Concentration Dependence of Optical Properties of Double-Doped LiTaO3:Cr3+:Nd3+ Crystals
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. X-Ray Phase Analysis
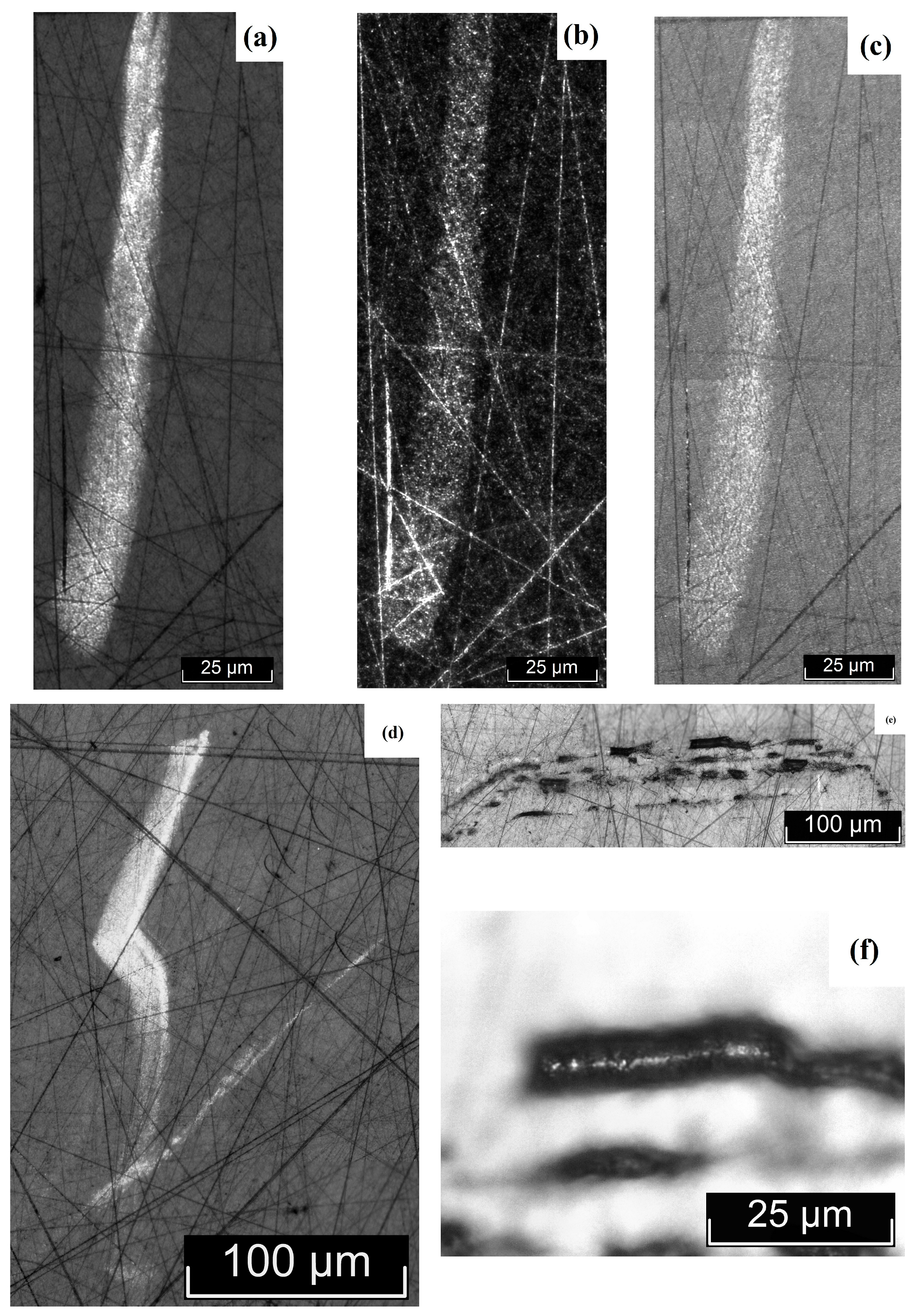
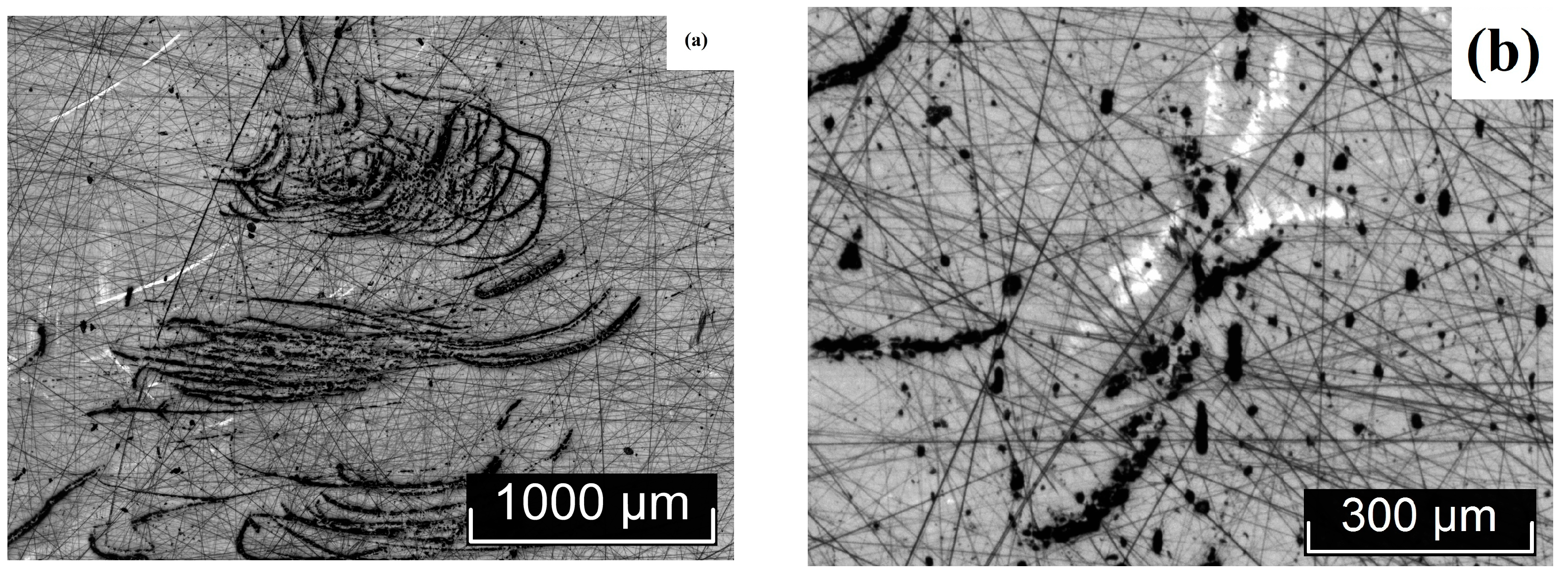
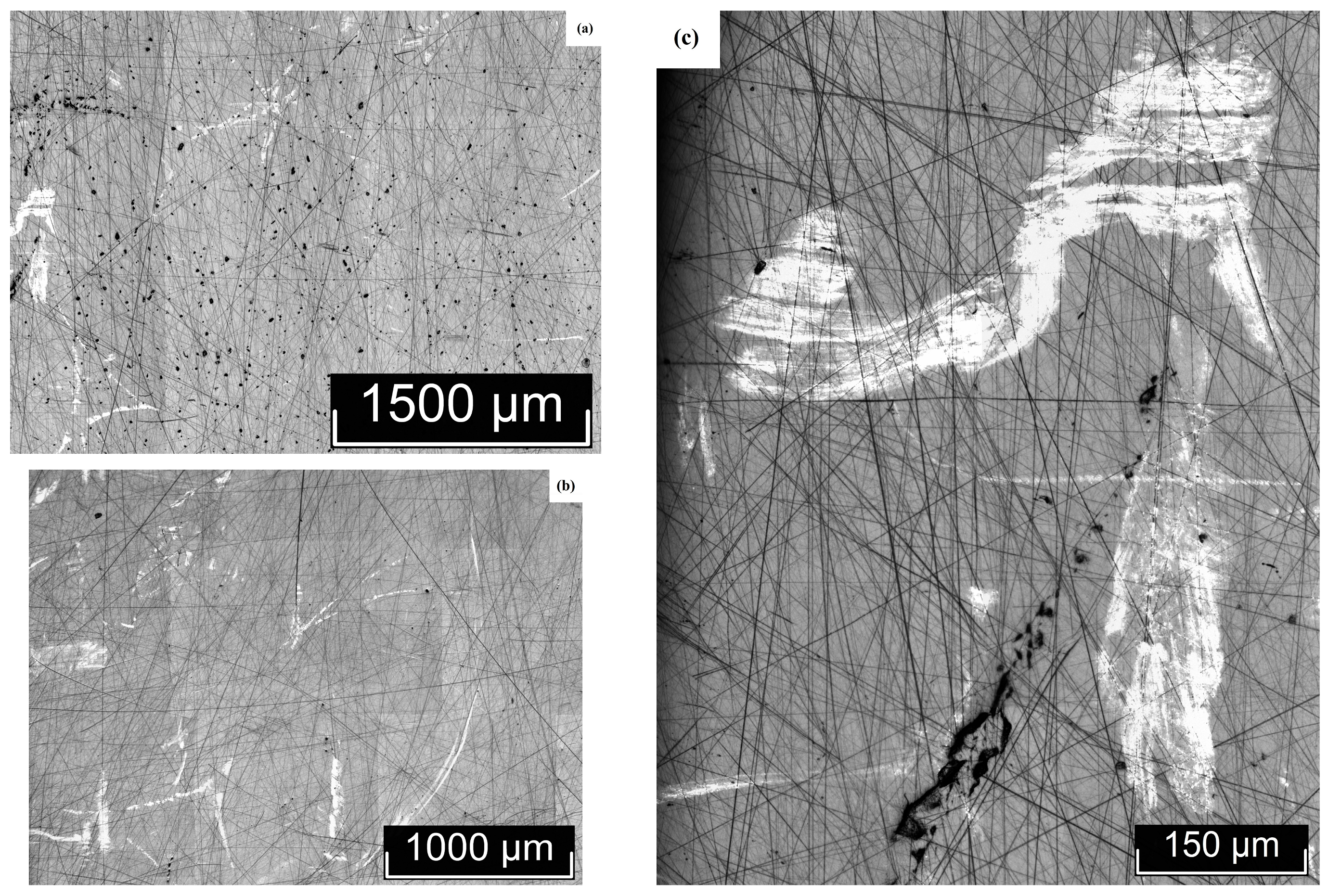
3.2. Macro- and Microdefect Structures of LiTaO3:Cr3+:Nd3+ Crystals
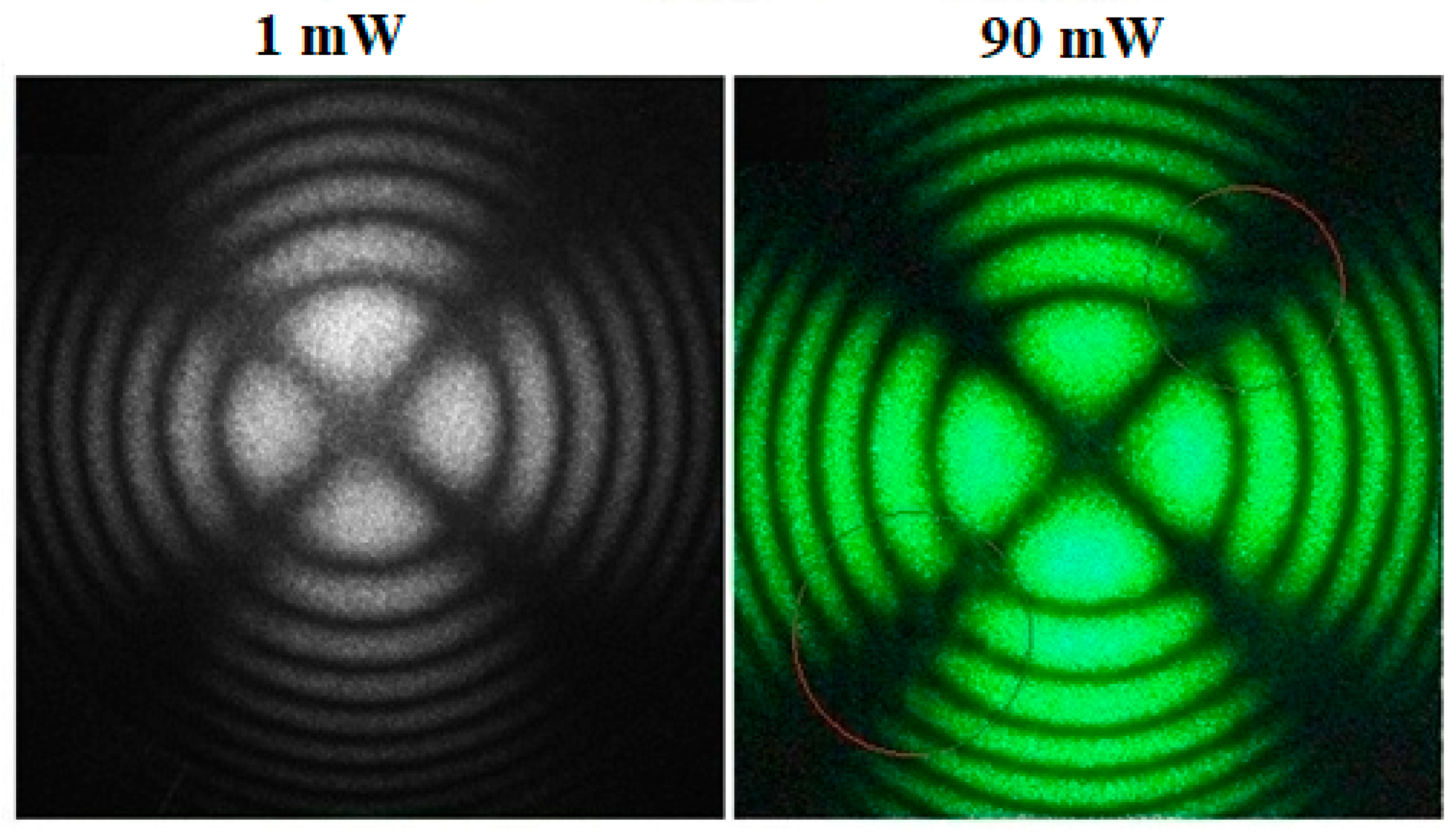
3.3. The Conoscopic Patterns of Studied Crystals
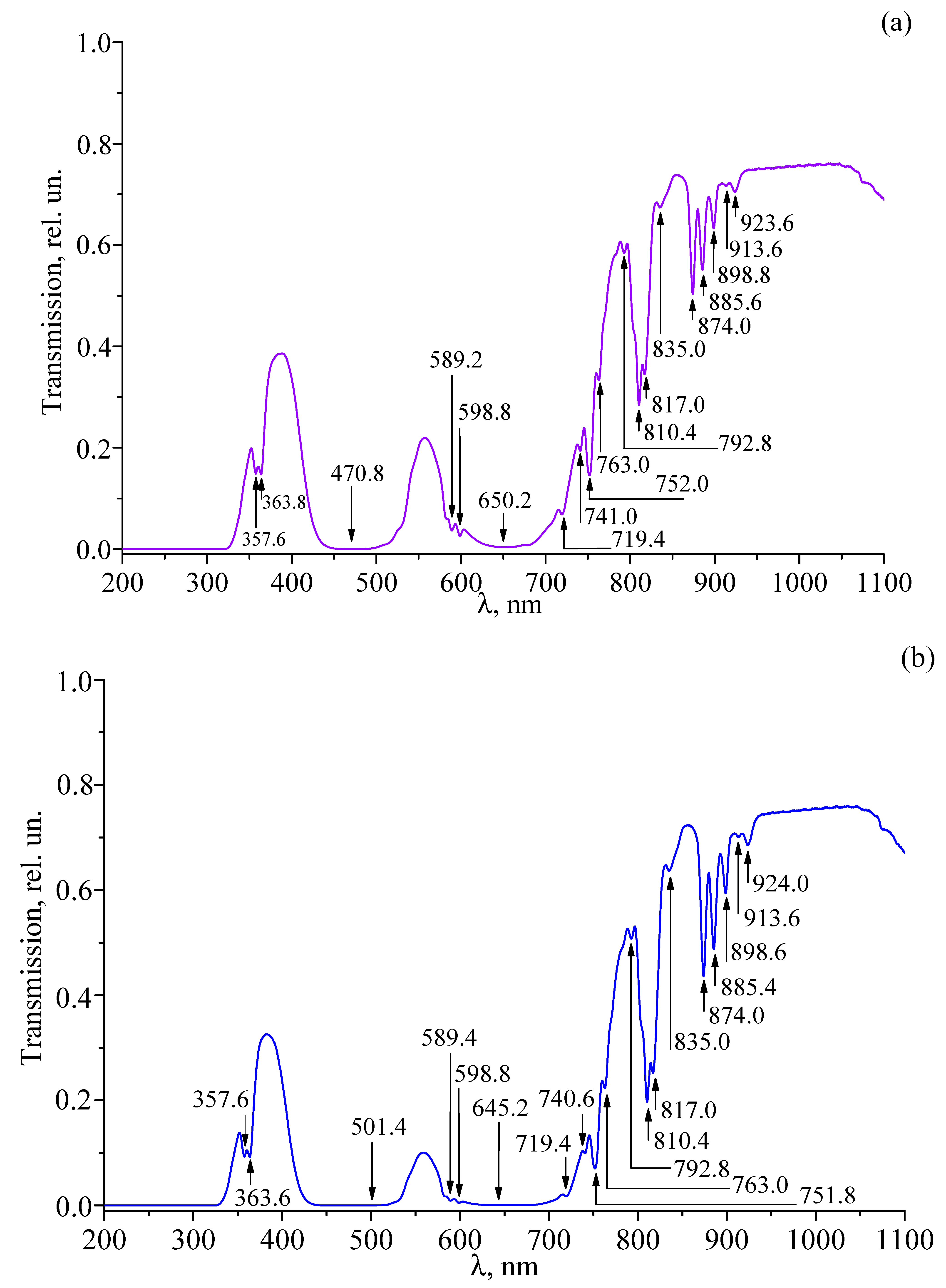
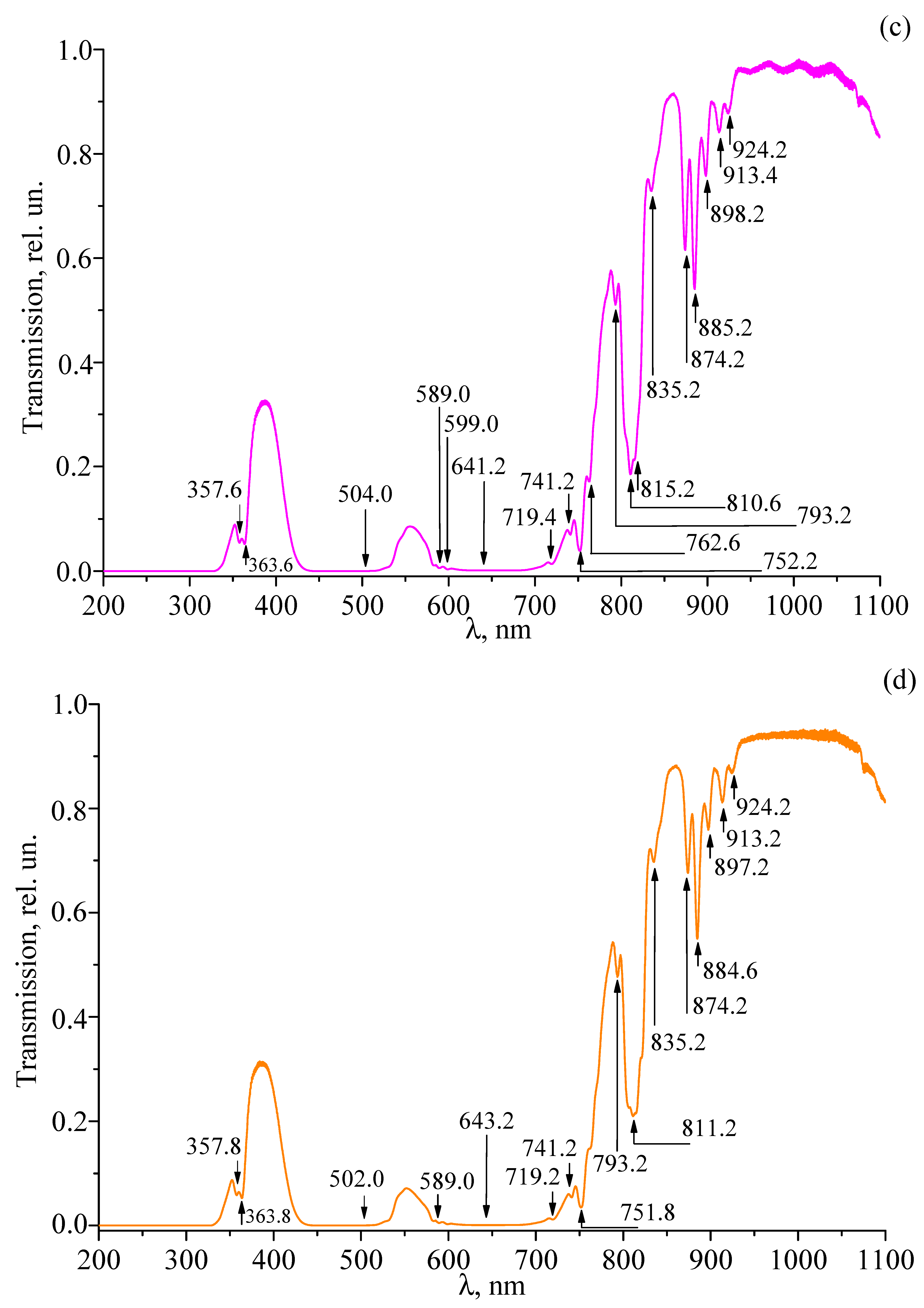
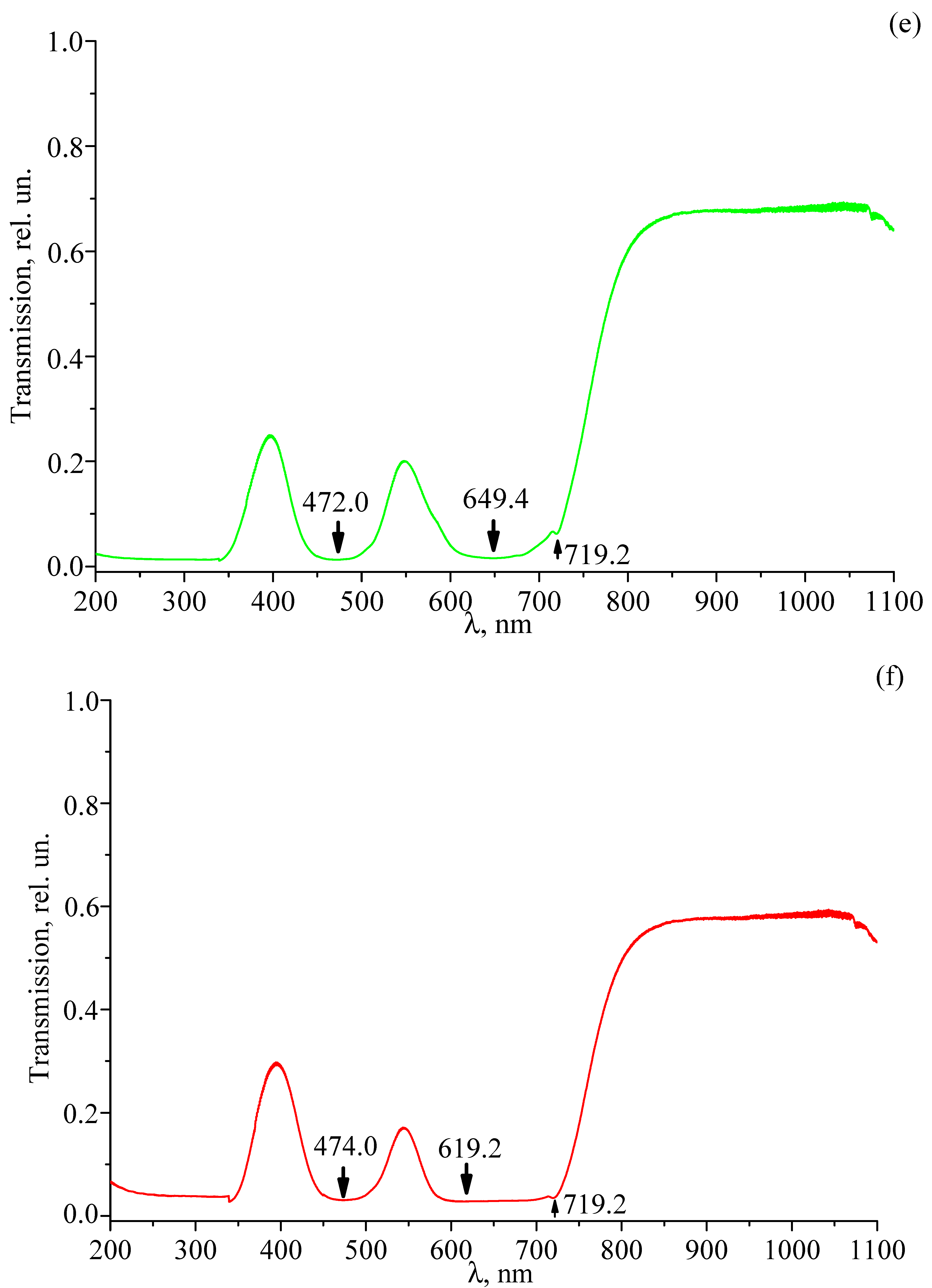
3.4. Transmission Spectra of Studied Crystals
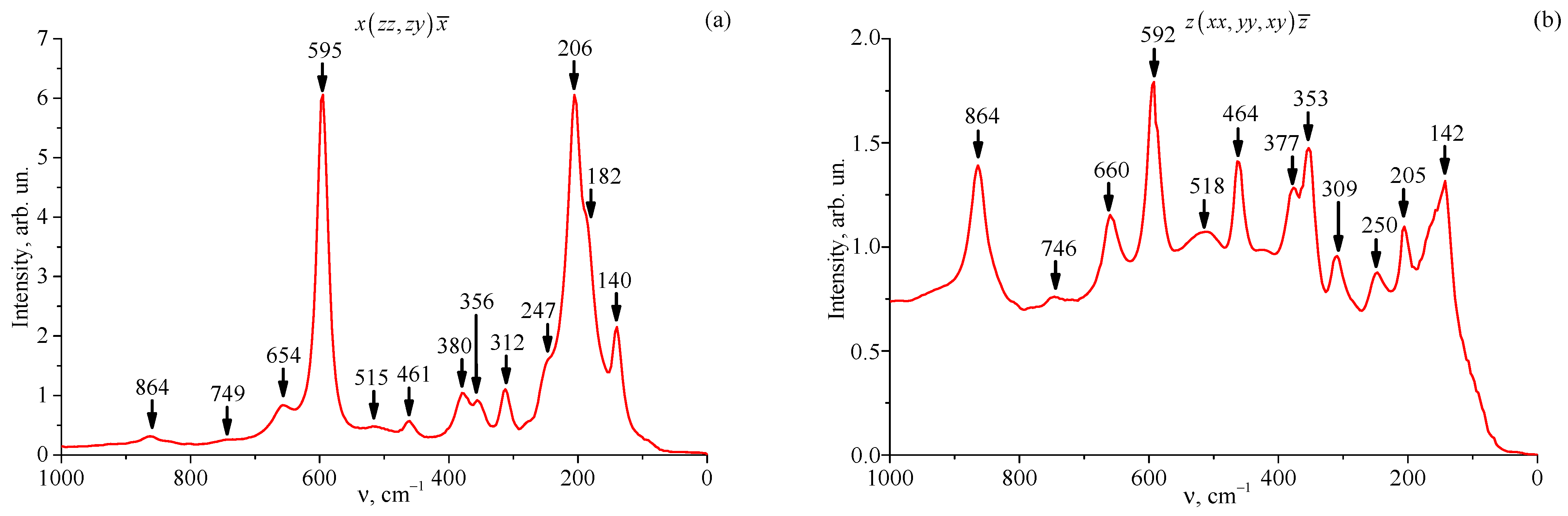
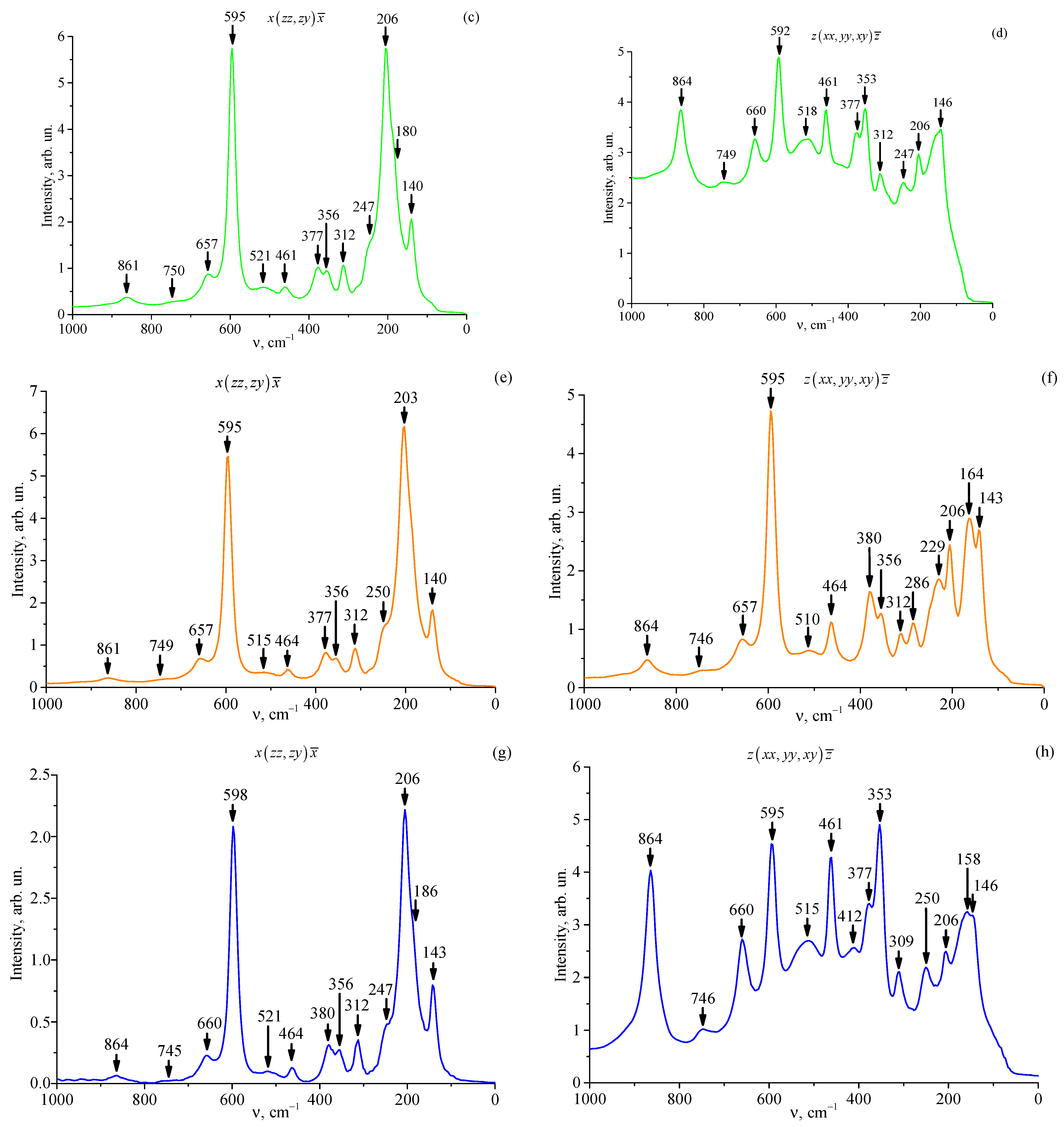
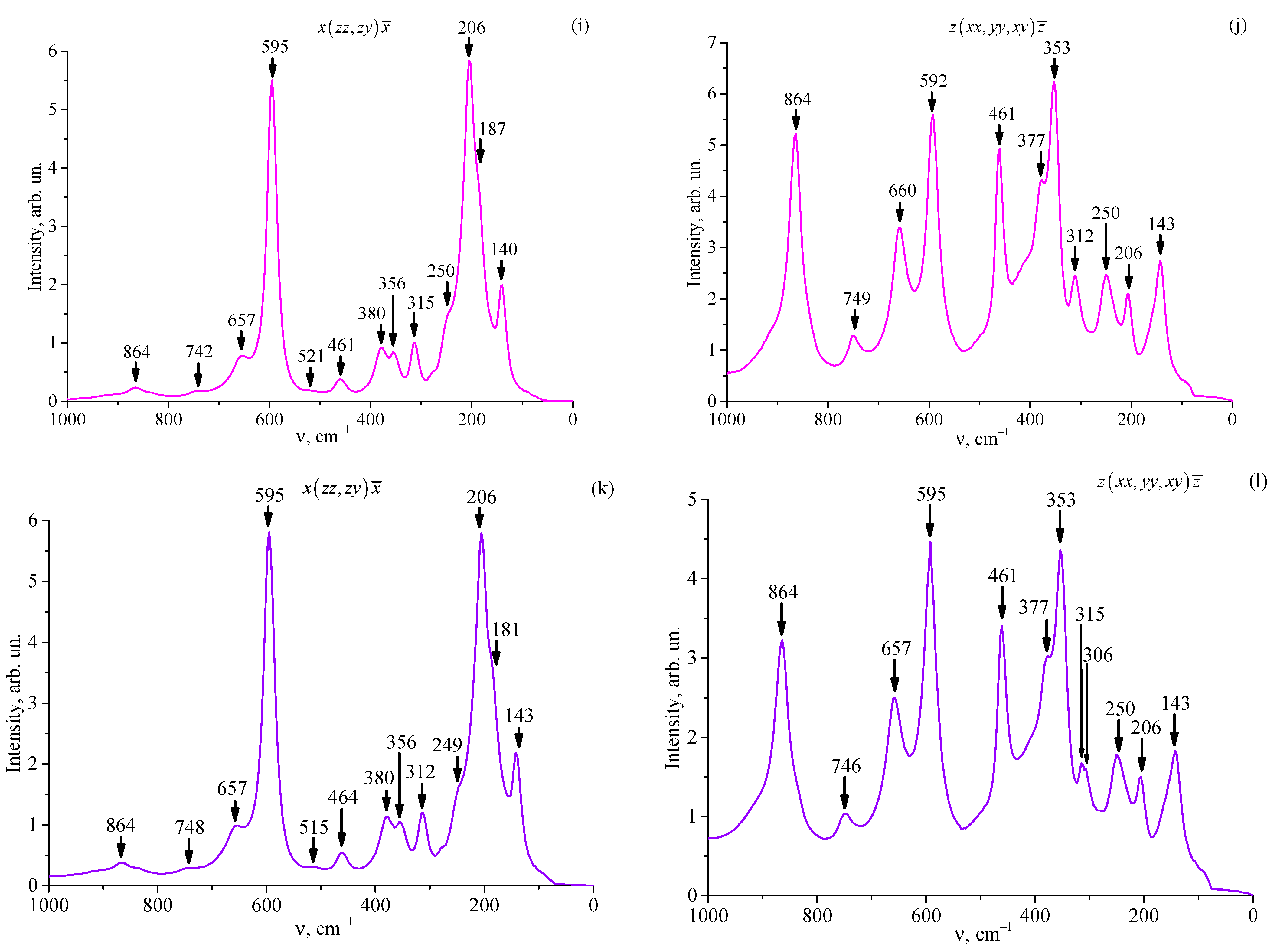
3.5. Raman Spectra of Studied Crystals
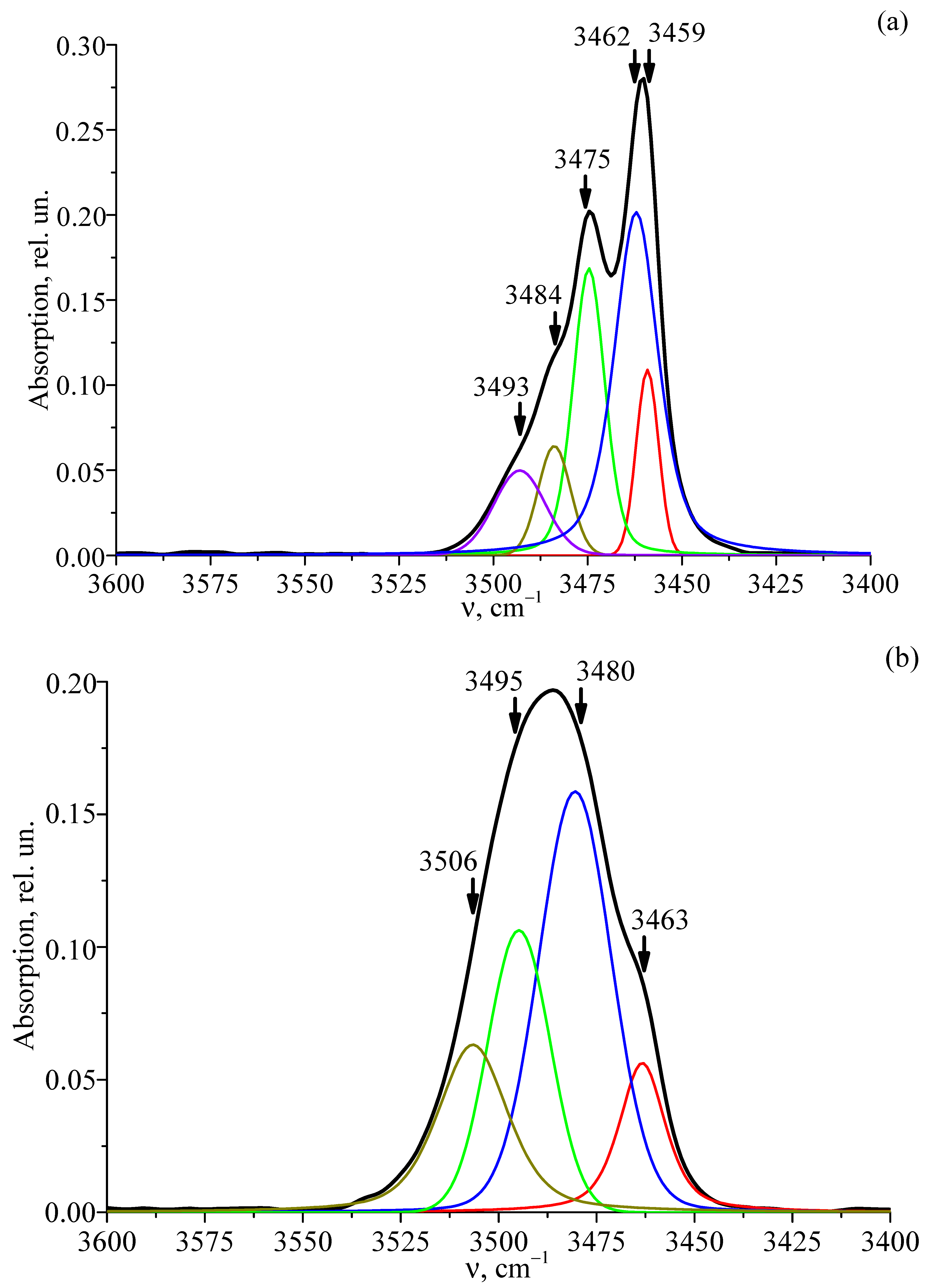
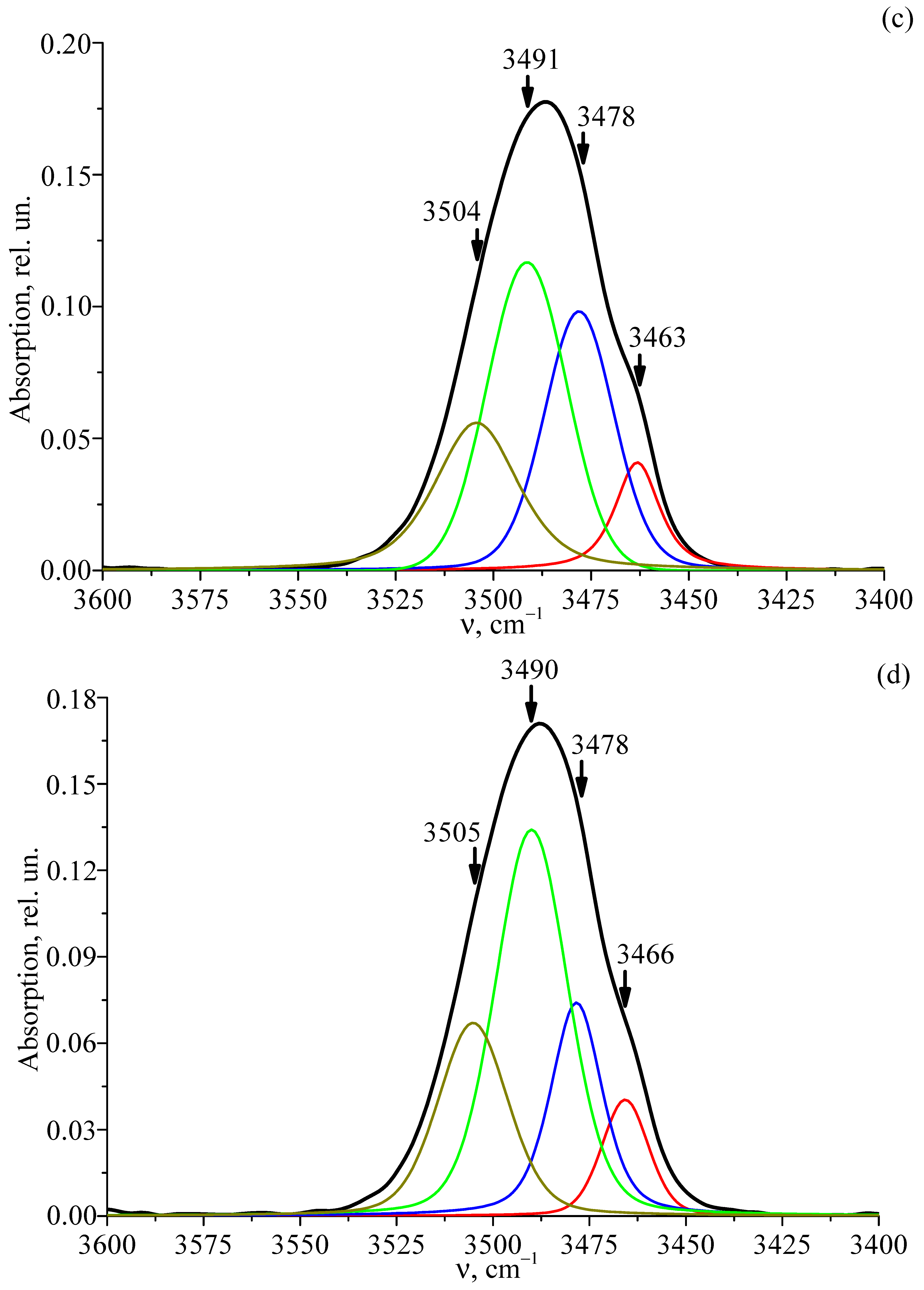
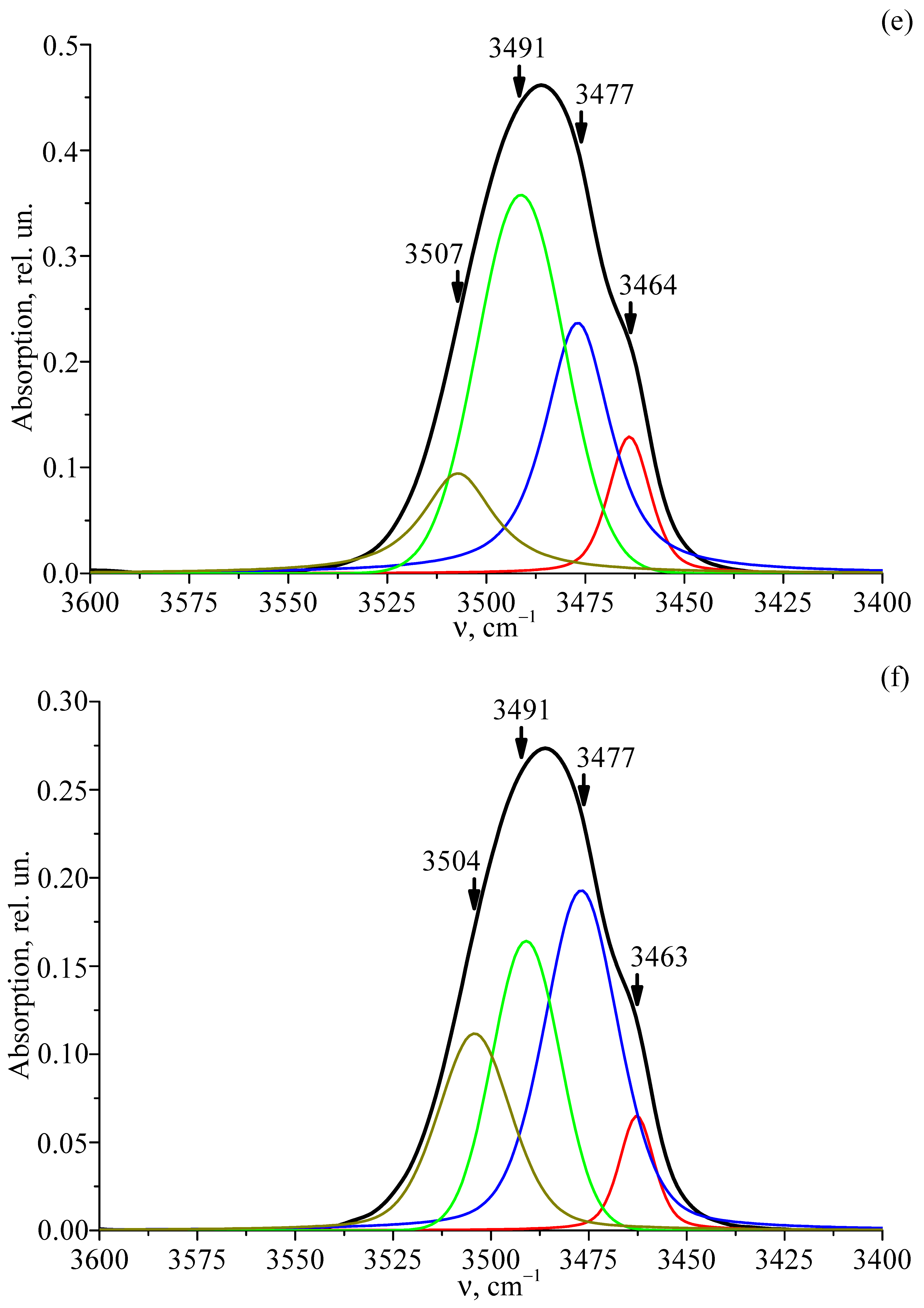
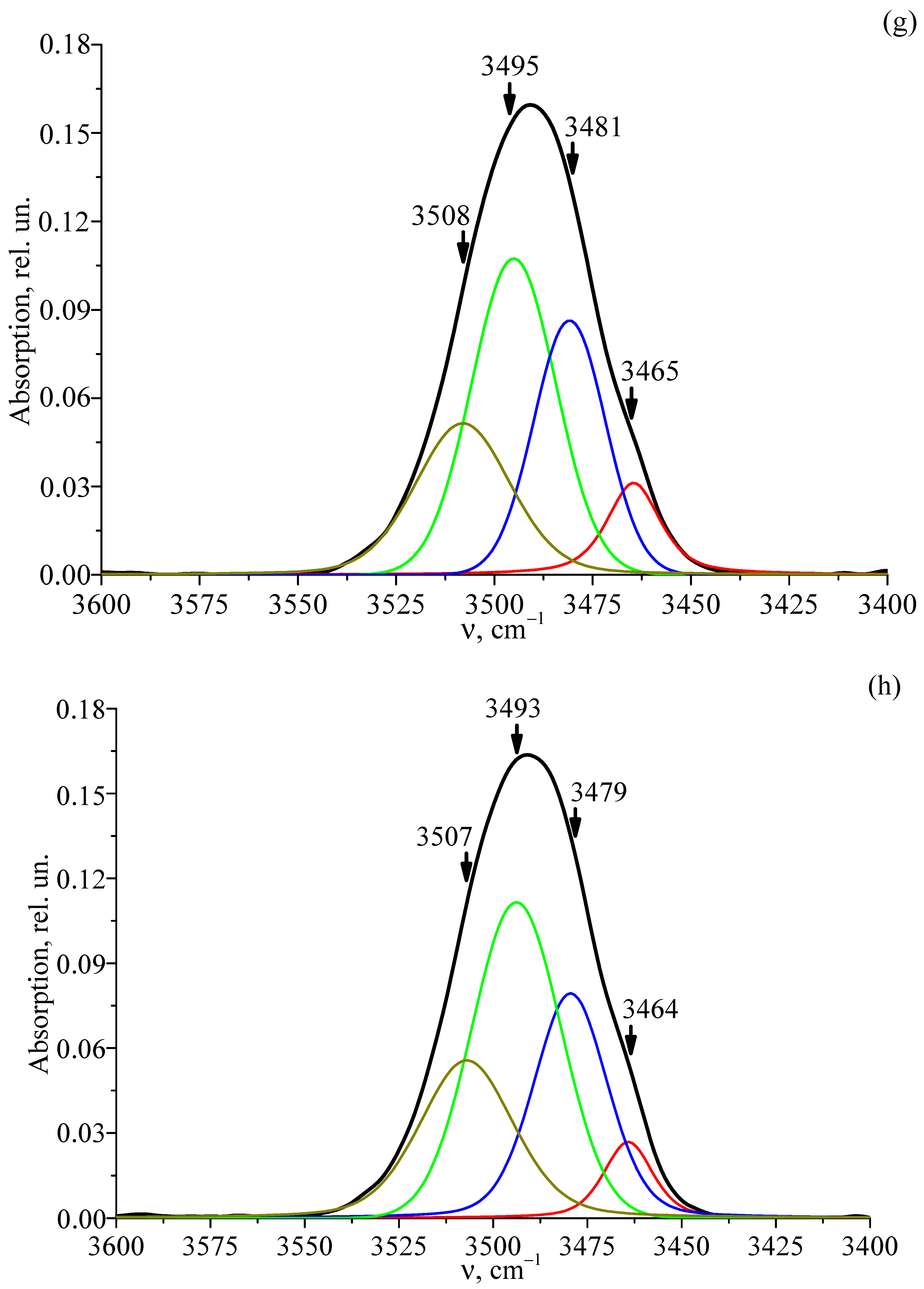
3.6. FTIR Absorption Spectra of Studied Crystals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sidorov, N.V.; Palatnikov, M.N.; Teplyakova, N.A.; Biryukova, I.V.; Titov, R.A.; Makarova, O.V.; Masloboeva, S.M. Single Crystals of Lithium Niobate and Tantalate of Different Composition and Genesis; RAS: Moscow, Russia, 2022; 288p. (In Russian) [Google Scholar]
- Katz, M.; Blau, P.; Shulga, B. Room temperature high power frequency conversion in periodically poled quasi-phase-matched crystals. Proc. SPIE 2008, 6875, 87504. [Google Scholar] [CrossRef]
- Hboub, H.; Fadlaoui, E.; Masaif, N. Effect of Nd doping on Curie temperature of nonstoichiometric lithium tantalate. Bull. Mater. Sci. 2024, 47, 90. [Google Scholar] [CrossRef]
- Abedin, K.S.; Tsuritani, T.; Sato, M.; Ito, H. Integrated intracavity quasi-phase-matched second harmonic generation based on periodically poled Nd:LiTaO3. Appl. Phys. Lett. 1997, 70, 10–12. [Google Scholar] [CrossRef]
- He, C.; Li, W.; Wang, J.; Gu, X.; Wu, T.; Liu, Y. Growth and optical properties of lithium tantalate single crystals doped with indium and neodymium. Acta Opt. Sin. 2018, 38, 0116003. [Google Scholar] [CrossRef]
- Zhang, P.X.; Hang, Y.; Gong, J.; Zhao, C.C.; Yin, J.G.; Zhang, L.H.; Zhu, Y.Y. Optical spectroscopy of Nd3+/Mg2+ co-doped LiTaO3 laser crystal. Laser Phys. 2013, 23, 015803. [Google Scholar] [CrossRef]
- Wu, C.C.; Hsu, W.T.; Chen, Z.B.; Choubey, R.K.; Lan, C.W. Crystal growth, VTE treatment, and characterizations of Nd-doped LiTaO3. J. Cryst. Growth 2011, 318, 649–652. [Google Scholar] [CrossRef]
- Sokólska, I.; Gołab, S.; Ryba-Romanowski, W. Optical spectroscopy of doped LiTaO3 crystals. Spectrochim. Acta A 1998, 54, 1685–1694. [Google Scholar] [CrossRef]
- Sokólska, I.; Kück, S. Optical characterization of Cr3+ doped LiTaO3 crystals relevant for laser application. Spectrochim. Acta A 1998, 54, 1695–1700. [Google Scholar] [CrossRef]
- Grinberg, M.; Sokólska, I.; Kück, S.; Jaskólski, W. Temperature dependence of the luminescence decay of Cr3+ ions in LiTaO3: Confined potential model. Phys. Rev. B 1999, 60, 959–965. [Google Scholar] [CrossRef]
