In Situ-Reinforced Phase Evolution and Mechanical Properties of CoCrFeNi High-Entropy Alloy Composite Coating on Q235B by Laser Cladding with Nb Addition
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. LC Technology
2.3. Text Procedure
3. Results
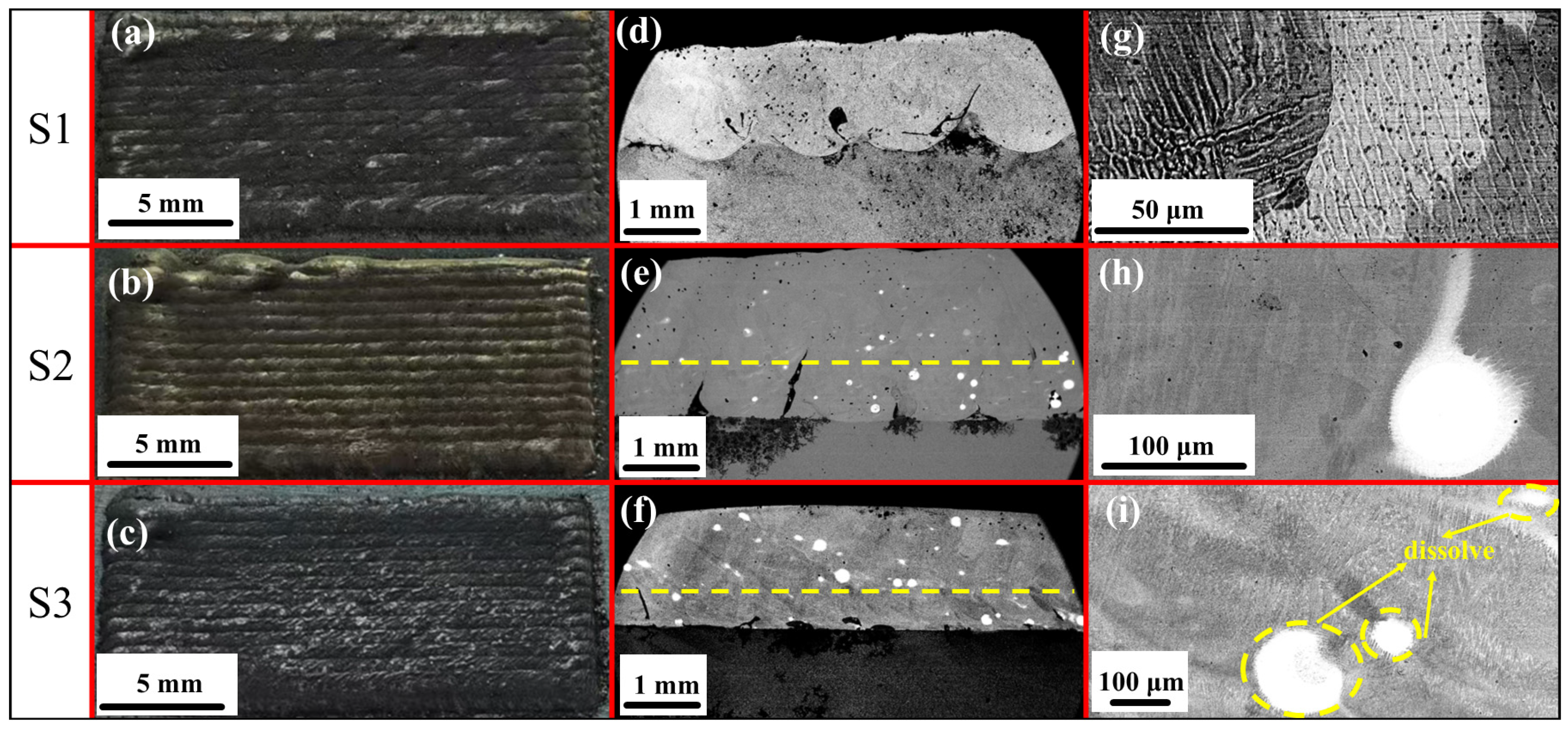
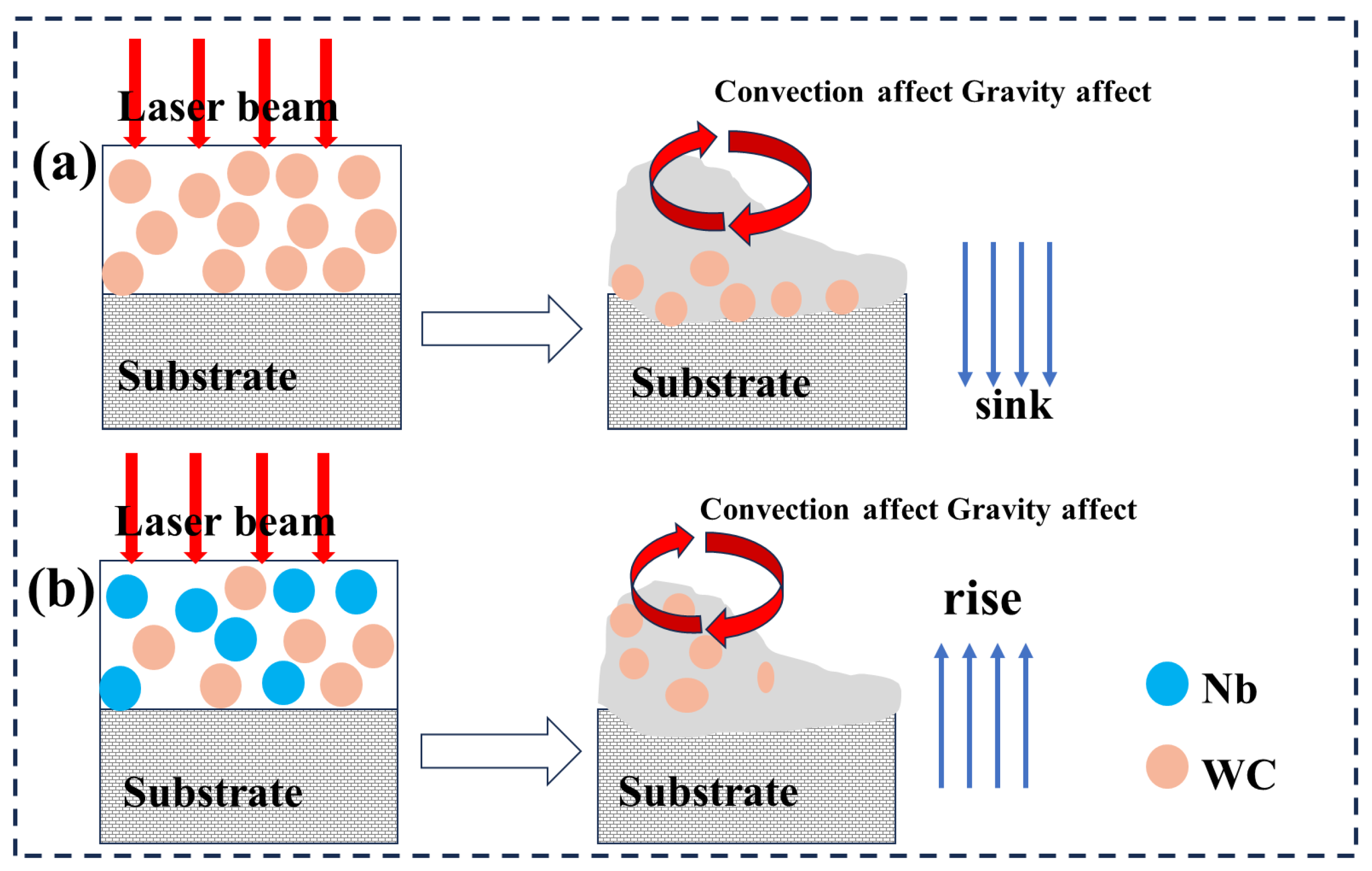
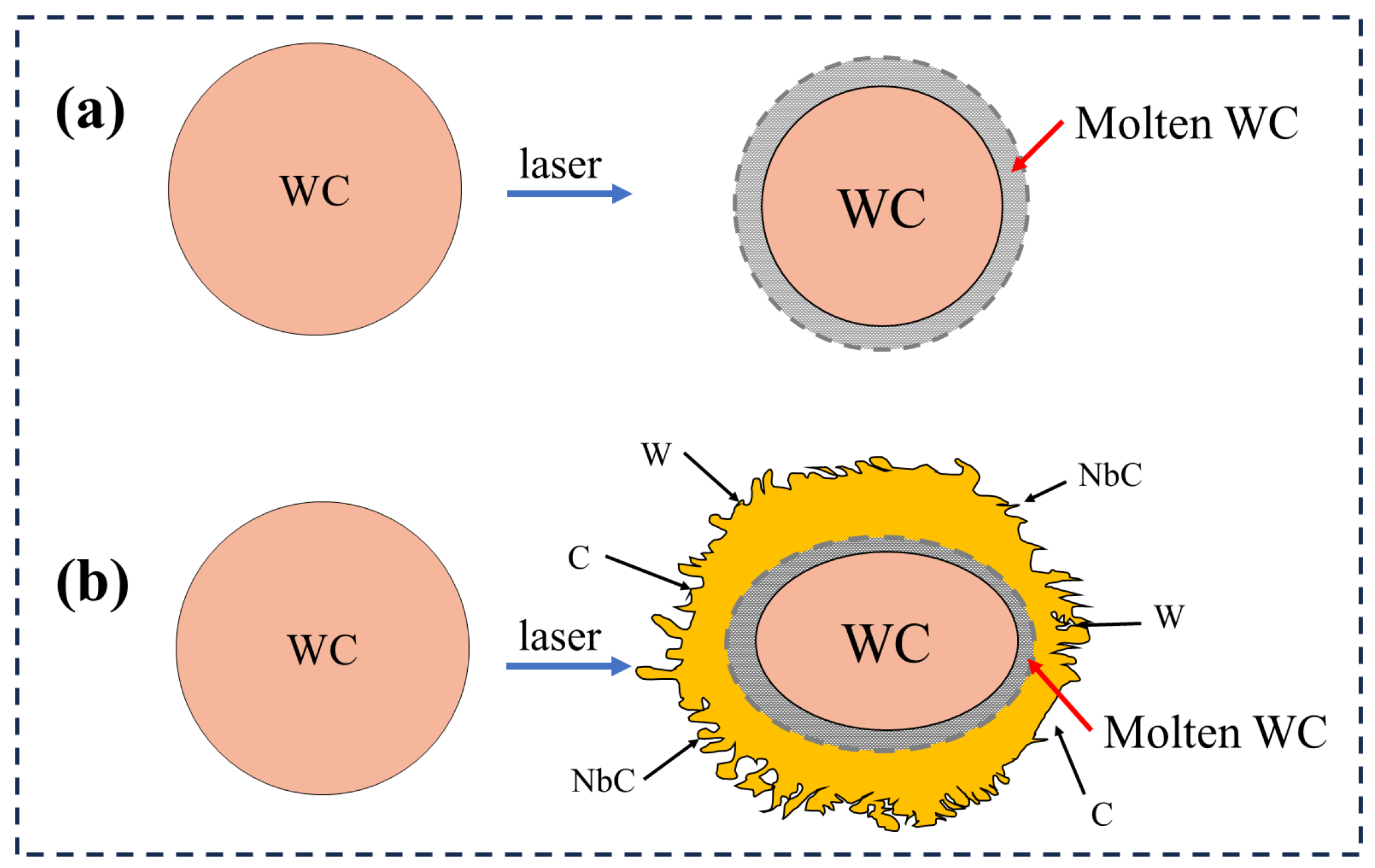
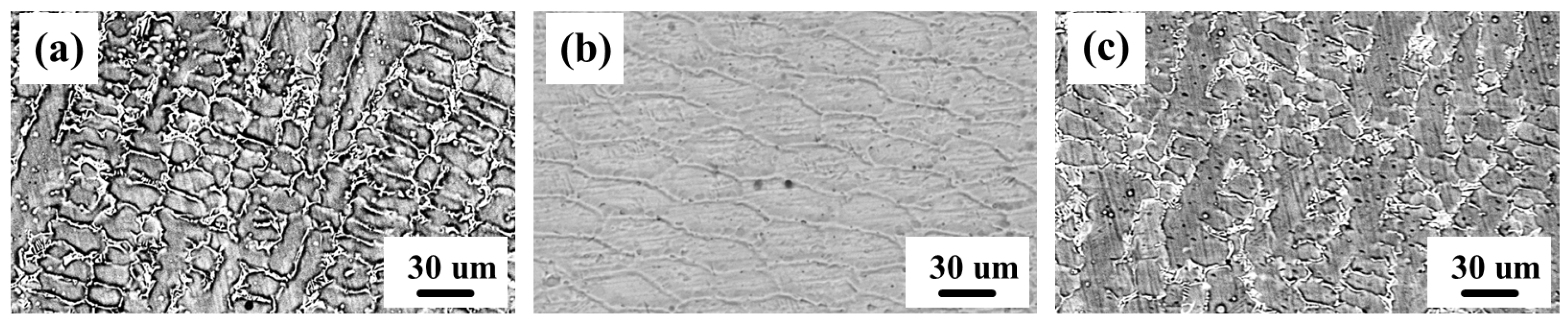
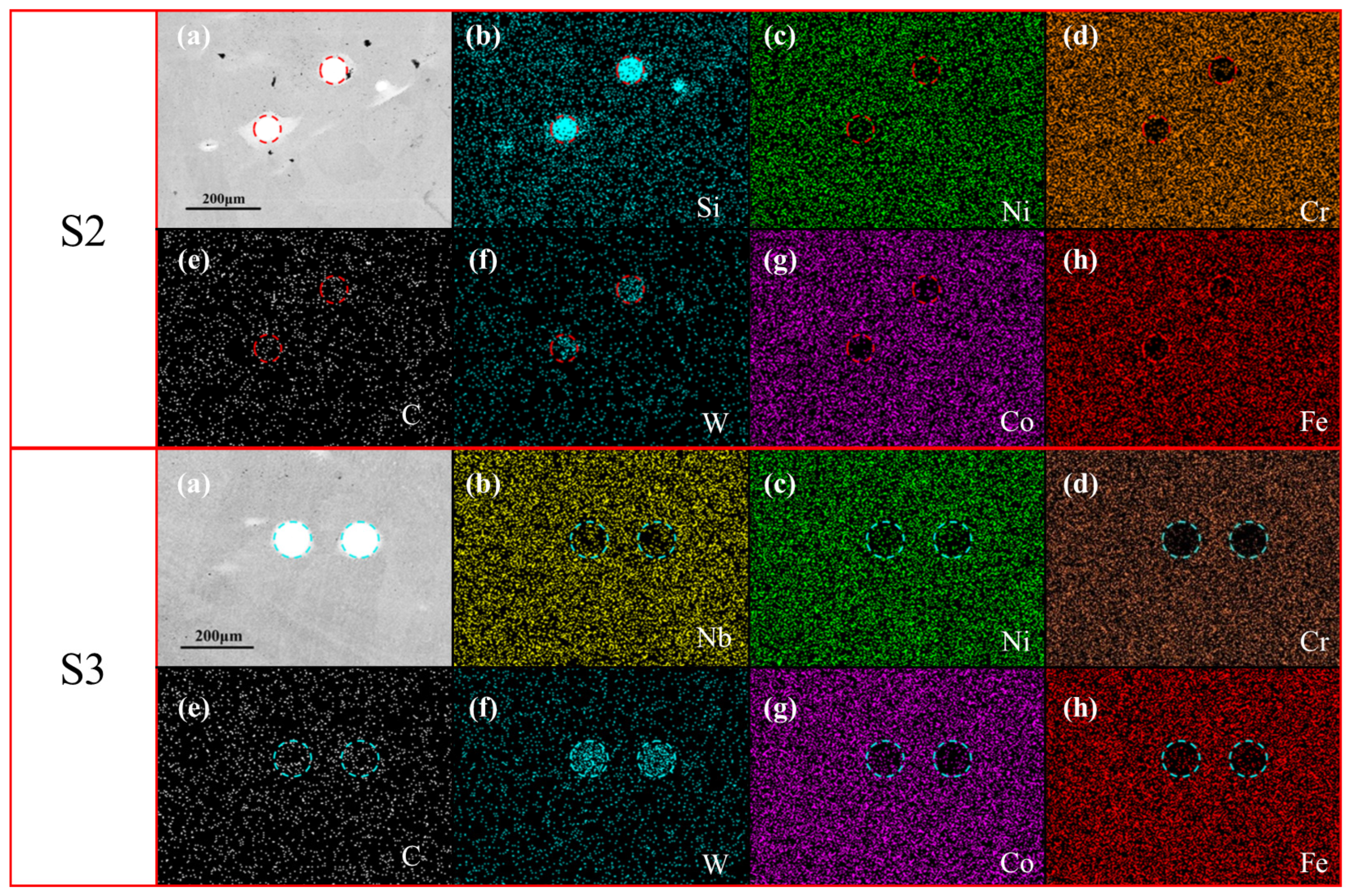
3.1. Macroscopic and Microscopic Observations
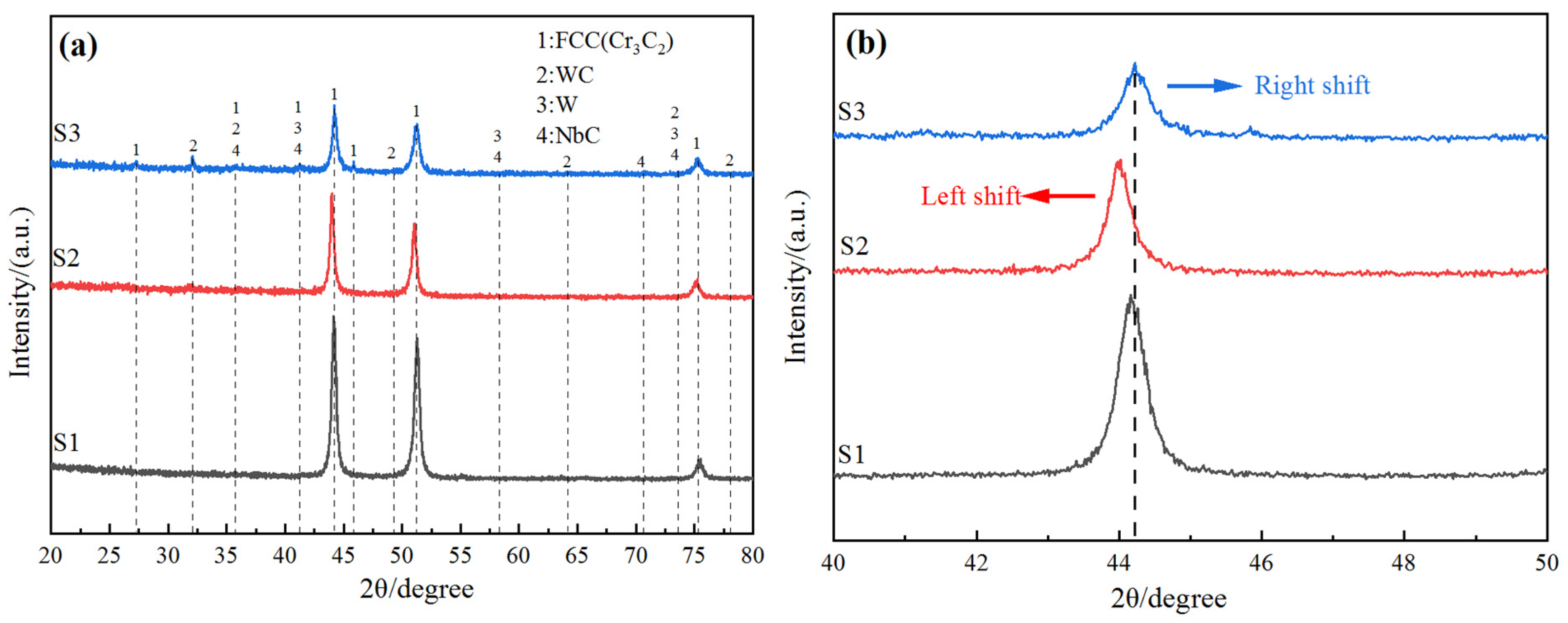
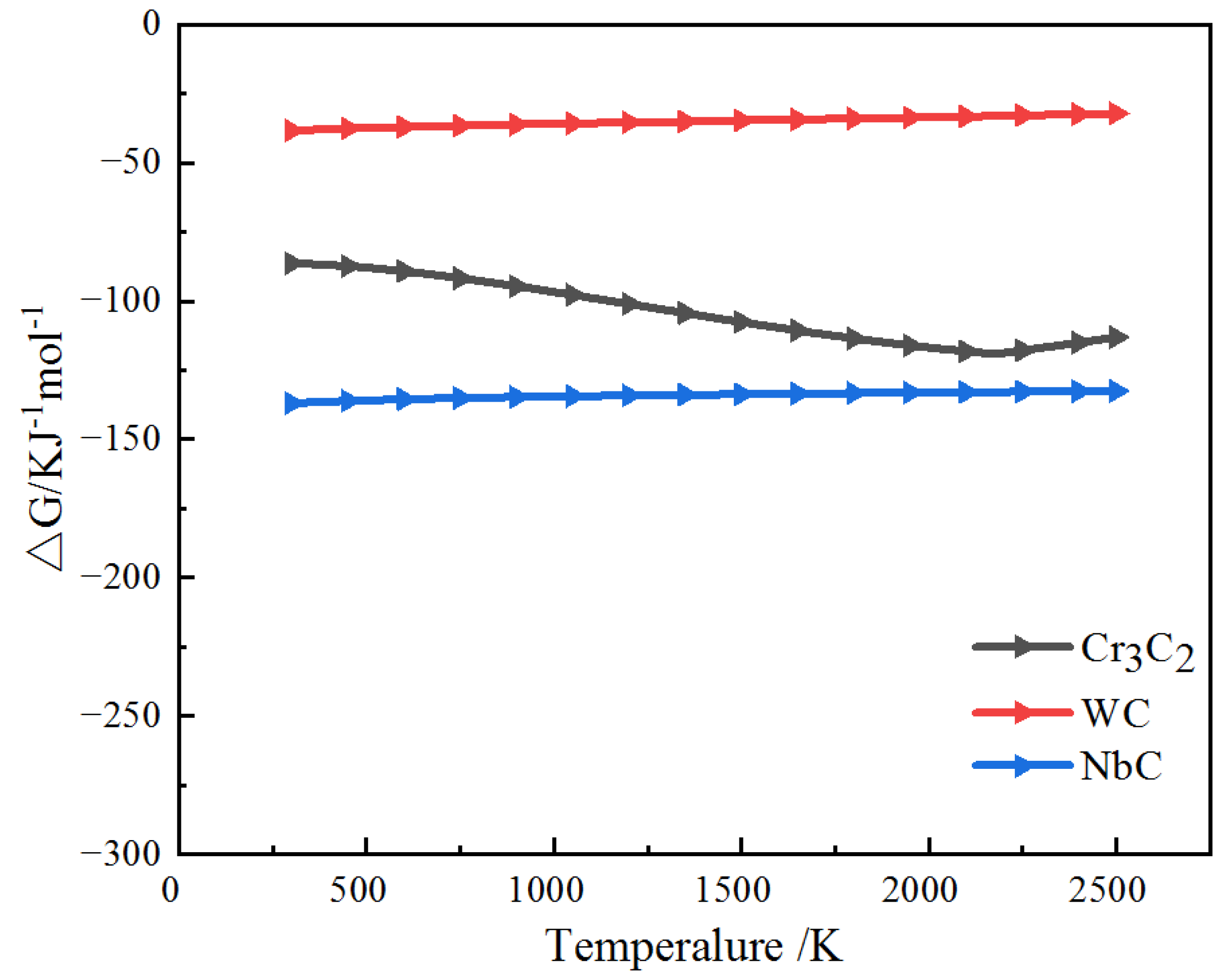
3.2. XRD Analysis
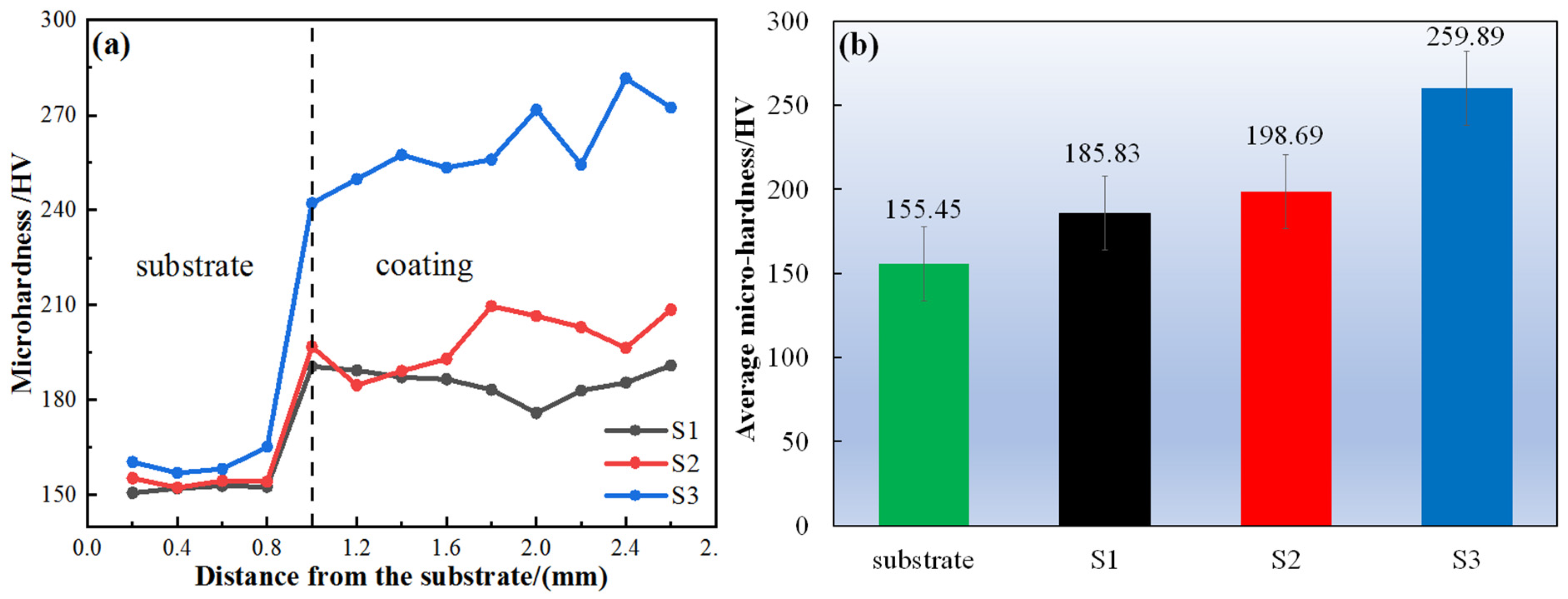
3.3. Hardness and Wear Properties
3.4. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, Z.; Wang, Y.; Zhai, X.; Zhou, H. Compressive mechanical behaviors of Q235B steel over a wide range of temperatures and strain rates. Int. J. Impact Eng. 2025, 198, 105222. [Google Scholar] [CrossRef]
- Yang, Q.; Xiong, Y.; Huang, Y.; Cheng, J.; Lou, D.; Chen, L.; Li, Q.; Liu, D. Nanosecond laser passivation mechanism of Q235B carbon steel surface. J. Mater. Eng. Perform. 2024, 34, 2371–2379. [Google Scholar] [CrossRef]
- Xia, Z.; Huang, Y.; Zhong, J.; Guan, K. Cracking failure analysis on a high-frequency electric resistance welding pipe in buried fire water pipeline. Eng. Fail. Anal. 2023, 146, 107072. [Google Scholar] [CrossRef]
- Si, Q.; Ding, Y.; Zong, L.; Meng, X. Low cycle fatigue properties of corroded Q355B steel. J. Mater. Civ. Eng. 2024, 36, 04023530. [Google Scholar] [CrossRef]
