Recycling Fiber-Reinforced Polyamide Waste from the Automotive Industry: Life Cycle Assessment (LCA) of an Advanced Pyrolysis Process to Reclaim Glass Fibers and Valuable Chemicals
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Experimental Procedure
2.3. Analytical Techniques
- –
- Thermogravimetric analysis
- –
- Proximate analysis
- –
- Elemental analysis (CHNO)
- –
- Lower Heating Value (LHV)
- –
- Analysis of halogens and sulfur
- –
- Composition of gases
- –
- Composition of liquids
2.4. Life Cycle Assessment (LCA)
3. Results and Discussion
3.1. Characterization of the Glass Fiber-Reinforced Polyamide Sample
3.2. Pyrolysis Yields
3.3. Pyrolysis Gases
3.4. Pyrolysis Liquids
3.5. Pyrolysis Solids
3.6. Life Cycle Assessment (LCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allied Market Research. Automotive Composites Market Size, Share, Competitive Landscape and Trend Analysis Report, by Fiber Type, by Resin Type, by Application: Global Opportunity Analysis and Industry Forecast, 2024–2033. Available online: https://www.alliedmarketresearch.com/automotive-composites-market-A09125 (accessed on 28 March 2025).
- Mason, H. Composites End Markets: Automotive (2024). Composites World. 2024. Available online: https://www.compositesworld.com/articles/composites-end-markets-automotive-(2024)?utm_source=Omeda&utm_medium=email&utm_campaign=CW+Spotlight+7%2F24%2F2024 (accessed on 28 March 2025).
- He, D.; Kim, H.C.; Sommacal, S.; Stojcevski, F.; Soo, V.K.; Lipinski, W.; Morozov, E.; Henderson, L.C.; Compston, P.; Doolan, M. Improving mechanical and life cycle environmental performances of recycled CFRP automotive component by fibre architecture preservation. Compos. Part A-Eng. 2023, 175, 107749. [Google Scholar] [CrossRef]
- García-Arrieta, S.; Elizetxea, C.; de la Calle, A.; Ollo, O.; Múgica, M.; Goikuria, E.; Sarlin, E.; García, M. Recycled fibers integration in automotive components manufactured by injection. Mater. Compuestos 2022, 07, 161. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Y.; Li, Y.; Wang, Y.; Li, G.; Liu, X. Wind turbine blade recycling: A review of the recovery and high-value utilization of decommissioned wind turbine blades. Resour. Conserv. Recycl. 2024, 210, 107813. [Google Scholar] [CrossRef]
- Utekar, S.; Suriya, V.K.; More, N.; Rao, A. Comprehensive study of recycling of thermosetting polymer composites—Driving force, challenges and methods. Compos. Part B-Eng. 2024, 275, 108596. [Google Scholar] [CrossRef]