- Sun, D.H.; Liu, S.D.; Wang, D.Z.; Sang, Y.H.; Kang, X.L.; Liu, H.; Bi, Y.; Yan, B.X.; He, J.L.; Wang, J.Y. High output power of differently cut Nd:MgO:LiTaO3 CW lasers. Laser Phys. 2013, 23, 045802. [Google Scholar] [CrossRef]
- Sun, D.; Leng, Y.; Sang, Y.; Kang, X.; Liu, S.; Qin, X.; Cui, K.; Hairul Anuar, B.K.W.; Liu, H.; Bi, Y. Nd:MgO:LiTaO3 crystal for self-doubling laser applications: Growth, structure, thermal and laser properties. CrystEngComm 2013, 15, 7468–7474. [Google Scholar] [CrossRef]
- Hsu, W.T.; Chen, Z.B.; Wu, C.C.; Choubey, R.K.; Lan, C.W. Optical properties of Mg, Fe, co-doped near-stoichiometric LiTaO3 single crystals. Materials 2012, 5, 227–238. [Google Scholar] [CrossRef]
- Palatnikov, M.N.; Sidorov, N.V.; Palatnikova, O.V.; Biryukova, I.V. Defect Structure of Single and Double Doped Lithium Niobate Crystals; RAS: Moscow, Russia, 2024; 332p. (In Russian) [Google Scholar]
- Yuan, L.; Wang, J.; Huang, Z.; Shen, K. The codoping of Bi3+ to enhance the long afterglow performance of LiTaO3:Er3+. Appl. Phys. A 2025, 131, 500. [Google Scholar] [CrossRef]
- Sun, B.; Li, T.; Shen, C.; Zhang, M.; Guang, Z. Tunable Photoluminescence of LiTaO3: Tb3+, Sm3+ for dynamic multimode optical anti-counterfeiting. Chem. Eur. J. 2025, 31, e202500249. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Guo, N.; Xiao, R.; Ma, Q.; Liu, R.; Yang, M.; Shao, B.; Ouyang, R. Tuning the thermal quenching properties of Ga3+-modified LiTaO3:Bi3+ phosphor through defect engineering strategy. J. Lumin. 2023, 255, 119609. [Google Scholar] [CrossRef]
- Vergara, I.; Camarillo, E.; Sanz-García, J.A.; García Solé, J.; Jaque, F.; Monteil, A.; Boulon, G. Radiative energy transfer from Cr3+ to Nd3+ in LiNbO3:Nd,Cr. Solid State Commun. 1992, 82, 733–737. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Wen, J.K.; Wang, H.F. Sensitization of Nd3+ luminescence by Cr3+ in Nd:LiNbO3 crystals. Opt. Commun. 1993, 98, 261–264. [Google Scholar] [CrossRef]
- Li, J.; Zhang, K.X.; Li, B.; Wang, H.F. Influence of Cr3+ concentration on Cr3+ sensitizing Nd3+ in Cr,Nd:MgO:LiNbO3. J. Lumin. 1995, 65, 99–104. [Google Scholar] [CrossRef]
- Mahpoud, S.; Chamiel, N.; Weiss, A.M.; Rosenbluh, M.; Herman, A.; Shoham, A.; Lipavsky, B.; Rotman, S.R. Luminescence from chromium–neodymium-doped lithium niobate. Opt. Mater. 1999, 13, 55–61. [Google Scholar] [CrossRef]
- Marciniak, L.; Bednarkiewicz, A.; Kowalska, D.; Strek, W. A new generation of highly sensitive luminescent thermometers operating in the optical window of biological tissues. J. Mater. Chem. C 2016, 4, 5559–5563. [Google Scholar] [CrossRef]
- Pontuschka, W.M.; Kanashiro, L.S.; Courrol, L.C. Luminescence mechanisms for borate glasses: The role of local structural units. Glass Phys. Chem. 2001, 27, 37–47. [Google Scholar] [CrossRef]
- Avanesov, A.G.; Voronko, Y.K.; Denker, B.I.; Maksimova, G.V.; Osiko, V.V.; Prokhorov, A.M.; Shcherbakov, I.A. Nonradiative energy transfer from Cr3+ to Nd3+ ions in glasses with high neodymium concentrations. Sov. J. Quantum Electron. 1979, 9, 935–936. [Google Scholar] [CrossRef]
