Precursor-Derived Mo2C/SiC Composites with a Two-Dimensional Sheet Structure for Electromagnetic Wave Absorption
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Mo2C/SiC Ceramics
2.3. Characterization
3. Result and Discussion
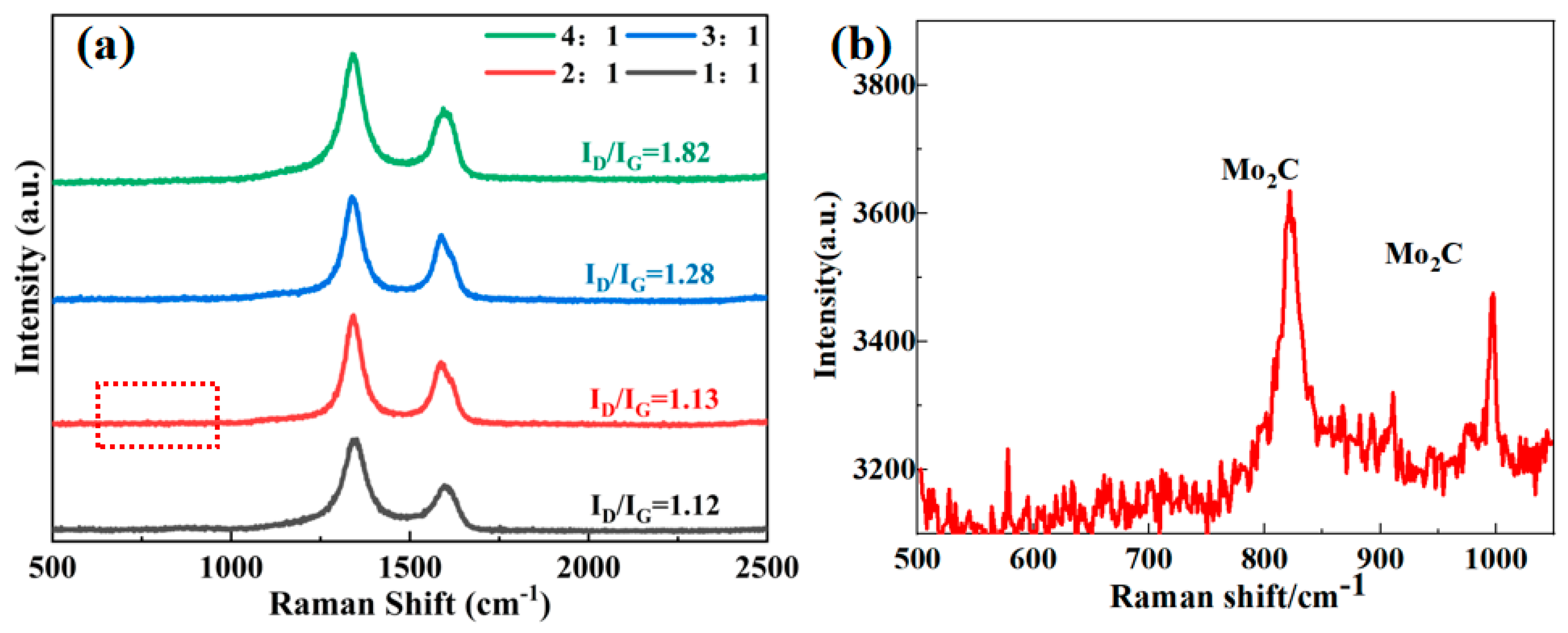
3.1. Characterizations of Mo2C/SiC
3.2. Microstructure of Mo2C/SiC
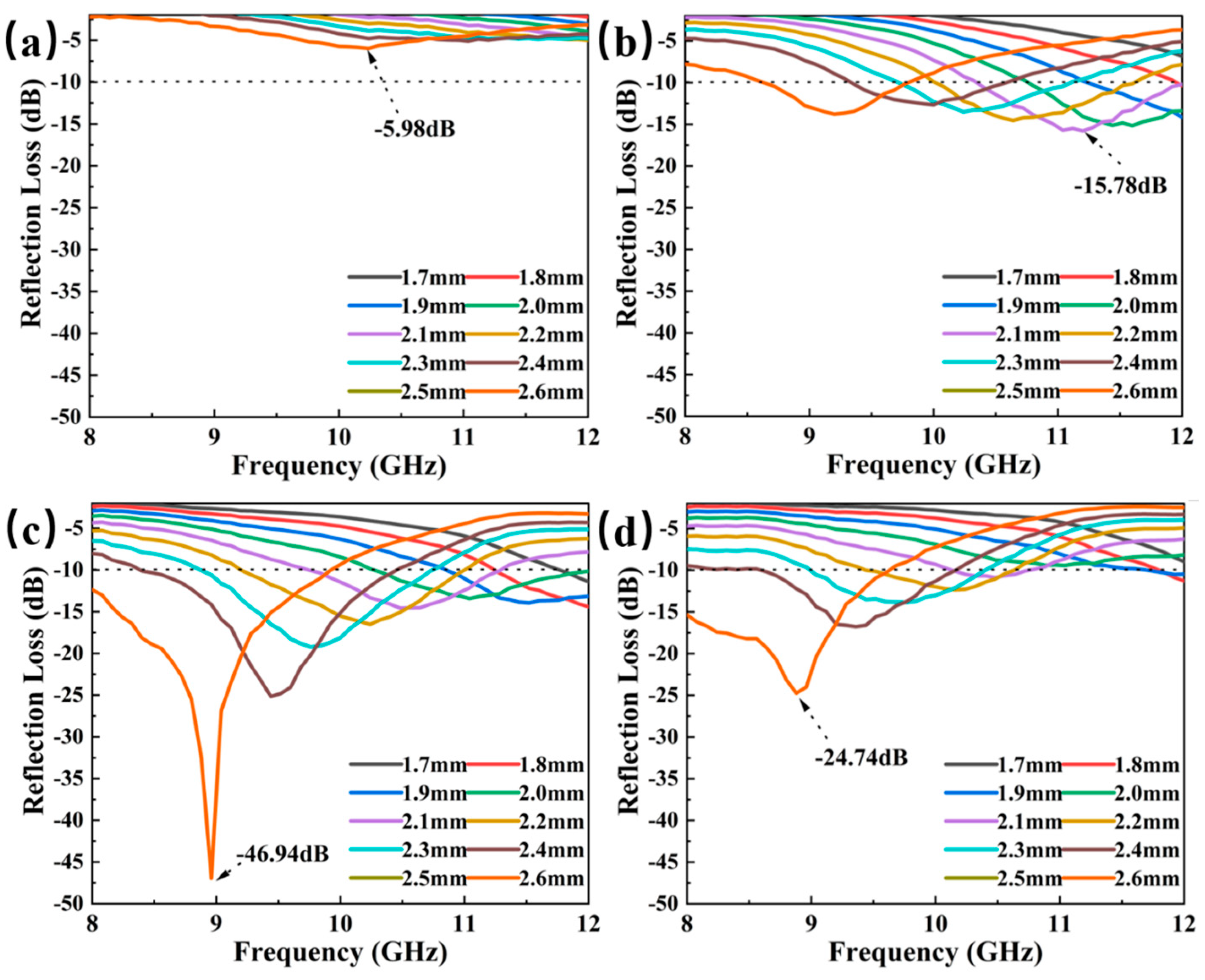
3.3. Electromagnetic Properties of the Mo2C/SiC
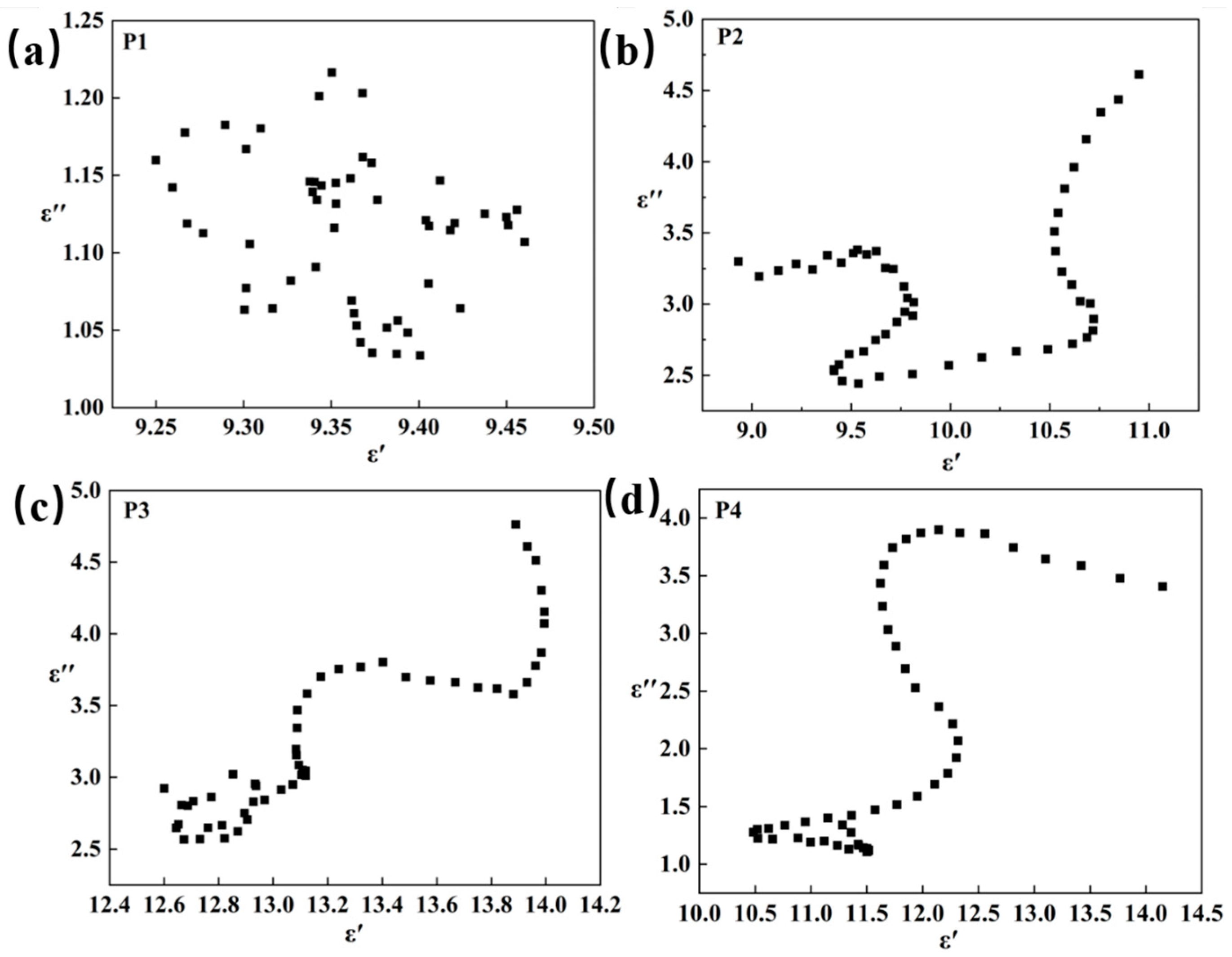
3.4. Absorbing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, J.; Yan, L.; Song, M.; Li, Y.; Guo, A.; Du, H.; Liu, J. Thermally insulated C/SiC/SiBCN composite ceramic aerogel with enhanced electromagnetic wave absorption performance. Ceram. Int. 2025, 51, 17–24. [Google Scholar] [CrossRef]
- Yan, Q.; Chen, S.; Shi, H.; Gao, B.; Li, J.; Meng, S. Novel anti-oxidation coating prepared by polymer-derived ceramic for harsh environments up to 1200 °C. Surf. Coat. Technol. 2024, 494, 131420. [Google Scholar]
- Zhao, H.; Chen, L.; Luan, X.; Zhang, X.; Yun, J.; Xu, T. Synthesis, pyrolysis of a novel liquid SiBCN ceramic precursor and its application in ceramic matrix composites. J. Eur. Ceram. Soc. 2017, 37, 1321–1329. [Google Scholar] [CrossRef]
- Li, Q.; Yin, X.; Kong, L.; Duan, W.; Zhang, L.; Cheng, L. High Temperature Dielectric and Microwave Absorption Properties of Polymer Derived SiCN Ceramic in X Band. High Temp. Ceram. Matrix Compos. 2014, 8, 193–202. [Google Scholar]
- Luo, C.; Jiao, T.; Tang, Y.; Kong, J. Excellent Electromagnetic Wave Absorption of Iron-Containing SiBCN Ceramics at 1158 K High-Temperature. Adv. Eng. Mater. 2018, 20, 1701168. [Google Scholar] [CrossRef]
- Yang, W.; Yang, D.; Mei, H.; Yao, L.; Xiao, S.; Yao, Y.; Chen, C.; Cheng, L. 3D printing of PDC-SiOC@SiC twins with high permittivity and electromagnetic interference shielding effectiveness. J. Eur. Ceram. Soc. 2021, 41, 5437–5444. [Google Scholar]
- Ye, F.; Cao, Y.; Liang, J.; Cheng, L. Fabrication and electromagnetic absorbing properties of CNTs modified PDCs-SiOC. Mater. Charact. 2023, 203, 113037. [Google Scholar] [CrossRef]
