Compatibility of Carbonate Mixtures to Be Used as Molten Salts with Different Metal Alloys to Be Used as Container Materials
Abstract
1. Introduction
1.1. Use of Carbonates in CCS
1.2. Use of Carbonates in TES
1.3. Literature Review on Use of Carbonates
1.4. Motivation of the Paper
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metz, B.; Davidson, O.; de Coninck, H.C.; Loos, M.; Meyer, L.A. IPCC Special Report on Carbon Dioxide Capture and Storage; Prepared by Working Group III of the Intergovernmental Panel on Climate Change, Cambridge, United Kingdom and New York, NY, USA; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Cabeza, L.F.; Martorell, I.; Miró, L.; Fernández, A.I.; Barreneche, C. Introduction to thermal energy storage (TES) systems. In Advances in Thermal Energy Storage Systems; Woodhead Publishing: Sawston, UK, 2014. [Google Scholar] [CrossRef]
- Remmel, A.-L.; Ratso, S.; Liivand, K.; Danilson, M.; Kaare, K.; Mikli, V.; Kruusenberg, I. CO2 transformed into highly active catalysts for the oxygen reduction reaction via low-temperature molten salt electrolysis. Electrochem. Commun. 2024, 166, 107781. [Google Scholar] [CrossRef]
- Remmel, A.-L.; Ratso, S.; Divitini, G.; Danilson, M.; Mikli, V.; Uibu, M.; Aruväli, J.; Kruusenberg, I. Nickel and Nitrogen-Doped Bifunctional ORR and HER Electrocatalysts Derived from CO2. ACS Sustain. Chem. Eng. 2022, 10, 134–145. [Google Scholar] [CrossRef]
- González-Roubaud, E.; Pérez-Osorio, D.; Prieto, C. Review of commercial thermal energy storage in concentrated solar power plants: Steam vs. molten salts. Renew. Sustain. Energy Rev. 2017, 80, 133–148. [Google Scholar] [CrossRef]
- Morales, M.; Cabezas, L.; Castro-Alloca, M.; Fargas, G.; Llanes, L.; Mateo, A. Corrosion Evaluation of Austenitic and Duplex Stainless Steels in Molten Carbonate Salts at 600 °C for Thermal Energy Storage. Metals 2022, 12, 2190. [Google Scholar] [CrossRef]