- Voronko, Y.K.; Gessen, S.B.; Es’kov, N.A.; Osiko, V.V.; Sobol, A.A.; Timoshechkin, M.I.; Ushakov, S.N.; Tsymbal, L.I. Spectroscopic and lasing properties of calcium niobium gallium garnet activated with Cr3+ and Nd3+. Sov. J. Quantum Electron. 1988, 18, 198–201. [Google Scholar] [CrossRef]
- Avanesov, A.G.; Voronko, Y.K.; Denker, B.I.; Maksimova, G.V.; Osiko, V.V.; Pirumov, S.S.; Shcherbakov, I.A. Investigation of the mechanisms of interaction between chromium and neodymium ions in phosphate glasses. Sov. J. Quantum Electron. 1981, 11, 873–877. [Google Scholar] [CrossRef]
- Dianov, E.M.; Dmitruk, M.V.; Karasik, A.Y.; Kirpichenkova, E.O.; Osiko, V.V.; Ostroumov, V.G.; Timoshechkin, M.I.; Shcherbakov, I.A. Synthesis and investigation of spectral, luminescence, and lasing properties of alumoborate crystals activated with chromium and neodymium ions. Sov. J. Quantum Electron. 1980, 10, 1222–1225. [Google Scholar] [CrossRef]
- Zharikov, E.V.; Lavrishchev, S.V.; Laptev, V.V.; Ostroumov, V.G.; Saidov, Z.S.; Smirnov, V.A.; Shcherbakov, I.A. New possibilities for Cr3+ ions as activators of the active media of solid-state lasers. Sov. J. Quantum Electron. 1984, 14, 332–336. [Google Scholar] [CrossRef]
- Balda, R.; Fernandez, J.; Illarramend, M.A. Steady-state and time-resolved laser spectroscopy of Cr3+ and Nd3+ singly and doubly doped calcium niobium gallium garnet. Phys. Rev. B 1993, 48, 9279–9290. [Google Scholar] [CrossRef]
- Balda, R.; Azkargota, J.; Iparraguirre, I.; Fernández, J.; Arriandiaga, M.A. Study of the Cr3+ sensitization and structural disorder effects on the Nd3+ laser action in Ca-gallogermanate-type codoped crystals. Opt. Mater. 1997, 8, 99–108. [Google Scholar] [CrossRef]
- Bovero, E.; Cavalli, E.; Jaque, D.; García Solé, J.; Speghini, A.; Bettinelli, M. Cr3+→Nd3+ energy transfer in the YAl3(BO3)4 nonlinear laser crystal. J. Appl. Phys. 2005, 98, 023103. [Google Scholar] [CrossRef]
- Hughes, M.A.; Li, H.; Curry, R.J.; Suzuki, T.; Ohishi, Y. Energy transfer in Cr and Nd co-doped Si-B-Na-Al-Ca-Zr-O glasses. J. Non-Cryst. Solids 2020, 530, 119769. [Google Scholar] [CrossRef]
- Kato, T.; Ito, H.; Hasegawa, K.; Ichikawa, T.; Ikesue, A.; Mizuno, S.; Takeda, Y.; Ichiki, A.; Motohiro, T. Effect of Cr content on the output of a solar-pumped laser employing a Cr-doped Nd:YAG ceramic laser medium operating in sunlight. Jpn. J. Appl. Phys. 2019, 58, 062007. [Google Scholar] [CrossRef]
- Saiki, T.; Iwashita, T.; Sakamoto, J.; Hayashi, T.; Nakamachi, T.; Fujimoto, Y.; Iida, Y.; Nakatsuka, M. Rod-type Ce/Cr/Nd: YAG ceramic lasers with white-light pump source. Int. J. Opt. 2022, 2022, 8480676. [Google Scholar] [CrossRef]
- Marletta, A.; Campos, K.R.; Nogueira, S.L.; Andrade, A.A.; Barbosa Neto, N.M.; Silva, R.A. Synthesis and optical characterization of poly(styrene sulphonate) films doped with neodymium (III) and co-doped with chromium (III). J. Non-Cryst. Solids 2010, 356, 2414–2416. [Google Scholar] [CrossRef]
