Photoluminescence and Crystal-Field Analysis of Reddish CaYAl3O7: Eu3+ Phosphors for White LEDs
Abstract
1. Introduction
2. Experimental Methods
3. Results and Discussion
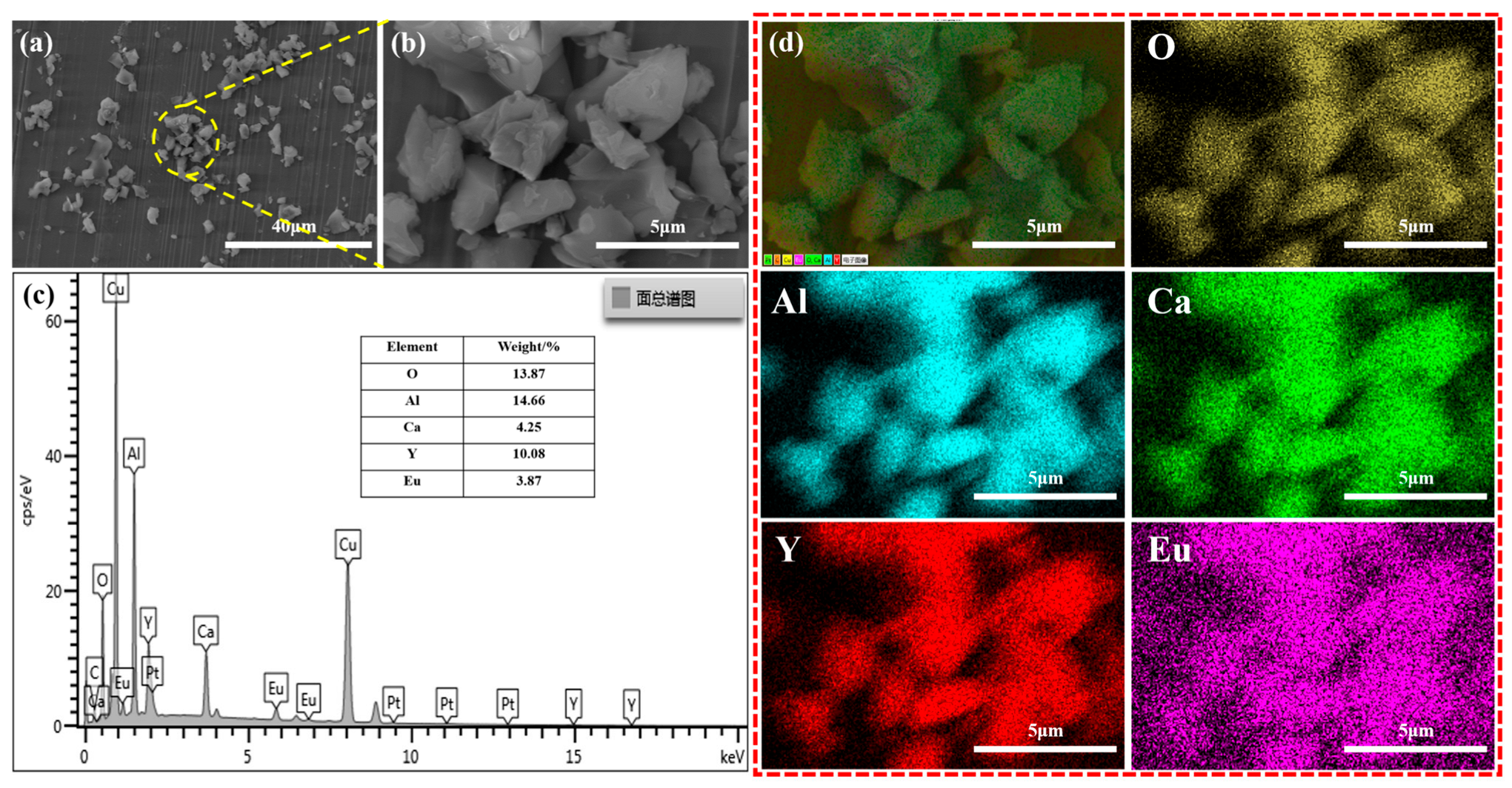
3.1. Crystal Structure and Morphology Characterization
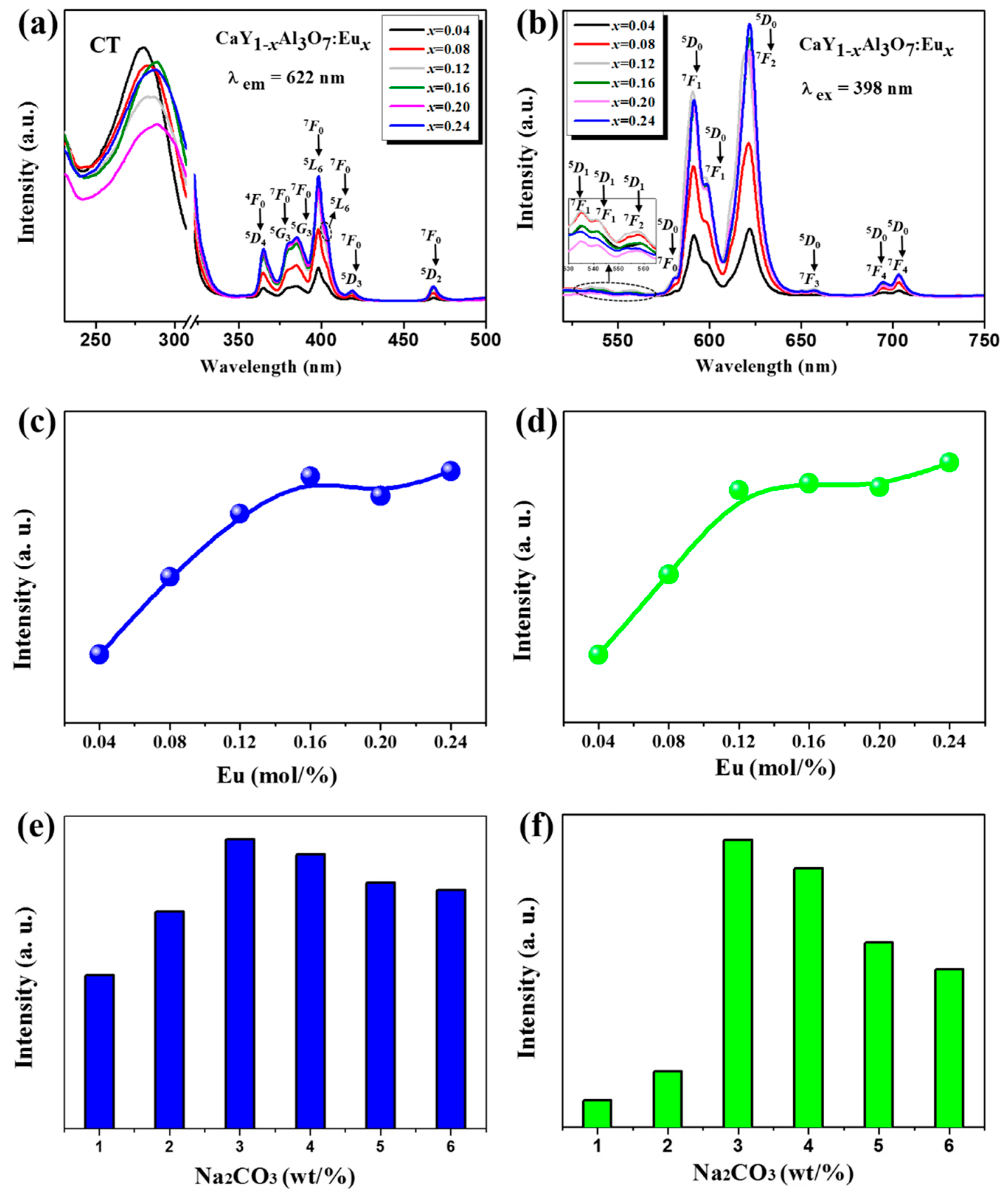
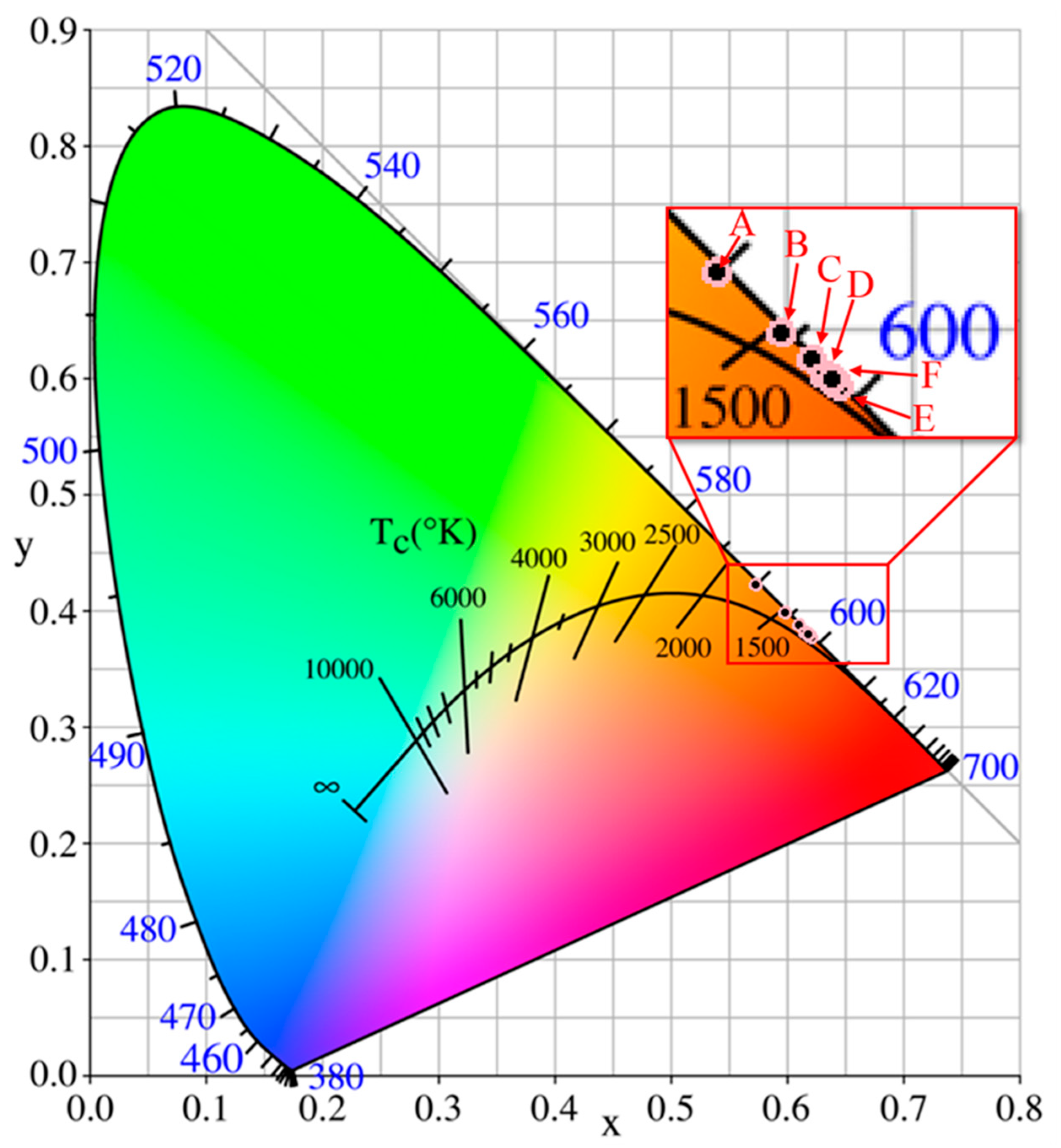
3.2. Photoluminescence Characteristics
3.3. Crystal Field Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, C.; Dong, L.; Zhu, G.; Niu, S.; Yu, R.; Yang, Q.; Liu, Y.; Wang, Z.L. High-resolution electroluminescent imaging of pressure distribution using a piezoelectric nanowire LED array. Nat. Photon. 2013, 7, 752–758. [Google Scholar] [CrossRef]
- Bao, R.; Wang, C.; Dong, L.; Yu, R.; Zhao, K.; Wang, Z.L.; Pan, C. Flexible and controllable piezo-phototronic pressure mapping sensor matrix by ZnO NW/p-polymer LED array. Adv. Funct. Mater. 2015, 25, 2884–2891. [Google Scholar] [CrossRef]
- Yang, W.; Liu, Z.; Chen, J.; Huang, L.; Zhang, L.; Pan, H.; Wu, B.; Lin, Y. A high-performance white-light-emitting-diodes based on nano-single crystal divanadates quantum dots. Sci. Rep. 2015, 5, 10460. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, M.; Vaidyanathan, S. High performance red/deep-red emitting phosphors for white LEDs. New J. Chem. 2020, 44, 5354–5365. [Google Scholar] [CrossRef]
- Yang, W.Q.; Liu, H.G.; Gao, M.; Bai, Y.; Zhao, J.T.; Xu, X.D.; Wu, B.; Zheng, W.C.; Liu, G.K.; Lin, Y. Dual-luminescence-center single-component white-light Sr2V2O7: Eu3+ phosphors for white LEDs. Acta Mater. 2013, 61, 5096–5104. [Google Scholar] [CrossRef]