- European Commission. Proposal for a Regulation on Circularity Requirements for Vehicle Design and on Management of End-of-Life Vehicles. 2023. Available online: https://environment.ec.europa.eu/publications/proposal-regulation-circularity-requirements-vehicle-design-and-management-end-life-vehicles_en (accessed on 28 March 2025).
- Walker, R.; Korey, M.; Hubbard, A.M.; Clarkson, C.M.; Corum, T.; Smith, T.; Hershey, C.J.; Lindahl, J.; Ozcan, S.; Duty, C. Recycling of CF-ABS machining waste for large format additive manufacturing. Compos. Part B-Eng. 2024, 275, 111291. [Google Scholar] [CrossRef]
- Dörr, D.; Singh-Heer, N.; Xu, C.; Chang, T.; Clement-Thorne, B.; Gergely, R.C.R.; Okonski, D.; Henning, F.; Straatman, A.G.; Hrymak, A. Experimental and predictive analysis of the molding behavior of a PA6 glass mat thermoplastic (GMT). Int. J. Adv. Manuf. Technol. 2023, 129, 1159–1173. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Jiang, A.; Chen, G. Experimental study of glass-fiber mat thermoplastic material impact properties and lightweight automobile body analysis. Mater. Des. 2004, 25, 579–585. [Google Scholar] [CrossRef]
- Teltschik, J.; Matter, J.; Woebbeking, S.; Jahn, K.; Adasme, Y.B.; Van Paepegem, W.; Drechsler, K.; Tallawi, M. Review on recycling of carbon fibre reinforced thermoplastics with a focus on polyetheretherketone. Compos. Part B-Eng. 2024, 184, 108236. [Google Scholar] [CrossRef]
- Bandaru, A.K.; Anderson, T.; Weaver, P.M. Recycling CF/PEEK offcut waste from laser assisted tape placement: Influence of overlaps and gaps. Compos. Part B-Eng. 2024, 180, 108104. [Google Scholar] [CrossRef]
- Vincent, G.A.; de Bruijn, T.A.; Wijskamp, S.; van Drongelen, M.; Akkerman, R. Process- and material-induced heterogeneities in recycled thermoplastic composites. J. Thermoplast. Compos. Mater. 2022, 35, 2530–2551. [Google Scholar] [CrossRef]
- Zhang, J.; Chevali, V.S.; Wang, H.; Wang, C.H. Current status of carbon fibre and carbon fibre composites recycling. Compos. Part B-Eng. 2020, 193, 108053. [Google Scholar] [CrossRef]
- Xu, M.; Yang, J.; Ji, H.; Wu, Y.; Li, J.; Di, J.; Meng, X.; Jiang, H.; Lu, Q. Recovering high-quality glass fibers from end-of-life wind turbine blades through swelling-assisted low-temperature pyrolysis. Waste Manag. 2024, 187, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ye, M.; Li, M.; Xi, B.; Hou, J.; Qi, X.; Zhang, J.; Wei, Y.; Meng, F. Characteristics, kinetics and product distribution on pyrolysis process for waste turbine blades. J. Anal. Appl. Pyrol. 2023, 169, 105859. [Google Scholar] [CrossRef]
- Ge, L.; Xu, C.; Feng, H.; Jiang, H.; Li, X.; Lu, Y.; Sun, Z.; Wang, Y.; Xu, C. Study on isothermal pyrolysis and product characteristics of basic components of waste turbine blades. J. Anal. Appl. Pyrol. 2023, 171, 105964. [Google Scholar] [CrossRef]