- Li, Q.; Yin, X.; Duan, W.; Kong, L.; Hao, B.; Ye, F. Electrical, dielectric and microwave-absorption properties of polymer derived SiC ceramics in X band. J. Alloys Compd. 2013, 565, 66–72. [Google Scholar] [CrossRef]
- Wei, H.; Zhou, C.; Feng, P.; Yu, Y.; Xue, J.; Zhao, F.; Wang, Q. Rear earth (Re: Sc, Y, and Ce) modified PDCs-SiC ceramics for efficient microwave absorption. Mater. Charact. 2022, 190, 112048. [Google Scholar]
- Xue, J.; Tang, Z.; Wang, C.; Wei, H. Microstructure and EMW absorption properties of PDCs-SiCN(Ti) ceramics with adjustable SiC nanowires and TiC nanocrystallines. Mater. Res. Bull. 2024, 176, 112804. [Google Scholar]
- Xue, J.; Hu, S.; Li, X.; Li, F.; Liu, Y.; Wei, H. Enhanced microwave absorbing properties of Y2O3 modified PDC SiCN ceramics with heterogeneous amorphous interface. J. Alloys Compd. 2023, 931, 167499. [Google Scholar]
- Lu, J.; Feng, Y.; Liu, J.; Liu, C.; Tong, Y.; Wu, S.; Sun, H.; Gong, H.; Guo, X. Improved electromagnetic wave absorbing performance of PDCs-SiCN(Ni) fibers with different nickel content. Ceram. Int. 2022, 48, 23578–23589. [Google Scholar] [CrossRef]
- Jiang, J.; Yan, L.; Li, J.; Xue, Y.; Zhang, C.; Hu, X.; Guo, A.; Du, H.; Liu, J. Lightweight, thermally insulating SiBCN/Al2O3 ceramic aerogel with enhanced high-temperature resistance and electromagnetic wave absorption performance. Chem. Eng. J. 2024, 501, 157656. [Google Scholar]
- Zhang, Y.; Yin, X.; Ye, F.; Kong, L. Effects of multi-walled carbon nanotubes on the crystallization behavior of PDCs-SiBCN and their improved dielectric and EM absorbing properties. J. Eur. Ceram. Soc. 2014, 34, 1053–1061. [Google Scholar]
- Liu, C.; Tong, Y.; Liu, C.; Liu, J.; Sun, H.; Hu, Q.; Wu, S.; Zhao, Y.; Li, J.; Guo, X.; et al. Novel and efficient electromagnetic wave absorption of SiBCN(Fe) nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2023, 679, 132605. [Google Scholar]