- Pust, P.; Weiler, V.; Hecht, C.; Tücks, A.; Wochnik, A.S.; Henß, A.K.; Wiechert, D.; Scheu, C.; Schmidt, P.J.; Schnick, W. Narrow-band red-emitting Sr [LiAl3N4]: Eu2+ as a next-generation LED-phosphor material. Nat. Mater. 2014, 13, 891. [Google Scholar]
- Li, H.; Jiao, J.; Luo, D.; Xiong, D.; Gao, L. Controllable synthesis of Cu-based quantum dots/nanocrystals and application in white light-emitting diodes. Dalton Trans. 2022, 51, 17883–17894. [Google Scholar]
- Zhang, L.; Pan, H.; Liu, H.; Zhang, B.; Jin, L.; Zhu, M.; Yang, W. Theoretical spectra identification and fluorescent properties of reddish orange Sm-doped BaTiO3 phosphors. J. Alloys Compd. 2015, 643, 247–252. [Google Scholar]
- Pan, H.; Zhang, L.; Jin, L.; Zhang, B.; Yang, W.J. High-performance simultaneous two-photon absorption upconverted stimulated single-component Sr2V2O7 phosphor for white LEDs. Electron. Mater. 2015, 44, 3465–3470. [Google Scholar] [CrossRef]
- Nakamura, S.; Mukai, T.; Senoh, M. Candela-class high-brightness InGaN/AlGaN double-heterostructure blue-light-emitting diodes. Appl. Phys. Lett. 1994, 64, 1687. [Google Scholar] [CrossRef]
- Huang, X.; Guo, H.; Liang, J.; Wang, S. Synthesis and photoluminescence properties of novel red-emitting KBaLu(MoO4)3: Eu3+ phosphors with high thermal stability and high color purity. Inorg. Chem. Commun. 2020, 116, 107938. [Google Scholar] [CrossRef]
- Mehare, M.; Mehare, C.; Swart, H.; Dhoble, S. Recent development in color tunable phosphors: A review. Prog. Mater. Sci. 2023, 133, 101067. [Google Scholar]