- Kolb, G. An Evaluation of Possible Next-Generation High Temperature Molten-Salt Power Towers; Sandia National Laboratories: Albuquerque, NM, USA; Livermore, CA, USA, 2011. [Google Scholar] [CrossRef]
- Wang, P.; Du, K.; Yin, H.; Wang, D. Corrosion and protection of metallic materials in molten carbonates for concentrating solar power and molten carbonate electrolysis applications. Corros. Commun. 2023, 11, 58–71. [Google Scholar] [CrossRef]
- Fernández, A.G.; Pineda, F.; Walczak, M.; Cabeza, L.F. Corrosion evaluation of alumina-forming alloys in carbonate molten salt for CSP plants. Renew Energy 2019, 140, 227–233. [Google Scholar] [CrossRef]
- de Miguel, M.T.; Encinas-Sánchez, V.; Lasanta, M.I.; García-Martín, G.; Pérez, F.J. Corrosion resistance of HR3C to a carbonate molten salt for energy storage applications in CSP plants. Sol. Energy Mater. Sol. Cells 2016, 157, 966–972. [Google Scholar] [CrossRef]
- Encinas-Sánchez, V.; de Miguel, M.T.; García-Martín, G.; Lasanta, M.I.; Pérez, F.J. Corrosion resistance of Cr/Ni alloy to a molten carbonate salt at various temperatures for the next generation high-temperature CSP plants. Sol. Energy 2018, 171, 286–292. [Google Scholar] [CrossRef]
- Sarvghad, M.; Steinberg, T.A.; Will, G. Corrosion of stainless steel 316 in eutectic molten salts for thermal energy storage. Sol. Energy 2018, 172, 198–203. [Google Scholar] [CrossRef]
- Gomez-Vidal, J.C.; Tirawat, R. Corrosion of alloys in a chloride molten salt (NaCl-LiCl) for solar thermal technologies. Sol. Energy Mater. Sol. Cells 2016, 157, 234–244. [Google Scholar] [CrossRef]
- Morales, M.; Gordon, S.; Fernández-Arana, Ó.; García-Marro, F.; Mateo, A.; Llanes, L.; Fargas, G. Duplex Stainless Steels for Thermal Energy Storage: Characterization of Oxide Scales Formed in Carbonate Salts at 500 °C. Metals 2022, 12, 2156. [Google Scholar] [CrossRef]
