Study on Mechanical Properties and Curing Reaction Mechanism of Alkali-Activated-Slag Solidified Port Soft Soil with Different Activators
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. AAS Matching Design
2.2.1. Mixing of Sodium Silicate Modulus
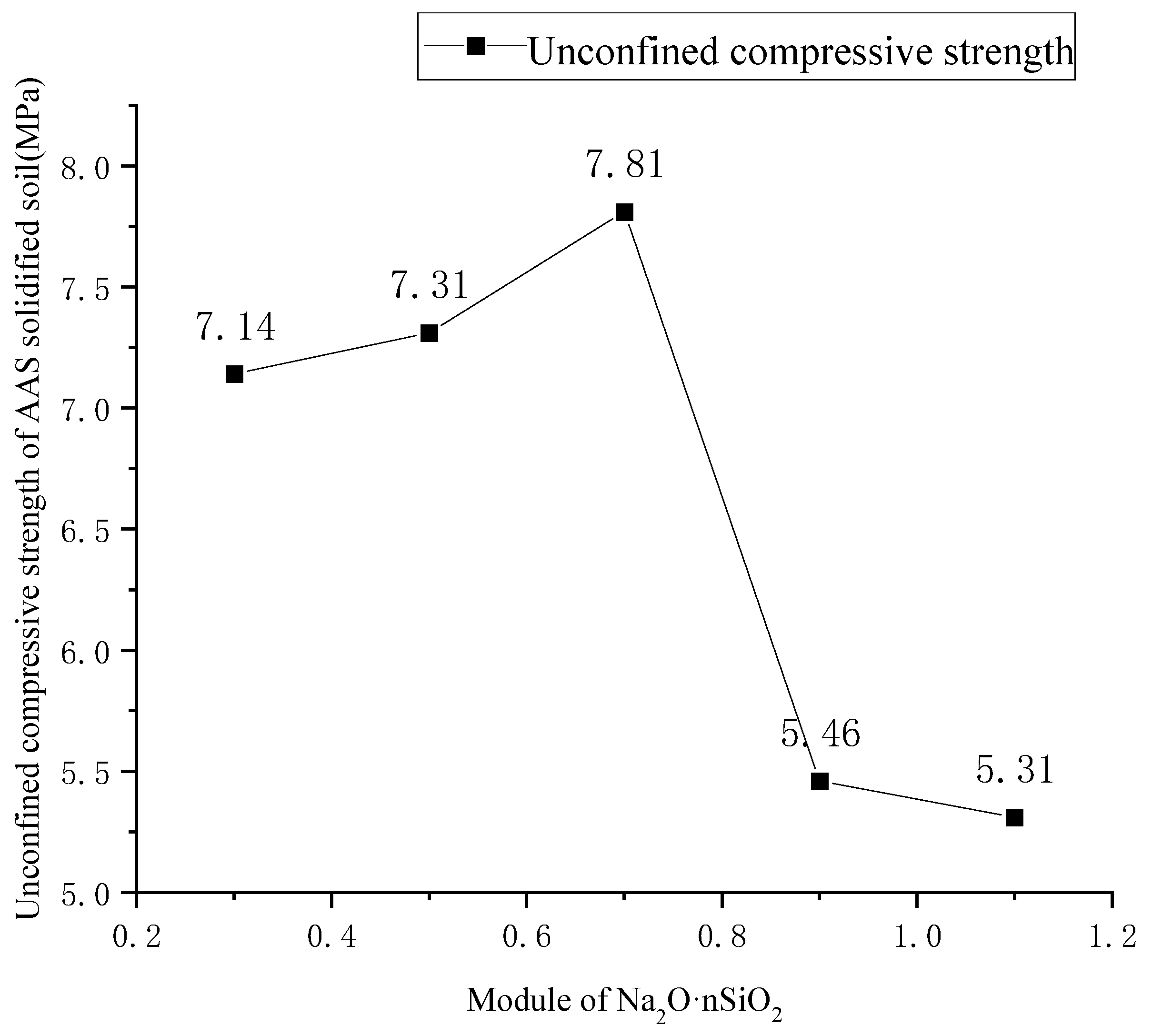
2.2.2. Optimum Modulus Test Scheme for Na2O·nSiO2
2.2.3. Orthogonal Test Design of AAS-Solidified Soil
2.3. Test Method
3. Results and Discussion
3.1. Optimum Modulus of Na2O·nSiO2
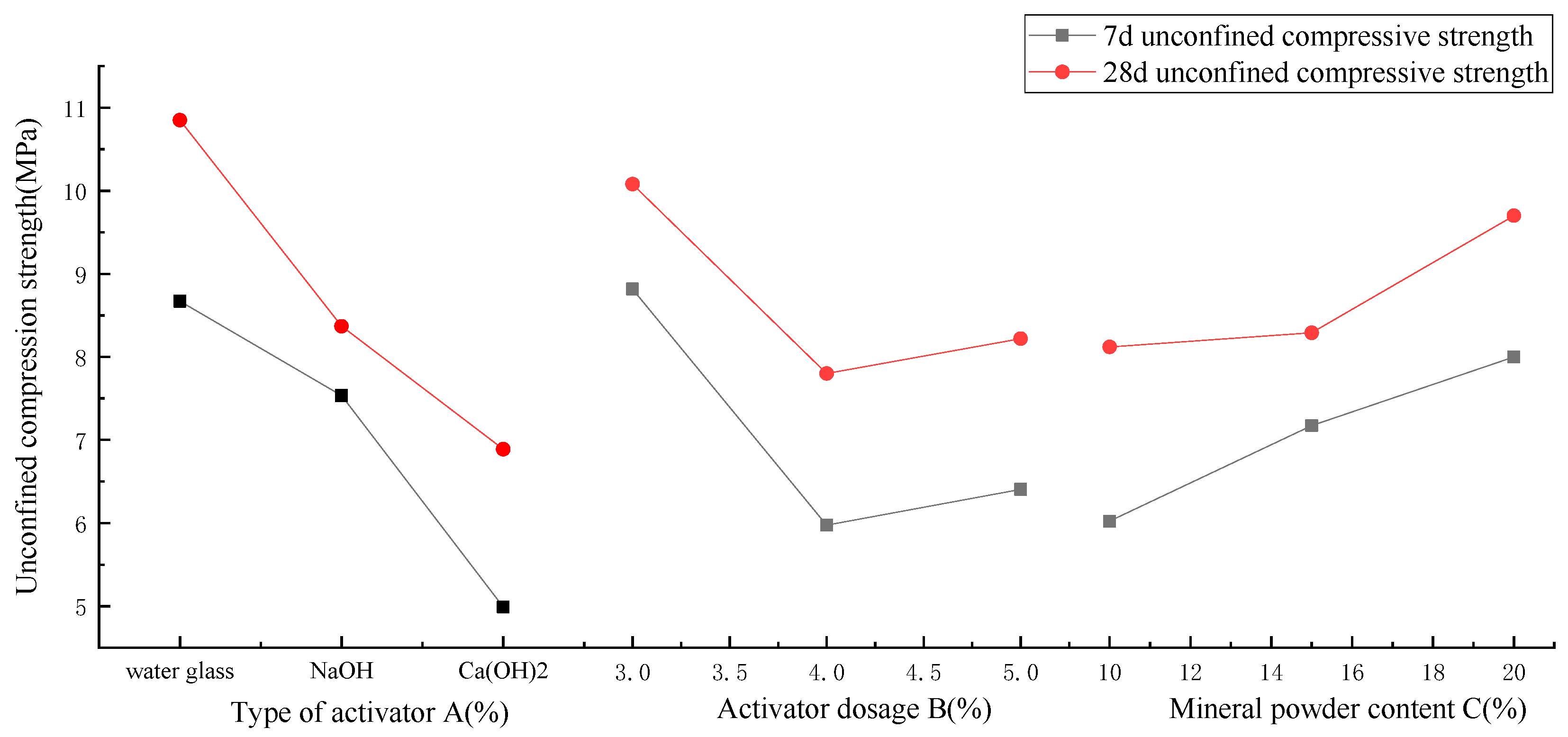
3.2. Analysis of Orthogonal Test Results
3.3. Mechanical Properties of AAS-Solidified Soils
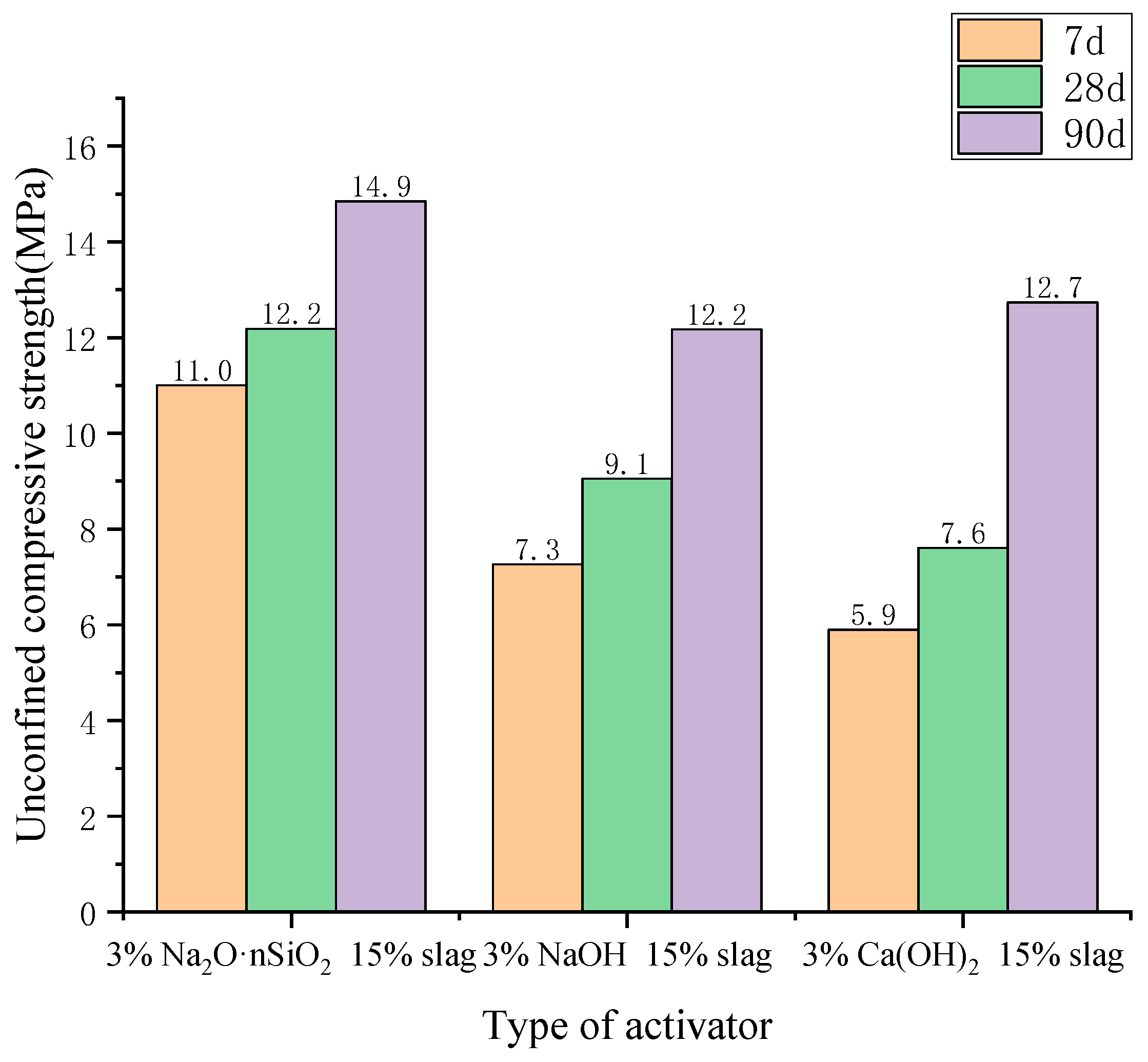
3.3.1. Unconfined Compressive Strength
3.3.2. Rebound Modulus
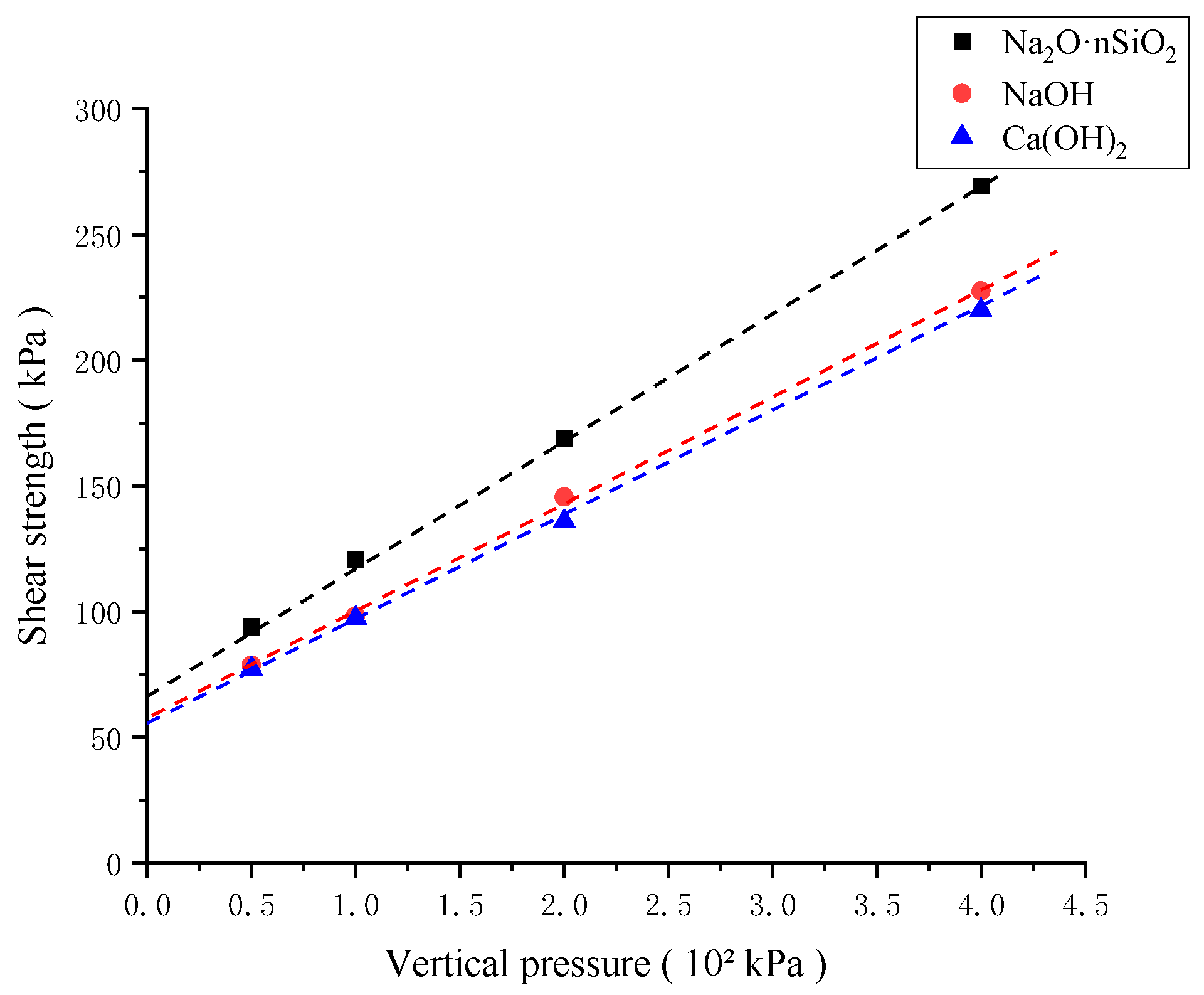
3.3.3. Shear Strength
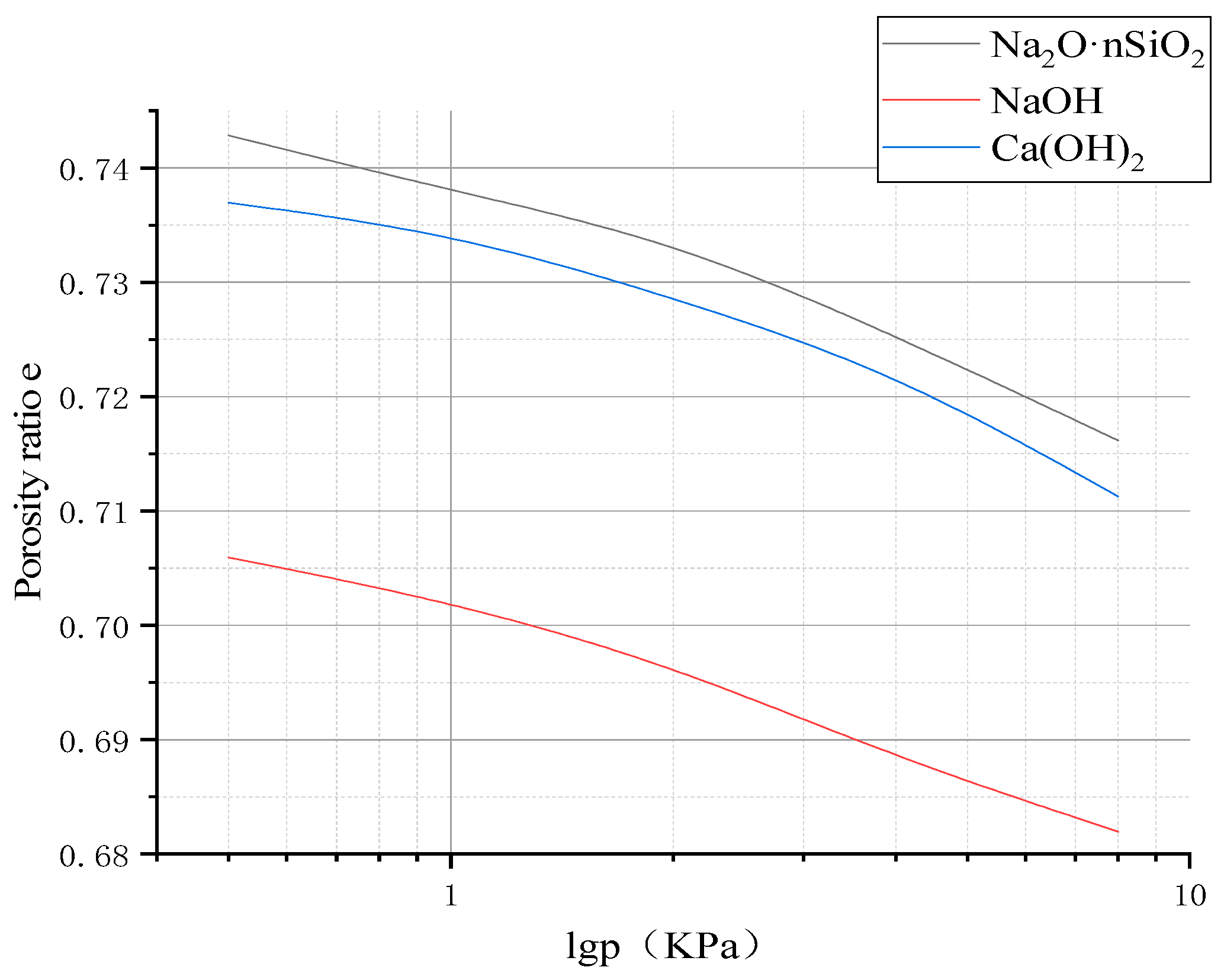
3.3.4. Compression Modulus
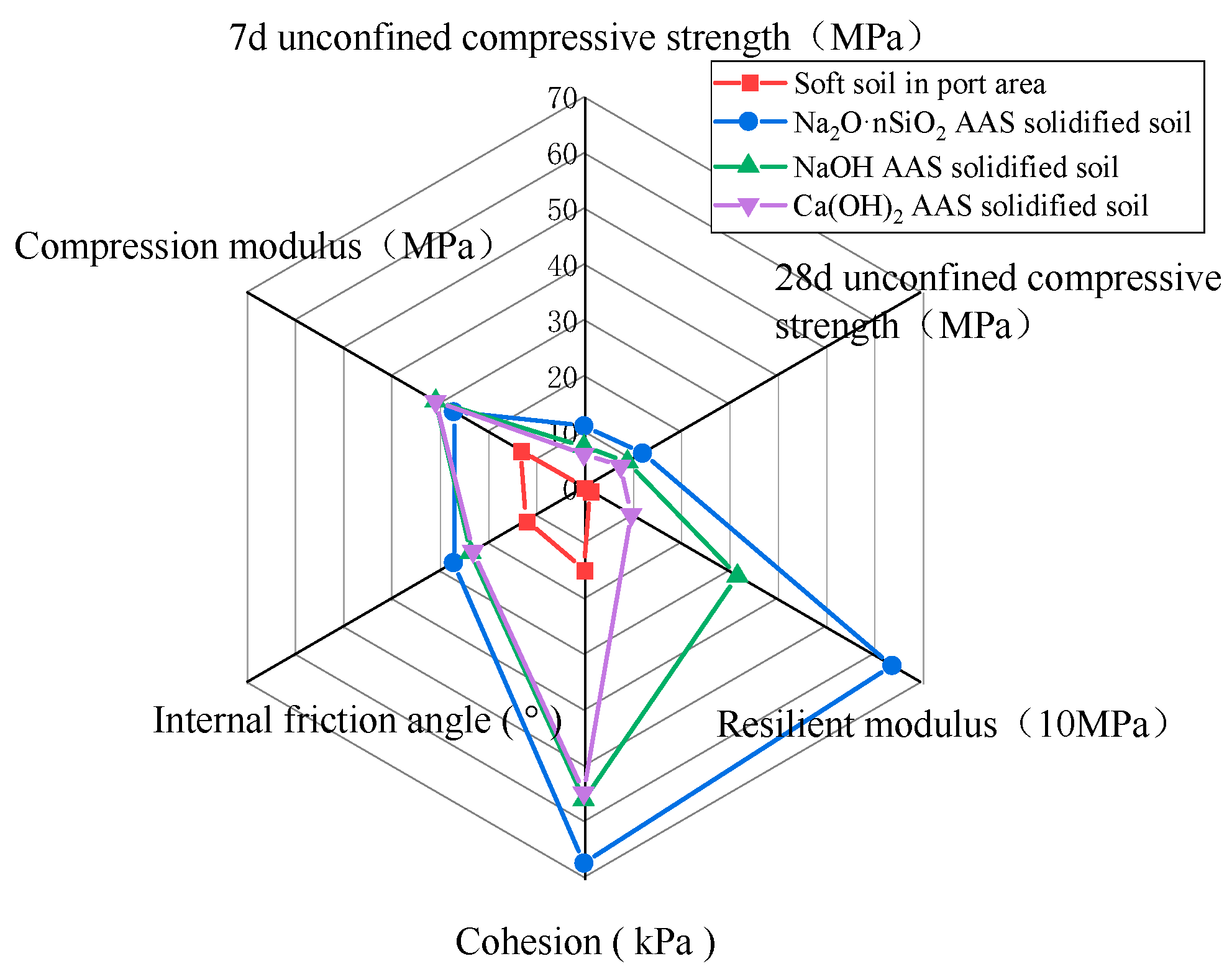
3.3.5. Evaluation of Macroscopic Mechanical Properties of AAS-Solidified Soil
3.4. Microscopic Analysis
3.4.1. Phase Composition Analysis
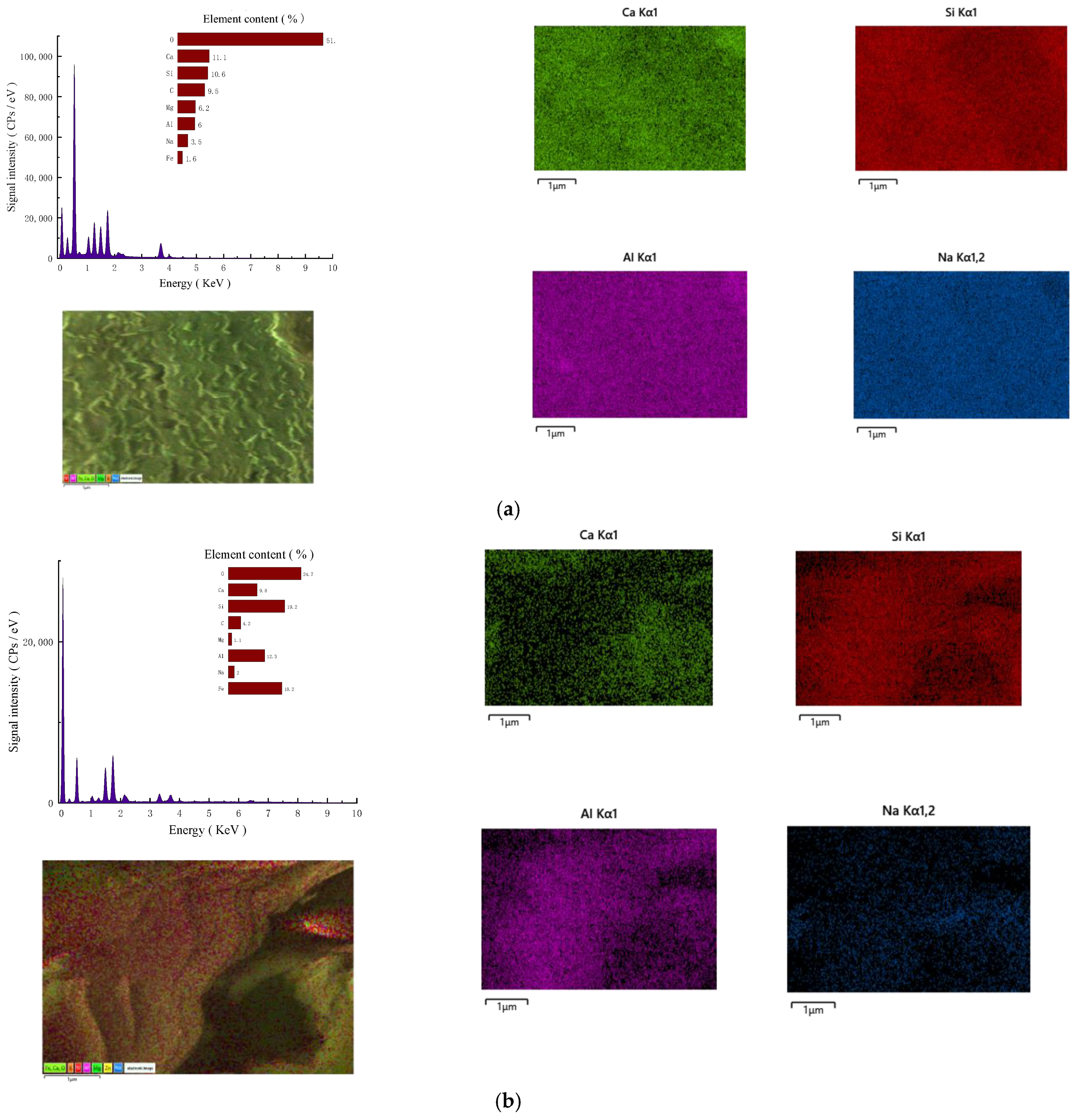
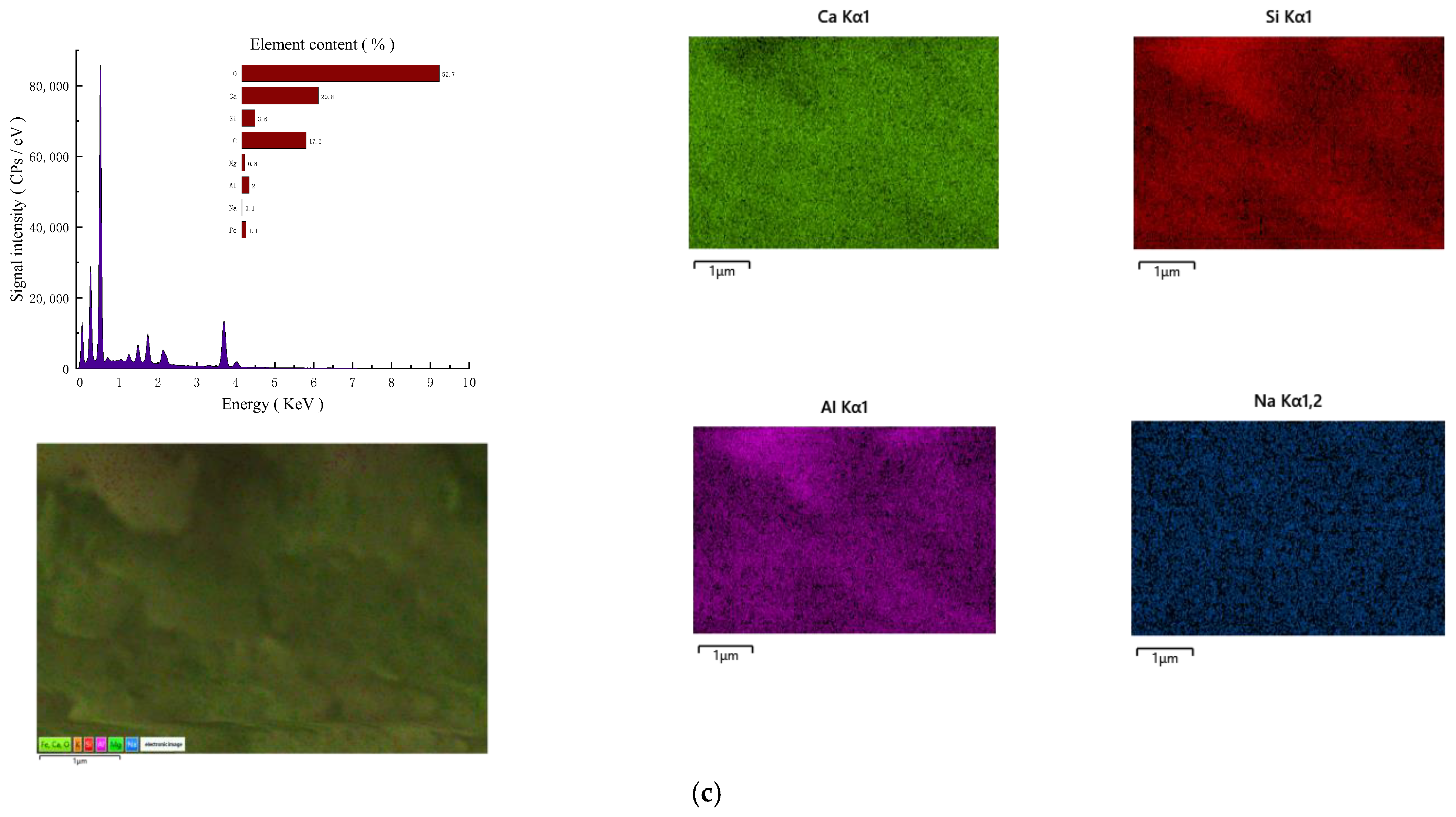
3.4.2. SEM-EDS Analysis
- (1)
- Micromorphology