- Jiang, L.; Jiang, X.; Zhang, Y.; Wang, C.; Liu, P.; Lv, G.; Su, Y. Multiobjective machine learning-assisted discovery of a novel cyan–green garnet: Ce phosphors with excellent thermal stability. ACS Appl. Mater. Interfaces 2022, 14, 15426–15436. [Google Scholar]
- Wang, Q.; Zhu, G.; Li, Y.; Wang, Y. Photoluminescent properties of Pr3+ activated Y2WO6 for light emitting diodes. Opt. Mater. 2015, 42, 385–389. [Google Scholar]
- He, X.; Liu, X.; Li, R.; Yang, B.; Yu, K.; Zeng, M.; Yu, R. Effects of local structure of Ce3+ ions on luminescent properties of Y3Al5O12: Ce nanoparticles. Sci. Rep. 2016, 6, 22238. [Google Scholar]
- Zhang, Z.; Ma, S.; Yu, H.J. Luminescence studies of Ba1−xMg2−y(PO4)2: xEu2+, yMn2+ phosphor. J. Alloys Compd. 2011, 509, 76. [Google Scholar]
- Yu, H.; Chung, W.; Park, S.; Kim, J.; Kim, S. Luminous properties of SrGa2S3Se phosphors doped with Eu2+ for LEDs application. Mater. Lett. 2011, 65, 474–476. [Google Scholar]
- Kadyan, S.; Singh, K.; Singh, S.; Sheoran, S.; Singh, J.; Singh, D. Photoluminescence and structural analysis of trivalent europium doped MLaAl3O7 (M = Ba, Ca, Mg and Sr) nanophosphors. Luminescence 2020, 35, 673–683. [Google Scholar]
- Zhang, W.; Shen, H.; Hu, X.L.; Wang, Y.; Li, J.F.; Zhu, Z.J.; You, Z.Y.; Tu, C.Y. Solid-state synthesis, structure and spectroscopic analysis of Dy: CaYAl3O7 phosphors. J. Alloys Compd. 2019, 781, 255–260. [Google Scholar]
- Wang, D.; Chen, L.; Jiang, L.; Yu, Y.; Lu, Y.; Li, H.; Li, Y.; Meng, Z.; Su, Q.; Zhang, Y.; et al. A high temperature macroscopically flexible inorganic CaYAl3O7: Eu3+ nanofiber luminescent membrane. J. Mater. Chem. C 2022, 10, 7594. [Google Scholar]