- Feng, R.; Pan, J.; Zhang, J.; Shao, Y.; Chen, B.; Fang, Z.; Roy, K.; Lim, J.B. Effects of corrosion morphology on the fatigue life of corroded Q235B and 42CrMo steels: Numerical modelling and proposed design rules. Structures 2023, 57, 105136. [Google Scholar] [CrossRef]
- Thornton, J.A. Magnetron sputtering: Basic physics and application to cylindrical magnetrons. J. Vac. Sci. Technol. 1978, 15, 171–177. [Google Scholar] [CrossRef]
- Pawlowski, L. The Science and Engineering of Thermal Spray Coatings; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Zhang, Y.; Huang, J.; Cheng, Z.; Ye, Z.; Chi, H.; Peng, L.; Chen, S. Study on MIG-TIG double-sided arc welding-brazing of aluminum and stainless steel. Mater. Lett. 2016, 172, 146–148. [Google Scholar] [CrossRef]
- Fang, Z.Z. (Ed.) Sintering of Advanced Materials; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Toyserkani, E.; Khajepour, A.; Corbin, S.F. Laser Cladding; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Wu, Q.; Long, W.; Zhang, L.; Zhao, H. A review on ceramic coatings prepared by laser cladding technology. Opt. Laser Technol. 2024, 176, 110993. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, K.; Li, J.; Geng, S.; Sun, L.; Skuratov, V. A review on cracking mechanism and suppression strategy of nickel-based superalloys during laser cladding. J. Alloys Compd. 2024, 1001, 175164. [Google Scholar] [CrossRef]
