Effect of Disulfide Bond Density on the Properties of Polyurethane/Epoxy Interpenetrating Networks
Highlights
- By partially and completely substituting 2-Aminophenyl disulfide(2-ADPS) with Di-octachlorodiphenylamine methane(MOCA) during the synthesis of polyurethane/epoxy resin interpenetrating networks (PU/EP IPNs), we have synthesized PU/EP IPNs with varying disulfide bond densities.
- The synthesized PU/EP IPNs exhibit excellent damping properties, with an effective damping temperature range reaching 61.4 °C and a loss factor of 0.859.
- The synthesized PU/EP IPNs demonstrate excellent shape memory and self-healing properties, with a shape fixation rate of up to 95.0% and a shape recovery rate of up to 99.7%.
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
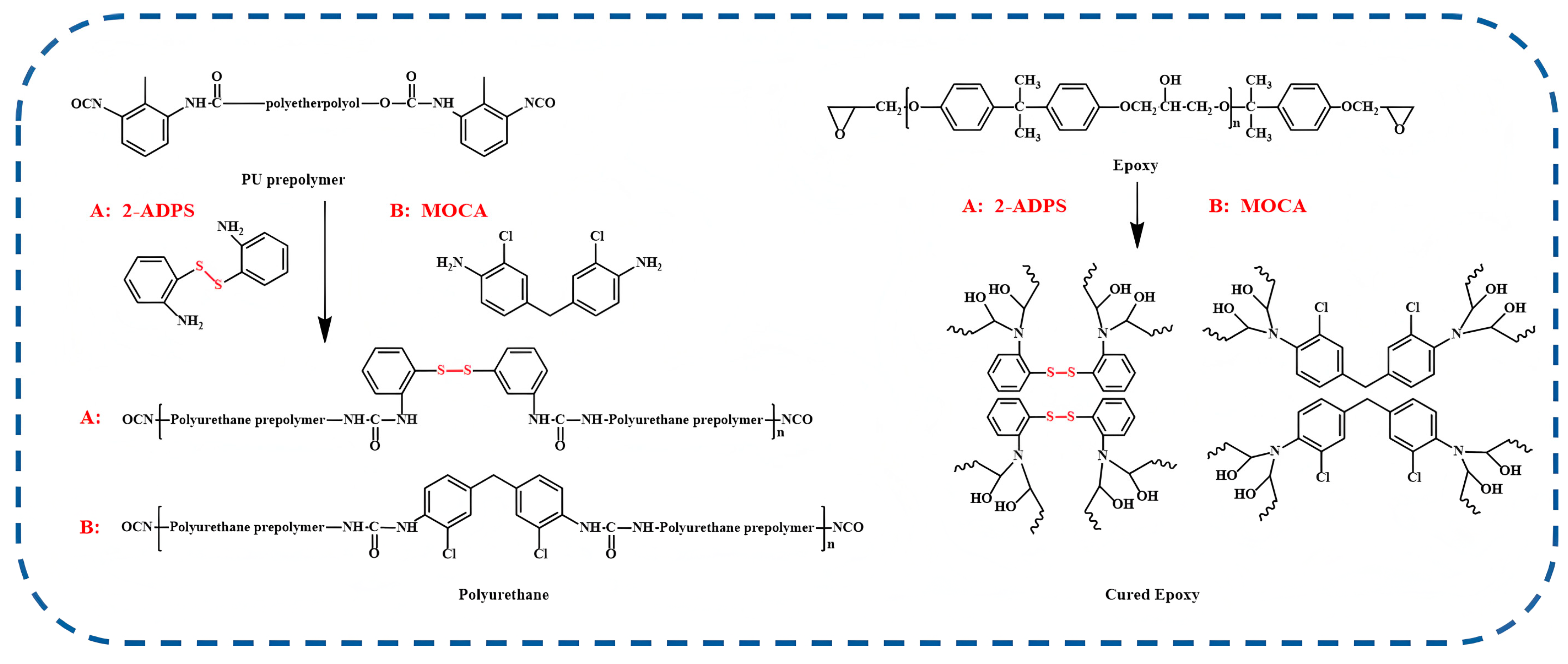
2.2. Synthesis of PU/EP-IPNs
2.2.1. Synthesis of Polyurethane Prepolymers and Epoxy Resin Precursors
2.2.2. Preparation of PU/EP-IPNs
2.3. Characterization
3. Results and Discussions
3.1. Structural Characterization
3.2. Thermal Performance
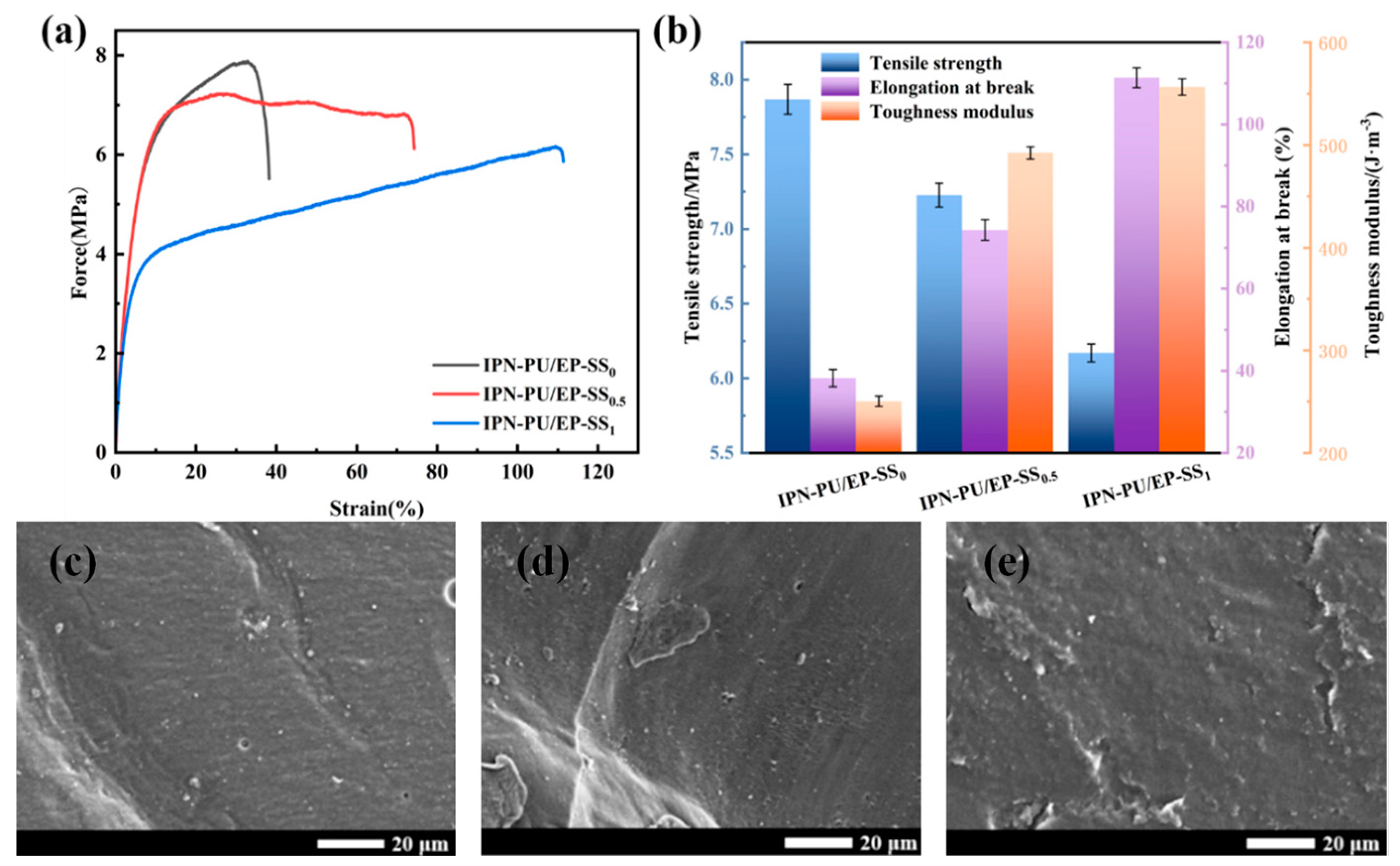
3.3. Mechanical Properties
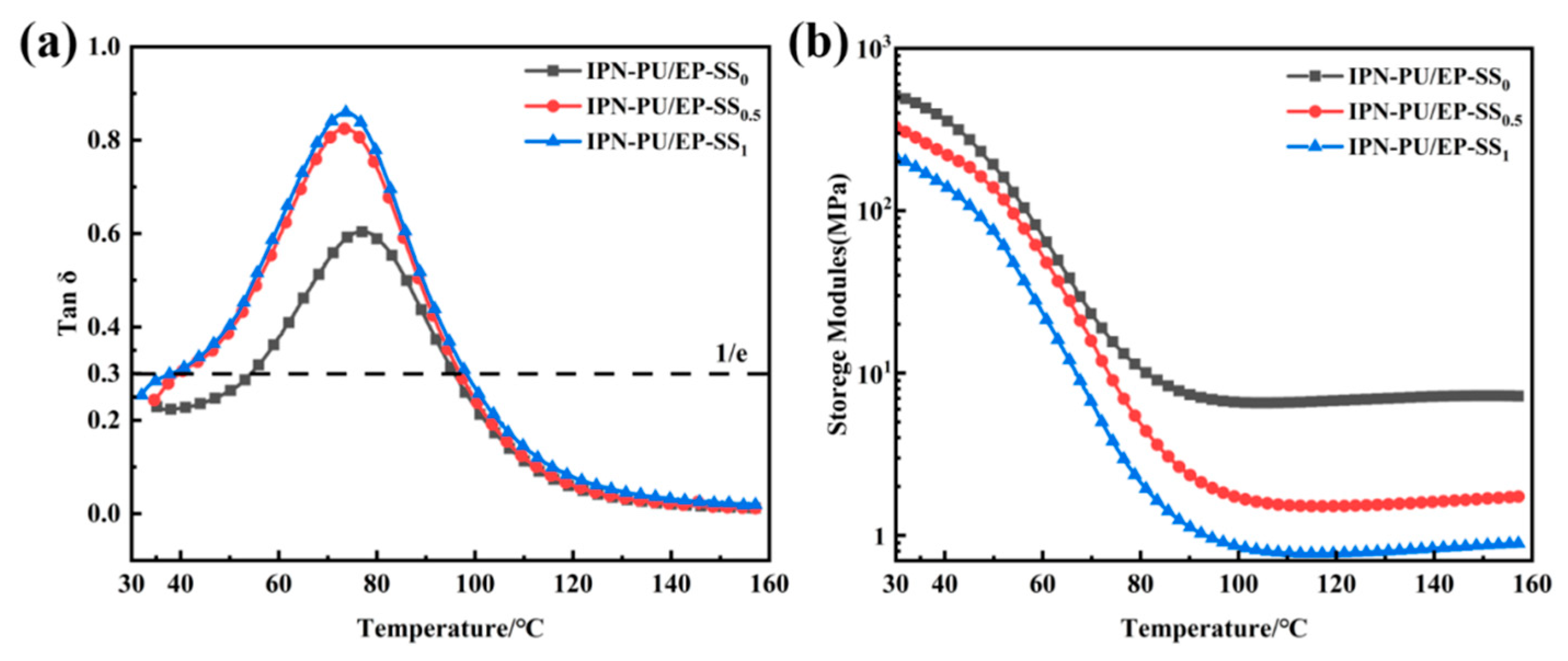
3.4. DMA Characterization
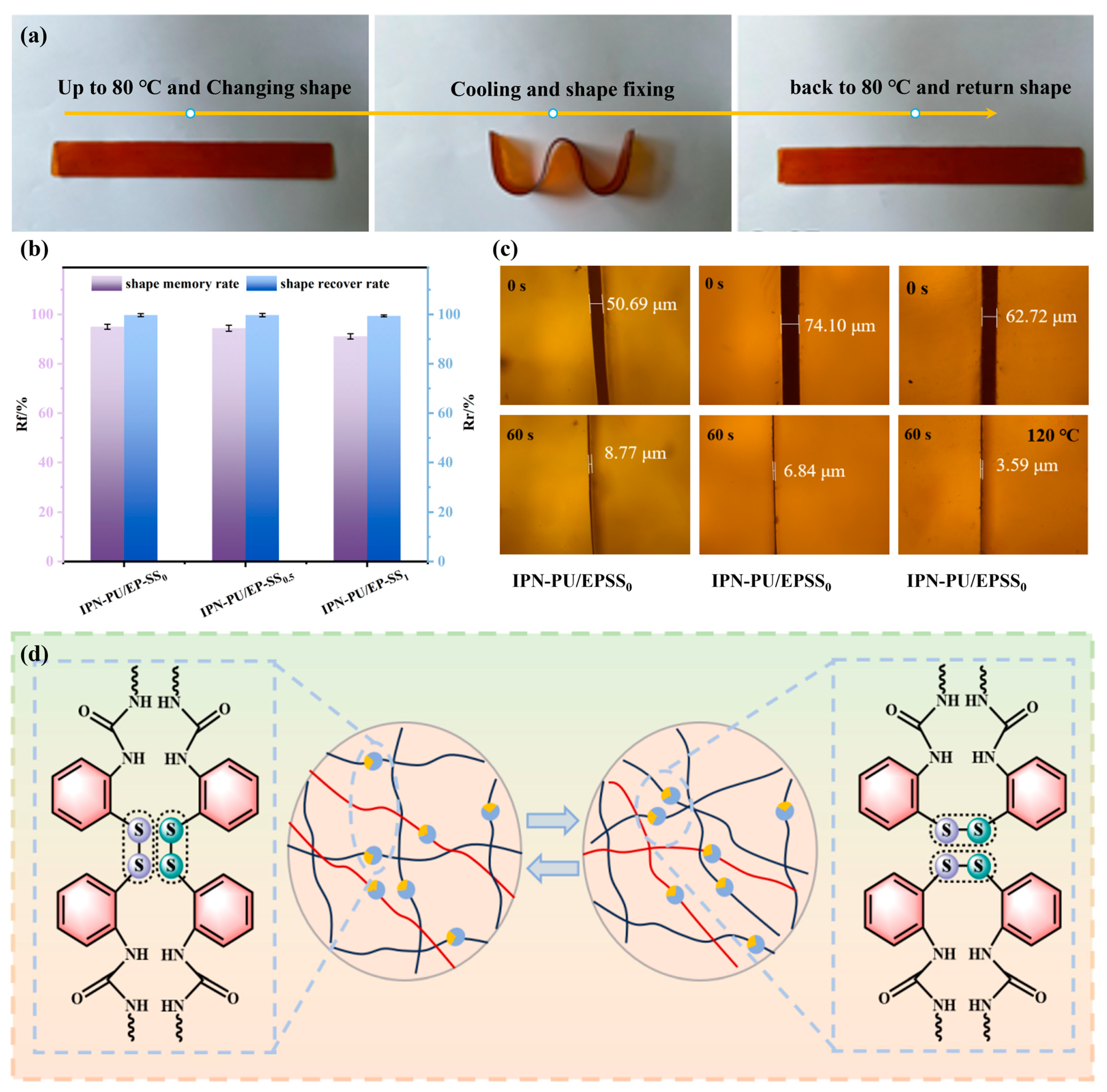
3.5. Shape Memory Performance and Self-Healing Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, X.; Qin, R.; Wang, H.; Xu, M.; Sheng, Y.; Lu, X. Controllable wide temperature range and high damping polyurethane elastomer based on disulfide bond and dangling chain. Polym. Adv. Technol. 2021, 32, 2185. [Google Scholar]