- Tang, H.; Ren, K.; Wang, Y. Polysiloxane encapsulating strategy to enhance the high-temperature electromagnetic wave absorption performance of carbon-rich SiOC ceramics. Ceram. Int. 2024, 50, 51392–51402. [Google Scholar]
- Wang, S.; Ashfaq, M.Z.; Qi, D.; Yue, X.; Gong, H. Electromagnetic wave absorption properties of polymer-derived magnetic carbon-rich SiCN-based composite ceramics. Ceram. Int. 2022, 48, 4986–4998. [Google Scholar]
- Song, Y.; He, L.; Zhang, X.; Liu, F.; Tian, N.; Tang, Y.; Kong, J. Highly Efficient Electromagnetic Wave Absorbing Metal-Free and Carbon-Rich Ceramics Derived from Hyperbranched Polycarbosilazanes. J. Phys. Chem. C 2017, 121, 24774–24785. [Google Scholar]
- Yang, L.; Liu, H.; Zu, M. Enhanced microwave-absorbing property of precursor infiltration and pyrolysis derived SiCf/SiC composites at X band: Role of carbon-rich interphase. J. Am. Ceram. Soc. 2018, 101, 3402–3413. [Google Scholar]
- Mo, P.; Shui, A.; Yu, H.; Qian, J. Synthesis and high electromagnetic wave absorption performance of carbon-enriched porous SiOC ceramics. J. Alloys Compd. 2025, 1010, 177120. [Google Scholar]
- Sádovská, G.; Honcová, P.; Morávková, J.; Jirka, I.; Vorokhta, M.; Pilař, R.; Rathouský, J.; Kaucký, D.; Mikysková, E.; Sazama, P. The thermal stability of carbon materials in the air: Quantitative structural investigation of thermal stability of carbon materials in air. Carbon 2023, 206, 211–225. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, X.; Gong, H.; Zhang, Y.; Liu, Y.; Lin, X.; Mao, J. Microwave absorption performance of PDCs-SiCN(Fe) ceramics with negative imaginary permeability. Ceram. Int. 2018, 44, 10420–10425. [Google Scholar] [CrossRef]
- Wei, H.; Yang, S.; Feng, P.; Zhou, C.; Xue, J.; Wang, C.; Chen, L.; Zhao, F.; Wang, Q. Optimization of Ti with modified SiC ceramics for electromagnetic absorption properties. Mater. Charact. 2023, 198, 112761. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Ning, Z.; Mao, S.; Xue, Z.; Zhu, Y.; Tao, Q.; Lan, S.; Yao, R. Design of lightweight and antioxidant SiCnws/SiC(Mo, rGO) nanocomposite from molybdenum-modified precursors for aerospace vehicle components. Ceram. Int. 2023, 49, 25998–26014. [Google Scholar] [CrossRef]