- (2)
- Element content
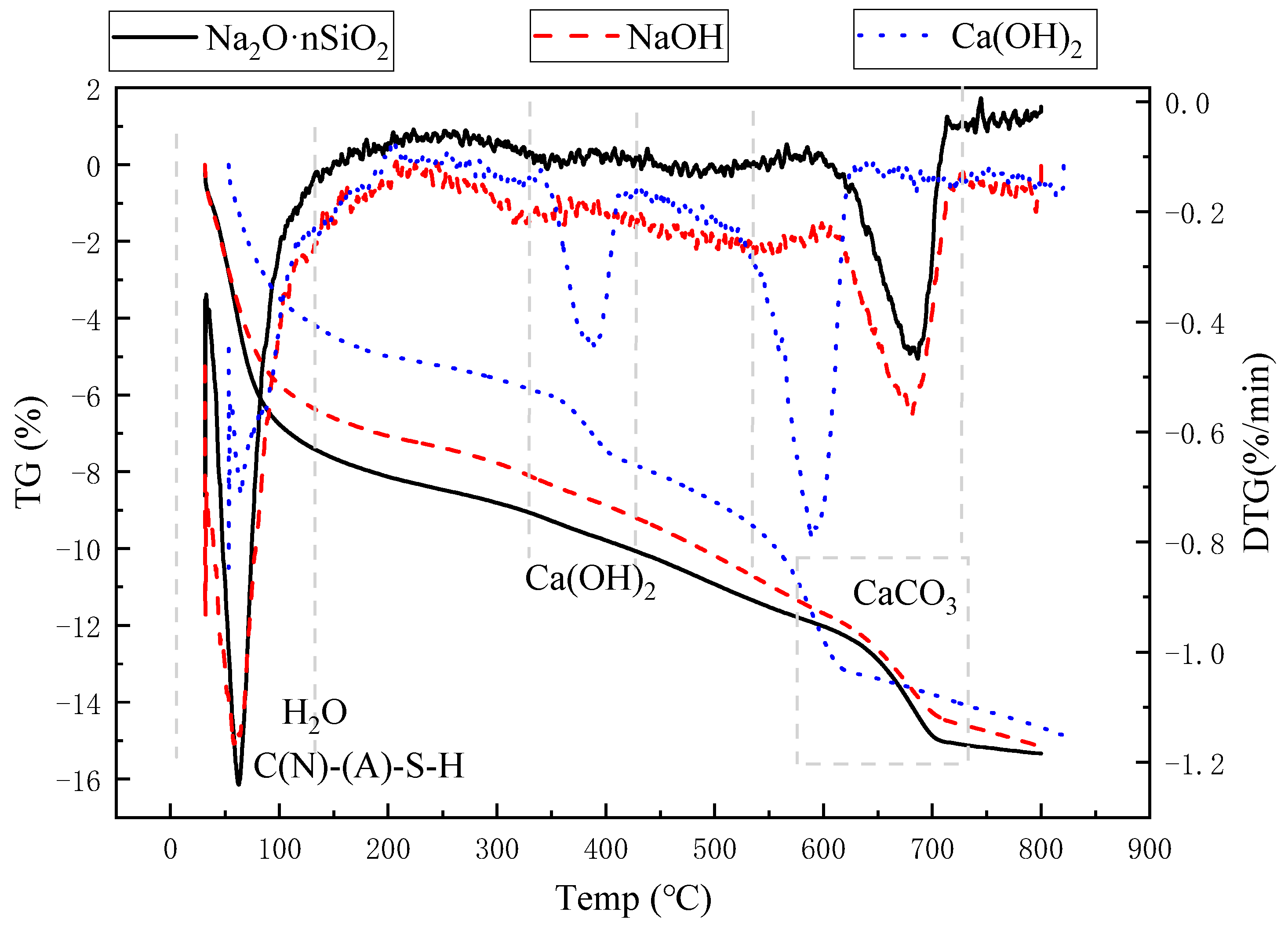
3.4.3. TG-DTG Analysis
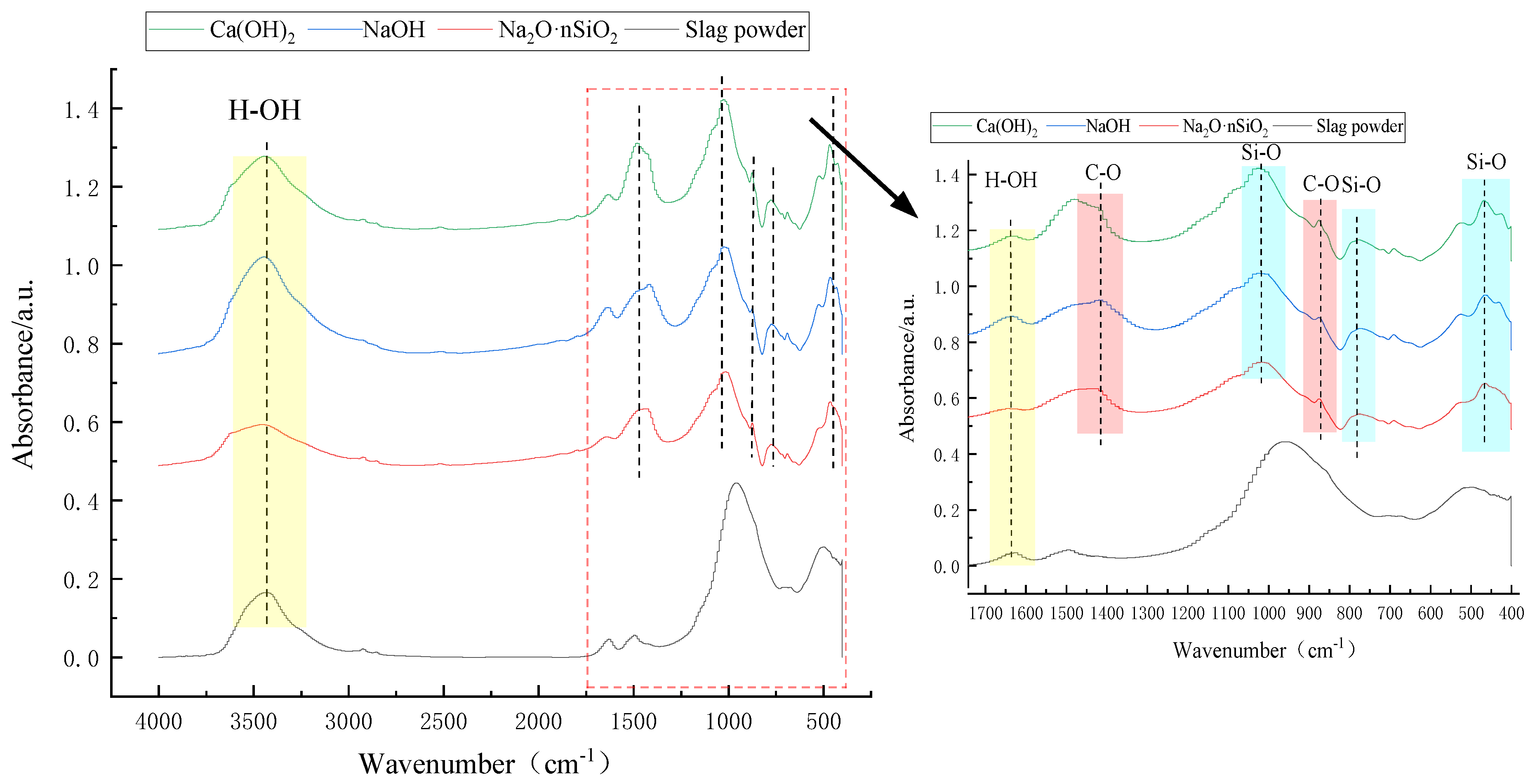
3.4.4. FTIR Analysis
3.4.5. AAS Reaction Mechanism
- (1)
- Vitreous deconstruction stage
- (2)
- Monomer polycondensation stage
- (3)
- Gel evolution stage
4. Conclusions
- (1)
- Orthogonal tests were carried out on three factors: the type of activator, the amount of activator, and the amount of slag powder in AAS-solidified soil. It was found that the main factor affecting the unconfined compressive strength of AAS-solidified soil was the type of activator, the secondary factor was the amount of activator, and the tertiary factor was the amount of slag powder. The optimal factors were as follows: Na2O·nSiO2 as the activator type, 3% for the activator amount, and 20% for the slag powder amount.
- (2)
- The unconfined compressive strength, resilience modulus, shear strength, and compression modulus of the three AAS-solidified soils were tested. It was concluded that the effects of the initiators can be ordered as follows: Na2O·nSiO2 > NaOH > Ca(OH)2. Here, the unconfined compressive strength of