- Liu, C.; Fan, J.; Chen, Y. Design of regulable chlorobutyl rubber damping materials with highdamping value for a wide temperature range. Polym. Test. 2019, 79, 106003. [Google Scholar] [CrossRef]
- Shin, J.; Choi, K.; Shiko, S.; Choi, H.; Bae, D. Mechanical damping behavior of Al/C60-fullerene composites with supersaturated Al-C phases. Compos. Part B Eng. 2015, 77, 194. [Google Scholar]
- Tang, X.; Yan, X. A review on the damping properties of fiber reinforced polymer composites. J. Ind. Text. 2020, 49, 693. [Google Scholar] [CrossRef]
- Khan, S.U.; Li, C.Y.; Siddiqui, N.A.; Kim, J.-K. Vibration damping characteristics of carbon fiber-reinforced composites containing multi-walled carbon nanotubes. Compos. Sci. Technol. 2011, 71, 1486. [Google Scholar] [CrossRef]
- Qin, C.-L.; Cai, W.-M.; Cai, J.; Tang, D.-Y.; Zhang, J.-S.; Qin, M. Damping properties and morphology of polyurethane/vinyl ester resin interpenetrating polymer network. Mater. Chem. Phys. 2004, 85, 402. [Google Scholar] [CrossRef]
- Lv, X.; Huang, Z.; Shi, M.; Fan, Y.; Gao, G. Self-gradient mechanism, morphology and damping analysis of a thickness continuous gradient epoxy-polyurethane interpenetrating polymer network. RSC Adv. 2016, 6, 111688. [Google Scholar] [CrossRef]
- Wang, T.; Chen, S.; Wang, Q.; Pei, X. Damping analysis of polyurethane/epoxy graft interpenetrating polymer network composites filled with short carbon fiber and micro hollow glass bead. Mater. Des. 2010, 31, 3810. [Google Scholar] [CrossRef]