- Morales, M.; Rezayat, M.; Mateo, A. Amorphous Carbon Film as a Corrosion Mitigation Strategy for Stainless Steel in Molten Carbonate Salts for Thermal Energy Storage Applications. Materials 2024, 17, 5619. [Google Scholar] [CrossRef]
- Spiegel, M.; Schraven, P. Corrosion of Commercial Alloys in Ternary Carbonate Melt at 700 and 750 °C—Role of LiFeO2 Formation. High Temp. Corros. Mater. 2024, 101, 1103–1116. [Google Scholar] [CrossRef]
- Gallardo-González, J.; Martínez, M.; Barreneche, C.; Fernández, A.I.; Liu, M.; Tay, N.H.S.; Bruno, F.; Segarra, M. Corrosion of AISI316 as containment material for latent heat thermal energy storage systems based on carbonates. Sol. Energy Mater. Sol. Cells 2018, 186, 1–8. [Google Scholar] [CrossRef]
- Bell, S.; Sarvghad, M.; Ong, T.-C.; Naylor, D.; Wang, X.; Yin, Y.; Rumman, R.; Andersson, G.; Will, G.; Lewis, D.A.; et al. Corrosion of iron–nickel–chromium alloys in high temperature carbonate salt under argon atmosphere. Sol. Energy Mater. Sol. Cells 2023, 256, 112317. [Google Scholar] [CrossRef]
- Xue, X.J.; Dong, J.; Zhao, C.Y. Corrosion evaluation of austenitic stainless steels in Li2CO3-K2CO3 eutectic salt for thermal energy storage. J. Energy Storage 2024, 99, 113312. [Google Scholar] [CrossRef]
- Bell, S.; de Bruyn, M.; Steinberg, T.; Will, G. Corrosion resistance of 625 nickel superalloy exposed to isothermal and thermal cycling conditions in a chloride/carbonate salt. Sol. Energy 2023, 249, 278–287. [Google Scholar] [CrossRef]
- Lippiatt, K.; Bell, S.; Will, G.; Steinberg, T. Corrosion effects of Na2CO3/NaCl molten salt eutectic on 316L stainless steel under isothermal and cycling conditions. Sol. Energy Mater. Sol. Cells 2024, 272, 112933. [Google Scholar] [CrossRef]
- Conecband. 2024. Available online: https://www.conecband.com (accessed on 22 January 2025).
