Pyrolysis and Combustion Kinetics of Garden Waste Pellets as Solid Biofuel for Thermochemical Energy Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Biomass Samples
| Properties | Standard | Biomass | ||
|---|---|---|---|---|
| PG0 | PG5 | WP [25] | ||
| Ultimate analysis [wt% dry base and ash free] | ||||
| C | ASTM D5373-08 [32] | 51.7 ± 0.5 | 49.7 ± 0.1 | 47.0 |
| H | ASTM D5373-08 | 7.1 ± 0.2 | 6.9 ± 0.1 | 5.7 |
| O | By difference | 41.2 ± 0.6 | 43.4 ± 0.1 | 47.3 |
| Proximate analysis [wt% dry base] | ||||
| Volatile matter (VM) | ASTM D5142-04 [33] | 69.0 | 72.1 | 84.6 |
| Fixed carbon (FC) | By difference | 10.3 | 9.9 | 14.1 |
| Ash | ASTM D5142-04 | 20.6 | 18.0 | 1.3 |
| Moisture [wt%] | ASTM D5142-04 | 5.0 ± 0.1 | 5.2 ± 0.2 | 7.9 |
| Physical properties | ||||
| Bulk density [kg/m3] | ASTM E873-82 [34] | 524.7 ± 5.6 | 461.5 ± 9.0 | 560.0 |
| Particle density [kg/m3] | Oil immersion | 1102.1 ± 32.2 | 980.2 ± 8.7 | 1153.6 |
| Packing factor [-] | - | 0.5 | 0.5 | 0.5 |
| Energy properties | ||||
| LHV (dry base) [MJ/kg] | ASTM D2015 [35] | 13.5 ± 0.1 | 14.0 ± 0.1 | 19.0 ± 0.1 |
| Fiber composition (wt%) | ||||
| Lignin a | - | 29.4 | 27.1 | 43.7 b |
| Cellulose a | - | 32.2 | 24.1 | 32.8 b |
| Hemicellulose a | - | 36.7 | 37.5 | 12.7 b |
2.2. Reactivity by Thermogravimetric Analysis
2.3. Kinetic of Pyrolysis and Combustion
3. Results and Discussion
3.1. Physicochemical Characteristics of Biomass Samples
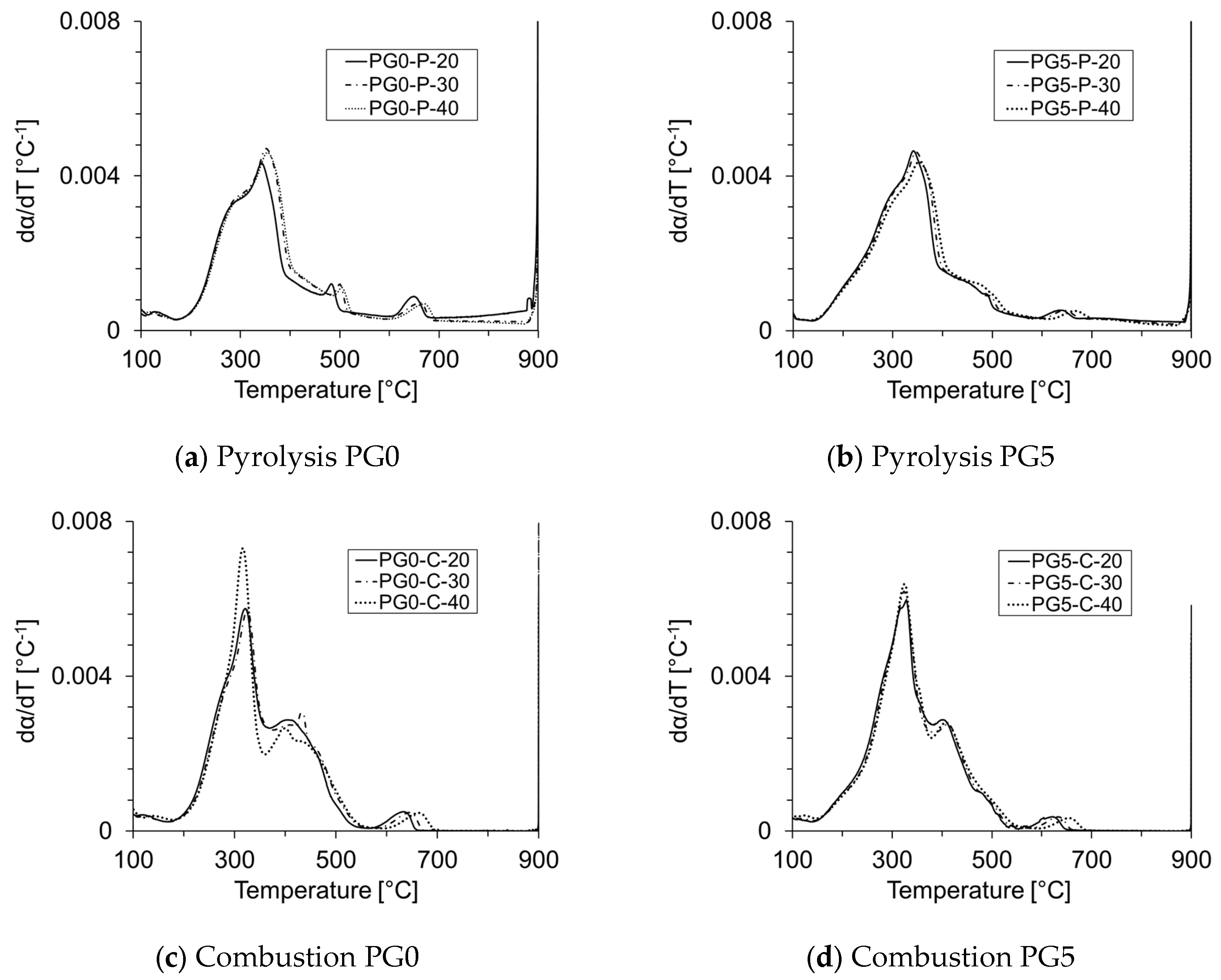
3.2. TG and DTG Analyses
3.3. Reactivity and Reaction Rate
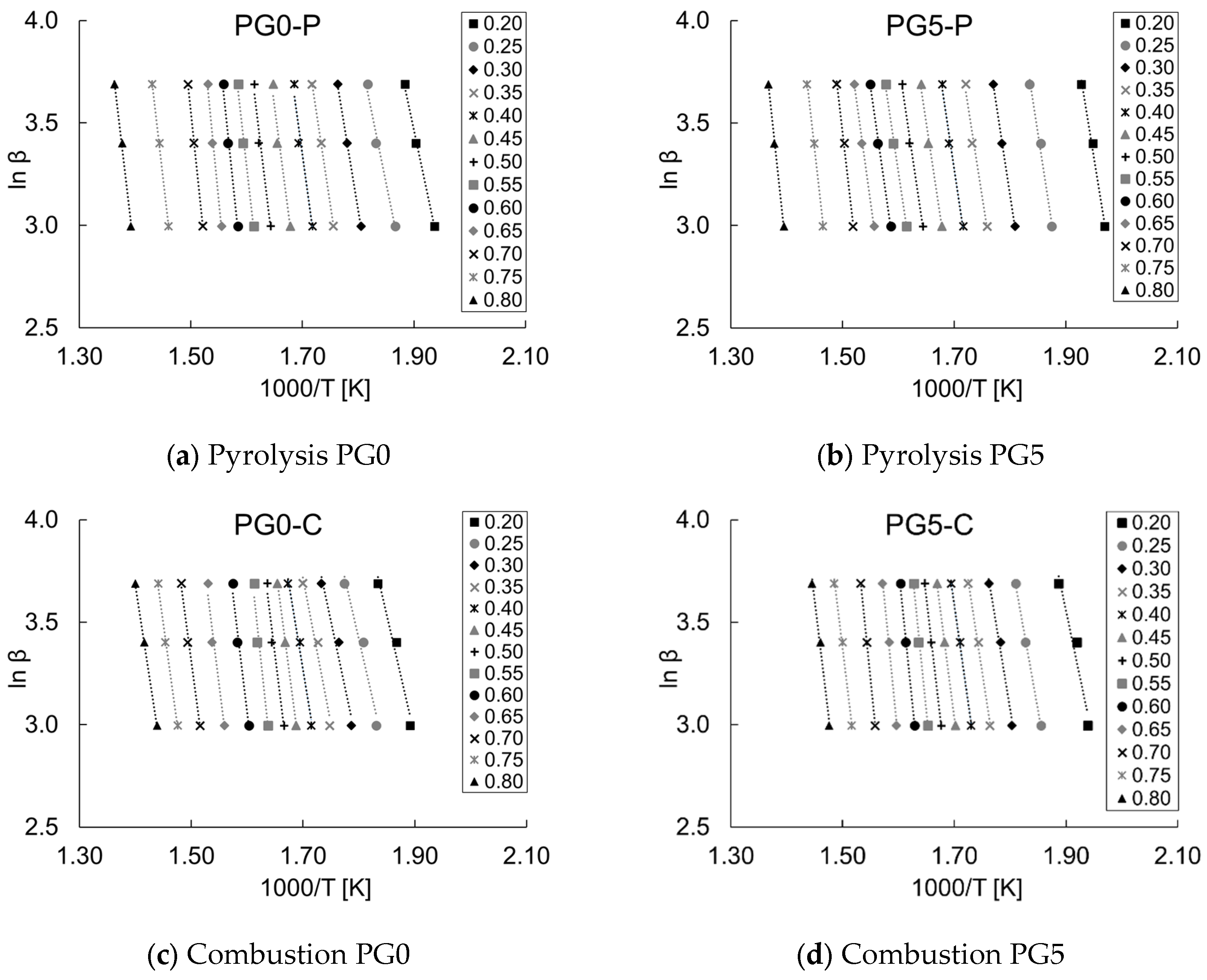
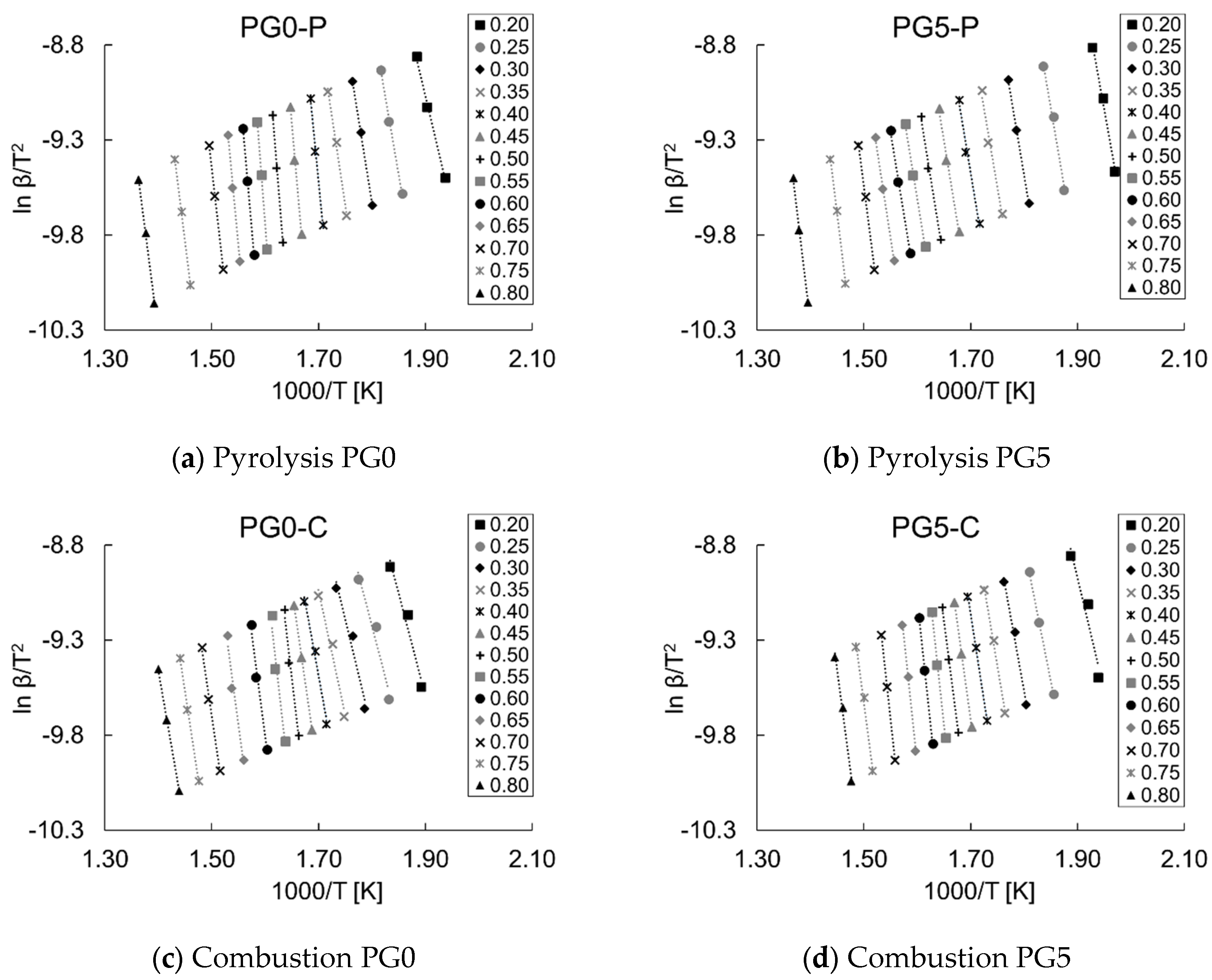
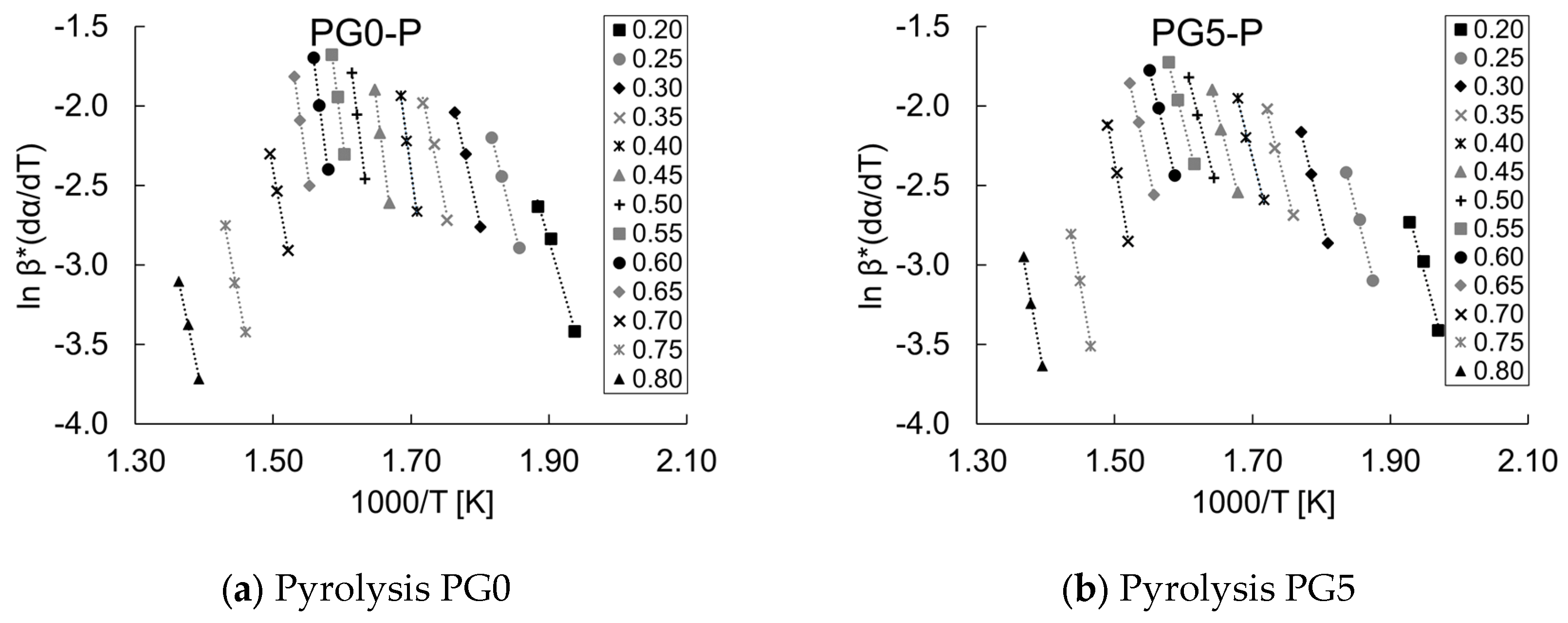
3.4. Pyrolysis and Combustion Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| α | Mass conversion fraction |
| βj | Heating rate |
| ϒmax | Maximum reaction rate |
| Aα | Pre-exponential factor |
| C | Combustion |
| DTG | Differential thermogravimetry |
| Eα | Activation energy |
| FC | Fixed carbon |
| FWO | Flynn–Wall–Ozawa |
| ICTAC | International Confederation for Thermal Analysis and Calorimetry |
| KAS | Kissinger–Akahira–Sunose |
| LHV | Lower heating value |
| m0 | Initial mass of the sample |
| m∞ | Final mass of the sample |
| MSW | Municipal solid waste |
| mt | Mass of the sample at a time t |
| P | Pyrolysis |
| PG0 | Pellets without glycerol |
| PG5 | Pellets with 5 wt% glycerol |
| R2 | Linear regression coefficient |
| R | Universal gas constant |
| Ra | Reaction rate |
| SEM | Scanning Electron Microscopy |
| t | Time |
| Tαi | Conversion temperature at a specified αi |
| Tb | Base temperature |
| Tf | End of reaction temperature |
| TG | Thermogravimetry |
| Ti | Reaction start temperature |
| Tm | Temperature corresponding to the maximum point of the plot dα/dT vs. T |
| Tmax | Temperature corresponding to the maximum reaction rate |
| UdeA | University of Antioquia |
| VM | Volatile matter |
| WP | Wood pellets |
Appendix A


References
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Woerden, F.V. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018. [Google Scholar] [CrossRef]