- Zhou, L.; Ma, G.; Zhao, H.; Mou, H.; Xu, J.; Wang, W.; Xing, Z.; Li, Y.; Guo, W.; Wang, H. Research status and prospect of extreme high-speed laser cladding technology. Opt. Laser Technol. 2024, 168, 109800. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, Y.; Yu, T.; Wang, D.; Leng, X.; Wang, K.; Liu, L.; Pan, J.; Yao, S.; Chen, Z. Review on wear resistance of laser cladding high-entropy alloy coatings. J. Mater. Res. Technol. 2024, 28, 911–934. [Google Scholar] [CrossRef]
- Zhang, Z.; Hua, K.; Cao, Y.; Song, Y.; Li, X.; Zhou, Q.; Wang, H. Microstructures and properties of FeCrAlMoSix high entropy alloy coatings prepared by laser cladding on a titanium alloy substrate. Surf. Coat. Technol. 2024, 478, 130437. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chang, S.Y.; Hong, Y.D.; Chen, S.K.; Lin, S.J. Anomalous decrease in X-ray diffraction intensities of Cu–Ni–Al–Co–Cr–Fe–Si alloy systems with multi-principal elements. Mater. Chem. Phys. 2007, 103, 41–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Liu, L.H.; Li, N.; Han, M.; Han, J.R.; Liang, H.Y. Scalable synthesis of nanoporous high entropy alloys for electrocatalytic oxygen evolution. Rare Met. 2022, 41, 125–131. [Google Scholar] [CrossRef]
- Krishna, S.A.; Noble, N.; Radhika, N.; Saleh, B. A comprehensive review on advances in high entropy alloys: Fabrication and surface modification methods, properties, applications, and future prospects. J. Manuf. Process. 2024, 109, 583–606. [Google Scholar] [CrossRef]
