In Vivo Degradation Behavior of AZ91 Magnesium Alloy: Comprehensive Microstructural and Crystallographic Characterization by TEM and NBED
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation and Mechanical Testing
2.2. Implantation In Vivo
2.3. Degradation Layer Characterization
3. Results
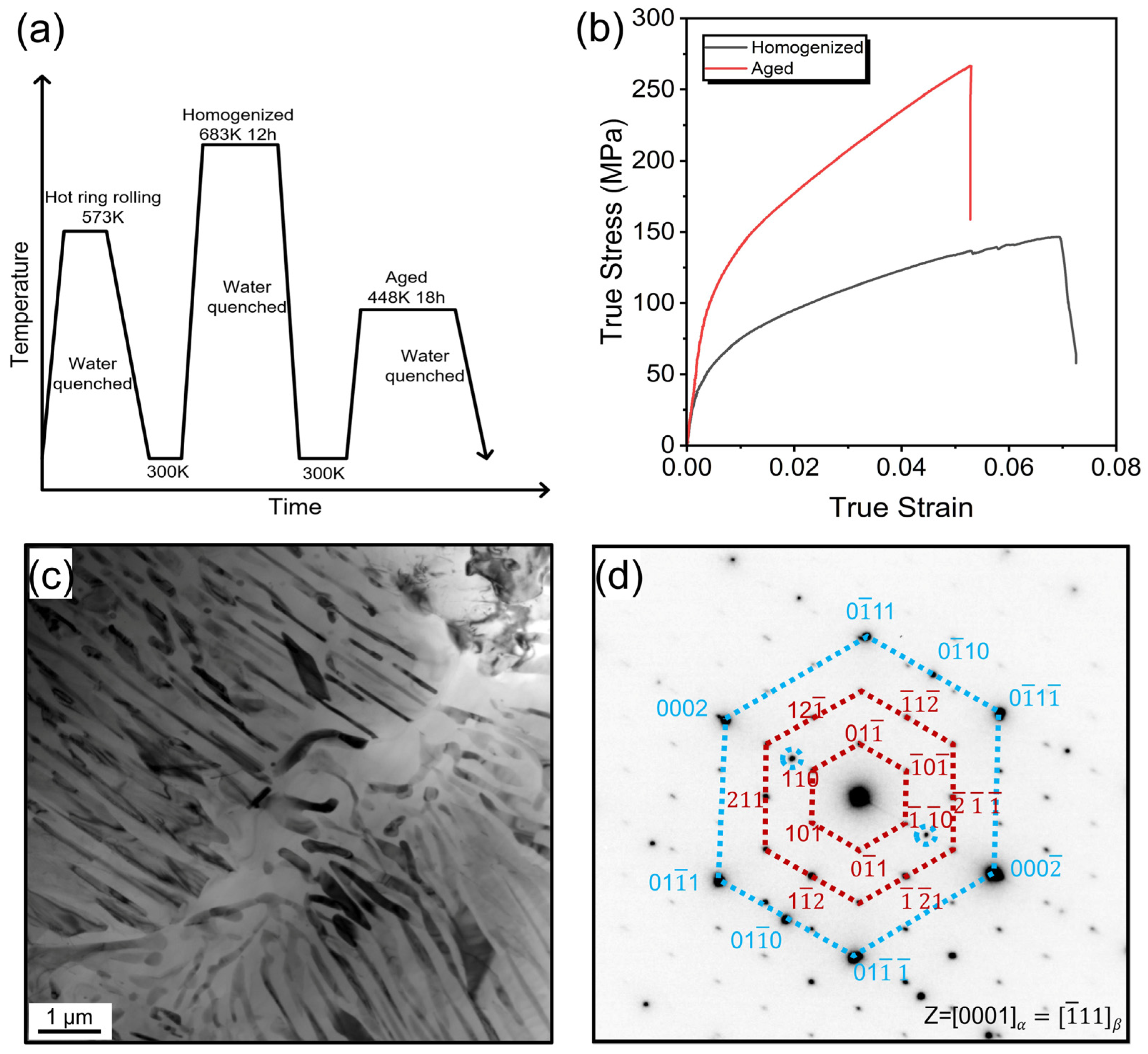