Na2O·nSiO2 AAS-solidified soil can reach 11 MPa after 7 days, and the early strength forms quickly. Regarding macroscopic mechanical properties, AAS-solidified soil can effectively improve the mechanical properties of plain soil, and AAS-solidified soil as a foundation has good engineering characteristics.
- (3)
- Through XRD, FTIR, and TG-DTG tests on AAS-solidified soil, it was concluded that the hydration products of AAS are mainly C-A-S-H gel, N-A-S-H gel, and C-S-H gel. The gel cementation of soil particles can enhance the strength and stability of soft soil foundations.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| XRF | X-Ray Fluorescence Spectrometer |
| XRD | X-Ray Diffraction |
| SEM | Scanning Electron Microscopy |
| EDS | Energy Dispersive Spectrometer |
| FTIR | Fourier Transform Infrared Spectrometer |
| TG | Thermal Gravimetric Analyzer |
| d | Day |
References
- Zhong, W. Study on Settlement Deformation Control of Shallow Solidified Soft Soil Foundation Under Traffic Load; Changsha University of Science and Technology: Changsha, China, 2022. [Google Scholar]
- Li, Y. Study on Settlement Law of Gully High Fill Foundation in Collapsible Loess Area; Lanzhou Jiaotong University: Lanzhou, China, 2020. [Google Scholar]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in understanding alkali-activated materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, Z.; Zhuang, S.; He, W. Hydration properties and microstructure characteristics of alkali–activated steel slag. Constr. Build. Mater. 2020, 241, 118141. [Google Scholar] [CrossRef]
- El-Yamany, H.E.; El-Salamawy, M.A.; El-Assal, N.T. Microstructure and mechanical properties of alkali-activated slag mortar modified with latex. Constr. Build. Mater. 2018, 191, 32–38. [Google Scholar] [CrossRef]
- Li, X.G.; Xu, J.S.; Jiang, D.B.; Lyu, Y.; He, C.C.; Zhang, C.; Fu, Q.Y. Effect of Internal Curing of SAP on Properties of Alkali-Activated Slag Cementitious Materials. Silic. Bull. 2021, 40, 3435–3441. [Google Scholar]
- Long, W.J.; Xiao, B.X.; Gu, Y.C.; Xing, F. Micro- and macro-scale characterization of nano-SiO2 reinforced alkali activated slag composites. Mater. Charact. 2017, 136, 111–121. [Google Scholar]