- Tian, C.; Ouyang, D.; Wang, P.; Zhang, L.; Cai, C.; Zhou, K.; Shi, Y. Strength-ductility synergy of an additively manufactured metastable high-entropy alloy achieved by transformation-induced plasticity strengthening. Int. J. Plast. 2024, 172, 103823. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Xiao, B.; Zhang, S.; Wang, Y.; Cui, H.; Li, C.; Hou, Y.; Guo, Y.; Yang, T.; et al. Phase Engineering of High-Entropy Alloy for Enhanced Electrocatalytic Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2024, 63, e202407589. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, J.; Luo, F.; Zhao, Y.; Shi, W. Fe-based amorphous reinforced CoCrFeNi HEA composite coating prepared by laser cladding. J. Mater. Res. Technol. 2024, 31, 1912–1923. [Google Scholar] [CrossRef]
- Huang, G.; Li, B.; Chen, Y.; Xuan, F. Nanotwining induced by tensile fatigue and dynamic impact of laser powder bed fusion additively manufactured CoCrFeNi high-entropy alloy. J. Mater. Sci. Technol. 2024, 183, 241–257. [Google Scholar]
- Cao, T.; Zhang, Q.; Wang, L.; Xiao, Y.; Yao, J.; Liu, H.; Ren, Y.; Liang, J.; Xue, Y.; Li, X. Dynamic deformation behaviors and mechanisms of CoCrFeNi high-entropy alloys. Acta Mater. 2023, 260, 119343. [Google Scholar] [CrossRef]