- Cabeza, L.F.; Roca, J.; Nogueés, M.; Mehling, H.; Hiebler, S. Long term immersion corrosion tests on metal-PCM pairs used for latent heat storage in the 24 to 29 °C temperature range. Mater. Corros. 2005, 56, 33–39. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Roca, J.; Nogués, M.; Mehling, H.; Hiebler, S. Immersion corrosion tests on metal-salt hydrate pairs used for latent heat storage in the 48 to 58 °C temperature range. Mater. Corros. 2002, 53, 902–907. [Google Scholar] [CrossRef]
- ASTM G1-03(2017)e1; Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM: West Conshohocken, PA, USA, 2017. [CrossRef]
- Marín, P.E.; Ushak, S.; de Gracia, A.; Grageda, M.; Cabeza, L.F. Assessing corrosive behaviour of commercial phase change materials in the 21–25 °C temperature range. J. Energy Storage 2020, 32, 101711. [Google Scholar] [CrossRef]
- Ushak, S.; Marín, P.; Galazutdinova, Y.; Cabeza, L.F.; Farid, M.M.; Grágeda, M. Compatibility of materials for macroencapsulation of inorganic phase change materials: Experimental corrosion study. Appl. Therm. Eng. 2016, 107, 410–419. [Google Scholar] [CrossRef]
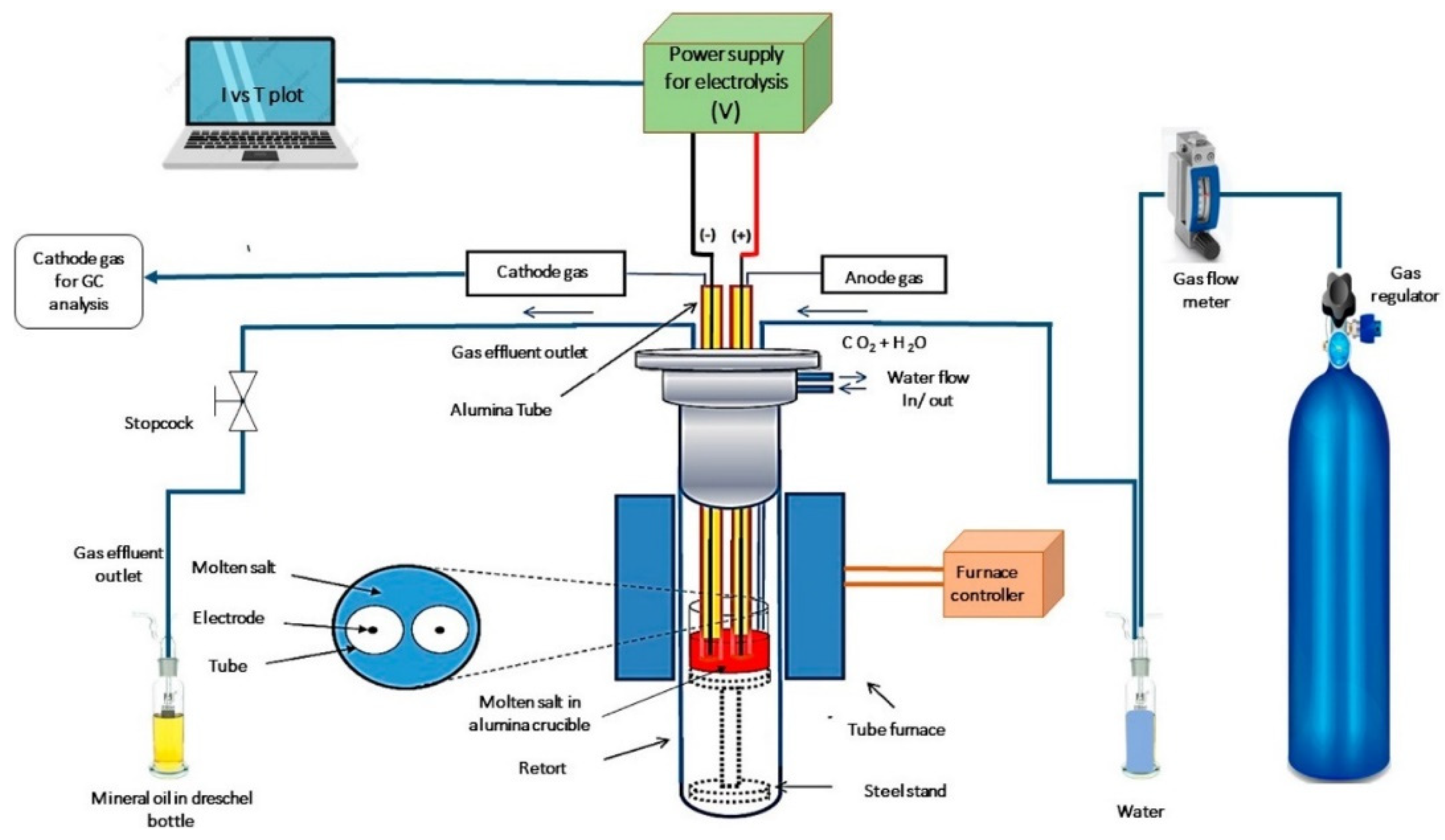
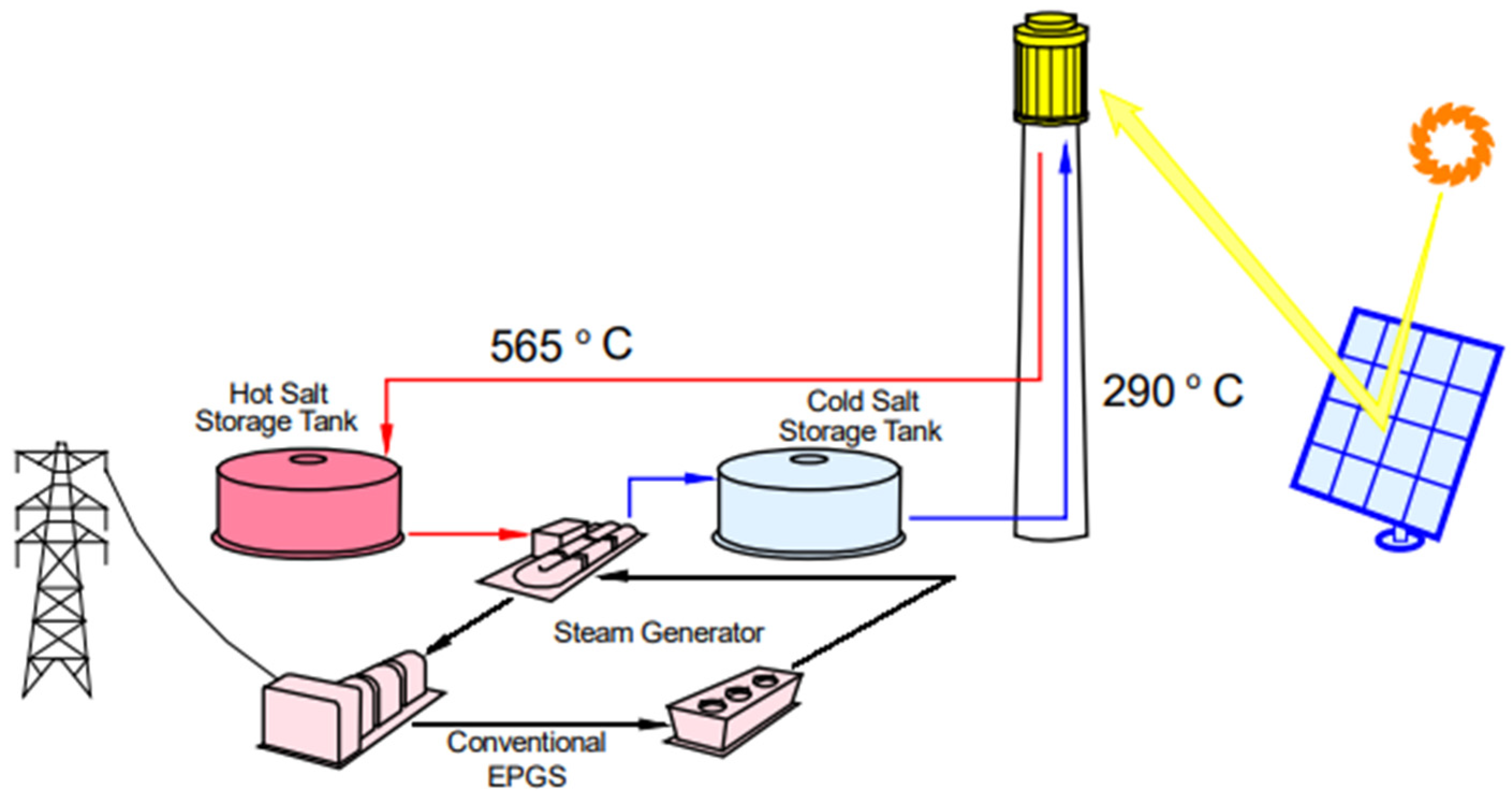
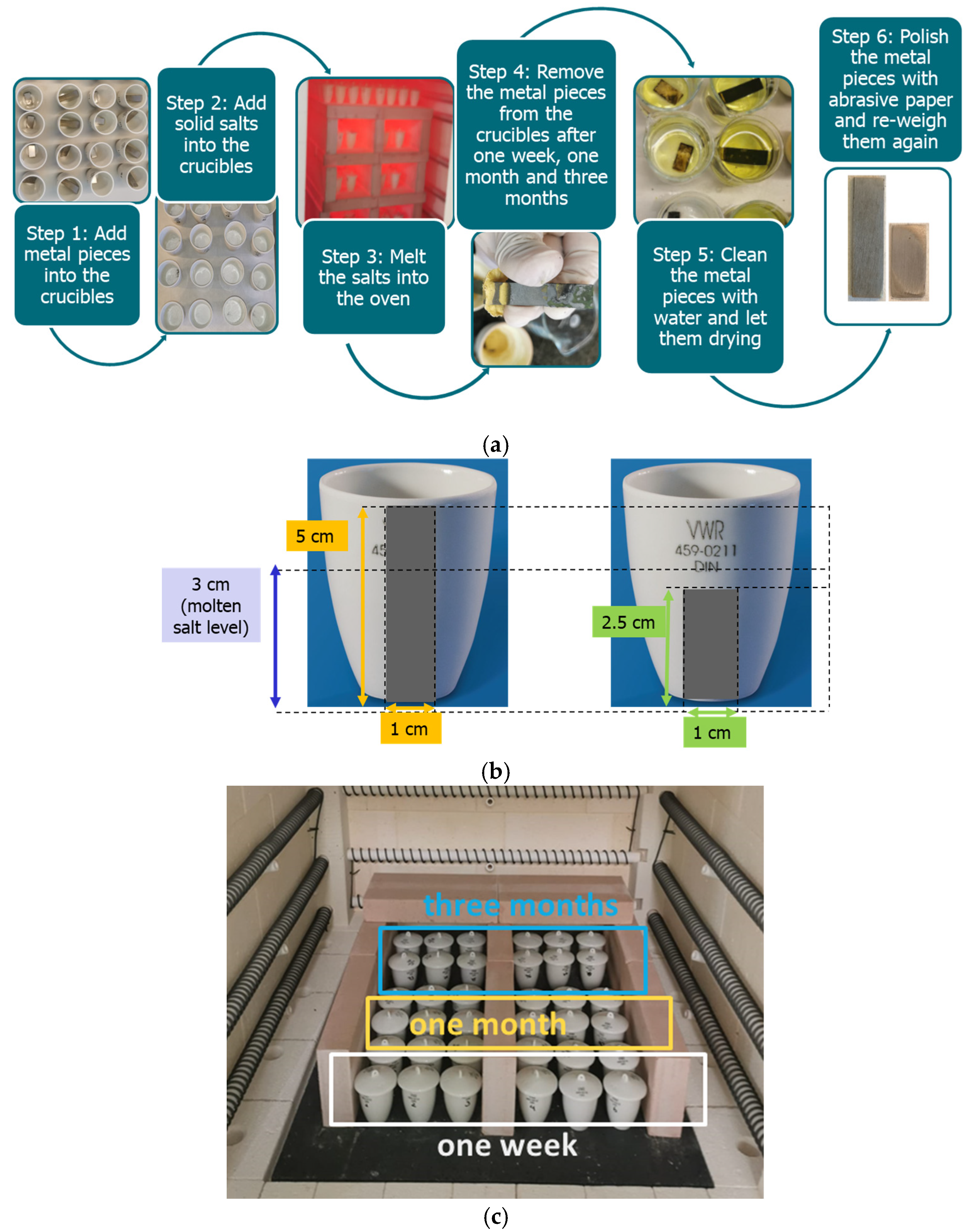
| Molten Carbonate Salt Mixture (wt.%) | Alloy | Temperature (°C) | Time of Exposure (h) | Metallographic Thickness (µm) | Corrosion Rate (mm/year) | Corrosion Rate (mpy) | Reference |
|---|---|---|---|---|---|---|---|
| Na2CO3-Li2CO3-K2CO3 (33.4-32.1-34.5) | HR3C | 700 | 2000 | 25.41 | n.a. | n.a. | [10] |
| 800 | 2000 | 56.66 | n.a. | n.a. | [11] | ||
| In601 | 450 | 120 | 10.5 | n.a. | n.a. | [12] | |
| In800H | 600 | 24 | n.a. | 0.451 | 17.77 | [13] | |
| 750 | --- | --- | 1.08 | 42.55 | [13] | ||
| 310SS | 600 | --- | --- | 0.632 | 24.90 | [13] | |
| 750 | --- | --- | 1.53 | 60.28 | [13] | ||
| 321SS | 750 | --- | --- | 4.64 | 182.82 | [13] | |
| 347SS | 750 | --- | --- | 2.36 | 92.98 | [13] | |