3.1. Mechanical Properties and Microstructures of AZ91 Alloy
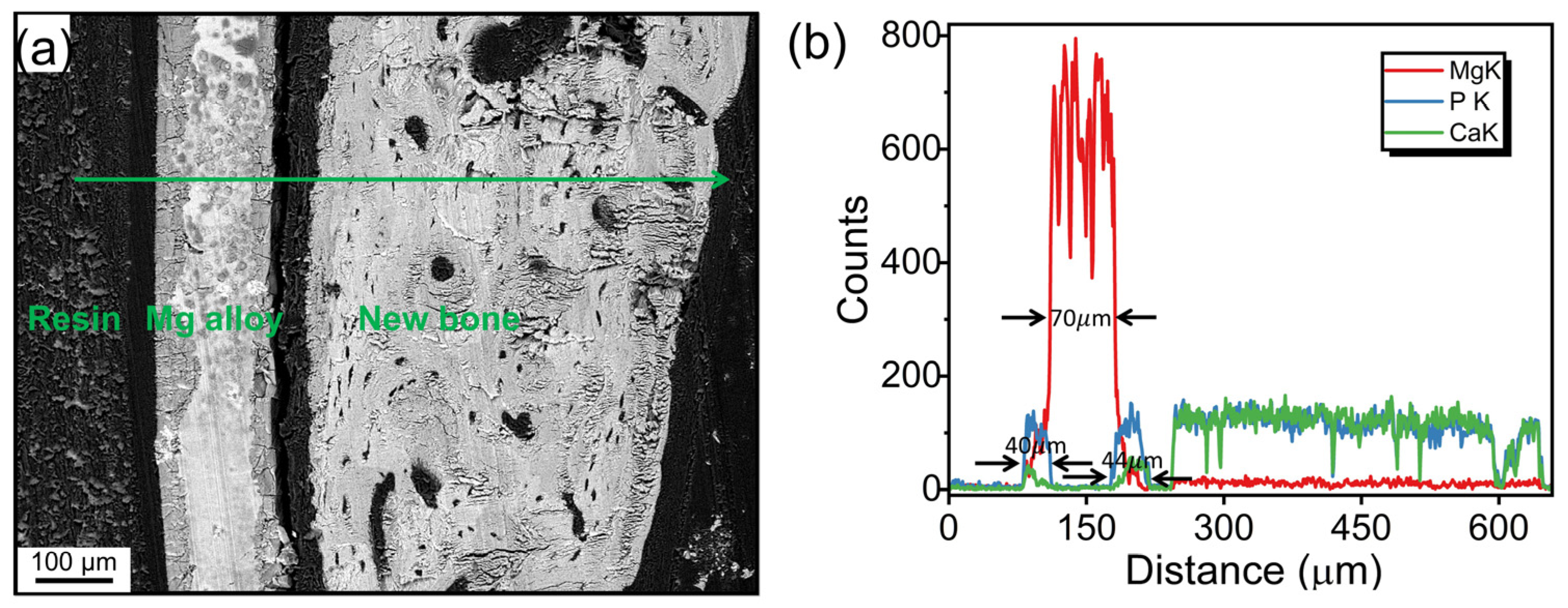
3.2. Degradation Layer Analysis via SEM and EDX
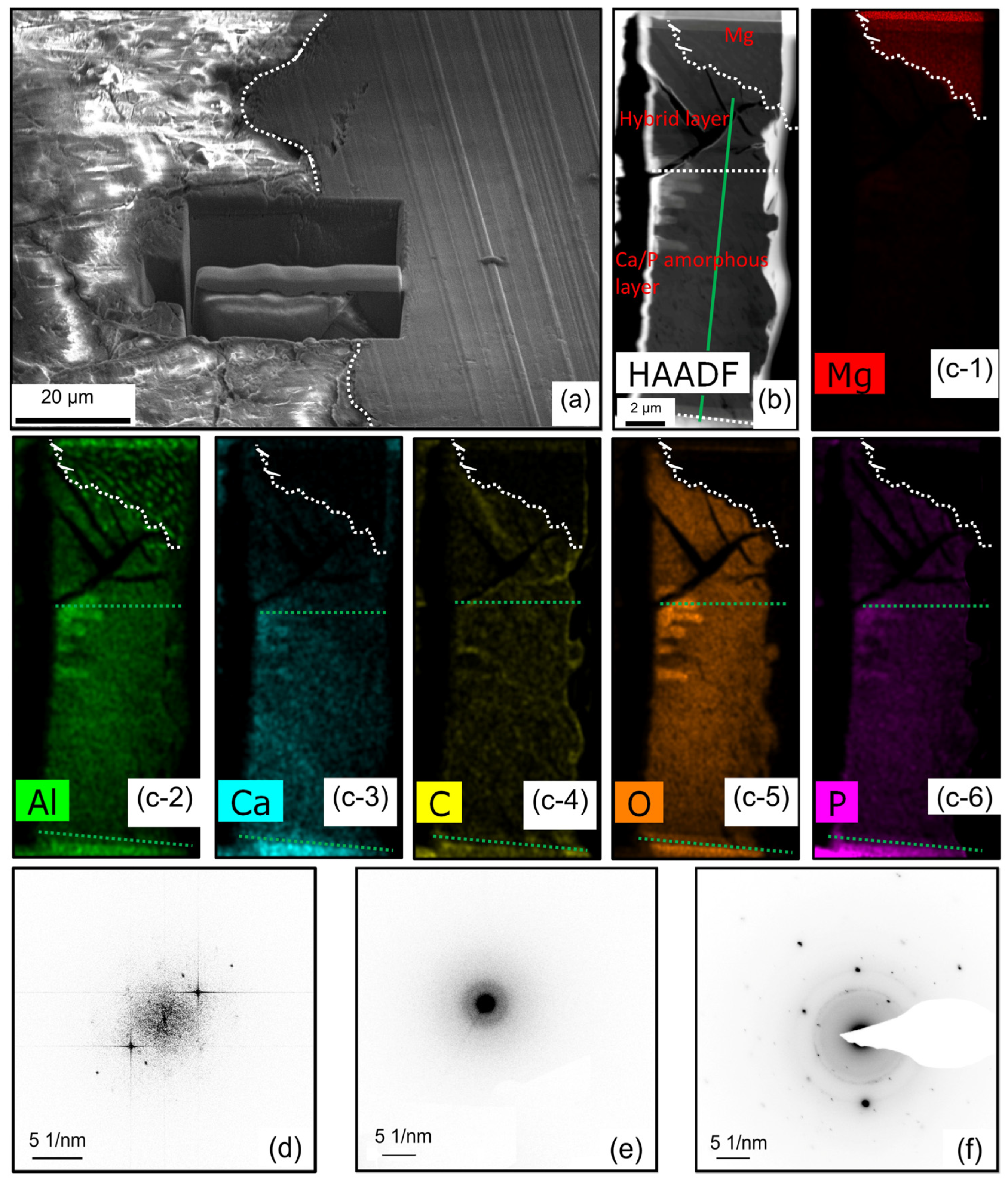
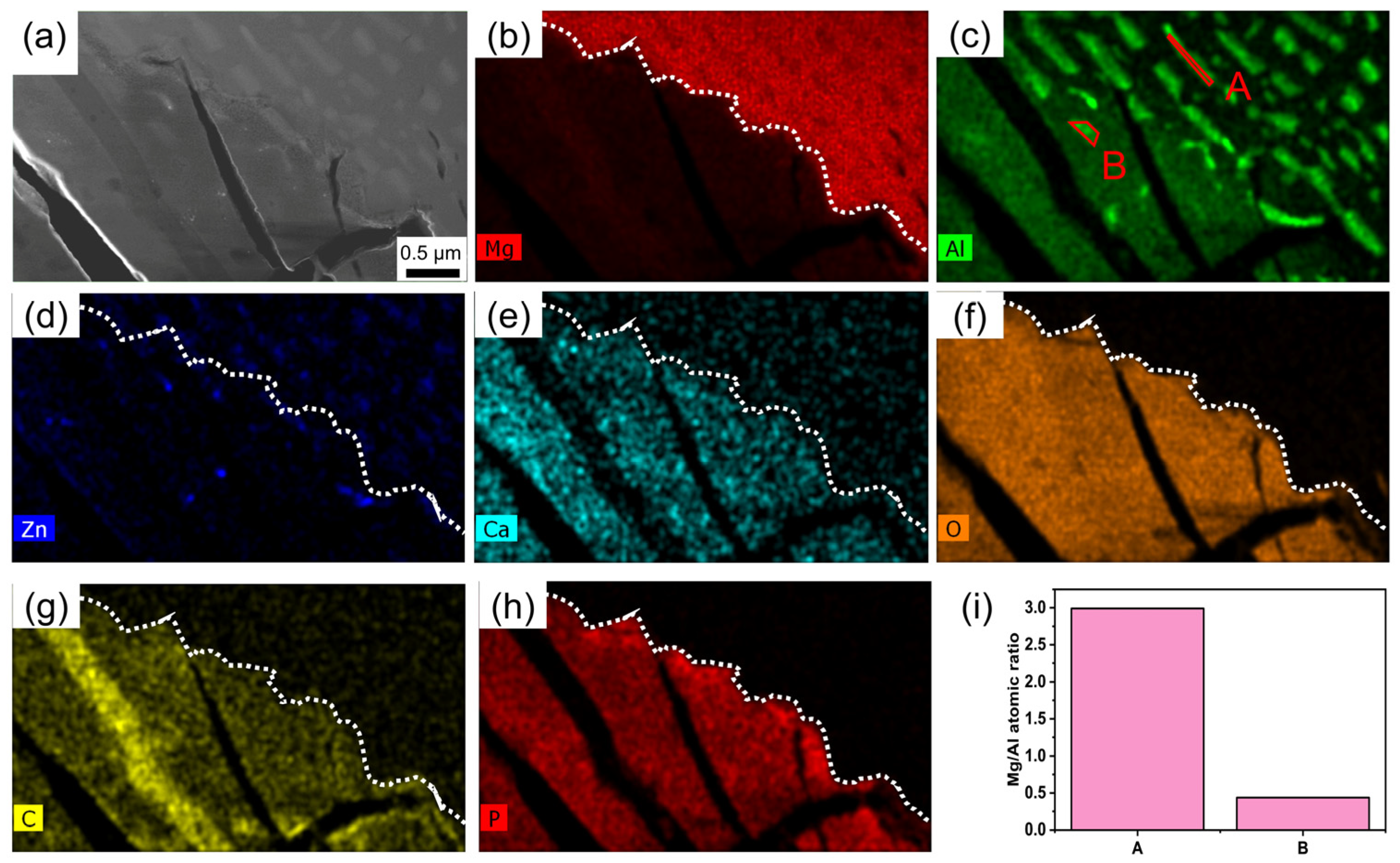
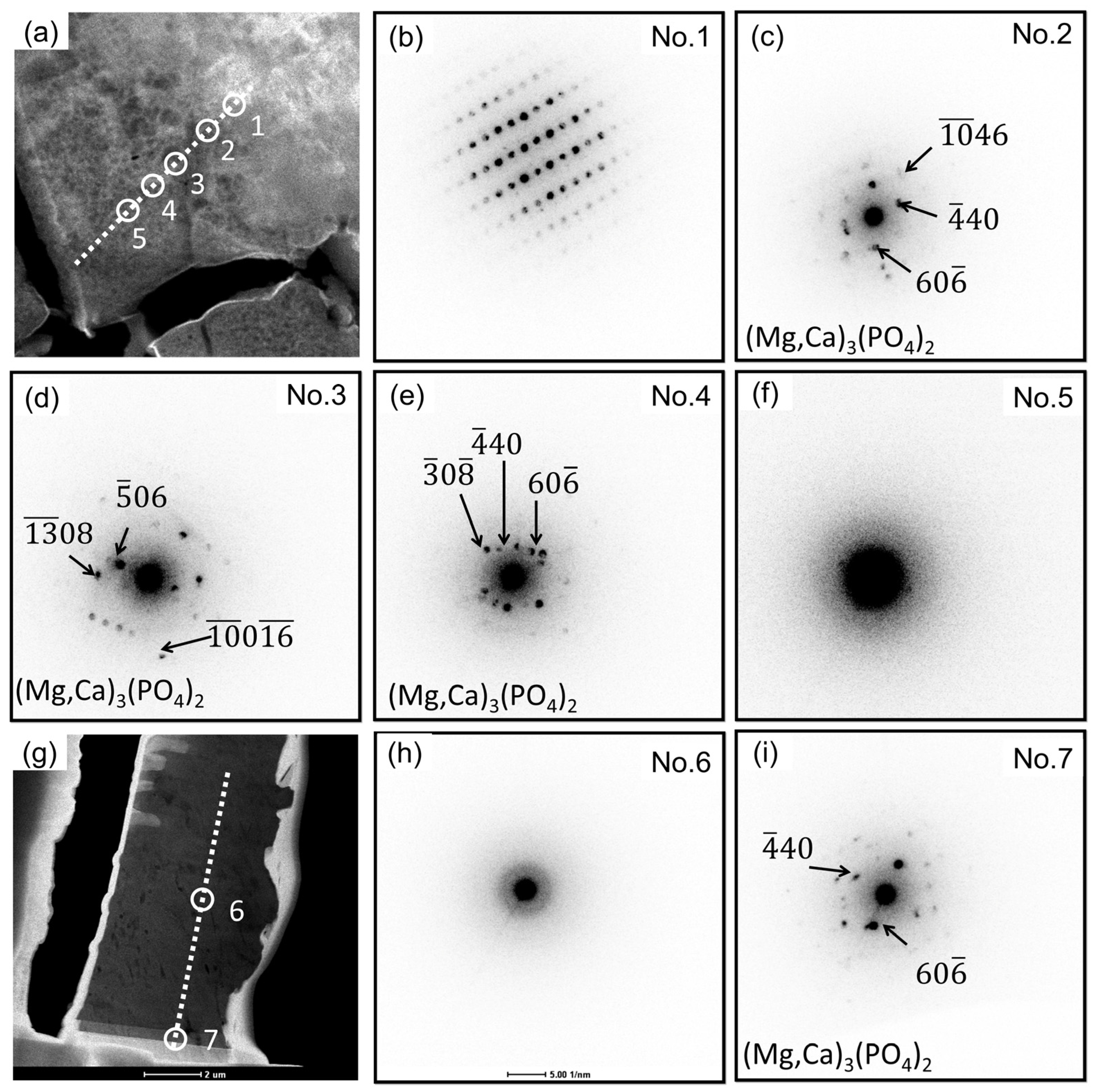
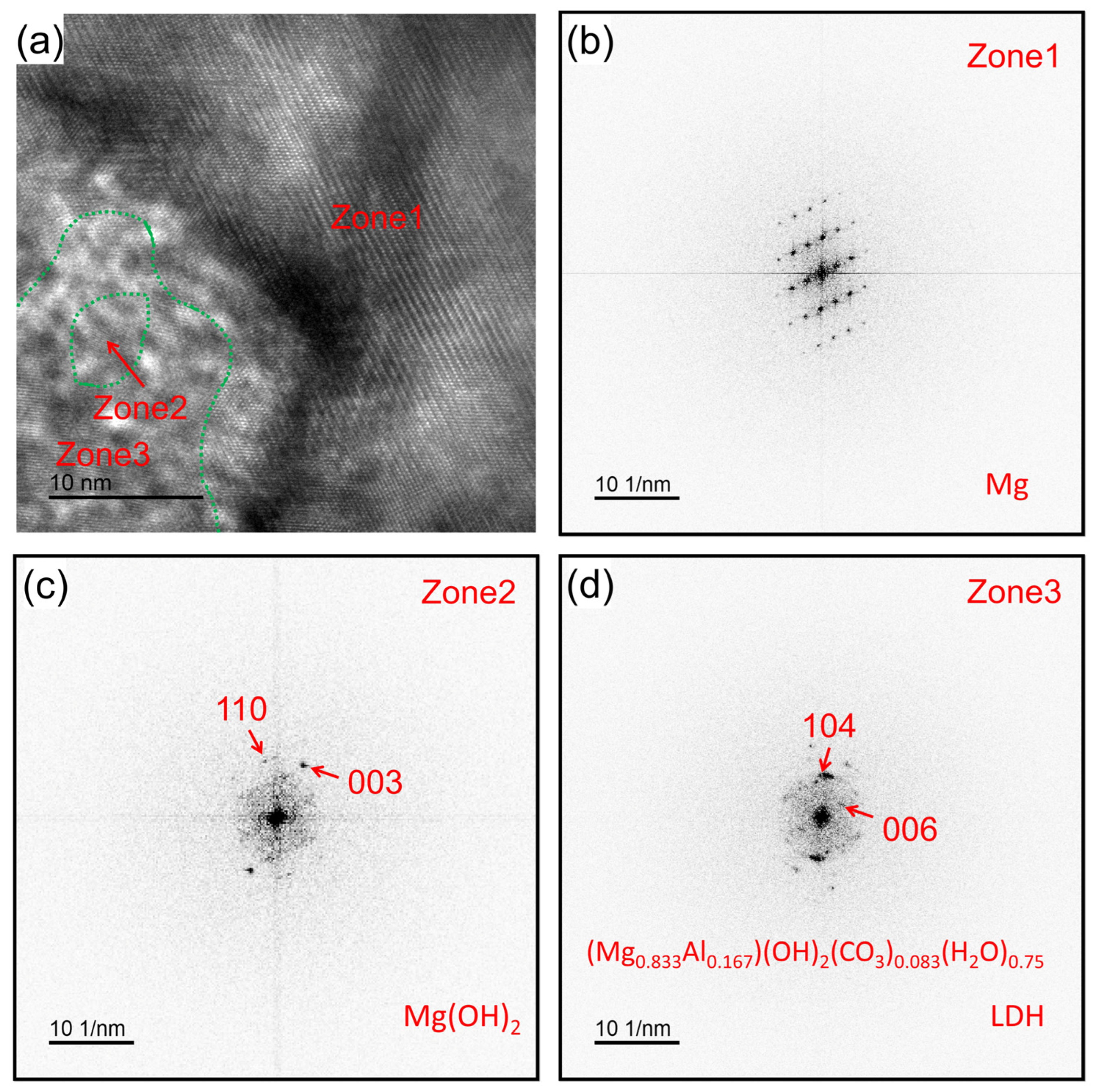
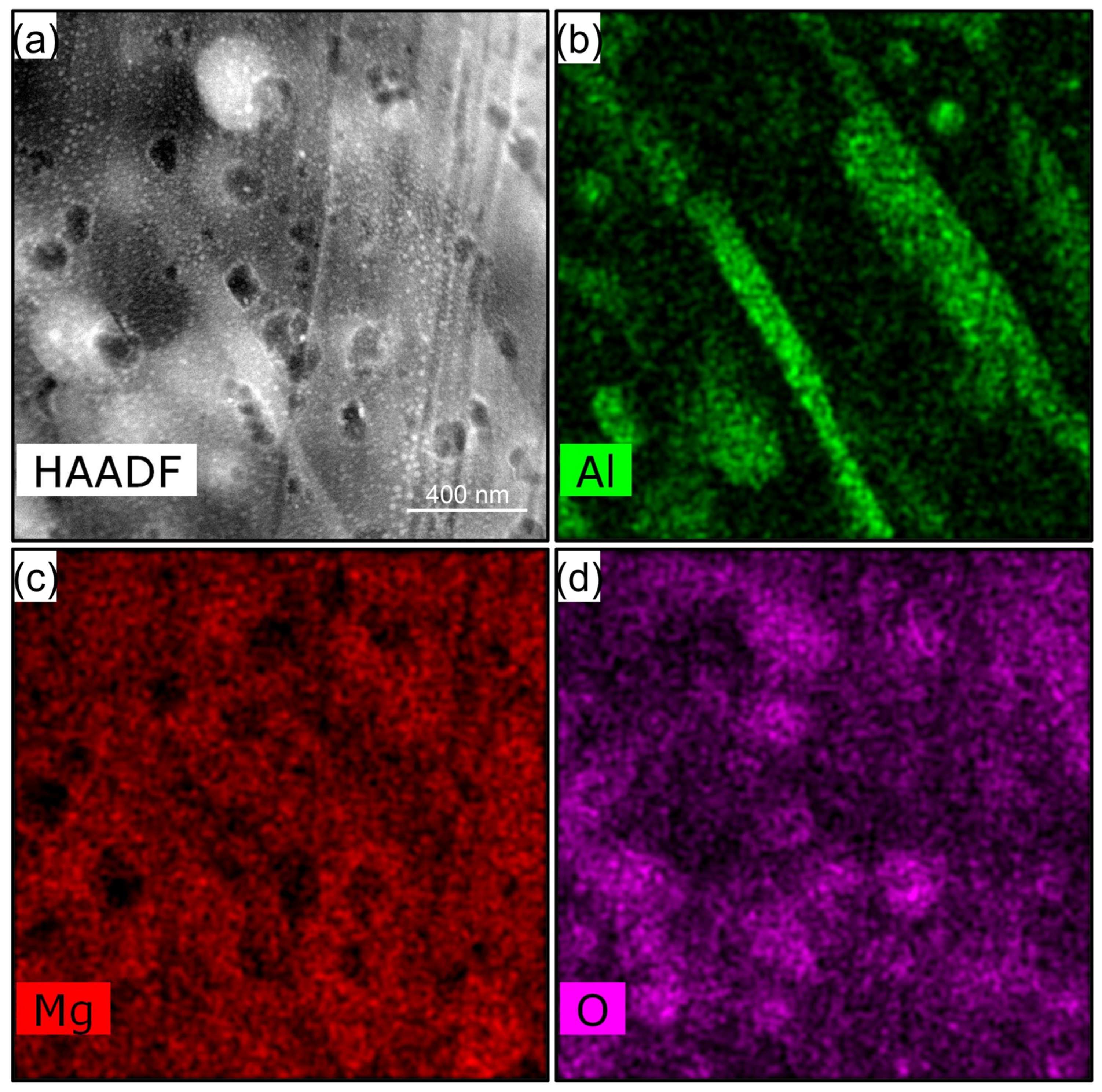