- Serras-Malillos, A.; Perez-Martinez, B.B.; Lopez-Urionabarrenechea, A.; Acha, E.; Caballero, B.M. Integral recycling of polyester based end-of-life fibre reinforced plastic waste towards syngas generation. Sustain. Mater. Technol. 2023, 38, e00773. [Google Scholar] [CrossRef]
- Serras-Malillos, A.; Perez-Martinez, B.B.; Lopez-Urionabarrenechea, A.; Acha, E.; Caballero, B.M. Integral recycling of epoxy based end-of-life fibre reinforced waste towards H2 rich gas generation. J. Ind. Eng. Chem. 2024, 129, 665–681. [Google Scholar] [CrossRef]
- Prado, L.O.; Souza, H.H.S.; Chiquito, G.M.; Paulo, P.L.; Boncz, M.A. A comparison of different scenarios for on-site reuse of blackwater and kitchen waste using the life cycle assessment methodology. Environ. Impact Assess. Rev. 2020, 82, 106362. [Google Scholar] [CrossRef]
- Kijo-Kleczkowska, A.; Szumera, M.; Gnatowski, A.; Sadkowski, D. Comparative thermal analysis of coal fuels, biomass, fly ash and polyamide. Energy 2022, 258, 124840. [Google Scholar] [CrossRef]
- Yun, Y.M.; Seo, M.W.; Ra, H.W.; Koo, G.H.; Oh, J.S.; Yoon, S.J.; Kim, Y.K.; Lee, J.G.; Kim, J.H. Pyrolysis characteristics of glass fiber-reinforced plastic (GFRP) under isothermal conditions. J. Anal. Appl. Pyrol. 2015, 114, 40–46. [Google Scholar] [CrossRef]
- Braun, U.; Bahr, H.; Schartel, B. Fire retardancy effect of aluminium phosphinate and melamine polyphosphate in glass fibre reinforced polyamide 6. e-Polymers 2010, 10, 041. [Google Scholar] [CrossRef]
- Perez, B.A.; Krishna, J.V.J.; Toraman, H.E. Insights into co-pyrolysis of polyethylene terephthalate and polyamide 6 mixture through experiments, kinetic modeling and machine learning. Chem. Eng. J. 2023, 468, 143637. [Google Scholar] [CrossRef]
- Pannase, A.M.; Singh, R.K.; Ruj, B.; Gupta, P. Decomposition of polyamide via slow pyrolysis: Effect of heating rate and operating temperature on product yield and composition. J. Anal. Appl. Pyrol. 2020, 151, 104886. [Google Scholar] [CrossRef]
- Royuela, D.; Martínez, J.D.; López, J.M.; Callén, M.S.; García, T.; Verdejo, R.; Murillo, R.; Veses, A. Pursuing the circularity of wind turbine blades: Thermochemical recycling by pyrolysis and recovery of valuable resources. J. Anal. Appl. Pyrol. 2024, 181, 106657. [Google Scholar] [CrossRef]
- Luberti, M.; Ahn, H. Review of Polybed pressure swing adsorption for hydrogen purification. Int. J. Hydrogen Energy 2022, 47, 10911–10933. [Google Scholar] [CrossRef]
- Zhu, X.; Shi, Y.; Li, S.; Cai, N. Two-train elevated-temperature pressure swing adsorption for high-purity hydrogen production. Appl. Energy 2018, 229, 1061–1071. [Google Scholar] [CrossRef]