- Yim, C.; Unithrattil, S.; Chung, W.; Im, W. Comparative study of optical and structural properties of electrospun 1-dimensional CaYAl3O7: Eu3+ nanofibers and bulk phosphor. Mater. Character. 2014, 95, 27–35. [Google Scholar]
- Yang, X.; Zhao, L.; Zeng, W.; Liu, Z.; Fan, X.; Tian, S.; Zhang, H.; Xu, X.; Qiu, J.; Yu, X. Multiple-response anti-counterfeiting realized in CaYAl3O7 host with the dual coexistence of Eu2+/Eu3+. J. Am. Ceram. Soc. 2020, 103, 2235. [Google Scholar]
- Liu, G.; Jacquier, B. (Eds.) Spectroscopic Properties of Rare Earths in Optical Materials; Science & Business Media Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Qu, B.; Zhang, B.; Wang, L.; Zhou, R.; Zeng, X. Mechanistic study of the persistent luminescence of CaAl2O4: Eu, Nd. Chem. Mater. 2015, 27, 2195–2202. [Google Scholar]
- Gschneidner, K.; Bunzli, J.; Pecharsky, V. (Eds.) Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Tanner, P.; Li, W.; Ning, L. Electronic spectra and crystal-field analysis of europium in hexanitritolanthanate systems. Inorg. Chem. 2012, 51, 2997–3006. [Google Scholar]
- Görller-Walrand, C.; Binnemans, K. (Eds.) Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Hufner, S. (Ed.) Optical Spectra of Transparent Rare Earth Compounds; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Wybourne, B.; Meggers, F. Spectroscopic properties of rare earths. Physics 1965, 18, 70–72. [Google Scholar] [CrossRef]
- Xie, L.; Yeung, Y. Study of the Defect Structure and Crystal-Field Parameters of α-Al2O3: Yb3+. Appl. Magn. Reson. 2013, 44, 917–925. [Google Scholar]