- Ouda, O.K.M.; Raza, S.A.; Nizami, A.S.; Rehan, M.; Al-Waked, R.; Korres, N.E. Waste to Energy Potential: A Case Study of Saudi Arabia. Renew. Sustain. Energy Rev. 2016, 61, 328–340. [Google Scholar] [CrossRef]
- The World Bank Urban Development: Overview 2023. Available online: https://www.worldbank.org/en/topic/urbandevelopment/overview (accessed on 18 February 2025).
- Vargas-Soplín, A.d.J.; Prochnow, A.; Herrmann, C.; Tscheuschner, B.; Kreidenweis, U. The Potential for Biogas Production from Autumn Tree Leaves to Supply Energy and Reduce Greenhouse Gas Emissions–A Case Study from the City of Berlin. Resour. Conserv. Recycl. 2022, 187, 106598. [Google Scholar] [CrossRef]
- Wysocka-Czubaszek, A.; Czubaszek, R. Fallen Leaves as a Substrate for Biogas Production. Energies 2024, 17, 6038. [Google Scholar] [CrossRef]
- González, W.A.; López, D.; Pérez, J.F. Biofuel Quality Analysis of Fallen Leaf Pellets: Effect of Moisture and Glycerol Contents as Binders. Renew. Energy 2020, 147, 1139–1150. [Google Scholar] [CrossRef]
- Andersen, J.K.; Boldrin, A.; Christensen, T.H.; Scheutz, C. Greenhouse Gas Emissions from Home Composting of Organic Household Waste. Waste Manag. 2010, 30, 2475–2482. [Google Scholar] [CrossRef]
- Zhang, J.; Smith, K.R.; Ma, Y.; Ye, S.; Jiang, F.; Qi, W.; Liu, P.; Khalil, M.A.K.; Rasmussen, R.A.; Thorneloe, S.A. Greenhouse Gases and Other Airborne Pollutants from Household Stoves in China: A Database for Emission Factors. Atmos. Environ. 2000, 34, 4537–4549. [Google Scholar] [CrossRef]
- Xu, X.; Pan, R.; Chen, R. Combustion Characteristics, Kinetics, and Thermodynamics of Pine Wood Through Thermogravimetric Analysis. Appl. Biochem. Biotechnol 2021, 193, 1427–1446. [Google Scholar] [CrossRef]
- Tin, K.K.; Taweepreda, W.; Singh, A.; Wagri, N.K.; Kumar, A. Fallen Leaves to Sustainable Energy Solution: Review on Hydrogen Production. RSC Sustain. 2024, 2, 2751–2767. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Mohanty, K. Kinetic Analysis and Pyrolysis Behavior of Low-Value Waste Lignocellulosic Biomass for Its Bioenergy Potential Using Thermogravimetric Analyzer. Mater. Sci. Energy Technol. 2021, 4, 136–147. [Google Scholar] [CrossRef]
- Wu, H.; Hanna, M.A.; Jones, D.D. Thermogravimetric Characterization of Dairy Manure as Pyrolysis and Combustion Feedstocks. Waste Manag. Res. 2012, 30, 1066–1071. [Google Scholar] [CrossRef]
- Liu, L.; Pang, Y.; Lv, D.; Wang, K.; Wang, Y. Thermal and Kinetic Analyzing of Pyrolysis and Combustion of Self-Heating Biomass Particles. Process Saf. Environ. Prot. 2021, 151, 39–50. [Google Scholar] [CrossRef]
- Boubacar Laougé, Z.; Merdun, H. Kinetic Analysis of Pearl Millet (Penissetum glaucum (L.) R. Br.) under Pyrolysis and Combustion to Investigate Its Bioenergy Potential. Fuel 2020, 267, 117172. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Ronda, A.; Blázquez, G.; Pérez, A.; Calero, M. Pyrolysis Kinetics of the Lead-Impregnated Olive Stone by Non-Isothermal Thermogravimetry. Process Saf. Environ. Prot. 2018, 113, 448–458. [Google Scholar] [CrossRef]