- Lee, J.H.; Driva, P.; Hadjichristidis, N.; Wright, P.J.; Rucker, S.P.; Lohse, D.J. Damping Behavior of Entangled Comb Polymers: Experiment. Macromolecules 2009, 42, 1392–1399. [Google Scholar] [CrossRef]
- Chu, H.; Lee, C.; Huang, W.G. Damping of Vinyl Acetate-n-Butyl Acrylate Copolymers. J. Appl. Polym. Sci. 2004, 91, 1396–1403. [Google Scholar] [CrossRef]
- Wu, J.-H.; Li, C.-H.; Wu, Y.-T.; Leu, M.-T.; Tsai, Y. Thermal resistance and dynamic damping properties of poly (styrene-butadiene-styrene)/thermoplastic polyurethane composites elastomer material. Compos. Sci. Technol. 2010, 70, 1258–1264. [Google Scholar]
- Liu, K.; Lv, Q.; Hua, J. Study on damping properties of HVBR/EVM blends prepared by in situ polymerization. Polym. Test. 2017, 60, 321. [Google Scholar] [CrossRef]
- Zhang, D.; He, M.; Qin, S.; Yu, J.; Guo, J.; Xu, G. Study on Dynamic Mechanical, Thermal, and Mechanical Properties of Long Glass Fiber Reinforced Thermoplastic Polyurethane/Poly (butylene terephthalate) Composites. Polym. Compos. 2018, 39, 63. [Google Scholar]
- Mrozek, R.A.; Berg, M.C.; Gold, C.S.; Leighliter, B.; Morton, J.T.; Lenhart, J.L. Highly compliant shape memory polymer gels for tunable damping and reversible adhesion. Smart Mater. Struct. 2016, 25, 025004. [Google Scholar]
- Cvek, M.; Mrlík, M.; Ilčíková, M.; Mosnáček, J.; Münster, L.; Pavlínek, V. Synthesis of Silicone Elastomers Containing Silyl-Based PolymerGrafted Carbonyl Iron Particles: An Efficient Way To Improve Magnetorheological, Damping, and Sensing Performances. Macromolecules 2017, 50, 2189. [Google Scholar]