- Wang, W.C.; Wang, H.Y.; Lo, M.H. The engineering properties of alkali-activated slag pastes exposed to high temperatures. Constr. Build. Mater. 2014, 68, 409–415. [Google Scholar] [CrossRef]
- Cao, W.W.; Lei, T.; Min, Z.H.; Xu, X.L.; D, P.P. Performance and Mechanism of Solid Alkali-Activated Slag/Fly Ash Grouting Material. Silic. Bull. 2021, 40, 4037–4043. [Google Scholar]
- Shen, W.; Zhou, M.; Ma, W.; Hu, J.; Cai, Z. Investigation on the application of steel slag–fly ash–phosphogypsum solidified material as road base material. J. Hazard. Mater. 2009, 164, 99–104. [Google Scholar] [PubMed]
- Liu, Y.; Wang, J.Z.; Lu, N.W.; Wang, B.W. Mechanical Properties of Alkali-Activated Slag-Fly Ash Geopolymers. China-Foreign Highw. 2024, 44, 67–74. [Google Scholar]
- Urinskas, D.; Vaiiukynien, D.; Stelmokaitis, G.; Doroševas, V. Clayey Soil Strength Improvement by Using Alkali Activated Slag Reinforcing. Minerals 2020, 10, 1076. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, A.P.; Li, P.; Chai, Y.B.; Wang, Z.Y. Research on Composite Improvement of Loess with Alkali Activated Coal Gangue Powder and Mineral Powder. Highway 2024, 69, 334–338. [Google Scholar]
