CsPbI3 Perovskite Nanorods: Enhancing Fluorescence Efficiency and Environmental Stability via Trioctylphosphine Ligand Coordination
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of CsOA Precursors
2.2.2. Synthesis of TOP-CsPbI3 NRs
2.3. Characterization
2.3.1. Characterization of Material Morphology
2.3.2. Structural and Surface Characterizations
2.3.3. Optical Characterization of Materials
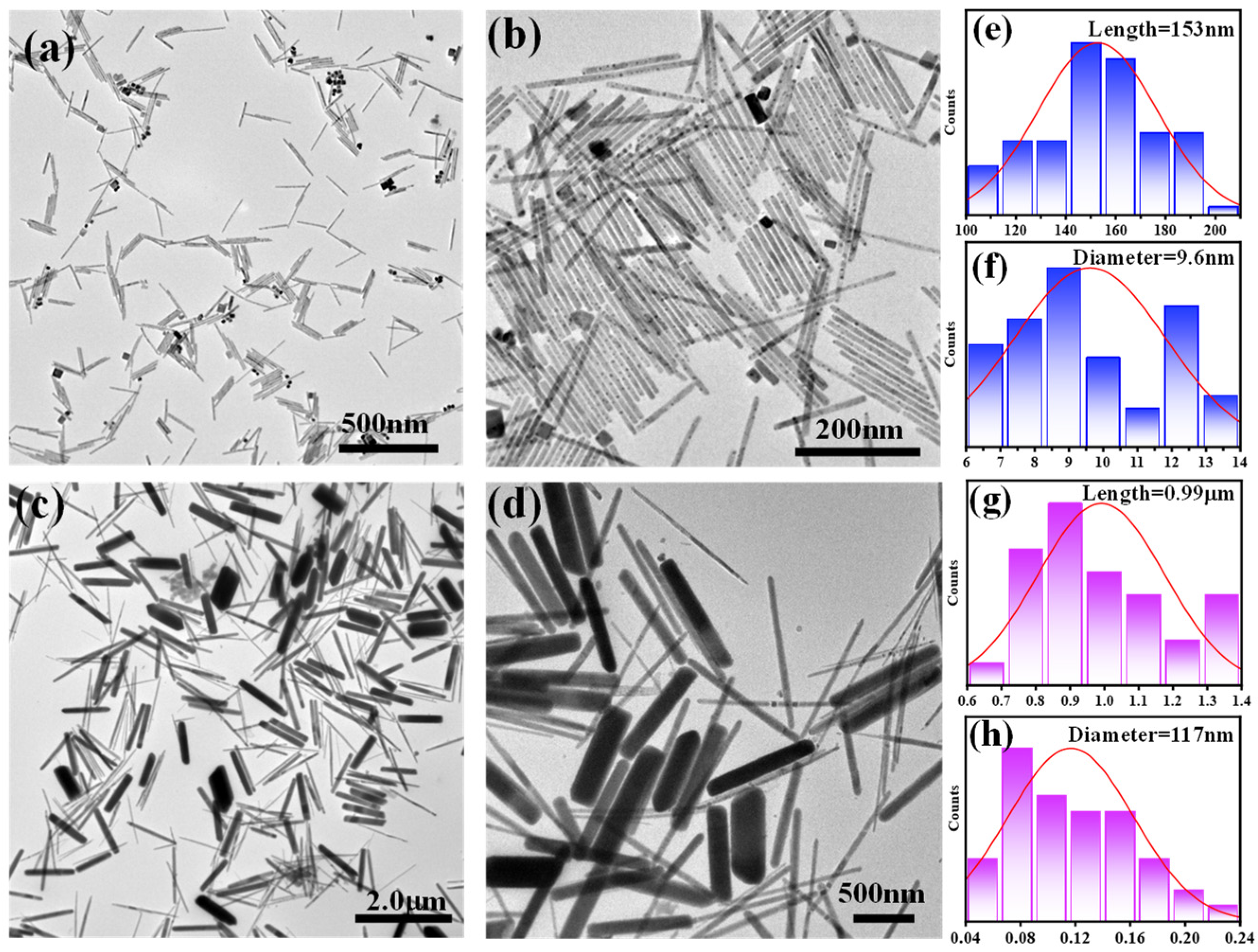
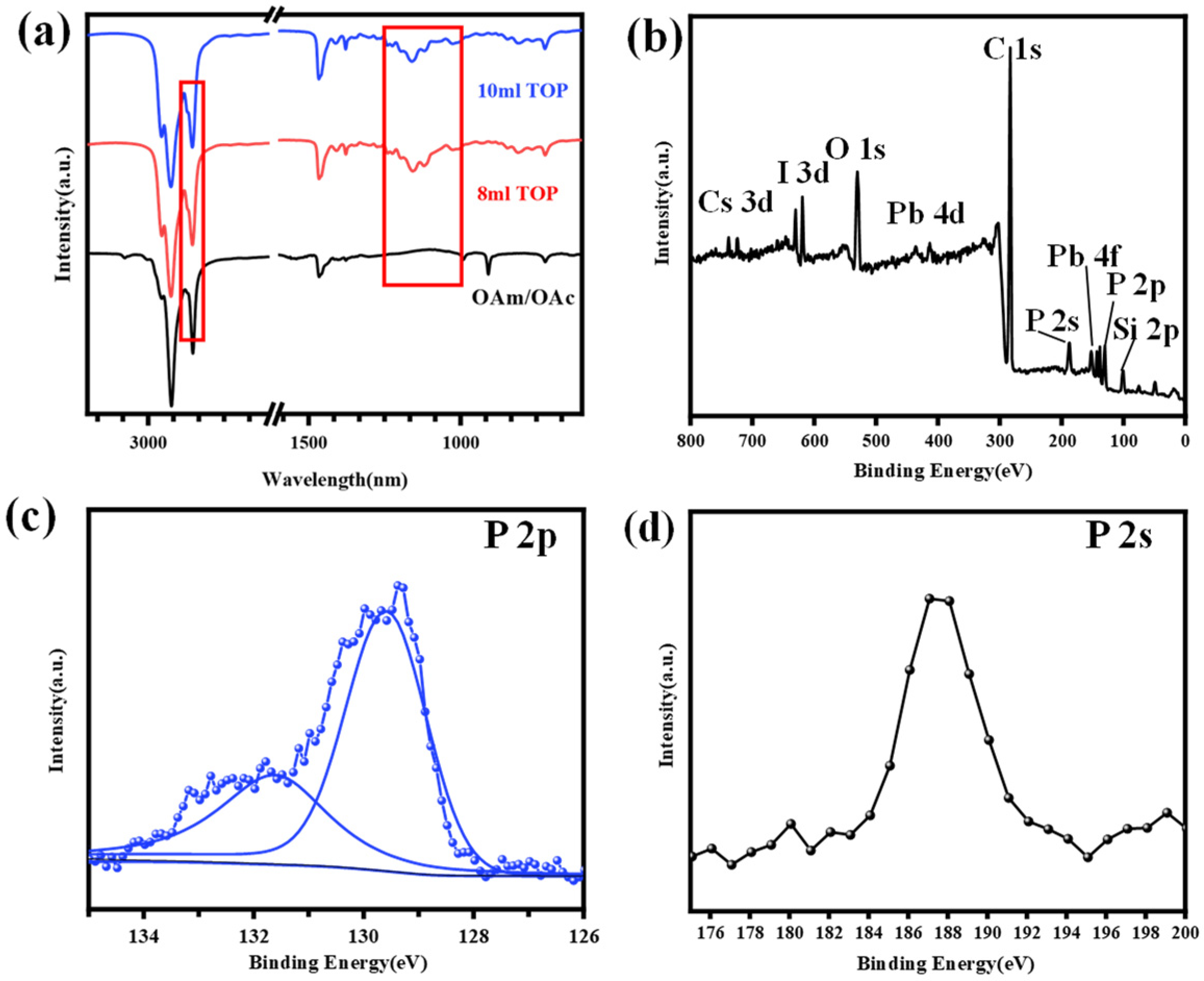
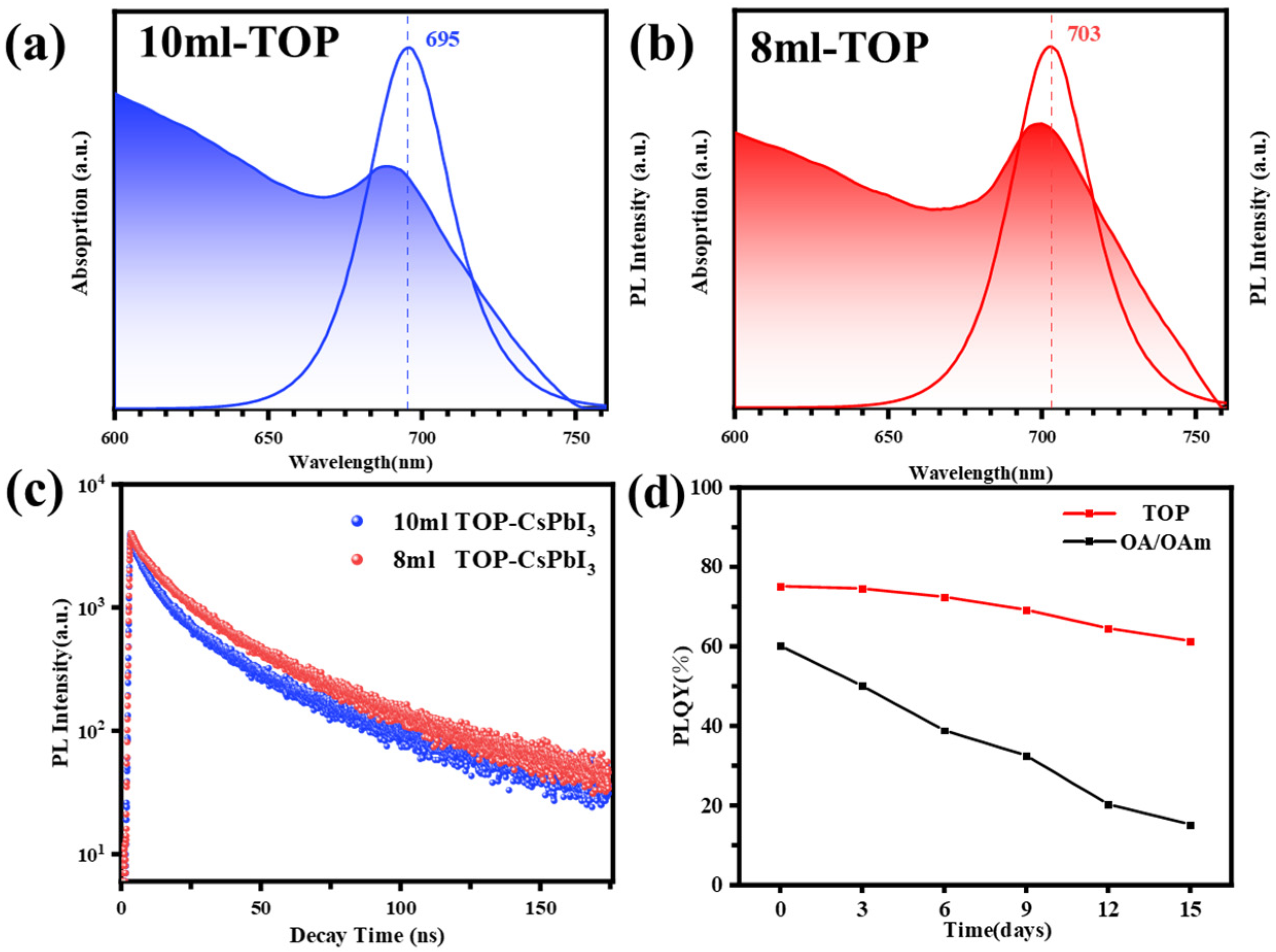
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Akkerman, Q.A.; D′Innocenzo, V.; Accornero, S.; Scarpellini, A.; Petrozza, A.; Prato, M.; Manna, L. Tuning the Optical Properties of Cesium Lead Halide Perovskite Nanocrystals by Anion Exchange Reactions. J. Am. Chem. Soc. 2015, 137, 10276–10281. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.; Oh, N.; Zhai, Y.; Shim, M. High Efficiency and Optical Anisotropy in Double-Heterojunction Nanorod Light-Emitting Diodes. ACS Nano 2015, 9, 878–885. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.M.; Song, J.Z.; Xiao, L.; Zeng, H.B.; Sun, H.D. All-Inorganic Colloidal Perovskite Quantum Dots: A New Class of Lasing Materials with Favorable Characteristics. Adv. Mater. 2015, 27, 7101–7108. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, N.; Tian, H.; Guo, J.; Wei, Y.; Chen, H.; Miao, Y.; Zou, W.; Pan, K.; He, Y.; et al. Perovskite light-emitting diodes based on spontaneously formed submicrometre-scale structures. Nature 2018, 562, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, C.; Liu, X.; Yao, J.; Zhao, Y.S. Materials chemistry and engineering in metal halide perovskite lasers. Chem. Soc. Rev. 2020, 49, 951–982. [Google Scholar] [CrossRef]
- Liang, S.; Zhang, M.; He, S.; Tian, M.; Choi, W.; Lian, T.; Lin, Z. Metal halide perovskite nanorods with tailored dimensions, compositions and stabilities. Nat. Synth. 2023, 2, 719–728. [Google Scholar] [CrossRef]