- Perez-Martinez, B.B.; Lopez-Urionabarrenechea, A.; Serras-Malillos, A.; Acha, A.; Martínez-Santos, M.; Caballero, B.M.; Iturrondobeitia, M.; Afonso, H. Recycling of plastic-rich streams from waste electrical and electronic equipment (WEEE) sorting plants: An in-depth study of pyrolysis potential through product characterization and life cycle assessment (LCA). Energy Conver. Manag. 2025, 329, 119633. [Google Scholar] [CrossRef]
- Serras-Malillos, A.; Perez-Martinez, B.B.; Iriondo, A.; Acha, E.; Lopez-Urionabarrenechea, A.; Caballero, B.M. Quantification of the composition of pyrolysis oils of complex plastic waste by gas chromatography coupled with mass spectrometer detector. RSC Adv. 2024, 14, 9892. [Google Scholar] [CrossRef]
- Czernik, S.; Elam, C.C.; Evans, R.J.; Meglen, R.R.; Moens, L.; Tatsumoto, K. Catalytic pyrolysis of nylon-6 to recover caprolactam. J. Anal. Appl. Pyrol. 1998, 46, 51–64. [Google Scholar] [CrossRef]
- Bockhorn, H.; Hornung, A.; Hornung, U.; Weichmann, J. Kinetic study on the non-catalysed and catalysed degradation of polyamide 6 with isothermal and dynamic methods. Thermochim. Acta 1999, 337, 97–110. [Google Scholar] [CrossRef]
- Kubatovics, F.; Blazsó, M. Thermal decomposition of polyamide-6 in the presence of PVC. Macromol. Chem. Phys. 2000, 201, 349–354. [Google Scholar] [CrossRef]
- de Rezende Locatel, W.; Mohabeer, C.; Laurenti, D.; Schuurman, Y.; Guilhaume, N. Co-pyrolysis of beech wood and polyamide-6: Effect of HZSM-5 catalyst on the properties of pyrolysis oils. J. Anal. Appl. Pyrol. 2022, 167, 105696. [Google Scholar] [CrossRef]
- López, F.A.; Rodríguez, O.; Alguacil, F.J.; García-Díaz, I.; Centeno, T.A.; García-Fierro, J.L.; González, C. Recovery of carbon fibres by the thermolysis and gasification of waste prepreg. J. Anal. Appl. Pyrol. 2013, 104, 675–683. [Google Scholar] [CrossRef]
- Nahil, M.A.; Williams, P.T. Recycling of carbon fibre reinforced polymeric waste for the production of activated carbon fibres. J. Anal. Appl. Pyrol. 2011, 91, 67–75. [Google Scholar] [CrossRef]
- Coralli, I.; Giorgi, V.; Vassura, I.; Rombolà, A.G.; Fabbri, D. Secondary reactions in the analysis of microplastics by analytical pyrolysis. J. Anal. Appl. Pyrol. 2021, 161, 105377. [Google Scholar] [CrossRef]