- Kiss, Z.J.; Duncan, R.C. Cross-pumped Cr3+−Nd3+:YAG laser system. Appl. Phys. Lett. 1964, 5, 200–202. [Google Scholar] [CrossRef]
- Hattendorff, H.D.; Huber, G.; Danielmeyer, H.G. Efficient cross pumping of Nd3+ by Cr3+ in Nd(Al,Cr)3(BO3)4 lasers. J. Phys. C Solid State Phys. 1978, 11, 2399. [Google Scholar] [CrossRef]
- Ostroumov, V.G.; Shcherbakov, I.A. Gadolinium scandium gallium garnet (GSGG: Cr: Nd) laser with a high specific output energy. Sov. J. Quantum Electron. 1989, 19, 1549–1551. [Google Scholar] [CrossRef]
- Gołąb, S.; Sokólska, I.; Dominiak-Dzik, G.; Palatnikov, M.N.; Sidorov, N.V.; Biryukova, I.V.; Kalinnikov, V.T.; Ryba-Romanowski, W. Optical absorption and luminescence of LiTaO3:Cr and LiTaO3:Cr,Nd crystals. Proc. SPIE 1999, 3724, 270–273. [Google Scholar] [CrossRef]
- Sidorov, N.; Palatnikov, M.; Pyatyshev, A. Raman scattering in a single crystal of double doping LiTaO3:Cr(0.2):Nd(0.45 wt%). Photonics 2022, 9, 712. [Google Scholar] [CrossRef]
- Cabrera, J.M.; Olivares, J.; Carrascosa, M.; Rams, J.; Müller, R.; Diéguez, E. Hydrogen in lithium niobate. Adv. Phys. 1996, 45, 349–392. [Google Scholar] [CrossRef]
- Lengyel, K.; Péter, Á.; Kovács, L.; Corradi, G.; Pálfalvi, L.; Hebling, J.; Unferdorben, M.; Dravecz, G.; Hajdara, I.; Szaller, Z.; et al. Growth, defect structure, and THz application of stoichiometric lithium niobate. Appl. Phys. Rev. 2015, 2, 040601. [Google Scholar] [CrossRef]
- Kovacs, L.; Rebouta, L.; Soares, J.C.; da Silva, M.F.; Hage-Ali, M.; Stoquert, J.P.; Siffert, P.; Sanz-Garcia, J.A.; Corradi, G.; Szaller, Z.; et al. On the lattice site of trivalent dopants and the structure of Mg2+-OH--M3+ defects in LiNbO3:Mg crystals. J. Phys. Condens. Matter 1993, 5, 781–794. [Google Scholar] [CrossRef]
- Wöhlecke, M.; Kovács, L. OH− ions in oxide crystals. Crit. Rev. Solid State Mater. Sci. 2001, 26, 1–86. [Google Scholar] [CrossRef]
- Bäumer, C.; David, C.; Betzler, K.; Hesse, H.; Lengyel, K.; Kovács, L.; Wöhlecke, M. Composition dependence of the OH-stretch-mode spectrum in lithium tantalate. Phys. Stat. Sol. (A) 2004, 201, R13–R16. [Google Scholar] [CrossRef]
- Shi, L.; Yan, W.; Kong, Y. OH− absorption band in LiTaO3 with varied composition and hydrogenation. Eur. Phys. J. Appl. Phys. 2007, 40, 77–81. [Google Scholar] [CrossRef]
- Shi, L.; Kong, Y.; Yan, W.; Sun, J.; Chen, S.; Zhang, L.; Zhang, W.; Liu, H.; Li, X.; Xie, X.; et al. Determination of the composition of lithium tantalate by means of Raman and OH− absorption measurements. Mater. Chem. Phys. 2006, 95, 229–234. [Google Scholar] [CrossRef]
- Hu, P.; Zhang, L.; Xiong, J.; Yin, J.; Zhao, C.; He, X.; Hang, Y. Optical properties of MgO doped near-stoichiometric LiTaO3 single crystals. Opt. Mater. 2011, 33, 1677–1680. [Google Scholar] [CrossRef]
- Ting, S.; Xiao-Dong, Z.; Liang, S.; Rui, W. Nonvolatile photorefractive properties in triply doped stoichiometric Mg:Fe:Mn:LiTaO3 crystals. Chin. Phys. B 2014, 23, 014204. [Google Scholar] [CrossRef]