- Zhu, Y.; Jin, C.; Ning, Z.; Huang, W.; Mao, S.; Xue, Z.; Lan, S.; Tao, Q.; Yao, R. In-situ construction of oxidation resistant porous Mo4.8Si3C0.6/SiC(rGO) composite PDCs served as thermal insulation components of hypersonic vehicles. Ceram. Int. 2023, 49, 37280–37292. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, W.; Zhong, C.; Wu, S.; Wu, Y.; Liu, F.; Huang, X.; Wen, G. Thermostable SiCO@BN sheets with enhanced electromagnetic wave absorption. Chem. Eng. J. 2019, 378, 122239. [Google Scholar] [CrossRef]
- Han, Y.; Zhong, L.; Zheng, Y.; Zhou, R.; Liao, L.; Chen, G.; Huang, W.; Lin, S.; Zhong, Y.; Li, J.; et al. Enhanced electrical and thermal conductivities of 3D-SiC(rGO, Gx) PDCs based on polycarbosilane-vinyltriethoxysilane-graphene oxide (PCS-VTES-GO) precursor containing graphene fillers. Ceram. Int. 2020, 46, 950–958. [Google Scholar] [CrossRef]
- Ren, B.; Deng, Y.; Jia, Y.; Wu, X.; Feng, G.; Wang, Q.; Li, H. Electromagnetic wave absorbing ceramics composites made of polymer-derived SiC with BN@CNTs pyrolyzed higher than 1200 °C. J. Mater. Sci. Technol. 2023, 167, 213–227. [Google Scholar] [CrossRef]
- Zheng, C.-m.; Li, X.-d.; Wang, H.; Zhu, B. Thermal stability and curing kinetics of polycarbosilane fibers. Trans. Nonferrous Met. Soc. China 2006, 16, 44–48. [Google Scholar] [CrossRef]
- Kita, K.I.; Usukawa, R.; Hotta, M. A polymer blend containing polycarbosilane and polysilaethylene for designing improved silicon carbide based fibers. Ceram. Int. 2025, 51, 1227–1232. [Google Scholar] [CrossRef]
- Hackbarth, H.G.; Key, T.S.; Ackley, B.J.; Opletal, G.; Rawal, A.; Gallington, L.; Yang, Y.; Thomsen, L.; Dickerson, M.B.; Pruyn, T.L.; et al. Uncovering atomic-scale polymer-to-ceramic transformations in SiC polymer derived ceramics from polycarbosilanes. J. Eur. Ceram. Soc. 2024, 44, 1932–1945. [Google Scholar]