- Dong, Q.F.; Fang, Y.J.; Shao, Y.C.; Mulligan, P.; Qiu, J.; Cao, L.; Huang, J.S. Electron-hole diffusion lengths > 175 μm in solution-grown CH3NH3PbI3 single crystals. Science 2015, 347, 967–970. [Google Scholar] [CrossRef]
- Xing, J.; Liu, X.F.; Zhang, Q.; Ha, S.T.; Yuan, Y.W.; Shen, C.; Sum, T.C.; Xiong, Q.H. Vapor Phase Synthesis of Organometal Halide Perovskite Nanowires for Tunable Room-Temperature Nanolasers. Nano Lett. 2015, 15, 4571–4577. [Google Scholar]
- Yakunin, S.; Protesescu, L.; Krieg, F.; Bodnarchuk, M.I.; Nedelcu, G.; Humer, M.; De Luca, G.; Fiebig, M.; Heiss, W.; Kovalenko, M.V. Low-threshold amplified spontaneous emission and lasing from colloidal nanocrystals of caesium lead halide perovskites. Nat. Commun. 2015, 6, 8. [Google Scholar]
- Ning, Y.; Pu, Y.; Wu, C.; Chen, Z.; Zhang, X.; Zhang, L.; Wang, B. Design strategy of high-entropy perovskite energy-storage ceramics: A review. J. Eur. Ceram. Soc. 2024, 44, 4831–4843. [Google Scholar] [CrossRef]
- Ouyang, R.; Xu, J.; Zhong, X.; Gong, Y.; Liu, Y.; Fang, X.; Shen, J.; Wang, X. SrSnO3 perovskite vs. Nd2Sn2O7 pyrochlores for oxidative coupling of methane: Deciphering the reactive sites difference. Chem. Synth. 2024, 4, 72. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Wang, S.; Kong, L.; Lewis, S.W.; Yang, X.; Rogach, A.L.; Jia, G. Metal Halide Perovskite Nanorods: Shape Matters. Adv. Mater. 2020, 32, 2002736. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, B.B.; Zhou, Q.; Ma, D.Q.; Han, X.; He, D.M.; Chen, S.; Li, Y.L.; Lu, S.R.; Xu, Z.X.; et al. Critical role of 1D materials in realizing efficient and stable perovskite solar cells. J. Mater. Chem. A 2023, 11, 18592–18604. [Google Scholar]