- Liu, L.; Chen, S.; Chen, F.; He, L.; Shen, S. Analysis of strength characteristics and microstructure of alkali-activated slag cement fluidized solidified soil. Mater. Res. Express 2024, 11, 085502. [Google Scholar] [CrossRef]
- Zhu, Y. Research on Mechanical Mechanism of Soft Soil Foundation Reinforced by Geopolymer Mixing Pile; Chang’an University: Xi’an, China, 2020. [Google Scholar]
- Yi, Y.L.; Li, C.; Sun, C.; Zhang, Z.F.; Liu, S.Y. Test on Alkali-activated Ground Granulated Blast-furnace Slag (GGBS) for Lianyungang Soft Soil Stabilization. J. Rock Mech. Eng. 2013, 32, 1820–1826. [Google Scholar]
- Liu, G.; Ding, M.W.; Liu, J.J.; WAN, H.W.; Xue, Y.J.; Jian, S.W. Hydration Behavior and Mechanical Properties of Alkaline Excited Slag-Fly Ash-Metakaolin Geopolymer. Silic. Bull. 2023, 42, 2106–2114. [Google Scholar]
- Yang, X.; Zhang, Y.; Lin, C. Compressive and Flexural Properties of Ultra-Fine Coal Gangue-Based Geopolymer Gels and Microscopic Mechanism Analysis. Gels 2022, 8, 145. [Google Scholar] [CrossRef] [PubMed]
- JTG 3430-2020; Test Methods of Soils for Highway Enginering. Ministry of Transport of the People’s Republic of China, China Communications Press: Beijing, China, 2020.