- Zhou, J.L.; Cheng, Y.H.; Wan, Y.X.; Chen, H.; Wang, Y.F.; Yang, J.Y. Strengthening by Ti, Nb, and Zr doping on microstructure, mechanical, tribological, and corrosion properties of CoCrFeNi high-entropy alloys. J. Alloys Compd. 2024, 984, 173819. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, Y.; Kim, M.K.; Duan, Z.; Yuan, Q.; Oh, E.; Suhr, J. In-situ TiCxNy nanoparticle reinforced crack-free CoCrFeNi medium-entropy alloy matrix nanocomposites with high strength and ductility via laser powder bed fusion. Compos. Part B Eng. 2024, 273, 111237. [Google Scholar]
- Wei, X.; Zhang, L.; Zhang, F.; Zhang, C.; Jia, Q.; Sun, K.; Duan, D.; Jiang, H.; Li, G. Effect of carbon addition on the microstructure and corrosion resistance of the CoCrFeNi high-entropy alloy. Corros. Sci. 2024, 231, 111965. [Google Scholar]
- Huang, J.; Zhu, Z.; Wang, H.; Li, K.; Shi, W.; Jiao, T. Effect of WC content on microstructure and properties of CoCrFeNi HEA composite coating on 316L surface via laser cladding. Materials 2023, 16, 2706. [Google Scholar] [CrossRef]
- Hong, S.; Ma, Q.; Liu, G.; Yang, H.; Hu, L.; Meng, W.; Xie, H.; Yin, X. In-situ reinforced phase evolution and wear resistance of nickel-based composite coatings fabricated by wide-band laser cladding with Nb addition. Opt. Laser Technol. 2023, 157, 108678. [Google Scholar]
- Wu, H.; Zhang, S.; Zhang, H.Y.; Wang, R.; Zhang, H.F.; Zhang, C.H.; Wu, C.L.; Chen, H.T. Exploration of wear and slurry erosion mechanisms of laser clad CoCrFeNi+ x (NbC) high entropy alloys composite coatings. Tribol. Int. 2024, 193, 109405. [Google Scholar]