- Iqbal, M.M.W.; Xie, Q.H.; Cai, M.Q.; Zou, X.M.; Zhang, Q.L.; Zeng, R.S.; Zou, B.S.; Liao, L.; Wan, Q. Surface steered aligned gradient inorganic lead halide perovskite CsPbBrxI3_x nanowires for use in photodetectors. Appl. Surf. Sci. 2023, 617, 7. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, N.W.; Saeed, M.A.; Jeong, S.Y.; Woo, H.Y.; Park, J.; Shim, J.W. Record indoor performance of organic photovoltaics with long-term stability enabled by self-assembled monolayer-based interface management. Nano Energy 2023, 112, 108429. [Google Scholar]
- Shen, W.; Dai, Y.J.; Cai, B.; Chen, S.; Yang, H.; Ma, Y.Z.; Chen, Y.F.; Su, Z.; Zhang, J.B.; Qiu, Y.; et al. Ligand-Assisted Breaking Crystal Symmetry to Achieve Stable γ-CsPbI3 Nanorods with Strong Polarization Response. ACS Energy Lett. 2023, 8, 2561–2569. [Google Scholar] [CrossRef]
- Sun, C.J.; Jiang, Y.Z.; Zhang, L.; Wei, K.Y.; Yuan, M.J. Toward the Controlled Synthesis of Lead Halide Perovskite Nanocrystals. ACS Nano 2023, 17, 17600–17609. [Google Scholar] [CrossRef]
- Wang, F.; Duan, D.W.; Zhou, K.; Xue, Y.Z.B.; Liang, X.; Zhou, X.F.; Ge, C.Y.; Zhou, C.; Xiang, J.; Zhu, J.J.; et al. Ionic liquid engineering enabled in-plane orientated 1D perovskite nanorods for efficient mixed-dimensional perovskite photovoltaics. InfoMat 2023, 5, e12459. [Google Scholar] [CrossRef]
- Ye, Z.; Kong, L.; Zhao, Y.; Zhang, C.; Yang, X.; Yan, K.; Zhang, Y.; Zhang, H.; Tang, Y. Alkalinity-controlled zeolite nucleation and growth: Ultrafast synthesis of total-morphology zeolite L mesocrystals and adsorption evaluation. Chem. Synth. 2022, 2, 20. [Google Scholar]
- Chen, K.Q.; Jin, W.; Zhang, Y.P.; Yang, T.Q.; Reiss, P.; Zhong, Q.H.; Bach, U.; Li, Q.T.; Wang, Y.W.; Zhang, H.; et al. High Efficiency Mesoscopic Solar Cells Using CsPbI3 Perovskite Quantum Dots Enabled by Chemical Interface Engineering. J. Am. Chem. Soc. 2020, 142, 3775–3783. [Google Scholar]
- Dong, Y.T.; Qiao, T.; Kim, D.; Parobek, D.; Rossi, D.; Son, D.H. Precise Control of Quantum Confinement in Cesium Lead Halide Perovskite Quantum Dots via Thermodynamic Equilibrium. Nano Lett. 2018, 18, 3716–3722. [Google Scholar] [CrossRef]