- Heydari, M.; Rahman, M.; Gupta, R. Kinetic Study and Thermal Decomposition Behavior of Lignite Coal. Int. J. Chem. Eng. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Pňakovič, Ľ.; Dzurenda, L. Combustion Characteristics of Fallen Fall Leaves from Ornamental Trees in City and Forest Parks. BioResources 2015, 10, 5563–5572. [Google Scholar] [CrossRef]
- Shi, S.; Zhou, X.; Chen, W.; Wang, X.; Nguyen, T.; Chen, M. Thermal and Kinetic Behaviors of Fallen Leaves and Waste Tires Using Thermogravimetric Analysis. BioResources 2017, 12, 4707–4721. [Google Scholar] [CrossRef]
- Mudryk, K.; Jewiarz, M.; Wróbel, M.; Niemiec, M.; Dyjakon, A. Evaluation of Urban Tree Leaf Biomass-Potential, Physico-Mechanical and Chemical Parameters of Raw Material and Solid Biofuel. Energies 2021, 14, 818. [Google Scholar] [CrossRef]
- Montiel-Bohórquez, N.D.; Pérez, J.F. Energy Valorization Strategies of Fallen Leaves and Woody Biomass in a Based Downdraft Gasification-Engine Power Plant. Sustain. Energy Technol. Assess. 2022, 49, 101749. [Google Scholar] [CrossRef]
- Senneca, O.; Cerciello, F. Kinetics of Combustion of Lignocellulosic Biomass: Recent Research and Critical Issues. Fuel 2023, 347, 128310. [Google Scholar] [CrossRef]
- Broström, M.; Nordin, A.; Pommer, L.; Branca, C.; Di Blasi, C. Influence of Torrefaction on the Devolatilization and Oxidation Kinetics of Wood. J. Anal. Appl. Pyrolysis 2012, 96, 100–109. [Google Scholar] [CrossRef]
- Branca, C.; Di Blasi, C. Thermal Degradation Behavior and Kinetics of Industrial Hemp Stalks and Shives. Thermochim. Acta 2021, 697, 178878. [Google Scholar] [CrossRef]
- Ashraf, M.; Ramzan, N.; Khan, R.U.; Durrani, A.K. Analysis of Mixed Cattle Manure: Kinetics and Thermodynamic Comparison of Pyrolysis and Combustion Processes. Case Stud. Therm. Eng. 2021, 26, 101078. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Rubio-Clemente, A.; Pérez, J.F. Effect of Main Solid Biomass Commodities of Patula Pine on Biochar Properties Produced under Gasification Conditions. Ind. Crops Prod. 2021, 160, 113123. [Google Scholar] [CrossRef]
- Sait, H.H.; Hussain, A.; Salema, A.A.; Ani, F.N. Pyrolysis and Combustion Kinetics of Date Palm Biomass Using Thermogravimetric Analysis. Bioresour. Technol. 2012, 118, 382–389. [Google Scholar] [CrossRef]
- González, W.A.; Zimmermann, F.; Pérez, J.F. Thermodynamic Assessment of the Fixed-Bed Downdraft Gasification Process of Fallen Leaves Pelletized with Glycerol as Binder. Case Stud. Therm. Eng. 2019, 14, 100480. [Google Scholar] [CrossRef]
- González, W.A.; Pérez, J.F. CFD Analysis and Characterization of Biochar Produced via Fixed-Bed Gasification of Fallen Leaf Pellets. Energy 2019, 186, 115904. [Google Scholar] [CrossRef]
- United Nations. Transforming Our World: The 2030 Agenda for Sustainable Development; 2015. Available online: https://docs.un.org/en/A/RES/70/1 (accessed on 18 February 2025).
- Duque-Uribe, D.; Montiel-Bohórquez, N.D.; Pérez, J.F. Technoeconomic Analysis of a Small-Scale Downdraft Gasification-Based Cogeneration Power Plant Using Green Wastes. J. Energy Resour. Technol. 2023, 145, 081401. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Chica, E.L.; Pérez, J.F. Parametric Analysis of a Gasification-Based Cookstove as a Function of Biomass Density, Gasification Behavior, Airflow Ratio, and Design. ACS Omega 2022, 7, 7481–7498. [Google Scholar] [CrossRef]