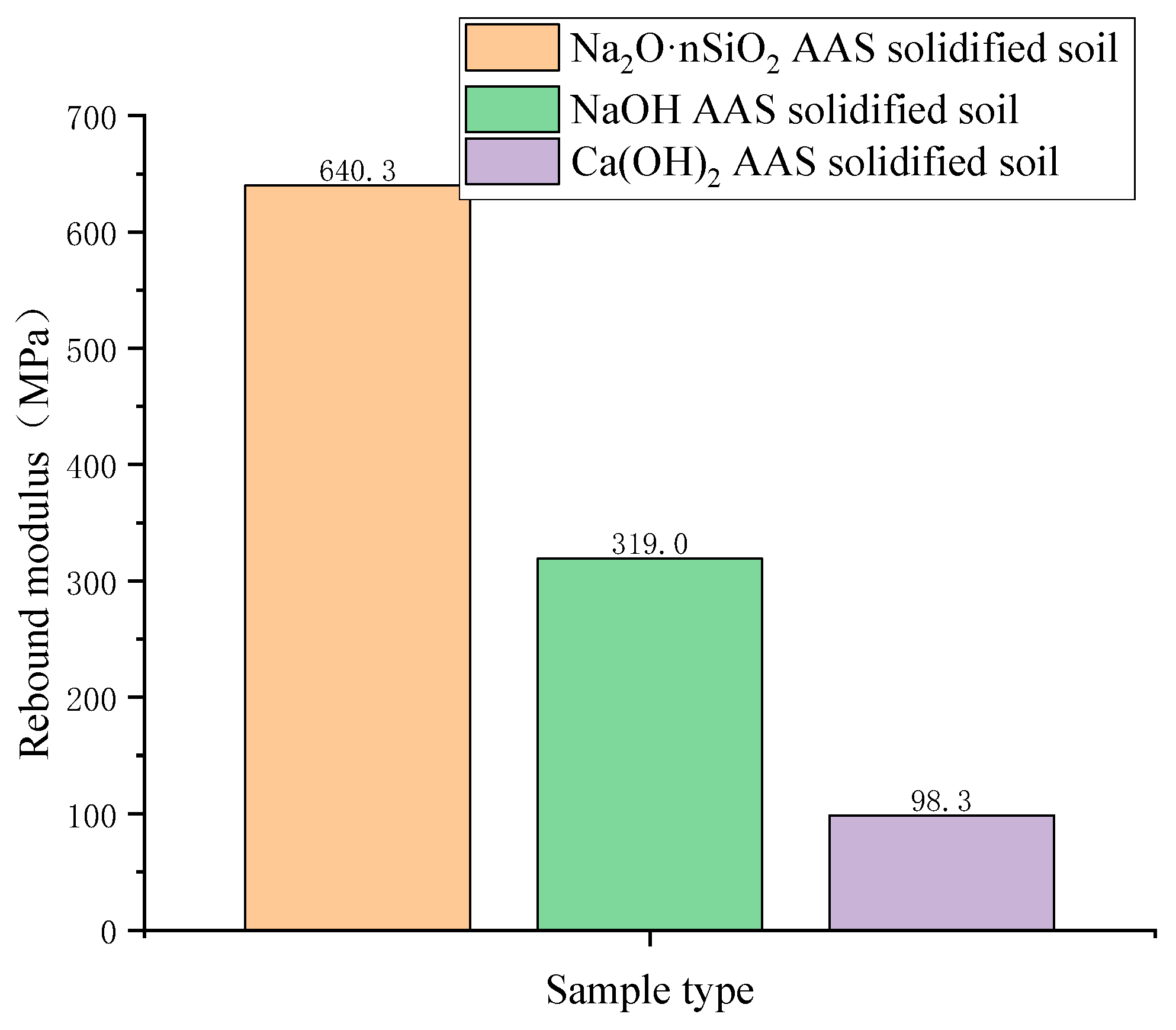
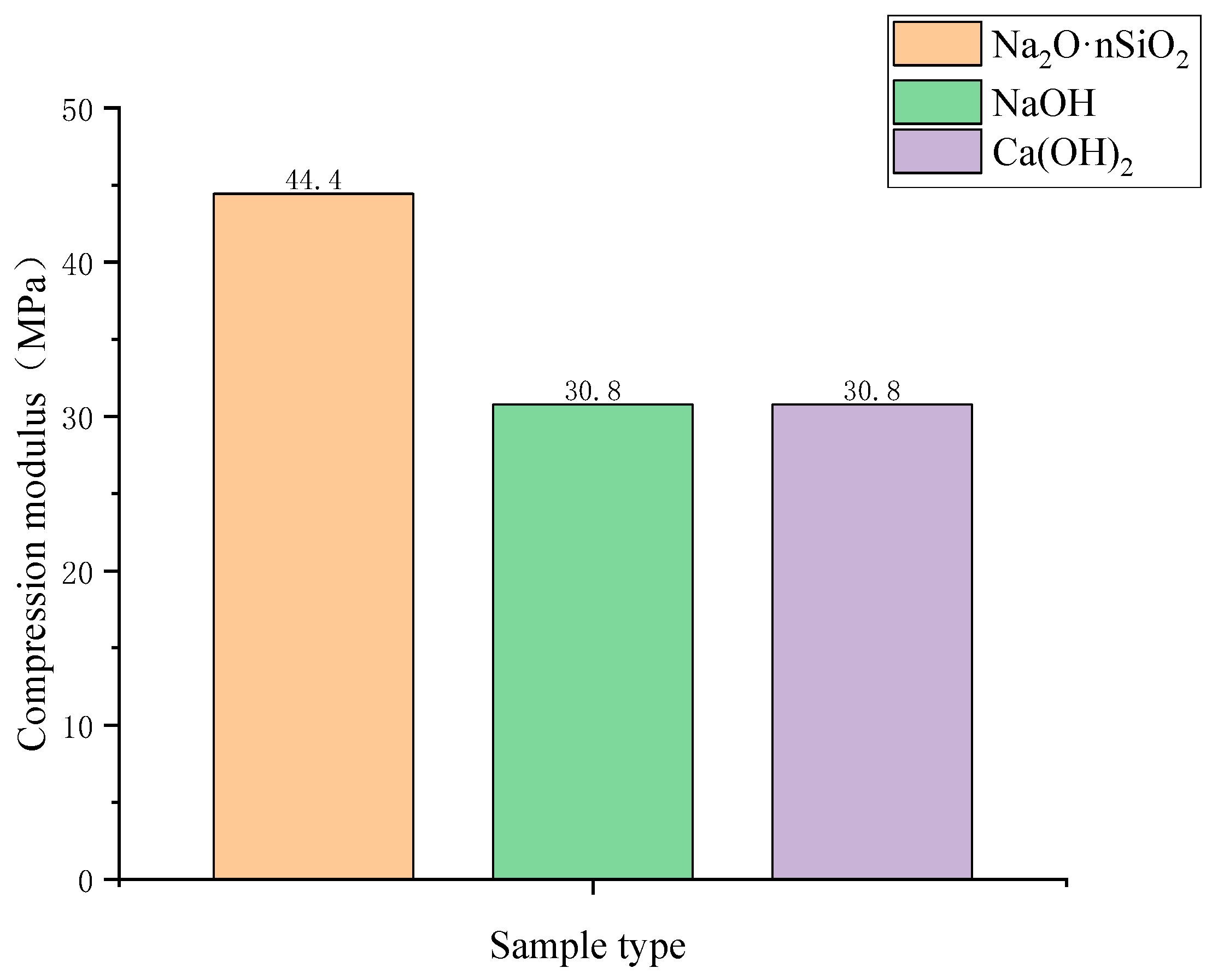


| Soil sample | Slag powder | ||
 |  | ||
| Na2O·nSiO2 | NaOH | Ca(OH)2 | |
 |  |  | |
| Natural Moisture Content/% | Maximum Dry Density/g/cm3 | Best Moisture Content/% | Liquid Limit/% | Plastic Limit/% | Plastic Index |
|---|---|---|---|---|---|
| 27.05 | 1.77 | 11.7 | 30.61 | 18.47 | 12.14 |
| Oxide | CaO | SiO2 | Al2O3 | MgO | SO3 | TiO2 | Fe2O3 | MnO | K2O | Na2O |
|---|---|---|---|---|---|---|---|---|---|---|
| Content/% | 37.17 | 26.66 | 17.29 | 12.18 | 3.42 | 1.51 | 0.58 | 0.37 | 0.31 | 0.29 |
| Modulus (nSiO2/nNa2O) | Solid Content/% | SiO2/% | Na2O/% | Baume Degree/°Bé | PH | Bulk Density/t/m3 |
|---|---|---|---|---|---|---|
| 3.3 | 35.5 | 26.98 | 8.53 | 38.5 | 10–13 | 1.35 |
| Na2O·nSiO2 Modulus | Activator Content/% | Slag Powder Content/% |
|---|---|---|
| 0.3/0.5/0.7/0.9/1.1 | 4 | 15 |
| No. | Activator Type A | Activator Content B/% | Slag Powder Content C/% |
|---|---|---|---|
| 1 | Na2O·nSiO2 | 5% | 20% |
| 2 | Na2O·nSiO2 | 4% | 10% |
| 3 | Na2O·nSiO2 | 3% | 15% |
| 4 | NaOH | 5% | 10% |
| 5 | NaOH | 4% | 15% |
| 6 | NaOH | 3% | 20% |
| 7 | Ca(OH)2 | 5% | 15% |
| 8 | Ca(OH)2 | 4% | 20% |
| 9 | Ca(OH)2 | 3% | 10% |
| Orthogonal Test Number | 7 d Unconfined Compressive Strength | 28 d Unconfined Compressive Strength |
|---|---|---|
| 1 | 9.159 | 10.958 |
| 2 | 5.851 | 9.399 |
| 3 | 11.004 | 12.188 |
| 4 | 6.304 | 7.682 |
| 5 | 6.764 | 6.643 |
| 6 | 9.535 | 10.781 |
| 7 | 3.753 | 6.025 |
| 8 | 5.304 | 7.368 |
| 9 | 5.913 | 7.272 |
| Range | 7 d Unconfined Compressive Strength | 28 d Unconfined Compressive Strength | ||||
|---|---|---|---|---|---|---|
| Activator Type A | Activator Content B/% | Slag Powder Content C/% | Activator Type A | Activator Content B/% | Slag Powder Content C/% | |