- Vyalikh, A.; Zschornak, M.; Köhler, T.; Nentwich, M.; Weigel, T.; Hanzig, J.; Zaripov, R.; Vavilova, E.; Gemming, S.; Brendler, E.; et al. Analysis of the defect clusters in congruent lithium tantalate. Phys. Rev. Mater. 2018, 2, 013804. [Google Scholar] [CrossRef]
- Wu, J.C.; Chen, Z.B.; Choubey, R.K.; Lan, C.W. On the study of zinc doping in congruent LiTaO3 crystals. Mater. Chem. Phys. 2012, 133, 813–817. [Google Scholar] [CrossRef]
- Pfannstiel, A.; Hehemann, T.; Schäfer, N.A.; Sanna, S.; Suhak, Y.; Vittadello, L.; Sauerwein, F.; Dömer, N.; Koelmann, J.; Fritze, H.; et al. Small electron polarons bound to interstitial tantalum defects in lithium tantalate. J. Phys. Condens. Matter 2024, 36, 355701. [Google Scholar] [CrossRef]
- Wu, X.L.; Bao, X.M.; Jiang, S.S.; Feng, D.; Tong, S. Phonon properties and microstructure of annealed LiTaO3:Nd. Appl. Phys. Lett. 1997, 70, 2401–2403. [Google Scholar] [CrossRef]
- Zhen, X.; Xu, Y.; Wang, R.; Zhao, C.; Shao, Z. Study on growth and optical property of Nd:Zn:LiNbO3 crystal. Ferroelectrics 2002, 265, 297–302. [Google Scholar] [CrossRef]
- Kaczmarek, S.M.; Świrkowiczb, M.; Jabłoński, R.; Łukasiewicz, T.; Kwaśny, M. Growth and characterization of lithium tantalate single crystals doped with Ho, Tm, Nd, Yb, Pr and doped by diffusion with Cr and Cu. J. Alloys Compd. 2000, 300–301, 322–328. [Google Scholar] [CrossRef]
- Wu, X.L.; Zhang, Z.Q.; Hu, A.; Zhang, M.S.; Jiang, S.S.; Feng, D. Microstructural study of Nd-doped LiTaO3. Appl. Phys. Lett. 1995, 67, 2450–2452. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Wang, H.F. Infrared absorption study of OH− in MgO:LiNbO3 doped with Cr and Nd. Phys. Lett. A 1995, 205, 112–116. [Google Scholar] [CrossRef]
- Tsuboi, T.; Kaczmarek, S.M.; Boulon, G. Spectral properties of Yb3+ ions in LiNbO3 single crystals: Influences of other rare-earth ions, OH− ions, and γ-irradiation. J. Alloys Compd. 2004, 380, 196–200. [Google Scholar] [CrossRef]
- Kovács, L.; Kocsor, L.; Szaller, Z.; Hajdara, I.; Dravecz, G.; Lengyel, K.; Corradi, G. Lattice site of rare-earth ions in stoichiometric lithium niobate probed by OH- vibrational spectroscopy. Crystals 2017, 7, 230. [Google Scholar] [CrossRef]
- Choubey, R.K.; Khattak, B.Q.; Kar, S.; Ramshankar, P.; Sen, P.; Bartwal, K.S. Influence of doping on OH absorption in LiNbO3 crystals. Cryst. Res. Technol. 2007, 42, 718–722. [Google Scholar] [CrossRef]
- Kösters, M.; Sturman, B.; Werheit, P.; Haertic, D.; Buse, K. Optical cleaning of congruent lithium niobate. Nat. Photonics 2009, 3, 310–313. [Google Scholar] [CrossRef]
- Sturman, B.; Kösters, M.; Haertic, D.; Decher, C.; Buse, K. Optical cleaning owing to the bulk photovoltaic effect. Phys. Rev. B 2009, 80, 245319. [Google Scholar] [CrossRef]
- Bera, A.; Muthu, D.V.S.; Sood, A.K. Enhanced Raman and photoluminescence response in monolayer MoS2 due to laser healing of defects. J. Raman Spectrosc. 2018, 49, 100–105. [Google Scholar] [CrossRef]
- Gunter, P.; Huignard, J.P. Photorefractive Materials and Their Applications, Part 1; Springer: Berlin/Heidelberg, Germany, 2006; 426p. [Google Scholar]