- Seth, S.; Ahmed, T.; De, A.; Samanta, A. Tackling the Defects, Stability, and Photoluminescence of CsPbX3 Perovskite Nanocrystals. ACS Energy Lett. 2019, 4, 1610–1618. [Google Scholar] [CrossRef]
- De Roo, J.; Ibanez, M.; Geiregat, P.; Nedelcu, G.; Walravens, W.; Maes, J.; Martins, J.; Van Driessche, I.; Kovalenko, M.; Hens, Z. Highly dynamic ligand binding and light absorption coefficient of cesium lead bromide perovskite nanocrystals. ACS Nano 2016, 10, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Ouedraogo, N.A.N.; Chen, Y.; Xiao, Y.Y.; Meng, Q.; Han, C.B.; Yan, H.; Zhang, Y. Stability of all-inorganic perovskite solar cells. Nano Energy 2020, 67, 104249. [Google Scholar] [CrossRef]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot-induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Butkus, J.; Vashishtha, P.; Chen, K.; Gallaher, J.K.; Prasad, S.K.K.; Metin, D.Z.; Laufersky, G.; Gaston, N.; Halpert, J.E.; Hodgkiss, J.M. The Evolution of Quantum Confinement in CsPbBr3 Perovskite Nanocrystals. Chem. Mat. 2017, 29, 3644–3652. [Google Scholar] [CrossRef]
- Raja, S.N.; Bekenstein, Y.; Koc, M.A.; Fischer, S.; Zhang, D.; Lin, L.; Ritchie, R.O.; Yang, P.; Alivisatos, A.P. Encapsulation of Perovskite Nanocrystals into Macroscale Polymer Matrices: Enhanced Stability and Polarization. ACS Appl. Mater. Interfaces 2016, 8, 35523–35533. [Google Scholar] [CrossRef]
- Konidakis, I.; Karagiannaki, A.; Stratakis, E. Advanced composite glasses with metallic, perovskite, and two-dimensional nanocrystals for optoelectronic and photonic applications. Nanoscale 2022, 14, 2966–2989. [Google Scholar] [CrossRef]
- Zhao, C.; Yan, J.; Ma, Z.; Zhang, Y.; Hu, T.; Zhang, H. Recent advances in conjugated ladder-type porous polymer networks for rechargeable batteries. Chem. Synth. 2025, 5, 2. [Google Scholar] [CrossRef]
- Almeida, G.; Goldoni, L.; Akkerman, Q.; Dang, Z.; Khan, A.H.; Marras, S.; Moreels, I.; Manna, L. Role of Acid–Base Equilibria in the Size, Shape, and Phase Control of Cesium Lead Bromide Nanocrystals. ACS Nano 2018, 12, 1704–1711. [Google Scholar] [CrossRef]