- Ghosh, R.; Sarkar, S.; Jana, Y.; Piwowarska, D.; Gnutek, P.; Rudowicz, C. Eu3+ ions as a crystal-field probe for low-symmetry sites in doped phosphors–a case study: Eu3+ at triclinic sites in Li6RE(BO3)3 (RE = Y, Gd), YBO3 and ZnO and at trigonal sites in YAl3(BO3)4. Phys. Chem. Chem. Phys. 2023, 25, 25537–25551. [Google Scholar]
- Liu, H.; Mei, Y.; Zheng, W. Reinvestigations of the optical spectra and EPR parameters for Yb3+ ions in AWO4 (A = Ca, Sr, Pb, Ba) scheelite crystals. Phys. Scr. 2012, 85, 035708. [Google Scholar]
- Liu, G.; Carnall, W.; Jones, R.; Cone, R.; Huang, J.J. Electronic energy level structure of Tb3+ in LiYF4. J. Alloys Compd. 1994, 207, 69–73. [Google Scholar]



| Ligand | [YO8]-CS | [YO8]-D4H | ||||
|---|---|---|---|---|---|---|
| R (nm) | θ (°) | ∅ (°) | R (nm) | θ (°) | ∅ (°) | |
| O1 | 2.524 | 67.57 | −4.12 | 2.460 | 60.44 | 0 |
| O2 | 2.397 | 53.32 | 90.85 | 2.460 | 60.44 | 90 |
| O3 | 2.397 | 53.32 | 179.15 | 2.460 | 60.44 | 180 |
| O4 | 2.521 | 67.54 | −85.95 | 2.460 | 60.44 | −90 |
| O5 | 2.789 | 123.28 | 34.55 | 2.588 | 123.79 | 45 |
| O6 | 2.394 | 116.34 | 135.06 | 2.588 | 123.79 | 135 |
| O7 | 2.789 | 123.28 | −124.55 | 2.588 | 123.79 | −135 |
| O8 | 2.38 | 132.26 | −45 | 2.588 | 123.79 | −45 |
| Eave | F2 | F4 | F6 | ζ4f | B20 | B40 | B44 | B60 | B64 |
|---|---|---|---|---|---|---|---|---|---|
| 63,302 | 80,351 | 68,113 | 37,363 | 1260 | −669 | 2668 | 1283 | 2265 | 2422 |
| Multiplet | Cal c. | Expt. | Multiplet | Cal c. | Expt. | ||
|---|---|---|---|---|---|---|---|
| 7F0 | 57 | 0 | −57 | 5D3 | 23,894 | 23,878 | −16 |
| 7F1 | 294 | 303 | 9 | 23,897 | |||
| 462 | 506 | 44 | 23,934 | ||||
| 7F2 | 824 | 23,968 | |||||
| 947 | 23,985 | ||||||
| 1060 | 1050 | −10 | 5L6 | 24,441 | |||