| AFA- | 750 | --- | --- | 1.75 | 68.95 | [13] | |
| OC6 | --- | --- | --- | --- | --- | [13] | |
| OC4 | 650 | 1000 | 29–55 | n.a. | 1.4 | [9] | |
| In625 | 750 | --- | --- | 2.60 | 102.44 | [13] | |
| HR224 | 650 | 1000 | 45 | n.a. | 0.2 | [9] | |
| Na2CO3-Li2CO3-K2CO3 (33-32-35) | DS2205 | 500 | 1600 | n.a. | n.a. | n.a. | [14] |
| DS2507 | 500 | 1600 | n.a. | n.a. | n.a. | [14] | |
| 301LNSS | 600 | 1000 | n.a. | n.a. | n.a. | [15] | |
| Na2CO3-Li2CO3-K2CO3 (34-33-33) | DMV310N | 700 | 1000 | 17 | n.a. | n.a. | [16] |
| 750 | 1000 | 45 | n.a. | n.a. | [16] | ||
| SZ 1183 | 700 | 1000 | 27 | n.a. | n.a. | [16] | |
| 750 | 1000 | 70 | n.a. | n.a. | [16] | ||
| In625 | 700 | 1000 | 48 | n.a. | n.a. | [16] | |
| 750 | 1000 | 78 | n.a. | n.a. | [16] | ||
| In617 | 700 | 1000 | 20 | n.a. | n.a. | [16] | |
| 750 | 1000 | 59 | n.a. | n.a. | [16] | ||
| Haynes230 | 700 | 1000 | 59 | n.a. | n.a. | [16] | |
| 750 | 1000 | 37 | n.a. | n.a. | [16] | ||
| Na2CO3-Li2CO3-K2CO3 (35.1-10.2-54.5) | 316SS | 600 | 1440 | n.a. | 1.71 | 67.37 | [17] |
| Na2CO3-Li2CO3-K2CO3 (31.2-15.5-53.3) | 316SS | 600 | 1440 | n.a. | 2.07 | 81.56 | [17] |
| Na2CO3-Li2CO3-K2CO3 (38-33-29) | 316SS | 600 | 1440 | n.a. | 1.03 | 40.58 | [17] |
| Na2CO3-K2CO3 (47.19-52.81) | 316LSS | 750 under Ar | 3024 | n.a. | 9.5 | n.a. | [18] |
| 347HSS | 750 under Ar | 3024 | n.a. | 0.98 ± 0.04 | n.a. | [18] | |
| In800H | 750 under Ar | 3024 | n.a. | 0.56 ± 0.025 | n.a. | [18] | |
| Li2CO3-K2CO3 (28-72) | 310SS | 600 | 500 | n.a. | 0.522 | n.a. | [19] |
| 316LSS | 600 | 500 | n.a. | 2.535 | n.a. | [19] | |
| In625 | 600 | 500 | n.a. | 1.098 | n.a. | [19] | |
| Na2CO3-NaCl (59.4-40.6) | In625 | 650 | 768 | n.a. | 0.12–0.17 | n.a. | [20] |
| 316LSS | 650 | 768 | n.a | 2.0 | n.a. | [21] |
| Element (%) | Alloy 600 | Alloy 601 | Alloy 625 | Alloy X1 | Alloy 214 |
|---|---|---|---|---|---|
| Ni | 72 | 58–63 | 58 | 40–50 | 74.64–69.61 |