- Dang, Z.; Dhanabalan, B.; Castelli, A.; Dhall, R.; Bustillo, K.C.; Marchelli, D.; Spirito, D.; Petralanda, U.; Shamsi, J.; Manna, L.; et al. Temperature-Driven Transformation of CsPbBr3 Nanoplatelets into Mosaic Nanotiles in Solution through Self-Assembly. Nano Lett. 2020, 20, 1808–1818. [Google Scholar] [PubMed]
- Nagaoka, Y.; Hills-Kimball, K.; Tan, R.; Li, R.; Wang, Z.; Chen, O. Nanocube Superlattices of Cesium Lead Bromide Perovskites and Pressure- Induced Phase Transformations at Atomic and Mesoscale Levels. Adv. Mater. 2017, 29, 1606666. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; et al. Highly Luminescent Phase-Stable CsPbI3 Perovskite Quantum Dots Achieving Near 100% Absolute Photoluminescence Quantum Yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef]
- Ye, J.; Ren, A.; Dai, L.; Baikie, T.K.; Guo, R.; Pal, D.; Gorgon, S.; Heger, J.E.; Huang, J.; Sun, Y.; et al. Direct linearly polarized electroluminescence from perovskite nanoplatelet superlattices. Nat. Photonics 2024, 18, 586–594. [Google Scholar] [CrossRef]
- Fanizza, E.; Cascella, F.; Altamura, D.; Giannini, C.; Panniello, A.; Triggiani, L.; Panzarea, F.; Depalo, N.; Grisorio, R.; Suranna, G.P.; et al. Post-synthesis phase and shape evolution of CsPbBr3 colloidal nanocrystals: The role of ligands. Nano Res. 2019, 12, 1155–1166. [Google Scholar] [CrossRef]
- Gao, M.; Liu, H.; Yu, S.; Louisia, S.; Zhang, Y.; Nenon, D.P.; Alivisatos, A.P.; Yang, P. Scaling Laws of Exciton Recombination Kinetics in Low Dimensional Halide Perovskite Nanostructures. J. Am. Chem. Soc. 2020, 142, 8871–8879. [Google Scholar] [PubMed]
- Liang, S.; Zhang, M.; Biesold, G.M.; Choi, W.; He, Y.; Li, Z.; Shen, D.; Lin, Z. Recent Advances in Synthesis, Properties, and Applications of Metal Halide Perovskite Nanocrystals/Polymer Nanocomposites. Adv. Mater. 2021, 33, 2005888. [Google Scholar] [CrossRef]