- ASTM D5373-08; Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2008.
- ASTM D5142-04; Standard Test Methods for Proximate Analysis of the Analysis Sample of Coal and Coke by Instrumental Procedures. ASTM International: West Conshohocken, PA, USA, 2004.
- ASTM E873-82; Standard Test Method for Bulk Density of Densified Particulate Biomass Fuels. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D2015-00; Standard Test Method for Gross Calorific Value of Coal and Coke by the Adiabatic Bomb Calorimeter. ASTM International: West Conshohocken, PA, USA, 2000.
- Zapata, R.B.; Bayer, J.F.P.; Jiménez, C.S. Colombian Coals: Classification and Thermochemical Characterization for Energy Applications. Rev. Ion 2014, 27, 43–54. [Google Scholar]
- Ramos-Carmona, S.; Martínez, J.D.; Pérez, J.F. Torrefaction of Patula Pine under Air Conditions: A Chemical and Structural Characterization. Ind. Crops Prod. 2018, 118, 302–310. [Google Scholar] [CrossRef]
- Nzioka, A.M.; Kim, M.G.; Hwang, H.U.; Kim, Y.J. Kinetic Study of the Thermal Decomposition Process of Municipal Solid Waste Using TGA. Waste Biomass Valorization 2019, 10, 1679–1691. [Google Scholar] [CrossRef]
- Elorf, A.; Kandasamy, J.; Belandria, V.; Bostyn, S.; Sarh, B.; Gökalp, I. Heating Rate Effects on Pyrolysis, Gasification and Combustion of Olive Waste. Biofuels 2021, 12, 1157–1164. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, F.; Niu, W.; Su, J.; Gao, Z.; Zhang, K. Effects of Heating Rate and Gas Atmosphere on the Pyrolysis and Combustion Characteristics of Different Crop Residues and the Kinetics Analysis. Energy 2019, 175, 320–332. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee Recommendations for Performing Kinetic Computations on Thermal Analysis Data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Bonilla, J.; Salazar, R.P.; Mayorga, M. Kinetic Triplet of Colombian Sawmill Wastes Using Thermogravimetric Analysis. Heliyon 2019, 5, e02723. [Google Scholar] [CrossRef]
- Kple, M.; Girods, P.; Anjorin, M.; Fagla, B.; Rogaume, Y. Thermal Degradation of Household Solid Waste in the Town of Abomey-Calavi in Benin: Kinetic Study. Waste and Biomass Valorization 2016, 7, 59–70. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Li, Z.; Feng, R.; Zhang, Y. Characterization of Corncob-Derived Biochar and Pyrolysis Kinetics in Comparison with Corn Stalk and Sawdust. Bioresour. Technol. 2014, 170, 76–82. [Google Scholar] [CrossRef]
- Cai, H.; Zou, H.; Liu, J.; Xie, W.; Kuo, J.; Buyukada, M.; Evrendilek, F. Thermal Degradations and Processes of Waste Tea and Tea Leaves via TG-FTIR: Combustion Performances, Kinetics, Thermodynamics, Products and Optimization. Bioresour. Technol. 2018, 268, 715–725. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, Y.S.; Kim, S.H. Investigation of Thermodynamic Parameters in the Thermal Decomposition of Plastic Waste−Waste Lube Oil Compounds. Environ. Sci. Technol. 2010, 44, 5313–5317. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, B. Investigation of Thermodynamic Parameters in the Pyrolysis Conversion of Biomass and Manure to Biochars Using Thermogravimetric Analysis. Bioresour. Technol. 2013, 146, 485–493. [Google Scholar] [CrossRef]