- Millar, T.R.; Marr, W.E. Kinetics and equilibria in a sulphonated secondary intermeshed copolymer. J. Chem. Soc. 1962, 1789. [Google Scholar] [CrossRef]
- Fang, L.; Chen, T.; Li, X.; Lu, C.; Xu, Z. Wide-temperature range damping polyurea-urethane blends with self-healing capability. Constr. Build. Mater. 2020, 262, 119991. [Google Scholar]
- Zeng, Y.; Yang, W.; Liu, S.; Shi, X.; Xi, A.; Zhang, F. Dynamic Semi IPNs with Duple Dynamic Linkers: Self-Healing, Reprocessing, Welding, and Shape Memory Behaviors. Polymers 2021, 13, 1679. [Google Scholar] [CrossRef]
- Chakma, P.; Konkolewicz, D. Dynamic Covalent Bonds in Polymeric Materials. Angew. Chem. Int. Ed. 2019, 58, 9682. [Google Scholar]
- Irigoyen, M.; Fernández, A.; Ruiz, A.; Ruipérez, F.; Matxain, J.M. Diselenide bonds as an alternative to out-perform the efficiency of disulfides in self-healing materials. J. Org. Chem. 2019, 84, 4200. [Google Scholar]
- Kim, S.; Jeon, H.; Shin, S.; Park, S.; Jegal, J.; Hwang, S.Y.; Oh, D.X.; Park, J. Superior Toughness and Fast Self-Healing at Room Temperature Engineered by Transparent Elastomers. Adv. Mater. 2018, 30, 1705145. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.T.; Thai, S.H.; Nguyen, H.T.; Phung, D.T.T.; Nguyen, L.T.; Pham, H.Q.; Nguyen, L.-T.T. Tailoring the Hard−Soft Interface with Dynamic Diels−Alder Linkages in Polyurethanes: Toward Superior Mechanical Properties and Healability at Mild Temperature. Chem. Mater. 2019, 31, 2347. [Google Scholar]