- Li, J.; Jin, C.; Jiang, R.; Su, J.; Tian, T.; Yin, C.; Meng, J.; Kou, Z.; Bai, S.; Müller-Buschbaum, P.; et al. Homogeneous coverage of the low-dimensional perovskite passivation layer for formamidinium–caesium perovskite solar modules. Nat. Energy 2024, 9, 1540–1550. [Google Scholar] [CrossRef]
- Wen, Z.; Zhai, W.; Liu, C.; Lin, J.; Yu, C.; Huang, Y.; Zhang, J.; Tang, C. Controllable synthesis of CsPbI3 nanorods with tunable photoluminescence emission. RSC Adv. 2019, 9, 24928–24934. [Google Scholar] [CrossRef]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 Quantum Dots for Lighting and Displays: Room Temperature Synthesis, Photoluminescence Superiorities, Underlying Origins and White Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Abdalla, Z.; Wang, X.; Liu, M.; Jiao, Y.; Tang, Z.; Zhang, Q.; Liu, Y. CsPbI3 Perovskite Nanorods: Enhancing Fluorescence Efficiency and Environmental Stability via Trioctylphosphine Ligand Coordination. Materials 2025, 18, 1518. https://doi.org/10.3390/ma18071518
Liu C, Abdalla Z, Wang X, Liu M, Jiao Y, Tang Z, Zhang Q, Liu Y. CsPbI3 Perovskite Nanorods: Enhancing Fluorescence Efficiency and Environmental Stability via Trioctylphosphine Ligand Coordination. Materials. 2025; 18(7):1518. https://doi.org/10.3390/ma18071518
Chicago/Turabian StyleLiu, Chengqi, Zahir Abdalla, Xiaoqian Wang, Manrui Liu, Yanhui Jiao, Zisheng Tang, Qi Zhang, and Yong Liu. 2025. "CsPbI3 Perovskite Nanorods: Enhancing Fluorescence Efficiency and Environmental Stability via Trioctylphosphine Ligand Coordination" Materials 18, no. 7: 1518. https://doi.org/10.3390/ma18071518
APA StyleLiu, C., Abdalla, Z., Wang, X., Liu, M., Jiao, Y., Tang, Z., Zhang, Q., & Liu, Y. (2025). CsPbI3 Perovskite Nanorods: Enhancing Fluorescence Efficiency and Environmental Stability via Trioctylphosphine Ligand Coordination. Materials, 18(7), 1518. https://doi.org/10.3390/ma18071518