- Maia, A.A.D.; De Morais, L.C. Kinetic Parameters of Red Pepper Waste as Biomass to Solid Biofuel. Bioresour. Technol. 2016, 204, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Bartocci, P.; Bidini, G.; Asdrubali, F.; Beatrice, C.; Frusteri, F.; Fantozzi, F. Batch Pyrolysis of Pellet Made of Biomass and Crude Glycerol: Mass and Energy Balances. Renew. Energy 2018, 124, 172–179. [Google Scholar] [CrossRef]
- Teixeira, P.; Lopes, H.; Gulyurtlu, I.; Lapa, N. Use of Chemical Fractionation to Understand Partitioning of Biomass Ash Constituents during Co-Firing in Fluidized Bed Combustion. Fuel 2012, 101, 215–227. [Google Scholar] [CrossRef]
- Lv, D.; Xu, M.; Liu, X.; Zhan, Z.; Li, Z.; Yao, H. Effect of Cellulose, Lignin, Alkali and Alkaline Earth Metallic Species on Biomass Pyrolysis and Gasification. Fuel Process. Technol. 2010, 91, 903–909. [Google Scholar] [CrossRef]
- Stelte, W.; Holm, J.K.; Sanadi, A.R.; Barsberg, S.; Ahrenfeldt, J.; Henriksen, U.B. A Study of Bonding and Failure Mechanisms in Fuel Pellets from Different Biomass Resources. Biomass Bioenergy 2011, 35, 910–918. [Google Scholar] [CrossRef]
- Kumar, P.; Subbarao, P.M.V.; Kala, L.D.; Vijay, V.K. Thermogravimetric Devolatilization Behavior of Agricultural Residue for Generation of Syngas. J. Energy Resour. Technol. 2021, 143, 112106. [Google Scholar] [CrossRef]
- Arteaga-Pérez, L.E.; Segura, C.; Bustamante-García, V.; Cápiro, O.G.; Jiménez, R. Torrefaction of Wood and Bark from Eucalyptus Globulus and Eucalyptus Nitens: Focus on Volatile Evolution vs Feasible Temperatures. Energy 2015, 93, 1731–1741. [Google Scholar] [CrossRef]
- Nyakuma, B.B.; Ahmad, A.; Johari, A.; Abdullah, T.A.T.; Oladokun, O.; Aminu, D.Y. Non-Isothermal Kinetic Analysis of Oil Palm Empty Fruit Bunch Pellets by Thermogravimetric Analysis. Chem. Eng. Trans. 2015, 45, 1327–1332. [Google Scholar] [CrossRef]
- Maache, M.; Kada, C.; Amano, R.S.; Kumano, H.; Selim, O.M.; Kada, K. Experimental and Mathematical Investigation of Thermochemical Conversion for Horse Manure. J. Energy Resour. Technol. 2024, 146, 121701. [Google Scholar] [CrossRef]
- Chen, R.; Li, Q.; Xu, X.; Zhang, D.; Hao, R. Combustion Characteristics, Kinetics and Thermodynamics of Pinus Sylvestris Pine Needle via Non-Isothermal Thermogravimetry Coupled with Model-Free and Model-Fitting Methods. Case Stud. Therm. Eng. 2020, 22, 100756. [Google Scholar] [CrossRef]
- Xiao, R.; Yang, W.; Cong, X.; Dong, K.; Xu, J.; Wang, D.; Yang, X. Thermogravimetric Analysis and Reaction Kinetics of Lignocellulosic Biomass Pyrolysis. Energy 2020, 201, 117537. [Google Scholar] [CrossRef]
- Epesse Misse, S.; Brillard, A.; Mayandyshev, P.; Brilhac, J.F.; Obonou, M. Comparative Pyrolysis, Combustion, and Kinetic Modeling of Twelve Cameroonian Woody Biomass. Biomass Conv. Bioref. 2022, 12, 3161–3181. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Yang, Y.; Zhang, Y.; Zhao, C.; Yu, Y.; Wang, S. Comparison of the Thermal Degradation Behaviors and Kinetics of Palm Oil Waste under Nitrogen and Air Atmosphere in TGA-FTIR with a Complementary Use of Model-Free and Model-Fitting Approaches. J. Anal. Appl. Pyrolysis 2018, 134, 12–24. [Google Scholar] [CrossRef]