- Delpierre, S.; Willocq, B.; Manini, G.; Lemaur, V.; Goole, J.; Gerbaux, P.; Cornil, J.; Dubois, P.; Raquez, J.-M. Simple Approach for a Self-Healable and Stiff Polymer Network from Iminoboronate-Based Boroxine Chemistry. Chem. Mater. 2019, 31, 3736. [Google Scholar]
- Tsedalu, A.A. A Review on Olefin Metathesis Reactions as a Green Method for the Synthesis of Organic Compounds. J. Chem. 2021, 2021, 1. [Google Scholar]
- Chen, M.; Si, H.; Zhang, H.; Zhou, L.; Wu, Y.; Song, L.; Kang, M.; Zhao, X.L. The Crucial Role in Controlling the Dynamic Properties of Polyester Based Epoxy Vitrimers: The Density of Exchangeable Ester Bonds (υ). Macromolecules 2021, 54, 10110. [Google Scholar]
- Ying, H.; Zhang, Y.; Cheng, J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 2014, 5, 3218. [Google Scholar]
- Cao, S.; Li, S.; Li, M.; Xu, L.; Ding, H.; Xia, J.; Zhang, M.; Huang, K. A thermal self-healing polyurethane thermoset based on phenolic urethane. Polymer 2017, 49, 775. [Google Scholar]
- Fang, Z.; Zheng, N.; Zhao, Q.; Xie, T. Healable, Reconfigurable, Reprocessable Thermoset Shape Memory Polymer with Highly Tunable Topological Rearrangement Kinetics. ACS Appl. Mater. Interfaces 2017, 9, 22077. [Google Scholar]
- Zechel, S.; Geitner, R.; Abend, M.; Siegmann, M.; Enke, M.; Kuhl, N.; Klein, M.; Vitz, J.; Gräfe, S.; Dietzek, B.; et al. Intrinsic self-healing polymers with a high E-modulus based on dynamic reversible urea bonds. NPG Asia Mater. 2017, 9, e420. [Google Scholar]