- Xu, M.; Ji, H.; Wu, Y.; Di, J.; Meng, X.; Jiang, H.; Lu, Q. The pyrolysis of end-of-life wind turbine blades under different atmospheres and their effects on the recovered glass fibers. Compos. Part B-Eng. 2023, 251, 110493. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Zakarauskas, K.; Striugas, N. Recovery of styrene-rich oil and glass fibres from fibres-reinforced unsaturated polyester resin end-of-life wind turbine blades using pyrolysis technology. J. Anal. Appl. Pyrol. 2023, 173, 106100. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Williams, P.T. Characterisation of products from the recycling of glass fibre reinforced polyester waste by pyrolysis. Fuel 2003, 82, 2223–2230. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Miskolczi, N.; Nagy, T.; Lipóczi, G. Recovery of glass fibre and carbon fibres from reinforced thermosets by batch pyrolysis and investigation of fibre re-using as reinforcement in LDPE matrix. Compos. Part B-Eng. 2016, 91, 154–161. [Google Scholar] [CrossRef]
- Du, C.; Zhang, J.; Li, L.; Wang, K.; Luo, G. Impurity Formation in the Beckmann Rearrangement of Cyclohexanone Oxime to Yield ε-Caprolactam. Ind. Eng. Chem. Res. 2017, 56, 14207–14213. [Google Scholar] [CrossRef]
- Romero, A.; Santos, A.; Yustos, P. Effect of Methyl-δ-valerolactams on the Quality of ε-Caprolactam. Ind. Eng. Chem. Res. 2004, 43, 7–1557. [Google Scholar] [CrossRef]
- Hu, B.; Wang, S.; Yan, J.; Zhang, H.; Qiu, L.; Liu, W.; Guo, Y.; Shen, J.; Chen, B.; Shi, C.; et al. Review of waste plastics treatment and utilization: Efficient conversion and high value utilization. Process Saf. Environ. Protect. 2024, 183, 378–398. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Stasiulaitiene, I.; Zakarauskas, K.; Striugas, N. Recovery of energy and carbon fibre from wind turbine blades waste (carbon fibre/unsaturated polyester resin) using pyrolysis process and its life-cycle assessment. Environ. Res. 2024, 245, 118016. [Google Scholar] [CrossRef]
- Pillain, B.; Loubeta, P.; Pestalozzic, F.; Woidasky, J.; Erriguible, A.; Aymonier, C.; Sonnemann, G. Positioning supercritical solvolysis among innovative recycling and current waste management scenarios for carbon fiber reinforced plastics thanks to comparative life cycle assessment. J. Supercrit. Fluids 2019, 154, 104607. [Google Scholar] [CrossRef]
- Tapper, R.J.; Longana, M.L.; Norton, A.; Potter, K.D.; Hamerton, I. An evaluation of life cycle assessment and its application to the closed-loop recycling of carbon fibre reinforced polymers. Compos. Part B-Eng. 2020, 184, 107665. [Google Scholar] [CrossRef]
- Cheng, H.; Guo, L.; Zheng, L.; Qian, Z.; Su, S. A closed-loop recycling process for carbon fiber-reinforced polymer waste using thermally activated oxide semiconductors: Carbon fiber recycling, characterization and life cycle assessment. Waste Manag. 2022, 153, 283–292. [Google Scholar] [CrossRef]