- Zhao, Y.; Chen, L.; Sun, J.; Wu, W.; Yu, T. Microstructure evolution and wear resistance of in-situ synthesized (Ti, Nb) C ceramic reinforced Ni204 composite coatings. Ceram. Int. 2022, 48, 17518–17528. [Google Scholar]
- Gao, Y.; Liu, Y.; Wang, L.; Yang, X.; Zeng, T.; Sun, L.; Wang, R. Microstructure evolution and wear resistance of laser cladded 316L stainless steel reinforced with in-situ VC-Cr7C3. Surf. Coat. Technol. 2022, 435, 128264. [Google Scholar]
- Wang, C.; Zhang, S.; Zhang, C.H.; Wu, C.L.; Zhang, J.B.; Abdullah, A.O. Phase evolution and wear resistance of in situ synthesized V8C7 particles reinforced Fe-based coating by laser cladding. Opt. Laser Technol. 2018, 105, 58–65. [Google Scholar] [CrossRef]
- Wang, K.; Du, D.; Liu, G.; Chang, B.; Ju, J.; Sun, S.; Fu, H. Microstructure and property of laser clad Fe-based composite layer containing Nb and B4C powders. J. Alloys Compd. 2019, 802, 373–384. [Google Scholar] [CrossRef]
- Chen, J.; Lian, G.; Lin, T.; Lu, H.; Wang, Y. Effects of the proportions of carbon on the microstructure and properties of NbC-reinforced Ni-WC composite coatings by laser cladding in-situ synthesis. Mater. Today Commun. 2024, 38, 107896. [Google Scholar] [CrossRef]
- Hsu, W.C.; Liao, W.H.; Liang, Y.C.; Shen, T.E.; Yeh, J.W.; Tzeng, Y.C.; Wu, T.F.; Tsai, C.W. Minor tungsten addition enhances corrosive resistance in CoCrFeNi high-entropy alloy at elevated seawater temperature. J. Mater. Res. Technol. 2024, 33, 2970–2980. [Google Scholar] [CrossRef]