- Lines, M.E.; Glass, A.M. Principles and Application of Ferroelectrics and Related Materials; Clarendon Press: Oxford, UK, 1977; 680p. [Google Scholar]
- Sidorov, N.V.; Volk, T.R.; Mavrin, B.N.; Kalinnikov, V.T. Lithium Niobate: Defects, Photorefraction, Vibrational Spectrum, Polaritons; Nauka: Moscow, Russia, 2003; 255p. (In Russian) [Google Scholar]
- Sidorov, N.V.; Yanichev, A.A.; Palatnikov, M.N.; Gabain, A.A. Effects of the ordering of structural units of the cationic sublattice of LiNbO3:Zn crystals and their manifestation in Raman spectra. Opt. Spectrosc. 2014, 116, 281–290. [Google Scholar] [CrossRef]
- Kar, S.; Bhatt, R.; Bartwal, K.S.; Wadhawan, V.K. Optimisation of chromium doping in LiNbO3 single crystals. Cryst. Res. Technol. 2004, 39, 230–234. [Google Scholar] [CrossRef]
- Xia, H.; Song, H.; Wang, J.; Zhang, J.; Wang, T.; Zhang, J.; Zhang, Y.; Nie, Q. Cr3+-doped LiNbO3 crystals grown by the Bridgman method. Cryst. Res. Technol. 2005, 40, 199–203. [Google Scholar] [CrossRef]
- Sanna, S.; Neufeld, S.; Rüsing, M.; Berth, G.; Zrenner, A.; Schmidt, W.G. Raman scattering efficiency in LiTaO3 and LiNbO3 crystals. Phys. Rev. B 2015, 91, 224302. [Google Scholar] [CrossRef]
- Margueron, S.; Bartasyte, A.; Glazer, A.M.; Simon, E.; Hlinka, J.; Gregora, I.; Gleize, J. Resolved E-symmetry zone-centre phonons in LiTaO3 and LiNbO3. J. Appl. Phys. 2012, 111, 104105. [Google Scholar] [CrossRef]
- Kostritskii, S.M.; Bourson, P.; Aillerie, M.; Fontana, M.D.; Kip, D. Quantitative evaluation of the electro-optic effect and second-order optical nonlinearity of lithium tantalate crystals of different compositions using Raman and infrared spectroscopy. Appl. Phys. B 2006, 82, 423–430. [Google Scholar] [CrossRef]
- Barker, A.S.; Ballman, A.A.; Ditzenberger, J.A. Infrared study of the lattice vibrations in LiTaO3. Phys. Rev. B 1970, 2, 4233–4239. [Google Scholar] [CrossRef]
- Xing-Long, W.; Ming-Sheng, Z.; Qiang, C.; Qun, Z.; Zhao-Hua, G.; Duan, F. Raman spectroscopic study of Nd-doped lithium tantalate. Acta Phys. Sin. 1994, 3, 493–500. [Google Scholar] [CrossRef]
- Glass, A.M. Optical spectra of Cr3+ impurity ions in ferroelectric LiNbO3 and LiTaO3. J. Chem. Phys. 1969, 50, 1501–1510. [Google Scholar] [CrossRef]
- Klauer, S.; Wöhlecke, M.; Kapphan, S. Influence of the H-D isotopic substitution on the protonic conductivity in LiNbO3 crystal. Phys. Rev. B 1992, 45, 2786–2799. [Google Scholar] [CrossRef] [PubMed]
- Salloum, M.Y.; Grunsky, O.S.; Man’shina, A.A.; Tver’yanovich, A.S.; Tver’yanovich, Y.S. Investigation of lithium niobate composition by optical spectroscopy methods. Russ. Chem. Bull. 2009, 73, 2228–2232. [Google Scholar] [CrossRef]
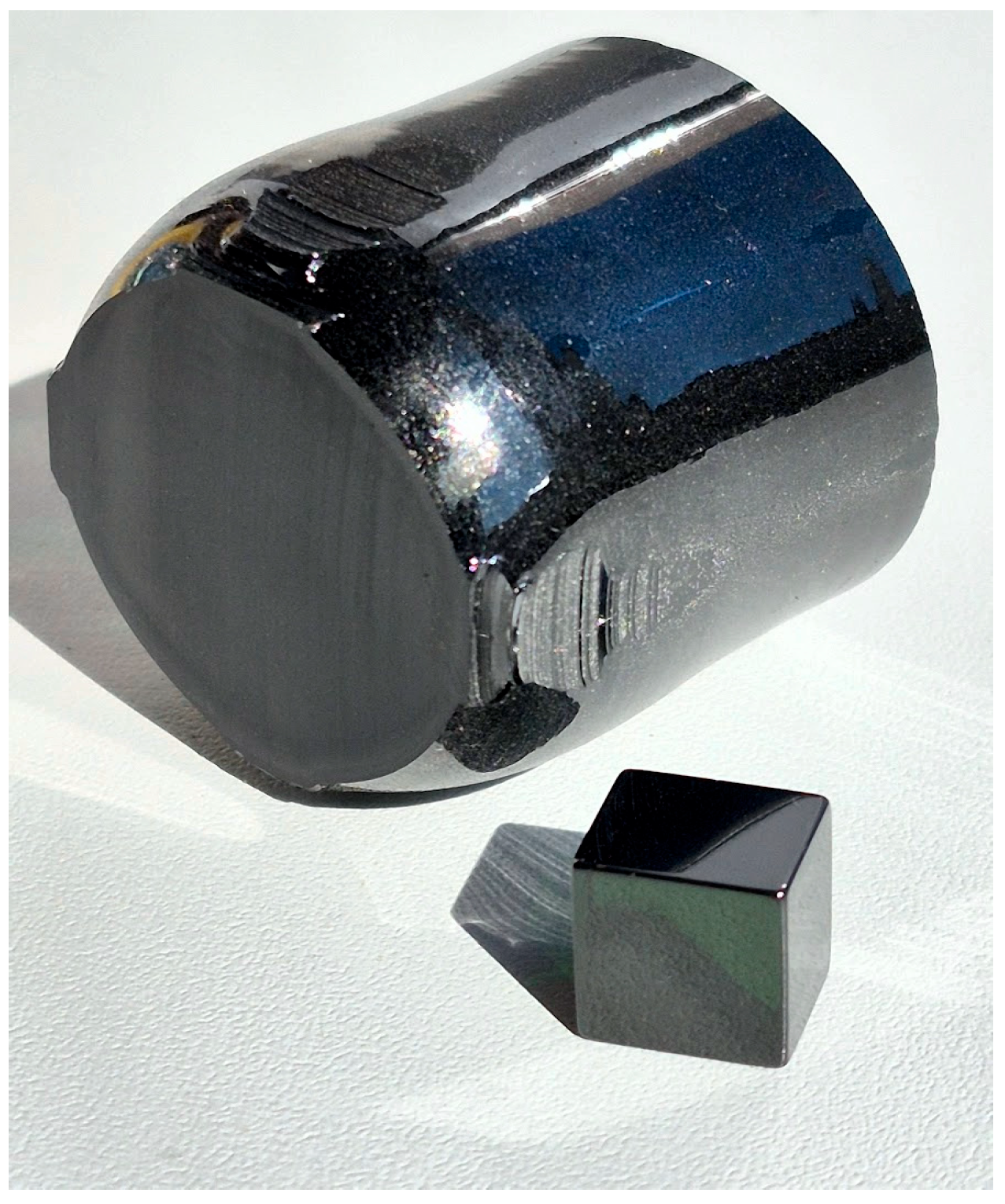
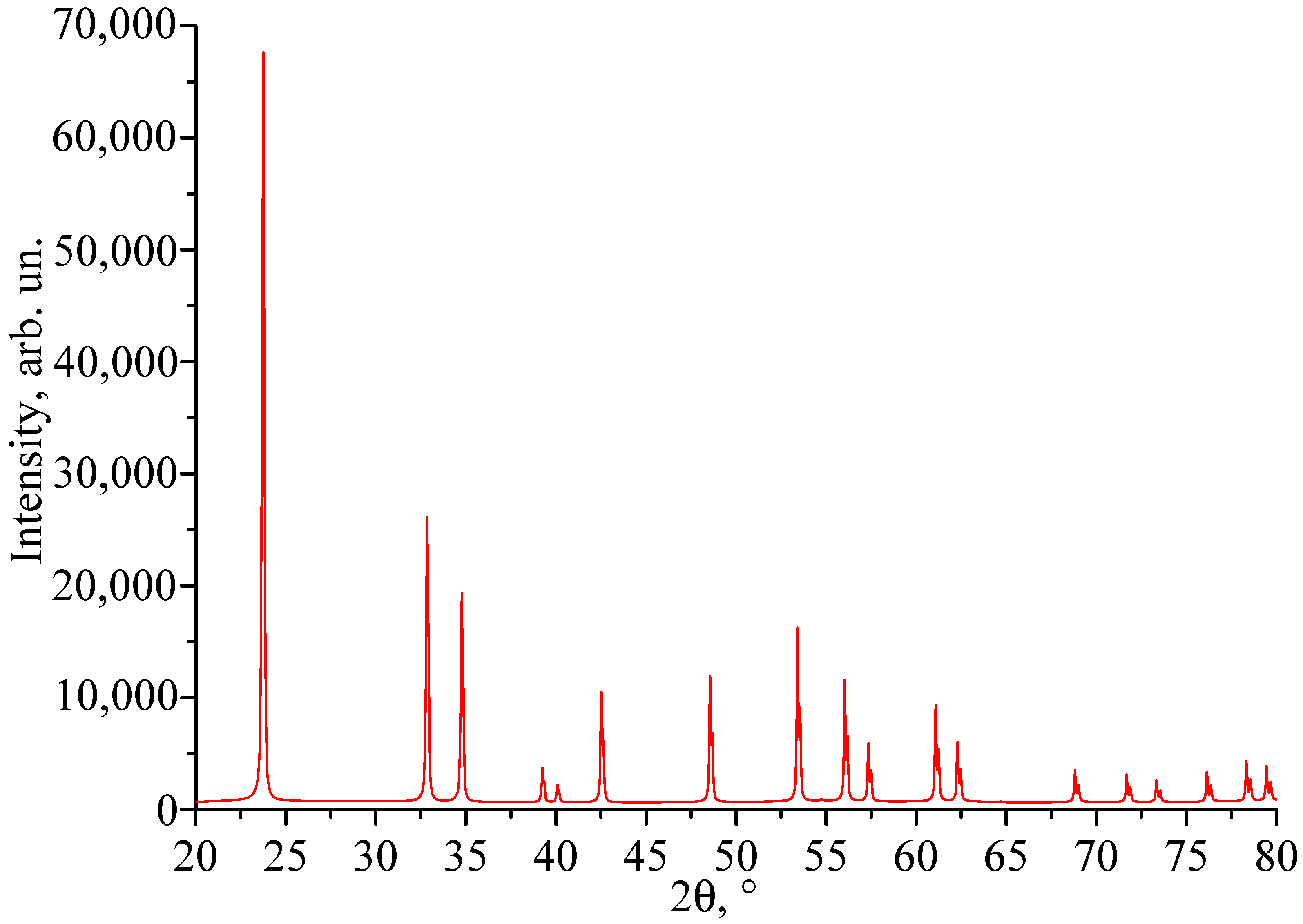
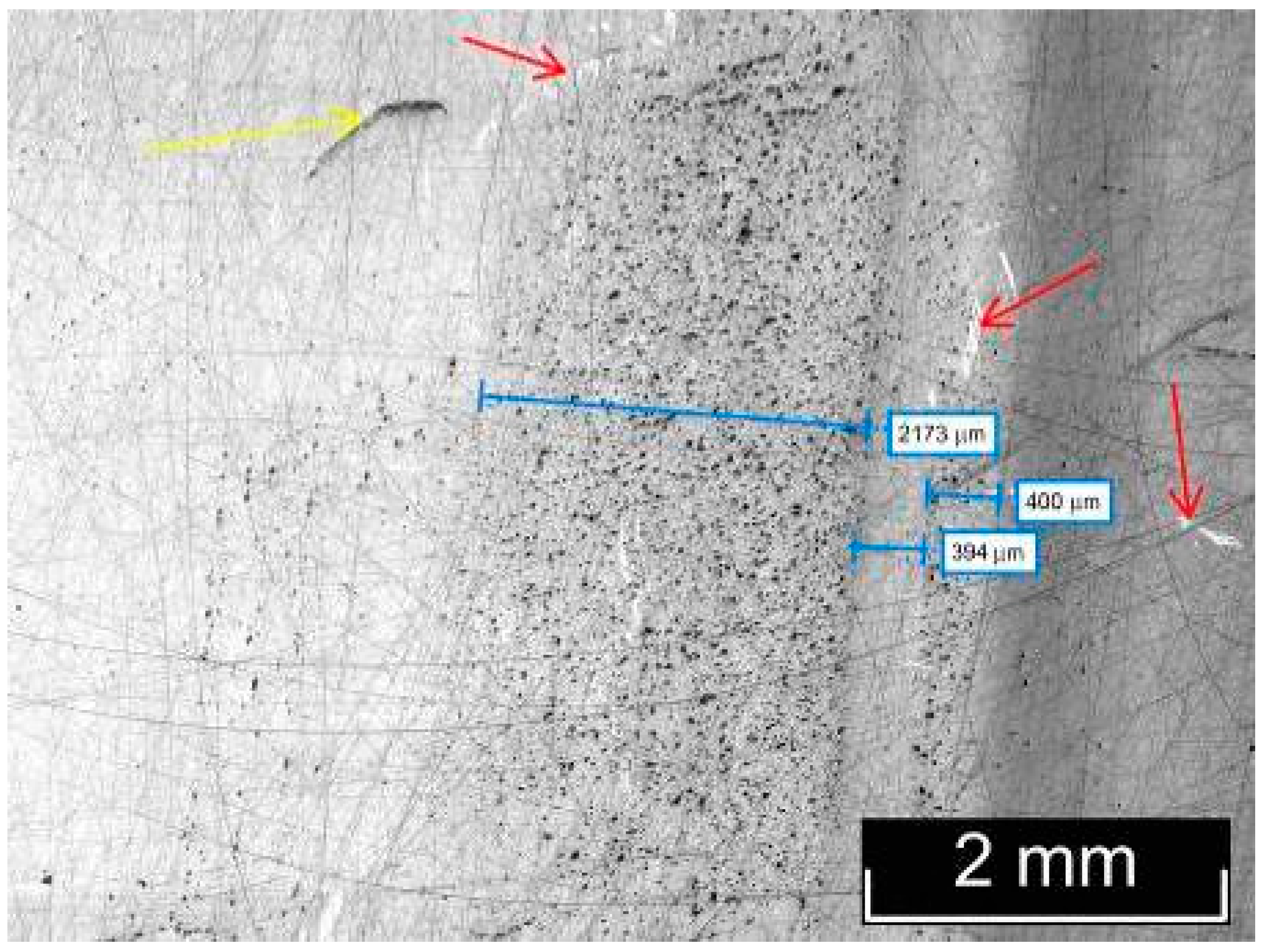
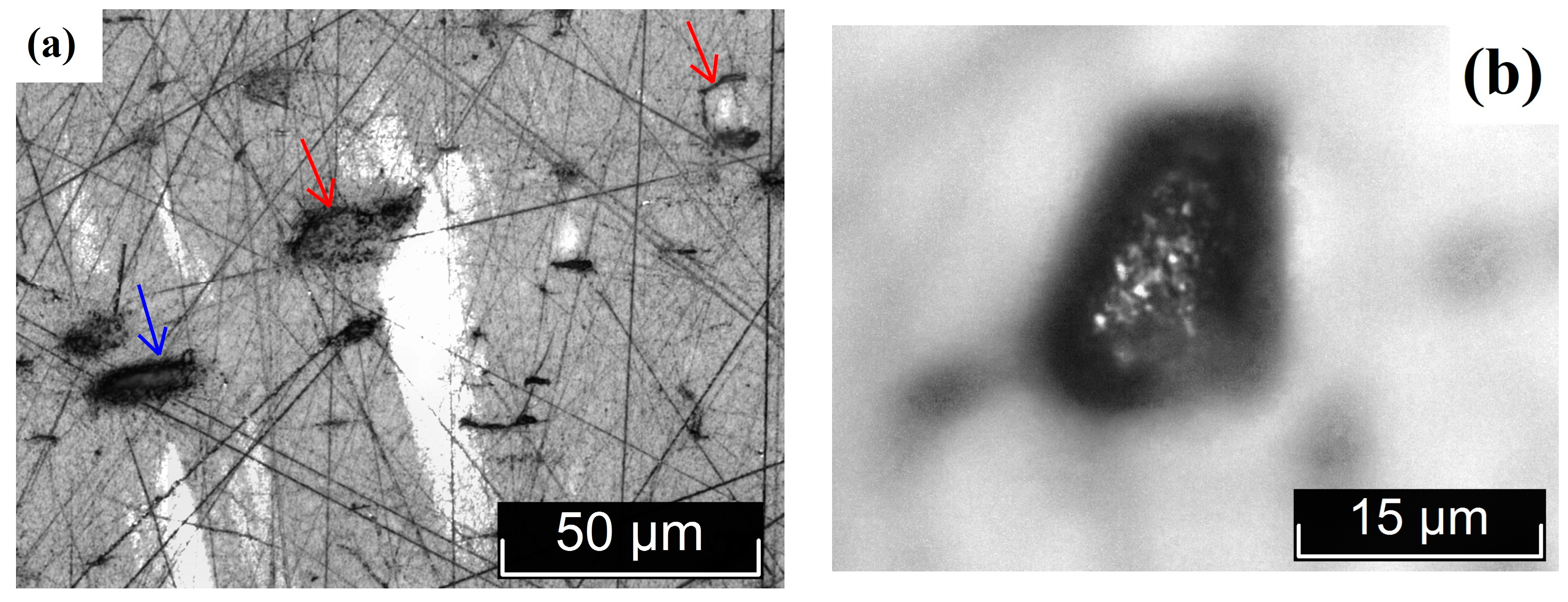
| Crystal | Crystal Composition | [Cr], wt% | [Nd], wt% |
|---|---|---|---|
| LT-1 | LiTaO3 | - | - |
| LT-2 | LiTaO3:Cr | 0.005 | - |
| LT-3 | LiTaO3:Cr:Nd | 0.06 | 0.2 |
| LT-4 | LiTaO3:Cr:Nd | 0.07 | 0.28 |