- Moutik, B.; Summerscales, J.; Graham-Jones, J.; Pemberton, R. Quality assessment of life cycle inventory data for fibre-reinforced polymer composite materials. Sustain. Prod. Consump. 2024, 49, 474–491. [Google Scholar] [CrossRef]

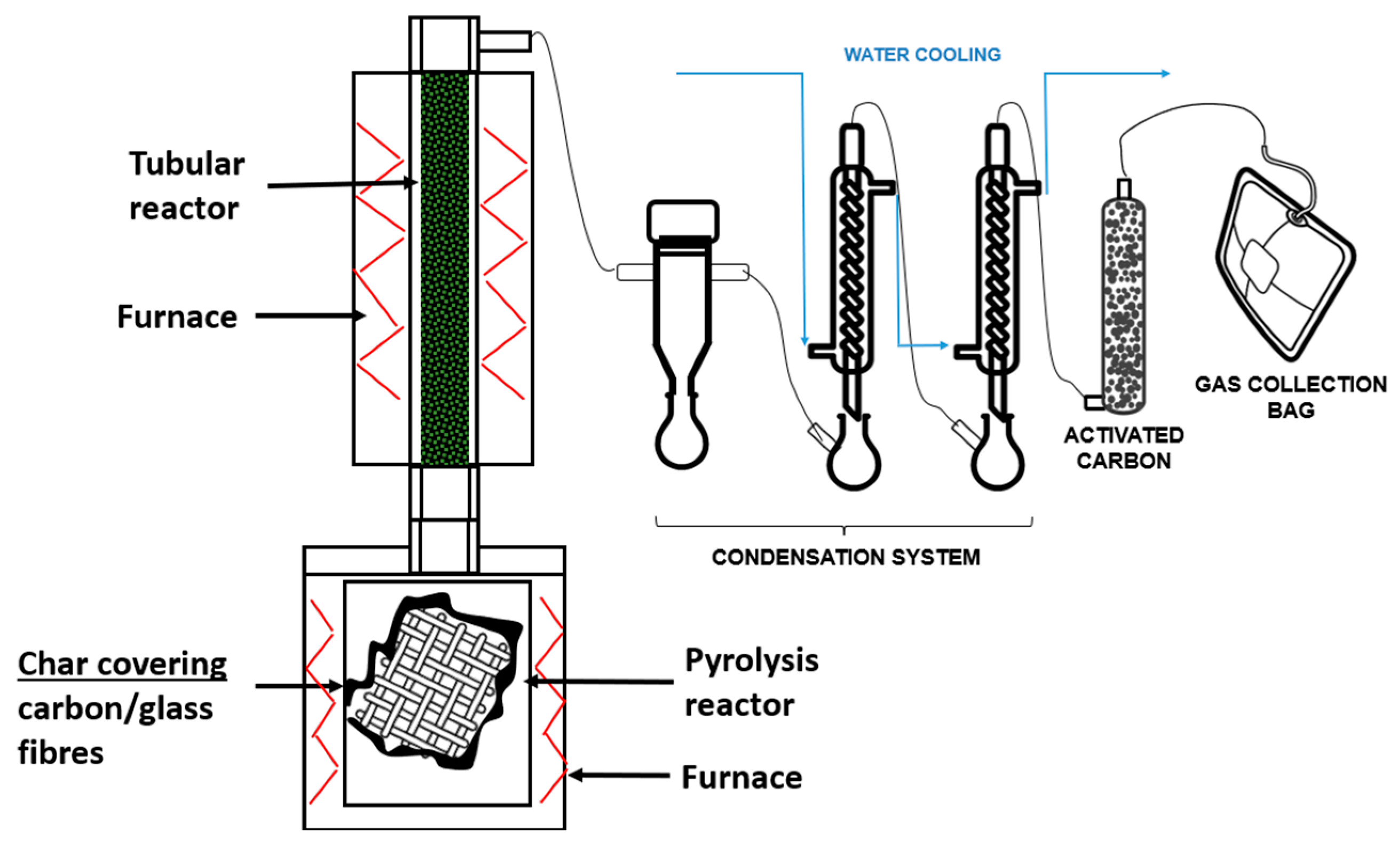




| Process | Input (I)/Output (O) | Flow | Amount | Unit | Avoided Waste | Provider |
|---|---|---|---|---|---|---|
| Conventional pyrolysis | I | Waste glass fiber PA | 1 | t | ||
| Natural gas, burned in gas turbine | 3650 | kWh | - | Natural gas, burned in gas turbine | natural gas, burned in gas turbine | cutoff, U—ES | ||
| Activated carbon, granular | 0.2 | t | - | Activated carbon production, granular from hard coal | activated carbon, granular | cutoff, U—RoW | ||
| Tap water | 20 | t | - | Tap water production, conventional treatment | tap water | cutoff, U—RoW | ||
| O | Waste refinery gas | 1458 | MJ | - | Treatment of waste refinery gas, burned in flare | waste refinery gas | cutoff, U—GLO | |
| Waste mineral oil | 0.598 | t | - | Clinker production | waste mineral oil | cutoff, U—Europe without Switzerland | ||
| Glass fiber | 0.27 | t | TRUE | Glass fiber production | glass fiber | cutoff, U—RoW | ||
| Spent activated carbon | 0.2 | t | - | Spent activated carbon, granular, recycled content cut-off | spent activated carbon, granular | cutoff, U—GLO | ||
| Advanced pyrolysis | I | Waste glass fiber PA | 1 | t | ||
| Natural gas, burned in gas turbine | 7058 | kWh | - | Natural gas, burned in gas turbine | natural gas, burned in gas turbine | cutoff, U—ES | ||
| Activated carbon, granular | 0.2 | t | - | Activated carbon production, granular from hard coal | activated carbon, granular | cutoff, U—RoW | ||
| Tap water | 20 | t | - | Tap water production, conventional treatment | tap water | cutoff, U—RoW | ||
| O | Refinery gas | 0.44 | t | TRUE | Refinery gas production, petroleum refinery operation | refinery gas | cutoff, U—RoW | |
| Wastewater, average | 0.211 | t | - | Treatment of wastewater, average, wastewater treatment | wastewater, average | cutoff, U—Europe without Switzerland | ||
| Glass fiber | 0.27 | t | TRUE | Glass fiber production | glass fiber | cutoff, U—RoW | ||
| Spent activated carbon | 0.2 | t | - | Spent activated carbon, granular, recycled content cut-off | spent activated carbon, granular | cutoff, U—GLO | ||
| Landfill | Process-specific burdens, residual material landfill | process-specific burdens, residual material landfill | cutoff, U | |||||
| Proximate Analysis (wt.%) | As Received (ar) | Dry and Ash Free Basis (daf) |
|---|---|---|
| Moisture | 2.3 ± 0.01 | - |
| Volatile matter | 69.3 ± 1.0 | 98.0 ± 1.0 |
| Ash | 27.0 ± 1.3 | - |
| Fixed carbon 1 | 1.4 | 2.0 |