| Cr | 14–17 | 21–25 | 20–23 | 20–23 | 15–17 |
| Fe | 6–10 | 18.39–7.69 | 5 | 17–20 | 2–4 |
| Mn | 1 | 1 | 0.50 | 1.00 | <0.50 |
| Cu | 0.50 | 1 | --- | 0.50 | --- |
| Si | 0.50 | 0.50 | 0.50 | 1.00 | <0.20 |
| C | 0.15 | 0.10 | 0.10 | 0.05–0.15 | <0.05 |
| S | 0.01 | 0.01 | 0.01 | 0.03 | <0.02 |
| Al | --- | 1.00–1.70 | --- | 0.50 | 4–5 |
| Mo | --- | --- | 8–10 | 8–10 | <0.50 |
| Nb + Ta | --- | --- | 3.15–4.15 | --- | --- |
| Co | --- | --- | 1 | 0.50–2.50 | <2.0 |
| Ti | --- | --- | 0.40 | 0.15 | <0.50 |
| Ag | --- | --- | 0.40 | --- | --- |
| P | --- | --- | 0.01 | 0.04 | <0.02 |
| W | --- | --- | --- | 0.20–1.00 | <0.50 |
| B | --- | --- | --- | 0.01 | <0.01 |
| Y | --- | --- | --- | --- | 0.01–0.04 |
| Zr | --- | --- | --- | --- | <0.50 |
| Metal Alloy | Before Testing | After One Week of Testing | After One Month of Testing | After Three Months of Testing | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Polishing | After Polishing | Before Polishing | After Polishing | Before Polishing | After Polishing | |||||||||
| Alloy 600 |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Alloy 601 |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Alloy 625 |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Alloy X1 |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Alloy 214 |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Metal Alloy | Before Testing | After One Week of Testing | After One Month of Testing | After Three Months of Testing | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Polishing | After Polishing | Before Polishing | After Polishing | Before Polishing | After Polishing | |||||||||
| Alloy 600 |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Alloy 601 |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Alloy 625 |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Alloy X1 |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Alloy 214 |  |  |  |  |  |  |  |  |  |  |  |  |  |  |