- Garcia Torrent, J.; Fernandez Anez, N.; Medic Pejic, L.; Montenegro Mateos, L. Assessment of Self-Ignition Risks of Solid Biofuels by Thermal Analysis. Fuel 2015, 143, 484–491. [Google Scholar] [CrossRef]
- Munir, S.; Daood, S.S.; Nimmo, W.; Cunliffe, A.M.; Gibbs, B.M. Thermal Analysis and Devolatilization Kinetics of Cotton Stalk, Sugar Cane Bagasse and Shea Meal under Nitrogen and Air Atmospheres. Bioresour. Technol. 2009, 100, 1413–1418. [Google Scholar] [CrossRef]
- Howell, A.; Beagle, E.; Belmont, E. Torrefaction of Healthy and Beetle Kill Pine and Co-Combustion With Sub-Bituminous Coal. J. Energy Resour. Technol. 2018, 140, 042002. [Google Scholar] [CrossRef]
- Arenas, C.N.; Navarro, M.V.; Martínez, J.D. Pyrolysis Kinetics of Biomass Wastes Using Isoconversional Methods and the Distributed Activation Energy Model. Bioresource Technology 2019, 288, 121485. [Google Scholar] [CrossRef]
- Islamova, S.; Tartygasheva, A.; Karaeva, J.; Panchenko, V.; Litti, Y. A Comprehensive Study on the Combustion of Sunflower Husk Pellets by Thermogravimetric and Kinetic Analysis, Kriging Method. Agriculture 2023, 13, 840. [Google Scholar] [CrossRef]
- Kaur, R.; Gera, P.; Jha, M.K.; Bhaskar, T. Pyrolysis Kinetics and Thermodynamic Parameters of Castor (Ricinus communis) Residue Using Thermogravimetric Analysis. Bioresour. Technol. 2018, 250, 422–428. [Google Scholar] [CrossRef]
- Vafakish, B.; Babaei-Ghazvini, A.; Ebadian, M.; Acharya, B. Pyrolysis and Combustion Behavior of Flax Straw as Biomass: Evaluation of Kinetic, Thermodynamic Parameters, and Qualitative Analysis of Degradation Products. Energies 2023, 16, 6932. [Google Scholar] [CrossRef]
- Aboyade, A.O.; Hugo, T.J.; Carrier, M.; Meyer, E.L.; Stahl, R.; Knoetze, J.H.; Görgens, J.F. Non-Isothermal Kinetic Analysis of the Devolatilization of Corn Cobs and Sugar Cane Bagasse in an Inert Atmosphere. Thermochim. Acta 2011, 517, 81–89. [Google Scholar] [CrossRef]
- Biagini, E.; Fantei, A.; Tognotti, L. Effect of the Heating Rate on the Devolatilization of Biomass Residues. Thermochim. Acta 2008, 472, 55–63. [Google Scholar] [CrossRef]
- Özsin, G.; Pütün, A.E. TGA/MS/FT-IR Study for Kinetic Evaluation and Evolved Gas Analysis of a Biomass/PVC Co-Pyrolysis Process. Energy Convers. Manag. 2019, 182, 143–153. [Google Scholar] [CrossRef]
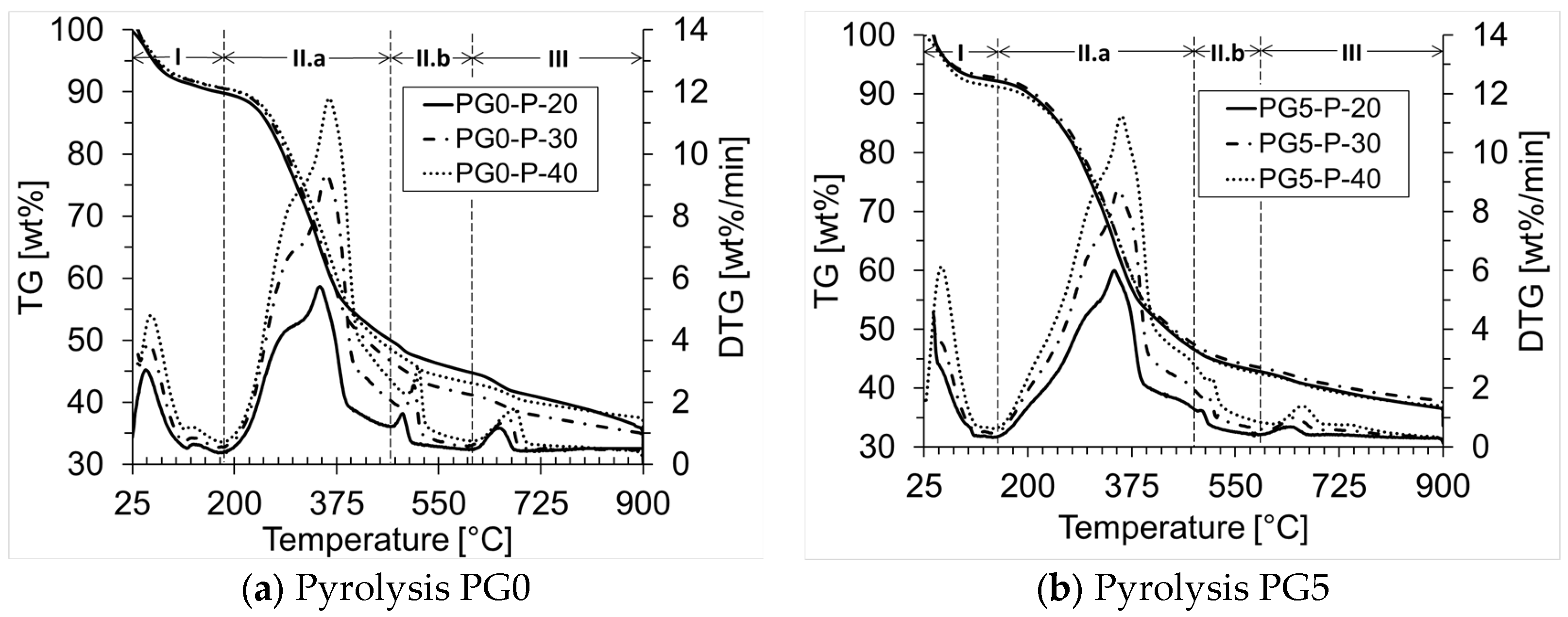
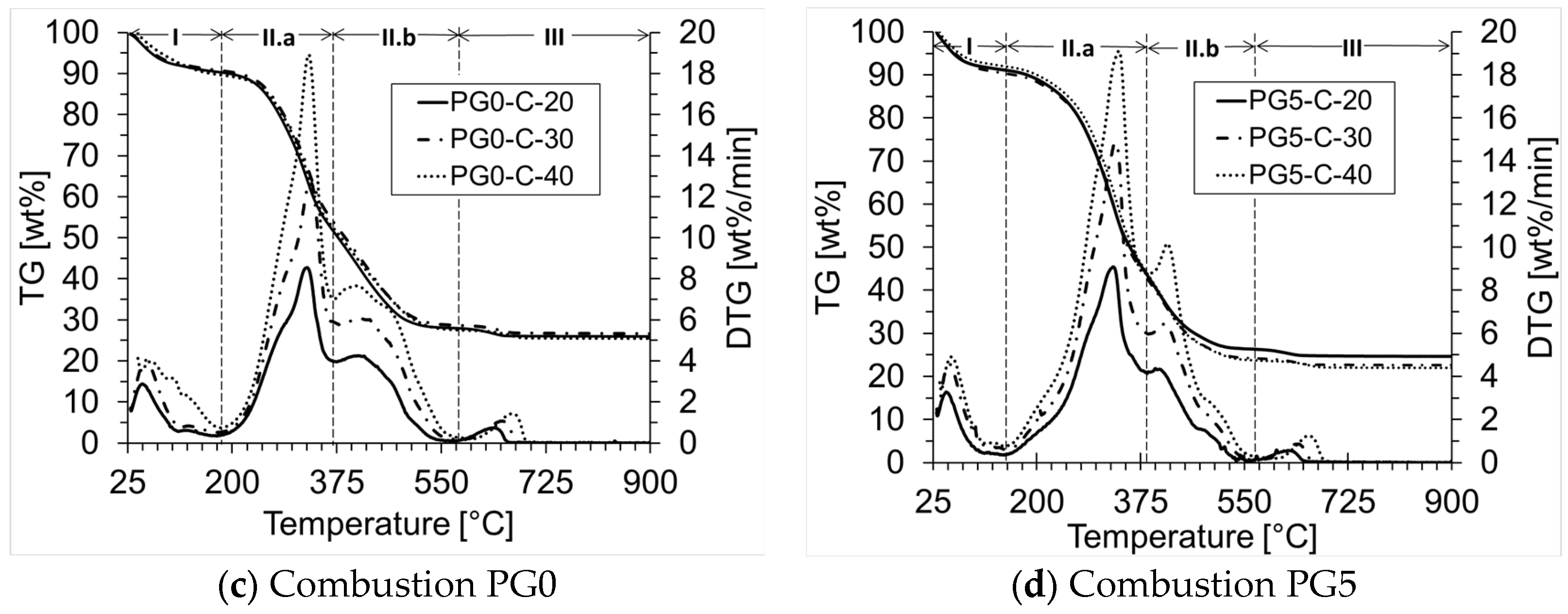
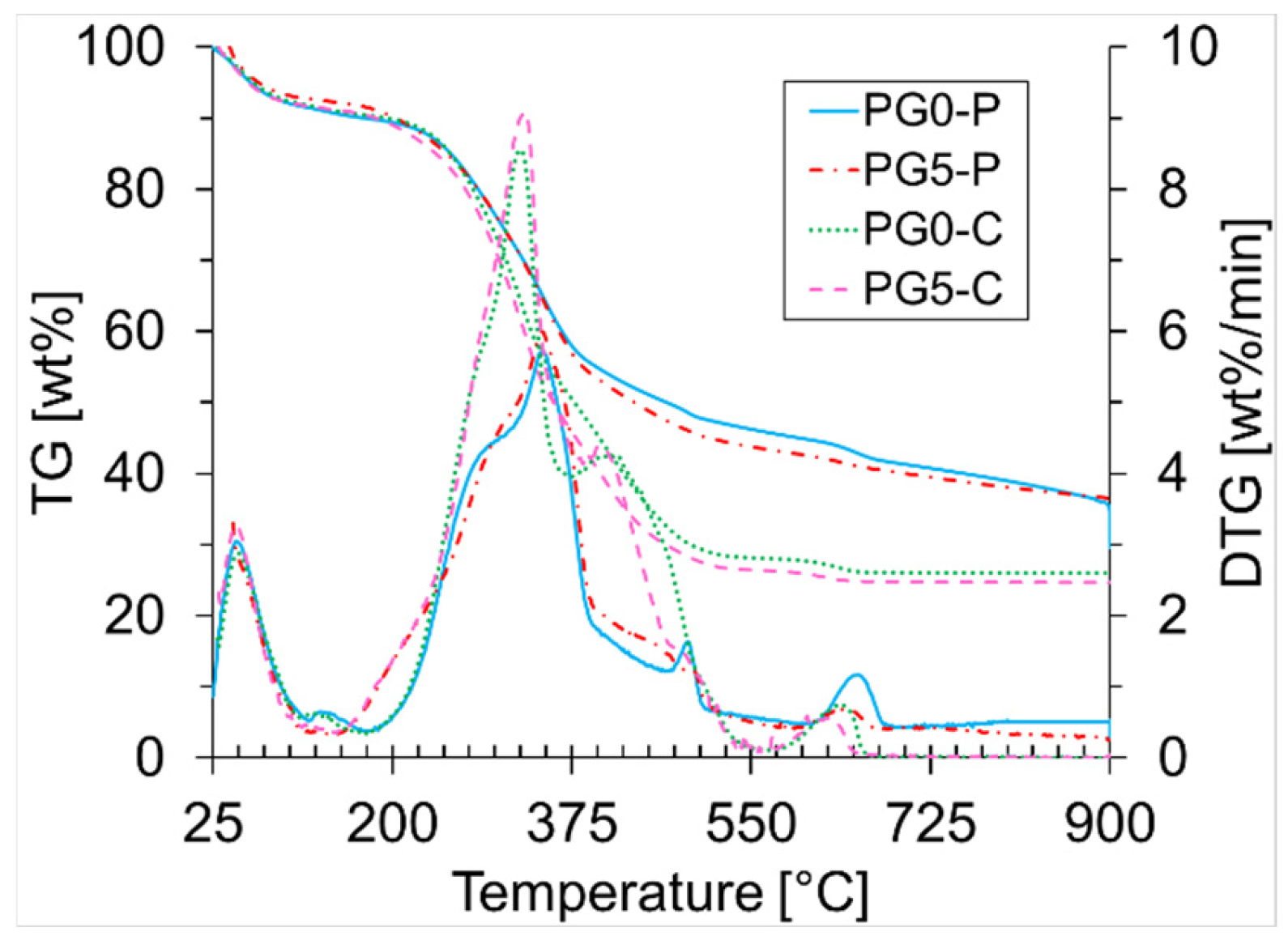

| Biomass | Ti (°C) | Tmax (°C) | Tf (°C) | ϒmax (wt%/min) | Tbase [°C] | Ra [min−1] |
|---|---|---|---|---|---|---|
| PG0-P | 225 | 347 | 898 | 5.73 | 219 | 0.057 |
| PG5-P | 228 | 346 | 858 | 6.00 | 186 | 0.060 |
| PG0-C | 242 | 325 | 614 | 8.55 | 217 | 0.086 |
| PG5-C | 238 | 329 | 586 | 9.09 | 183 | 0.091 |
| Atmosphere | α | PG0/Model | PG5/Model | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FWO | KAS | Friedman | FWO | KAS | Friedman | ||||||||||||||
| Eα | Aα | R2 | Eα | Aα | R2 | Eα | Aα | R2 | Eα | Aα | R2 | Eα | Aα | R2 | Eα | Aα | R2 | ||