| Elemental Analysis (wt.%) | As Received (ar) | Dry and Ash Free Basis (daf) |
| Carbon | 42.9 ± 2.8 | 60.7 ± 2.8 |
| Hydrogen | 6.4 ± 0.4 | 9.0 ± 0.4 |
| Nitrogen | 8.4 ± 0.1 | 11.9 ± 0.1 |
| Oxygen | 8.6 ± 1.8 | 12.2 ± 1.8 |
| Fluorine, chlorine, bromine | u.q.l. 2 | u.q.l. 2 |
| Sulfur | u.q.l. 2 | u.q.l. 2 |
| Others 1 | 4.4 | 6.2 |
| LHV (MJ kg−1) | 20.6 ± 0.34 | 29.1 ± 0.34 |
| Conventional Pyrolysis | Advanced Pyrolysis | |
|---|---|---|
| Solid | 34.8 ± 3.6 | 34.9 ± 2.4 |
| Liquid | 59.8 ± 3.1 | 21.1 ± 0.3 |
| Gas 1 | 5.4 ± 0.4 | 44.0 ± 0.1 |
| Conventional Pyrolysis | Advanced Pyrolysis | |||||
|---|---|---|---|---|---|---|
| vol.% | wt.% | Total Quantity (g) 1 | vol.% | wt.% | Total Quantity (g) 1 | |
| H2 | 47.4 | 4.6 | 0.25 | 39.9 | 5.0 | 2.20 |
| CO | 8.4 | 11.4 | 0.62 | 19.5 | 33.8 | 14.87 |
| CO2 | 18.8 | 40.2 | 2.17 | 5.6 | 15.2 | 6.69 |
| CH4 | 6.6 | 5.1 | 0.28 | 22.5 | 22.8 | 10.03 |
| C2H4 | 4.5 | 6.1 | 0.33 | 10.8 | 18.7 | 8.23 |
| C2H6 | 4.5 | 6.7 | 0.36 | 0.7 | 1.4 | 0.62 |
| C3H6 | 2.9 | 6.0 | 0.32 | 0.2 | 0.5 | 0.22 |
| C3H8 | 1.6 | 3.4 | 0.18 | 0.3 | 0.8 | 0.35 |
| C4 | 3.3 | 9.5 | 0.51 | 0.2 | 0.7 | 0.31 |
| C5 | 2.0 | 6.9 | 0.37 | 0.2 | 1.0 | 0.44 |
| LHV | 20.9 MJ/Nm3 | 27.0 MJ/kg | 19.1 MJ/Nm3 | 31.7 MJ/kg | ||
| H2 yield 2 | 7.8% | 68.8% | ||||
| Conventional Pyrolysis | Advanced Pyrolysis | |
|---|---|---|
| GC-MS analysis (area %) | ||
| Caprolactam | 90.7 (43.9 wt.%) | n.d. 2 |
| Water | n.d. 2 | 100 |
| Other identified | 1.3 | n.d. 2 |
| Not identified | 8.0 | n.d. 2 |
| Elemental analysis | ||
| Carbon | 57.3 ± 0.1 | 9.9 ± 9.1 |
| Hydrogen | 9.6 ± 0.4 | 9.8 ± 0.1 |
| Nitrogen | 10.9 ± 0.1 | 11.1 ± 0.1 |
| Oxygen | 10.9 ± 0.4 | 47.2 ± 2.5 |
| Others 1 | 11.3 | 22.0 |
| LHV (MJ kg−1) | 42.5 ± 4.01 | - 3 |
| Proximate Analysis | Conventional Pyrolysis | Advanced Pyrolysis |
|---|---|---|
| Moisture | 0.4 ± 0.0 | 0.3 ± 0.2 |
| Volatile matter | 4.6 ± 0.2 | 4.3 ± 0.3 |
| Ash | 88.0 ± 0.2 | 88.8 ± 4.1 |
| Fixed carbon 1 | 7.0 | 6.5 |
| Elemental Analysis | Conventional Pyrolysis | Advanced Pyrolysis |
| Carbon | 8.9 ± 0.2 | 8.6 ± 0.0 |
| Hydrogen | 0.4 ± 0.0 | 0.2 ± 0.0 |
| Nitrogen | 0.6 ± 0.1 | 0.6 ± 0.0 |
| Others 2 | 1.7 | 1.5 |
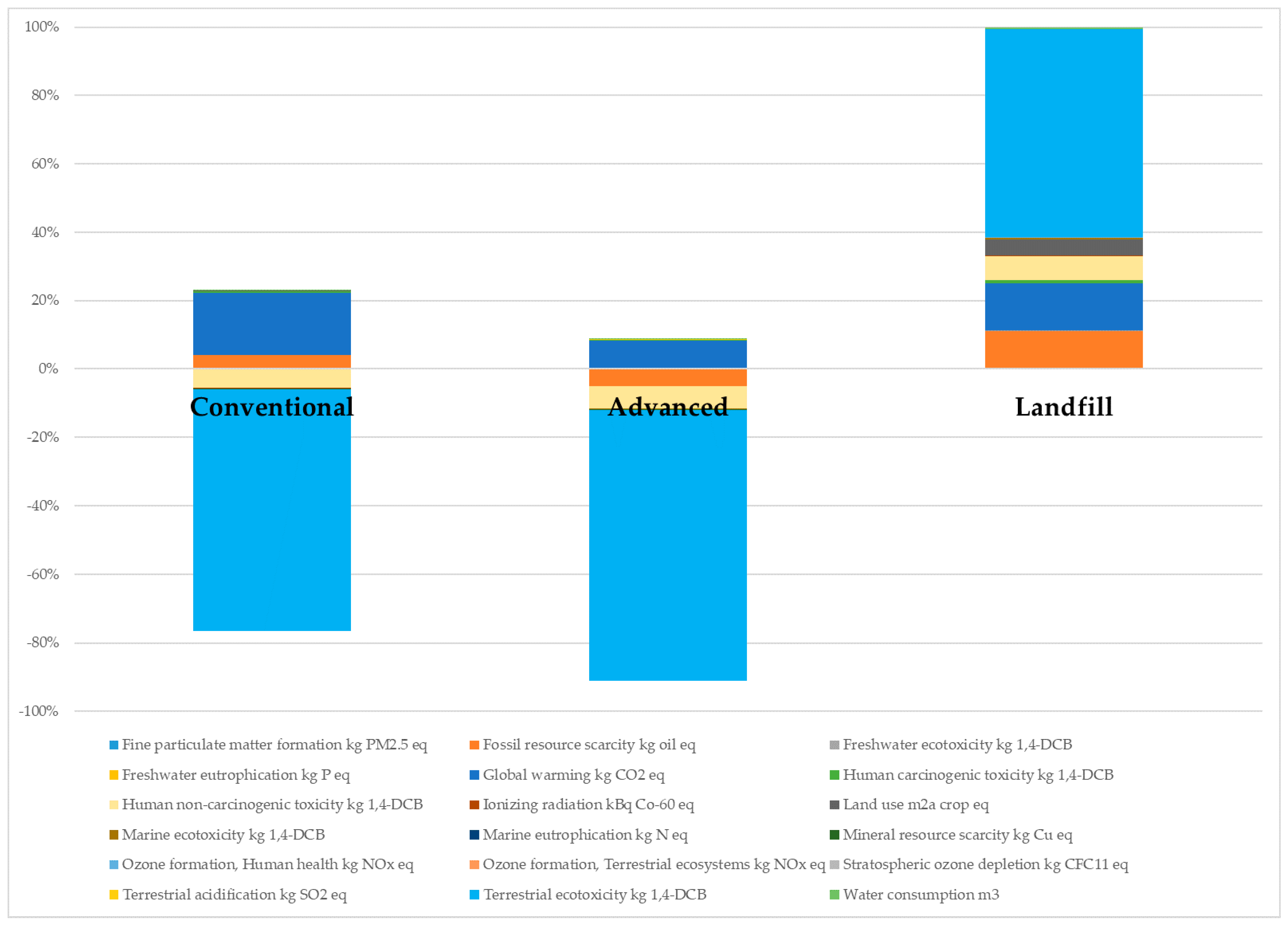
| Impact Categories | Conventional | Advanced | Landfill |
|---|---|---|---|
| Fine particulate matter formation (kg PM2.5 eq) | 2.165 | 1.216 | 4597 |
| Fossil resource scarcity (kg oil eq) | 248.8 | −335.3 | 2.44 × 106 |
| Freshwater ecotoxicity (kg 1,4-DCB) | −5.998 | −9.289 | 6.04 × 104 |
| Freshwater eutrophication (kg P eq) | 0.505 | 0.488 | 339 |
| Global warming (kg CO2 eq) | 1104 | 566.8 | 3.06 × 106 |