- Chang, K.; Jia, H.; Gu, S.-Y. A transparent, highly stretchable, self-healing polyurethane based on disulfide bonds. Eur. Polym. J. 2019, 112, 822. [Google Scholar]
- Wang, L.; Cai, Y.; Zhang, H.; Zou, H.; Chen, Y.; Liang, M.; Heng, Z. Room-temperature self-healing polysiloxane elastomer with reversible cross-linked network. Polymer 2022, 256, 125272. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, H.; Hu, Y.; Ding, D.; Cai, F.; Wu, Y. Electric tree intrinsic self-healing epoxy insulating materials based on disulfide bond. Polym. Degrad. Stab. 2023, 218, 110567. [Google Scholar] [CrossRef]
- Wan, K.; Cai, Y.; Chen, Q.; Hong, M.; Zhou, Z.; Fu, H. Self-healing polyurethane composite films loaded with Ni@C nanoparticles. Eur. Polym. J. 2023, 199, 112479. [Google Scholar] [CrossRef]
- Liu, M.; Zhong, J.; Li, Z.; Rong, J.; Yang, K.; Zhou, J.; Shen, L.; Gao, F.; Huang, X.; He, H. A high stiffness and self-healable polyurethane based on disulfide bonds and hydrogen bonding. Eur. Polym. J. 2020, 124, 109475. [Google Scholar] [CrossRef]
- Li, X.; Niu, Y.; Ai, T.; Li, X.; Yang, P.; Wang, X.; Zhang, Z. Preparation and Performance Study of Polyurethane/Epoxy Resin Interpenetrating Networks with Self-Healing, Shape Memory, and High Damping Properties. Macromol. Chem. Phys. 2024, 225, 2300311. [Google Scholar] [CrossRef]
- GB/T1040.3-2006; Plastics—Determination of Tensile Properties—Part 3: Test Conditions for Films and Sheets. Chinese Standard Press: Beijing, China, 2006.