| N2 | 0.20 | 53.17 | 2.13 × 102 | 0.997 | 51.38 | 1.46 × 102 | 0.997 | 65.12 | 2.57 × 103 | 0.985 | 67.86 | 4.28 × 103 | 0.991 | 66.99 | 3.58 × 103 | 0.990 | 70.16 | 7.43 × 103 | 0.976 |
| 0.25 | 58.45 | 6.38 × 102 | 0.982 | 57.63 | 5.38 × 102 | 0.982 | 62.58 | 1.50 × 103 | 0.997 | 75.41 | 2.03 × 104 | 0.988 | 74.49 | 1.68 × 104 | 0.987 | 77.81 | 3.58 × 104 | 0.993 | |
| 0.30 | 73.35 | 1.39 × 104 | 1.000 | 72.54 | 1.17 × 104 | 1.000 | 80.86 | 6.42 × 104 | 0.999 | 79.33 | 4.54 × 104 | 0.999 | 78.34 | 3.71 × 104 | 0.999 | 84.71 | 1.46 × 105 | 1.000 | |
| 0.35 | 83.34 | 1.06 × 105 | 0.999 | 82.59 | 9.13 × 104 | 0.999 | 93.38 | 8.13 × 105 | 0.989 | 80.28 | 5.53 × 104 | 0.981 | 78.96 | 4.21 × 104 | 0.978 | 82.55 | 9.42 × 104 | 0.992 | |
| 0.40 | 92.47 | 6.70 × 105 | 0.957 | 93.41 | 8.10 × 105 | 0.956 | 103.34 | 5.99 × 106 | 0.968 | 84.56 | 1.33 × 105 | 0.987 | 83.17 | 9.99 × 104 | 0.985 | 82.88 | 1.01 × 105 | 0.993 | |
| 0.45 | 100.97 | 3.72 × 106 | 0.962 | 102.28 | 4.84 × 106 | 0.961 | 110.34 | 2.44 × 107 | 0.975 | 87.57 | 2.45 × 105 | 0.993 | 86.06 | 1.80 × 105 | 0.992 | 86.37 | 2.05 × 105 | 0.997 | |
| 0.50 | 110.07 | 2.31 × 107 | 0.970 | 111.83 | 3.28 × 107 | 0.970 | 112.50 | 3.75 × 107 | 0.978 | 89.41 | 3.56 × 105 | 0.993 | 87.81 | 2.57 × 105 | 0.992 | 86.58 | 2.15 × 105 | 0.998 | |
| 0.55 | 118.92 | 1.35 × 108 | 0.983 | 121.03 | 2.07 × 108 | 0.983 | 112.59 | 3.82 × 107 | 0.976 | 90.79 | 4.73 × 105 | 0.997 | 89.00 | 3.28 × 105 | 0.996 | 89.00 | 3.51 × 105 | 1.000 | |
| 0.60 | 131.80 | 1.77 × 109 | 0.984 | 132.77 | 2.15 × 109 | 0.984 | 140.02 | 9.06 × 109 | 0.981 | 94.22 | 9.49 × 105 | 0.997 | 92.33 | 6.46 × 105 | 0.996 | 95.73 | 1.37 × 106 | 1.000 | |
| 0.65 | 143.65 | 1.87 × 1010 | 0.991 | 144.76 | 2.32 × 1010 | 0.990 | 150.18 | 6.79 × 1010 | 0.994 | 100.47 | 3.36 × 106 | 0.998 | 98.67 | 2.33 × 106 | 0.998 | 108.41 | 1.76 × 107 | 0.999 | |
| 0.70 | 137.17 | 5.17 × 109 | 0.999 | 135.75 | 3.90 × 109 | 1.000 | 127.21 | 7.13 × 108 | 1.000 | 123.60 | 3.51 × 108 | 0.996 | 122.73 | 2.95 × 108 | 0.995 | 137.16 | 5.49 × 109 | 0.995 | |
| 0.75 | 129.59 | 1.15 × 109 | 0.997 | 130.00 | 1.25 × 109 | 0.997 | 130.82 | 1.47 × 109 | 0.993 | 132.67 | 2.16 × 109 | 0.998 | 131.66 | 1.76 × 109 | 0.998 | 142.27 | 1.52 × 1010 | 0.998 | |
| 0.80 | 135.06 | 3.43 × 109 | 0.995 | 133.25 | 2.39 × 109 | 0.997 | 125.75 | 5.38 × 108 | 0.998 | 143.94 | 2.04 × 1010 | 0.999 | 142.69 | 1.59 × 1010 | 0.999 | 149.39 | 6.23 × 1010 | 0.997 | |
| Average | 105.23 | 2.34 × 109 | 0.986 | 105.33 | 2.55 × 109 | 0.986 | 108.82 | 6.13 × 109 | 0.987 | 96.16 | 1.76 × 109 | 0.994 | 94.84 | 1.38 × 109 | 0.993 | 99.46 | 6.39 × 109 | 0.995 | |
| Air | 0.20 | 49.86 | 1.59 × 102 | 0.965 | 47.68 | 9.81 × 101 | 0.958 | 45.10 | 6.08 × 101 | 0.915 | 53.41 | 2.91 × 102 | 0.941 | 51.62 | 1.98 × 102 | 0.931 | 57.45 | 7.52 × 102 | 0.991 |
| 0.25 | 52.17 | 2.64 × 102 | 0.958 | 49.77 | 1.56 × 102 | 0.949 | 53.27 | 3.68 × 102 | 0.946 | 59.05 | 9.79 × 102 | 0.995 | 57.69 | 7.32 × 102 | 0.995 | 61.59 | 1.81 × 103 | 0.943 | |
| 0.30 | 57.40 | 8.26 × 102 | 0.963 | 55.00 | 4.90 × 102 | 0.956 | 65.35 | 5.03 × 103 | 0.981 | 66.48 | 4.77 × 103 | 0.998 | 65.28 | 3.69 × 103 | 0.997 | 72.31 | 1.75 × 104 | 0.999 | |