- Mohammadnejad, A.; Bahrami, A.; Sajadi, M.; Karimi, P.; Fozveh, H.R.; Mehr, M.Y. Microstructure and mechanical properties of spark plasma sintered nanocrystalline Ni3Al-xB (0.0< x< 1.5 at%) alloy. Mater. Today Commun. 2018, 17, 161–168. [Google Scholar]
- Ye, D.L.; Hu, J.H. Practical Inorganic Thermodynamics Manual; Metallurgical Industry Press: Beijing, China, 2002. [Google Scholar]
- Luo, F.; Wang, S.; Shi, W.; Zhao, Y.; Xiong, Z.; Huang, J. Corrosion resistance and wear behavior of CoCrFeNiMn@ Gr high entropy alloy-based composite coatings prepared by laser cladding. J. Mater. Res. Technol. 2024, 33, 3769–3789. [Google Scholar] [CrossRef]
- Luo, F.; Wang, S.; Shi, W.; Zhao, Y.; Xiong, Z.; Huang, J. Wear behavior and corrosion resistance of laser-clad Ni60-1% carbon nanotubes coating. Surf. Coat. Technol. 2024, 482, 130686. [Google Scholar] [CrossRef]
- Huang, J.; Luo, F.; Zhao, Y.; Shi, W. Preparation of AlCoCrFeNi/W-TiC HEA composite coating by laser cladding. Mater. Today Commun. 2024, 39, 108677. [Google Scholar] [CrossRef]
- Gu, Z.; Xi, S.; Sun, C. Microstructure and properties of laser cladding and CoCr2.5FeNi2Tix high-entropy alloy composite coatings. J. Alloys Compd. 2020, 819, 152986. [Google Scholar] [CrossRef]





| Material | Chemical Composition (Mass Fraction) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | Fe | S | P | Cr | Co | |
| Q235B | 0.15 | 0.15 | 0.25 | Bal | 0.11 | 0.12 | - | - |
| CoCrFeNi HEA | 26.16 | - | - | 24.79 | - | - | 22.98 | 26.16 |
| Number of Coating | CoCrFeNi Powder | WC Powder | Nb Powder |