- Li, C.; Wang, P.; Zhang, D.; Wang, S. Near-Infrared Responsive Smart Superhydrophobic Coating with Self-Healing and Robustness Enhanced by Disulfide-Bonded Polyurethane. ACS Appl. Mater. Interfaces 2022, 14, 45988–46000. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, F.; Luo, Y. Self-Healing Mechanism of Microcracks on Waterborne Polyurethane with Tunable Disulfide Bond Contents. ACS Omega 2019, 4, 1703–1714. [Google Scholar] [CrossRef]
- Zhang, T.; Gao, S.; Yu, J.; He, Y.; Han, X.; Zhuang, R. Preparation and performance of self-healing SBS modified bitumen based on dynamic disulfide bonds. Constr. Build. Mater. 2023, 397, 132394. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, Y.; Li, H.; Liu, H. Facile fabrication of polyurethane/epoxy IPNs filled graphene aerogel with improved damping, thermal and mechanical properties. RSC Adv. 2018, 8, 27390. [Google Scholar] [CrossRef]
- Li, B.; Zhu, G.; Hao, Y.; Ren, T. Shape reconfiguration and functional self-healing of thermadapt shape memory epoxy vitrimers by exchange reaction of disulfide bonds. Smart Mater. Struct. 2022, 31, 095047. [Google Scholar] [CrossRef]
- Irigoyen, M.; Matxain, J.M.; Ruipérez, F. Effect of Molecular Structure in the Chain Mobility of Dichalcogenide-Based Polymers with Self-Healing Capacity. Polymers 2019, 11, 1960. [Google Scholar] [CrossRef] [PubMed]



| Samples | Polyurethane Prepolymers (g) | E51 (g) | 2-ADPS (g) | MOCA (g) | Cashew Phenol (g) |
|---|---|---|---|---|---|
| IPN-PU/EP-SS0 | 10.1 | 9.0 | / | 5.3 | 2.24 |
| IPN-PU/EP-SS0.5 | 10.1 | 9.0 | 2.5 | 2.6 | 2.22 |
| IPN-PU/EP-SS1 | 10.1 | 9.0 | 5.1 | / | 2.22 |
| Sample | Damping Temperature Range (°C) tan δ ≥ 0.3 | ΔT at tan δ ≥ 0.3/°C | tan δ (max) | Tg (°C) |
|---|---|---|---|---|
| IPNs-PU/EP-SS0 | 54.3–95.8 | 41.5 | 0.604 | 76.2 |
| IPNs-PU/EP-SS0.5 | 39.7–97.0 | 57.3 | 0.824 | 73.2 |
| IPNs-PU/EP-SS1 | 37.0–98.4 | 61.4 | 0.859 | 73.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, G.; Li, X.; Ren, K.; Ai, T.; Niu, Y. Effect of Disulfide Bond Density on the Properties of Polyurethane/Epoxy Interpenetrating Networks. Materials 2025, 18, 1636. https://doi.org/10.3390/ma18071636
You G, Li X, Ren K, Ai T, Niu Y. Effect of Disulfide Bond Density on the Properties of Polyurethane/Epoxy Interpenetrating Networks. Materials. 2025; 18(7):1636. https://doi.org/10.3390/ma18071636
Chicago/Turabian StyleYou, Gudong, Xi Li, Kaiwen Ren, Tao Ai, and Yanhui Niu. 2025. "Effect of Disulfide Bond Density on the Properties of Polyurethane/Epoxy Interpenetrating Networks" Materials 18, no. 7: 1636. https://doi.org/10.3390/ma18071636
APA StyleYou, G., Li, X., Ren, K., Ai, T., & Niu, Y. (2025). Effect of Disulfide Bond Density on the Properties of Polyurethane/Epoxy Interpenetrating Networks. Materials, 18(7), 1636. https://doi.org/10.3390/ma18071636