- Wang, J.; Wang, L.; Zhu, Y.; Luo, R.; Dong, X.; Song, J.; Wu, X. Microwave absorption properties of PDCs-SiC(N) based on heat treatment at different temperatures. Ceram. Int. 2024, 50, 40666–40677. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Wang, Z.; Wang, Y.; Yang, J.; Bordia, R.K. Influence of microstructure evolution on temperature-dependent dielectric and electromagnetic wave absorption properties of PDCs-SiC. Ceram. Int. 2022, 48, 8596–8604. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Y.; Hou, X.; Wen, J.; Chen, J.; Wang, S.; Cao, K.; Ye, W.; Zhao, R.; Wang, W.; et al. Construction of three-dimensional mesh porous Mo2C/carbon composites by chitosan salting-out for efficient microwave absorption. Carbon 2023, 214, 118323. [Google Scholar] [CrossRef]
- Roy, D.; Kanojia, S.; Mukhopadhyay, K.; Eswara Prasad, N. Analysis of carbon-based nanomaterials using Raman spectroscopy: Principles and case studies. Bull. Mater. Sci. 2021, 44, 31. [Google Scholar] [CrossRef]
- Wang, L.; Lu, J.; Zhang, J.; Zhu, J. Facile preparation and high microwave absorption of flower-like carbon nanosheet aggregations embedded with ultrafine Mo2C. J. Colloid Interface Sci. 2023, 641, 729–736. [Google Scholar]
- Koverga, A.A.; Gómez-Marín, A.M.; Dorkis, L.; Flórez, E.; Ticianelli, E.A. Role of Transition Metals on TM/Mo2C Composites: Hydrogen Evolution Activity in Mildly Acidic and Alkaline Media. ACS Appl. Mater. Interfaces 2020, 12, 27150–27165. [Google Scholar] [CrossRef]
- Yang, Q.; Qiu, R.; Ma, X.; Hou, R.; Sun, K. Surface reconstruction and the effect of Ni-modification on the selective hydrogenation of 1,3-butadiene over Mo2C-based catalysts. Catal. Sci. Technol. 2020, 10, 3670–3680. [Google Scholar]
- Zhou, W.; Li, Y.; Long, L.; Luo, H.; Wang, Y. High-temperature electromagnetic wave absorption properties of Cf/SiCNFs/Si3N4 composites. J. Am. Ceram. Soc. 2020, 103, 6822–6832. [Google Scholar]