|---|---|---|---|
| S1 | 100 | 0 | 0 |
| S2 | 95 | 5 | 0 |
| S3 | 90 | 5 | 5 |
| Materials’ | Ecorr (V/SCE) | Icorr (A/cm2) |
|---|---|---|
| S1 | 1.91821 × 10−1 | 2.76671 × 10−9 |
| S2 | 2.03847 × 10−1 | 3.62645 × 10−9 |
| S3 | 2.48573 × 10−1 | 2.67767 × 10−−9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Xiao, Z.; Chen, Z.; Jin, H.; Gao, C.; Huang, J. In Situ-Reinforced Phase Evolution and Mechanical Properties of CoCrFeNi High-Entropy Alloy Composite Coating on Q235B by Laser Cladding with Nb Addition. Materials 2025, 18, 1572. https://doi.org/10.3390/ma18071572
Yang F, Xiao Z, Chen Z, Jin H, Gao C, Huang J. In Situ-Reinforced Phase Evolution and Mechanical Properties of CoCrFeNi High-Entropy Alloy Composite Coating on Q235B by Laser Cladding with Nb Addition. Materials. 2025; 18(7):1572. https://doi.org/10.3390/ma18071572
Chicago/Turabian StyleYang, Feimuyun, Zhixuan Xiao, Zehuan Chen, Hongtao Jin, Chao Gao, and Jiang Huang. 2025. "In Situ-Reinforced Phase Evolution and Mechanical Properties of CoCrFeNi High-Entropy Alloy Composite Coating on Q235B by Laser Cladding with Nb Addition" Materials 18, no. 7: 1572. https://doi.org/10.3390/ma18071572
APA StyleYang, F., Xiao, Z., Chen, Z., Jin, H., Gao, C., & Huang, J. (2025). In Situ-Reinforced Phase Evolution and Mechanical Properties of CoCrFeNi High-Entropy Alloy Composite Coating on Q235B by Laser Cladding with Nb Addition. Materials, 18(7), 1572. https://doi.org/10.3390/ma18071572