| 1119 | 1123 | 4 | 24,515 | ||||
| 7F3 | 1616 | 24,521 | |||||
| 1781 | 24,539 | ||||||
| 1868 | 24,639 | ||||||
| 1880 | 24,765 | 24,789 | 24 | ||||
| 1969 | 1990 | 21 | 25,009 | ||||
| 7F4 | 2375 | 25,115 | 25,113 | −2 | |||
| 2415 | 25,206 | ||||||
| 2855 | 2809 | −46 | 25,316 | ||||
| 2871 | 5G3 | 25,599 | |||||
| 2943 | 2981 | 38 | 25,695 | ||||
| 3204 | 25,760 | ||||||
| 3263 | 25,835 | ||||||
| 5D0 | 17,208 | 17,206 | −2 | 25,961 | 25,961 | 0 | |
| 5D1 | 18,963 | 18,994 | 31 | 26,302 | 26,274 | −28 | |
| 18,985 | 19,004 | 19 | 26,800 | ||||
| 5D2 | 21,321 | 27,002 | |||||
| 21,333 | 27,193 | ||||||
| 21,386 | 21,358 | −28 | 27,235 | ||||
| 21,388 | 27,300 | ||||||
| 27,421 | 27,421 | −9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Yi, D.; Xu, T.; Ao, Y.; Yang, W. Photoluminescence and Crystal-Field Analysis of Reddish CaYAl3O7: Eu3+ Phosphors for White LEDs. Materials 2025, 18, 1578. https://doi.org/10.3390/ma18071578
Li Z, Yi D, Xu T, Ao Y, Yang W. Photoluminescence and Crystal-Field Analysis of Reddish CaYAl3O7: Eu3+ Phosphors for White LEDs. Materials. 2025; 18(7):1578. https://doi.org/10.3390/ma18071578
Chicago/Turabian StyleLi, Zhaoyu, Da Yi, Tianpei Xu, Yong Ao, and Weiqing Yang. 2025. "Photoluminescence and Crystal-Field Analysis of Reddish CaYAl3O7: Eu3+ Phosphors for White LEDs" Materials 18, no. 7: 1578. https://doi.org/10.3390/ma18071578
APA StyleLi, Z., Yi, D., Xu, T., Ao, Y., & Yang, W. (2025). Photoluminescence and Crystal-Field Analysis of Reddish CaYAl3O7: Eu3+ Phosphors for White LEDs. Materials, 18(7), 1578. https://doi.org/10.3390/ma18071578