| LT-5 | LiTaO3:Cr:Nd | 0.09 | 0.25 |
| LT-6 | LiTaO3:Cr:Nd | 0.1 | 0.25 |
| LT-7 | LiTaO3:Cr:Nd | 0.11 | 0.41 |
| LT-8 | LiTaO3:Cr:Nd | 0.2 | 0.45 |
| Admixture | Concentration of Impurities in the Batch LiTaO3, wt% | Impurity Content in the Crystal LT-5, wt% |
|---|---|---|
| Mn, V, Mg, Sn | <5·10−4 | <5·10−4 |
| Pb, Ni, Co | <1·10−3 | <1·10−3 |
| Mo | <1·10−3 | <1·10−3 |
| Si, Fe | <1·10−3 | <1·10−3 |
| Ti | <1·10−3 | <1·10−3 |
| Al | <5·10−4 | <5·10−4 |
| Zr | <1·10−2 | <1·10−2 |
| Ca | <5·10−3 | <5·10−3 |
| Cu | <5·10−4 | <5·10−4 |
| TC, °C | 1145.0 | 1145.0 |
| LT-3 | LT-4 | LT-5 | LT-6 | LT-7 | LT-8 | Transition |
|---|---|---|---|---|---|---|
| 357.6 | 357.6 | 357.6 | 357.8 | |||
| 363.8 | 363.6 | 363.6 | 363.8 | |||
| 470.8 | ||||||
| 472.0 | ||||||
| 474.0 | ||||||
| 501.4 | ||||||
| 502.0 | ||||||
| 504.0 | ||||||
| 589.2 | 589.4 | 589.0 | 589.0 | |||
| 598.8 | 598.8 | 599.0 | 599.0 | |||
| 619.2 | ||||||
| 641.2 | ||||||
| 643.2 | ||||||
| 645.2 | ||||||
| 650.2 | 649.4 | |||||
| 719.4 | 719.4 | 719.4 | 719.2 | 719.2 | 719.2 | |
| 741.0 | 740.6 | 741.2 | 741.2 | |||
| 752.0 | 751.8 | 752.2 | 751.8 | |||
| 763.0 | 763.0 | 762.6 | ||||
| 792.8 | 792.8 | 793.2 | 793.2 | |||
| 810.4 | 810.4 | 810.6 | 811.2 | |||
| 817.0 | 817.0 | 815.2 | ||||
| 835.0 | 835.0 | 835.2 | 835.2 | |||
| 874.0 | 874.0 | 874.2 | 874.2 | |||
| 885.6 | 885.4 | 885.2 | 884.6 | |||
| 898.8 | 898.6 | 898.2 | 897.2 | |||
| 913.6 | 913.6 | 913.4 | 913.2 | |||
| 923.6 | 924.0 | 924.2 | 924.2 |
| Crystal | Frequency, cm−1 | Assignment [70,71,72] | |
| LT-3 | 140 | 142 | 1E(TO) |
| 182 | 1E(LO) | ||
| 206 | 205 | 1A1(TO) | |
| 247 | 250 | 3E(TO) | |
| 312 | 309 | 4E(TO) | |
| 356 | 353 | 2A1(LO) (3A1(TO)) | |
| 380 | 377 | 5E(TO) (6E(TO)) | |
| 461 | 464 | 7E(TO) | |
| 515 | 518 | ||
| 595 | 592 | 8E(TO) (4A1(TO)) | |
| 654 | 660 | 9E(TO) (8E(LO)) | |
| 749 | 746 | ||
| 864 | 864 | 9E(LO) (4A1(TO)) | |
| LT-4 | 140 | 146 | 1E(TO) |
| 180 | 1E(LO) | ||
| 206 | 206 | 1A1(TO) | |
| 247 | 247 | 3E(TO) | |
| 312 | 312 | 4E(TO) | |
| 356 | 353 | 2A1(LO) (3A1(TO)) | |
| 377 | 377 | 5E(TO) | |
| 461 | 461 | 7E(TO) | |
| 521 | 518 | ||
| 595 | 592 | 8E(TO) (4A1(TO)) | |
| 657 | 660 | 9E(TO) (8E(LO)) | |
| 750 | 749 | ||
| 861 | 864 | 9E(LO) (4A1(LO)) | |
| LT-5 | 140 | 143 | 1E(TO) |
| 164 | |||
| 203 | 206 | 1A1(TO) | |
| 229 | |||
| 250 | |||
| 286 | |||
| 312 | 312 | 4E(TO) | |
| 356 | 356 | 2A1(LO) (3A1(TO)) | |
| 377 | 380 | 5E(TO) (6E(TO)) | |
| 464 | 464 | 7E(TO) | |
| 515 | 510 | ||
| 595 | 595 | 8E(TO) (4A1(TO)) | |
| 657 | 657 | 9E(TO) (8E(LO)) | |
| 749 | 746 | ||
| 861 | 864 | 9E(LO) (4A1(LO)) | |
| LT-6 | 143 | 146 | 1E(TO) |
| 158 | |||
| 186 | 1E(LO) | ||
| 206 | 206 | 1A1(TO) | |
| 247 | 250 | 3E(TO) | |
| 312 | 309 | 4E(TO) | |
| 356 | 353 | 2A1(LO) (3A1(TO)) | |
| 380 | 377 | 5E(TO) (6E(TO)) | |
| 412 | |||
| 464 | 461 | 7E(TO) | |
| 521 | 515 | ||
| 598 | 595 | 8E(TO) (4A1(TO)) | |
| 660 | 660 | 9E(TO) (8E(LO)) | |
| 745 | 746 | ||
| 864 | 864 | 9E(LO) (4A1(LO)) | |
| LT-7 | 140 | 143 | 1E(TO) |