| 0.35 | 65.35 | 4.61 × 103 | 0.971 | 63.15 | 2.87 × 103 | 0.966 | 84.03 | 2.69 × 105 | 0.998 | 71.39 | 1.35 × 104 | 1.000 | 70.28 | 1.07 × 104 | 1.000 | 79.00 | 7.12 × 104 | 0.999 | |
| 0.40 | 77.82 | 6.62 × 104 | 0.988 | 76.07 | 4.57 × 104 | 0.986 | 109.89 | 6.08 × 107 | 0.997 | 77.78 | 5.17 × 104 | 0.999 | 76.88 | 4.28 × 104 | 0.999 | 88.96 | 5.68 × 105 | 0.993 | |
| 0.45 | 98.25 | 4.95 × 106 | 1.000 | 97.38 | 4.12 × 106 | 1.000 | 122.08 | 7.59 × 108 | 0.988 | 85.92 | 2.84 × 105 | 0.995 | 85.37 | 2.54 × 105 | 0.995 | 105.92 | 1.89 × 107 | 0.985 | |
| 0.50 | 106.47 | 2.76 × 107 | 0.969 | 106.56 | 2.81 × 107 | 0.966 | 133.70 | 8.36 × 109 | 0.982 | 93.54 | 1.39 × 106 | 0.989 | 93.33 | 1.33 × 106 | 0.988 | 118.74 | 2.63 × 108 | 0.989 | |
| 0.55 | 127.29 | 2.12 × 109 | 0.950 | 127.61 | 2.26 × 109 | 0.945 | 115.27 | 1.82 × 108 | 0.999 | 106.21 | 1.92 × 107 | 0.975 | 106.66 | 2.10 × 107 | 0.974 | 121.57 | 4.67 × 108 | 0.964 | |
| 0.60 | 113.70 | 1.26 × 108 | 0.970 | 113.02 | 1.09 × 108 | 0.967 | 103.85 | 1.69 × 107 | 0.927 | 108.62 | 3.15 × 107 | 0.982 | 109.04 | 3.43 × 107 | 0.981 | 118.84 | 2.67 × 108 | 0.999 | |
| 0.65 | 113.04 | 1.10 × 108 | 0.962 | 111.97 | 8.77 × 107 | 0.958 | 99.14 | 6.34 × 106 | 0.985 | 115.77 | 1.37 × 108 | 1.000 | 116.33 | 1.54 × 108 | 1.000 | 100.65 | 6.33 × 106 | 0.972 | |
| 0.70 | 107.79 | 3.68 × 107 | 0.994 | 105.96 | 2.51 × 107 | 0.993 | 96.58 | 3.73 × 106 | 0.999 | 114.12 | 9.81 × 107 | 0.997 | 114.30 | 1.02 × 108 | 0.997 | 91.49 | 9.52 × 105 | 1.000 | |
| 0.75 | 107.63 | 3.57 × 107 | 0.998 | 105.35 | 2.21 × 107 | 0.997 | 104.05 | 1.79 × 107 | 0.995 | 101.75 | 7.67 × 106 | 1.000 | 100.72 | 6.20 × 106 | 0.999 | 84.92 | 2.45 × 105 | 0.992 | |
| 0.80 | 98.92 | 5.79 × 106 | 1.000 | 95.80 | 3.00 × 106 | 1.000 | 73.82 | 3.07 × 104 | 0.983 | 105.60 | 1.70 × 107 | 1.000 | 104.45 | 1.34 × 107 | 0.999 | 108.16 | 3.01 × 107 | 0.996 | |
| Average | 90.44 | 1.90 × 108 | 0.976 | 88.87 | 1.95 × 108 | 0.972 | 92.78 | 7.24 × 108 | 0.976 | 89.20 | 2.41 × 107 | 0.99 | 88.61 | 2.56 × 107 | 0.99 | 93.05 | 8.11 × 107 | 0.99 | |
| Biomass | Eα [kJ/mol] | Aα [s−1] | ||
|---|---|---|---|---|
| Pyrolysis | Combustion | Pyrolysis | Combustion | |
| PG0 | 106.46 | 90.70 | 3.67 × 109 | 3.70 × 108 |
| PG5 | 96.82 | 90.29 | 3.18 × 109 | 4.36 × 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, J.; Pérez, J.F. Pyrolysis and Combustion Kinetics of Garden Waste Pellets as Solid Biofuel for Thermochemical Energy Recovery. Materials 2025, 18, 1634. https://doi.org/10.3390/ma18071634
Gutiérrez J, Pérez JF. Pyrolysis and Combustion Kinetics of Garden Waste Pellets as Solid Biofuel for Thermochemical Energy Recovery. Materials. 2025; 18(7):1634. https://doi.org/10.3390/ma18071634
Chicago/Turabian StyleGutiérrez, Jonatan, and Juan F. Pérez. 2025. "Pyrolysis and Combustion Kinetics of Garden Waste Pellets as Solid Biofuel for Thermochemical Energy Recovery" Materials 18, no. 7: 1634. https://doi.org/10.3390/ma18071634
APA StyleGutiérrez, J., & Pérez, J. F. (2025). Pyrolysis and Combustion Kinetics of Garden Waste Pellets as Solid Biofuel for Thermochemical Energy Recovery. Materials, 18(7), 1634. https://doi.org/10.3390/ma18071634







