparticles with Mild Photothermal-Enhanced Cascade Enzyme-Mimic Reactions for Tumor Therapy. Materials, 18(7), 1649. https://doi.org/10.3390/ma18071649