| K1 | 26.01 | 26.45 | 18.07 | 32.55 | 30.24 | 24.35 |
| K2 | 22.60 | 17.92 | 21.52 | 25.11 | 23.41 | 24.86 |
| K3 | 14.97 | 19.22 | 24.00 | 20.67 | 24.67 | 29.11 |
| k1 | 8.67 | 8.82 | 6.02 | 10.85 | 10.08 | 8.12 |
| k2 | 7.53 | 5.97 | 7.17 | 8.37 | 7.80 | 8.29 |
| k3 | 4.99 | 6.41 | 8.00 | 6.89 | 8.22 | 9.70 |
| Superior level | A1 | B1 | C3 | A1 | B1 | C3 |
| R | 3.68 | 2.84 | 1.98 | 3.96 | 2.28 | 1.58 |
| Type of Solidified Soil | Cohesion C (KPa) | Internal Friction Angle φ (°) |
|---|---|---|
| Na2O·nSiO2 AAS-solidified soil | 67.45 | 27.1 |
| NaOH AAS-solidified soil | 56.13 | 23.5 |
| Ca(OH)2 AAS-solidified soil | 54.88 | 23.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Zhang, H.; Cheng, Y.; Xue, Y.; Han, Y.; Jia, J.; Li, K.; Zhang, Z. Study on Mechanical Properties and Curing Reaction Mechanism of Alkali-Activated-Slag Solidified Port Soft Soil with Different Activators. Materials 2025, 18, 1583. https://doi.org/10.3390/ma18071583
Hu W, Zhang H, Cheng Y, Xue Y, Han Y, Jia J, Li K, Zhang Z. Study on Mechanical Properties and Curing Reaction Mechanism of Alkali-Activated-Slag Solidified Port Soft Soil with Different Activators. Materials. 2025; 18(7):1583. https://doi.org/10.3390/ma18071583
Chicago/Turabian StyleHu, Wenjun, Han Zhang, Yu Cheng, Yi Xue, Yutong Han, Jianghua Jia, Kun Li, and Zhifeng Zhang. 2025. "Study on Mechanical Properties and Curing Reaction Mechanism of Alkali-Activated-Slag Solidified Port Soft Soil with Different Activators" Materials 18, no. 7: 1583. https://doi.org/10.3390/ma18071583
APA StyleHu, W., Zhang, H., Cheng, Y., Xue, Y., Han, Y., Jia, J., Li, K., & Zhang, Z. (2025). Study on Mechanical Properties and Curing Reaction Mechanism of Alkali-Activated-Slag Solidified Port Soft Soil with Different Activators. Materials, 18(7), 1583. https://doi.org/10.3390/ma18071583