| 187 | 1E(LO) | ||
| 206 | 206 | 1A1(TO) | |
| 250 | 250 | 3E(TO) | |
| 315 | 312 | 4E(TO) | |
| 356 | 353 | 2A1(LO) (3A1(TO)) | |
| 380 | 377 | 5E(TO) (6E(TO)) | |
| 461 | 461 | 7E(TO) | |
| 521 | |||
| 595 | 592 | 8E(TO) (4A1(TO)) | |
| 657 | 660 | 9E(TO) (8E(LO)) | |
| 742 | 749 | ||
| 864 | 864 | 9E(LO) (4A1(LO)) | |
| LT-8 | 143 | 143 | 1E(TO) |
| 184 | 1E(LO) | ||
| 206 | 206 | 1A1(TO) | |
| 250 | 250 | 3E(TO) | |
| 306 | |||
| 312 | 315 | 4E(TO) | |
| 356 | 353 | 2A1(LO) (3A1(TO)) | |
| 380 | 377 | 5E(TO) (6E(TO)) | |
| 464 | 461 | 7E(TO) | |
| 515 | |||
| 595 | 595 | 8E(TO) (4A1(TO)) | |
| 657 | 657 | ||
| 748 | 746 | ||
| 864 | 864 | 9E(LO) (4A1(LO)) | |
| Crystal | ν | I | S | C (OH− Groups), cm−3 |
|---|---|---|---|---|
| LT-1 | 3459 | 0.09 | 6.97 | 3.90·1017 |
| 3462 | 0.197 | 13.78 | ||
| 3474 | 0.16 | 10.01 | ||
| 3483 | 0.06 | 10.08 | ||
| 3493 | 0.04 | 16.12 | ||
| LT-2 | 3463 | 0.05 | 13.93 | 4.22·1017 |
| 3480 | 0.15 | 21.91 | ||
| 3493 | 0.102 | 18.67 | ||
| 3506 | 0.05 | 21.16 | ||
| LT-3 | 3463 | 0.03 | 13.49 | 3.73·1017 |
| 3478 | 0.09 | 21.09 | ||
| 3482 | 0.11 | 23.85 | ||
| 3504 | 0.05 | 25.08 | ||
| LT-4 | 3465 | 0.04 | 14.29 | 4.42·1017 |
| 3483 | 0.07 | 15.74 | ||
| 3489 | 0.13 | 22.15 | ||
| 3505 | 0.06 | 21.32 | ||
| LT-5 | 3463 | 0.12 | 12.47 | 10.6·1017 |
| 3486 | 0.23 | 19.66 | ||
| 3489 | 0.34 | 25.93 | ||
| 3507 | 0.09 | 21.80 | ||
| LT-6 | 3462 | 0.05 | 10.52 | 6.03·1017 |
| 3486 | 0.18 | 22.46 | ||
| 3489 | 0.15 | 20.38 | ||
| 3504 | 0.10 | 22.03 | ||
| LT-7 | 3464 | 0.02 | 15.93 | 3.46·1017 |
| 3480 | 0.08 | 21.42 | ||
| 3490 | 0.10 | 25.15 | ||
| 3508 | 0.04 | 28.38 | ||
| LT-8 | 3464 | 0.02 | 15.32 | 3.78·1017 |
| 3479 | 0.07 | 22.96 | ||
| 3493 | 0.10 | 27.69 | ||
| 3507 | 0.04 | 29.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sidorov, N.V.; Bobreva, L.A.; Pyatyshev, A.Y.; Palatnikov, M.N.; Palatnikova, O.V.; Skrabatun, A.V.; Teslenko, A.A.; Tarabrin, M.K. Concentration Dependence of Optical Properties of Double-Doped LiTaO3:Cr3+:Nd3+ Crystals. Materials 2025, 18, 3218. https://doi.org/10.3390/ma18143218
Sidorov NV, Bobreva LA, Pyatyshev AY, Palatnikov MN, Palatnikova OV, Skrabatun AV, Teslenko AA, Tarabrin MK. Concentration Dependence of Optical Properties of Double-Doped LiTaO3:Cr3+:Nd3+ Crystals. Materials. 2025; 18(14):3218. https://doi.org/10.3390/ma18143218
Chicago/Turabian StyleSidorov, Nikolay V., Lyubov A. Bobreva, Alexander Yu. Pyatyshev, Mikhail N. Palatnikov, Olga V. Palatnikova, Alexander V. Skrabatun, Andrei A. Teslenko, and Mikhail K. Tarabrin. 2025. "Concentration Dependence of Optical Properties of Double-Doped LiTaO3:Cr3+:Nd3+ Crystals" Materials 18, no. 14: 3218. https://doi.org/10.3390/ma18143218
APA StyleSidorov, N. V., Bobreva, L. A., Pyatyshev, A. Y., Palatnikov, M. N., Palatnikova, O. V., Skrabatun, A. V., Teslenko, A. A., & Tarabrin, M. K. (2025). Concentration Dependence of Optical Properties of Double-Doped LiTaO3:Cr3+:Nd3+ Crystals. Materials, 18(14), 3218. https://doi.org/10.3390/ma18143218