- Zhang, N.; Chen, P.; Wang, Y.; Zong, M.; Chen, W. Supramolecular self-assembly derived Mo2C/FeCo/NC hierarchical nanostructures with excellent wideband microwave absorption properties. Compos. Sci. Technol. 2022, 221, 109325. [Google Scholar]
- Zeng, X.; Ning, Y.; Guo, H.; Xie, N.; Yu, R. Dual template induced assembly of 2D nanosheets to 3D porous Mo2C/NiFe-NC networkers for electromagnetic wave absorption. Mater. Today Phys. 2023, 34, 101077. [Google Scholar]
- Dong, Y.; Yuan, X.; Wu, H.; Wang, W.; Zhou, M. Synthesis of Mo2C/C nanoclusters attached on rGO nanosheets for high-Efficiency electromagnetic wave absorption. Appl. Surf. Sci. 2024, 672, 160886. [Google Scholar]
- Huang, J.; Mahariq, I.; Kumar, S.M.; Abdullaev, S.; Kannan, S.; Thi Xuan Dieu, N.; Fouad, Y. Facile fabrication of bilayer electromagnetic wave absorber via hierarchical Mo2C/La0.6Sr0.4MnO3 nanocomposite with multi-heterointerfaces for efficient low-frequency absorption. Colloids Surf. A Physicochem. Eng. Asp. 2024, 689, 133664. [Google Scholar]
- Yang, X.; Qiang, R.; Shao, Y.; Ren, F.; Rong, L.; Fang, J.; Xue, R.; Liu, Z. Hollow hydrangea-like Mo2C/MoO2/C composites with tunable phase compositions as highly efficient microwave absorbers. Appl. Surf. Sci. 2025, 682, 161633. [Google Scholar]
- Liu, J.; Wei, X.; Gao, L.; Tao, J.; Xu, L.; Peng, G.; Jin, H.; Wang, Y.; Yao, Z.; Zhou, J. An overview of C-SiC microwave absorption composites serving in harsh environments. J. Eur. Ceram. Soc. 2023, 43, 1237–1254. [Google Scholar]
- Du, H.; Zhang, W.; Wang, L.; Shen, S.; Dong, W.; Hu, Y.; Rehman, S.U.; Zou, H.; Liang, T. Heterostructured C@Fe3O4@FeSiCr composite absorbing material derived from MIL-88(Fe)@FeSiCr. J. Alloys Compd. 2023, 968, 172129. [Google Scholar]
- Xue, J.; Wu, C.; Du, X.; Ma, W.; Wen, K.; Huang, S.; Liu, Y.; Liu, Y.; Zhao, G. Preparation and properties of functional particle Fe3O4-rGO and its modified fiber/epoxy composite for high-performance microwave absorption structure. Mater. Res. Express 2020, 7, 045303. [Google Scholar]
- Wang, X.; Lu, Y.; Zhu, T.; Chang, S.; Wang, W. CoFe2O4/N-doped reduced graphene oxide aerogels for high-performance microwave absorption. Chem. Eng. J. 2020, 388, 124317. [Google Scholar]