| Corrosion Test Procedure | Mass Loss | Corrosion Rate | |
|---|---|---|---|
| mg/cm2·yr | mm/yr | ||
| Li2CO3-Na2CO3-K2CO3-LiOH∙H2O (56.65-12.19-26.66-4.51wt.%) | |||
| Samples completely immersed | 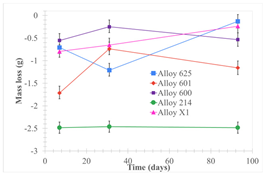 | 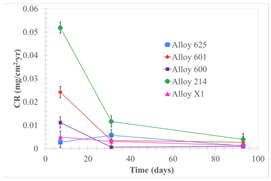 | 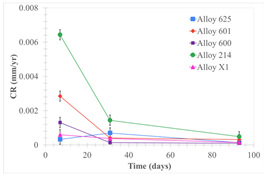 |
| Samples half immersed | 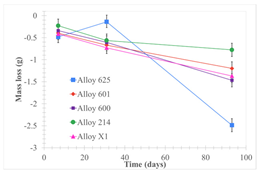 | 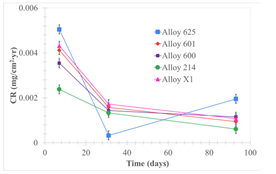 | 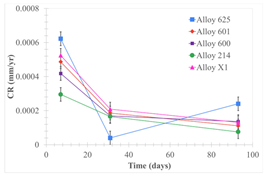 |
| Li2CO3 | |||
| Samples completely immersed | 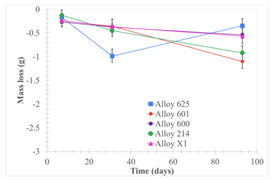 |  | 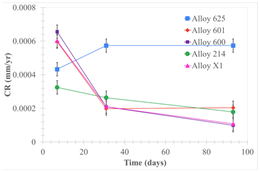 |
| Samples half immersed | 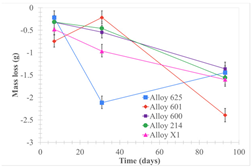 | 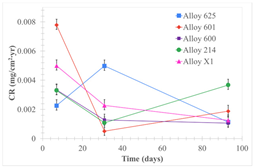 | 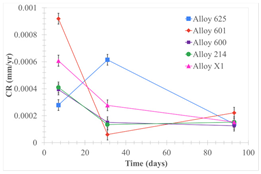 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabeza, L.F.; Martínez, F.R.; Borri, E. Compatibility of Carbonate Mixtures to Be Used as Molten Salts with Different Metal Alloys to Be Used as Container Materials. Materials 2025, 18, 1541. https://doi.org/10.3390/ma18071541
Cabeza LF, Martínez FR, Borri E. Compatibility of Carbonate Mixtures to Be Used as Molten Salts with Different Metal Alloys to Be Used as Container Materials. Materials. 2025; 18(7):1541. https://doi.org/10.3390/ma18071541
Chicago/Turabian StyleCabeza, Luisa F., Franklin R. Martínez, and Emiliano Borri. 2025. "Compatibility of Carbonate Mixtures to Be Used as Molten Salts with Different Metal Alloys to Be Used as Container Materials" Materials 18, no. 7: 1541. https://doi.org/10.3390/ma18071541
APA StyleCabeza, L. F., Martínez, F. R., & Borri, E. (2025). Compatibility of Carbonate Mixtures to Be Used as Molten Salts with Different Metal Alloys to Be Used as Container Materials. Materials, 18(7), 1541. https://doi.org/10.3390/ma18071541