| Human carcinogenic toxicity (kg 1,4-DCB) | 21.72 | 7.638 | 1.67 × 105 |
| Human non-carcinogenic toxicity (kg 1,4-DCB) | −331.2 | −436.1 | 1.55 × 106 |
| Ionizing radiation (kg Bq Co-60 eq) | −5.735 | −9.116 | 5.89 × 104 |
| Land use (m2a crop eq) | 9.962 | 6.743 | 1.07 × 106 |
| Marine ecotoxicity (kg 1,4-DCB) | −7.992 | −13.86 | 8.87 × 104 |
| Marine eutrophication (kg N eq) | 0.022 | −0.012 | 171.8 |
| Mineral resource scarcity (kg Cu eq) | −5.068 | −5.744 | 7520 |
| Ozone formation, Human health (kg NOx eq) | 1.394 | −0.252 | 1.42 × 104 |
| Ozone formation, Terrestrial ecosystems (kg NOx eq) | 1.383 | −0.544 | 1.54 × 104 |
| Stratospheric ozone depletion (kg CFC11 eq) | −6.1 × 10−4 | −7.3 × 10−4 | 0.844 |
| Terrestrial acidification (kg SO2 eq) | 6.289 | 3.416 | 1.10 × 104 |
| Terrestrial ecotoxicity (kg 1,4-DCB) | −4303 | −5385 | 1.35 × 107 |
| Water consumption (m3) | 18.73 | 17.72 | 1.41 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caballero, B.M.; Lopez-Urionabarrenechea, A.; Gonzalez-Arcos, J.P.; Perez-Martinez, B.B.; Acha, E.; Iturrondobeitia, M.; Ibarretxe, J.; Esnaola, A.; Baskaran, M. Recycling Fiber-Reinforced Polyamide Waste from the Automotive Industry: Life Cycle Assessment (LCA) of an Advanced Pyrolysis Process to Reclaim Glass Fibers and Valuable Chemicals. Materials 2025, 18, 1594. https://doi.org/10.3390/ma18071594
Caballero BM, Lopez-Urionabarrenechea A, Gonzalez-Arcos JP, Perez-Martinez BB, Acha E, Iturrondobeitia M, Ibarretxe J, Esnaola A, Baskaran M. Recycling Fiber-Reinforced Polyamide Waste from the Automotive Industry: Life Cycle Assessment (LCA) of an Advanced Pyrolysis Process to Reclaim Glass Fibers and Valuable Chemicals. Materials. 2025; 18(7):1594. https://doi.org/10.3390/ma18071594
Chicago/Turabian StyleCaballero, Blanca María, Alexander Lopez-Urionabarrenechea, Jean Paul Gonzalez-Arcos, Borja Benjamín Perez-Martinez, Esther Acha, Maider Iturrondobeitia, Julen Ibarretxe, Aritz Esnaola, and Maider Baskaran. 2025. "Recycling Fiber-Reinforced Polyamide Waste from the Automotive Industry: Life Cycle Assessment (LCA) of an Advanced Pyrolysis Process to Reclaim Glass Fibers and Valuable Chemicals" Materials 18, no. 7: 1594. https://doi.org/10.3390/ma18071594
APA StyleCaballero, B. M., Lopez-Urionabarrenechea, A., Gonzalez-Arcos, J. P., Perez-Martinez, B. B., Acha, E., Iturrondobeitia, M., Ibarretxe, J., Esnaola, A., & Baskaran, M. (2025). Recycling Fiber-Reinforced Polyamide Waste from the Automotive Industry: Life Cycle Assessment (LCA) of an Advanced Pyrolysis Process to Reclaim Glass Fibers and Valuable Chemicals. Materials, 18(7), 1594. https://doi.org/10.3390/ma18071594