- Huang, Y.; Ji, J.; Chen, Y.; Li, X.; He, J.; Cheng, X.; He, S.; Liu, Y.; Liu, J. Broadband microwave absorption of Fe3O4BaTiO3 composites enhanced by interfacial polarization and impedance matching. Compos. Part B Eng. 2019, 163, 598–605. [Google Scholar]








| Absorbers | RLmin/Thickness | EBW/Thickness | Refs. |
|---|---|---|---|
| Mo2C/SiC | −46.94 dB/2.6 mm | 4 GHz/2.6 mm | This work |
| Mo2C/FeCo/NC | −56.03 dB/3.4 mm | 10.27 GHz/3.4 mm | [40] |
| FCN-Mo2C | −36.80 dB/2.9 mm | 7.04 GHz/2.9 mm | [25] |
| Mo2C/NiFe-NC | −51.56 dB/1.4 mm | 3.7 GHz/1.4 mm | [41] |
| Mo2C/C composites | −20.38 dB/1.8 mm | 5.04 GHz/1.8 mm | [34] |
| Mo2C/C-rGO | −30.00 dB/1.6 mm | 5.12 GHz/1.6 mm | [42] |
| Mo2C/La0.6Sr0.4MnO3 | −39.00 dB/1.2 mm | 5.40 GHz/1.2 mm | [43] |
| Mo2C/MoO2/C | −62.9 dB/1.9 mm | 6.20 GHz/1.9 mm | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Yang, W.; Zhang, J.; Hou, Y.; Wen, G.; Xin, G.; Jiang, M.; Ma, Y. Precursor-Derived Mo2C/SiC Composites with a Two-Dimensional Sheet Structure for Electromagnetic Wave Absorption. Materials 2025, 18, 1573. https://doi.org/10.3390/ma18071573
Li Y, Yang W, Zhang J, Hou Y, Wen G, Xin G, Jiang M, Ma Y. Precursor-Derived Mo2C/SiC Composites with a Two-Dimensional Sheet Structure for Electromagnetic Wave Absorption. Materials. 2025; 18(7):1573. https://doi.org/10.3390/ma18071573
Chicago/Turabian StyleLi, Yang, Wen Yang, Jipeng Zhang, Yongzhao Hou, Guangwu Wen, Guodong Xin, Meixian Jiang, and Yongqiang Ma. 2025. "Precursor-Derived Mo2C/SiC Composites with a Two-Dimensional Sheet Structure for Electromagnetic Wave Absorption" Materials 18, no. 7: 1573. https://doi.org/10.3390/ma18071573
APA StyleLi, Y., Yang, W., Zhang, J., Hou, Y., Wen, G., Xin, G., Jiang, M., & Ma, Y. (2025). Precursor-Derived Mo2C/SiC Composites with a Two-Dimensional Sheet Structure for Electromagnetic Wave Absorption. Materials, 18(7), 1573. https://doi.org/10.3390/ma18071573