3.3. Degradation Layer Analysis via TEM and STEM
4. Discussion
4.1. Structure and Composition of Degradation Layer
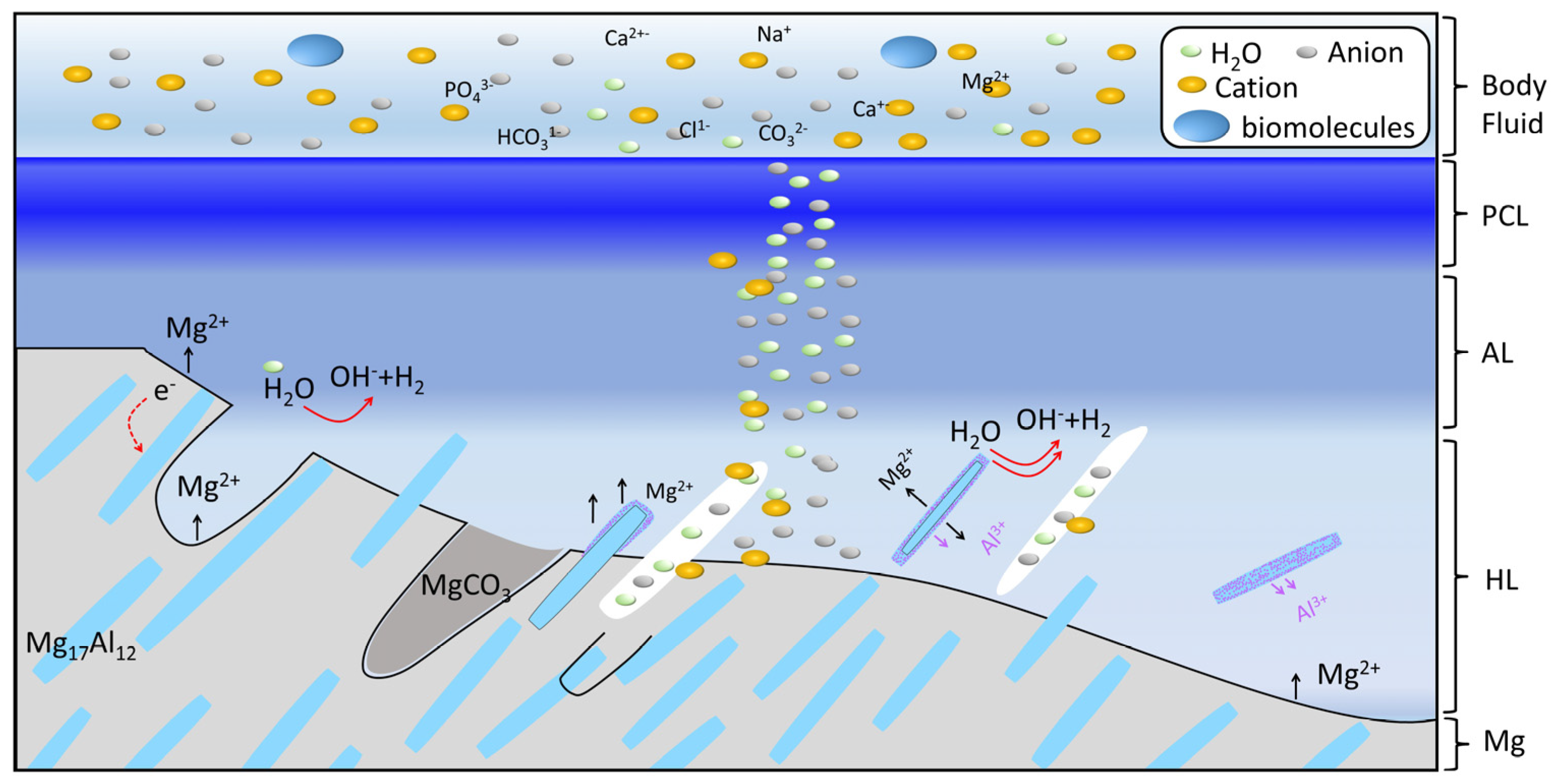
4.2. Degradation Mechanism of AZ91 Alloy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and Its Alloys as Orthopedic Biomaterials: A Review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable Metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Witte, F. The History of Biodegradable Magnesium Implants: A Review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current Status on Clinical Applications of Magnesium-Based Orthopaedic Implants: A Review from Clinical Translational Perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Emerging Magnesium-Based Biomaterials for Orthopedic Implantation. Emerg. Mater. Res. 2020, 8, 305–319. [Google Scholar] [CrossRef]
- Virtanen, S. Biodegradable Mg and Mg Alloys: Corrosion and Biocompatibility. Mater. Sci. Eng. B 2011, 176, 1600–1608. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and Advances in Magnesium Alloy Corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Popa, M.; Stefan, L.M.; Prelipcean, A.M.; Drob, S.I.; Anastasescu, M.; Calderon Moreno, J.M. Inhibition of Mg Corrosion in Physiological Fluids by Carbonate Coating. Corros. Sci. 2022, 209, 110775. [Google Scholar] [CrossRef]
- Witte, F.; Kaese, V.; Haferkamp, H.; Switzer, E.; Meyer-Lindenberg, A.; Wirth, C.J.; Windhagen, H. In Vivo Corrosion of Four Magnesium Alloys and the Associated Bone Response. Biomaterials 2005, 26, 3557–3563. [Google Scholar] [CrossRef]
- Xue, D.; Yun, Y.; Tan, Z.; Dong, Z.; Schulz, M.J. In Vivo and In Vitro Degradation Behavior of Magnesium Alloys as Biomaterials. J. Mater. Sci. Technol. 2012, 28, 261–267. [Google Scholar] [CrossRef]
- Yang, L.; Hort, N.; Laipple, D.; Höche, D.; Huang, Y.; Kainer, K.U.; Willumeit, R.; Feyerabend, F. Element Distribution in the Corrosion Layer and Cytotoxicity of Alloy Mg-10Dy during in Vitro Biodegradation. Acta Biomater. 2013, 9, 8475–8487. [Google Scholar] [CrossRef] [PubMed]
- Grillo, C.A.; Alvarez, F.; Fernández Lorenzo de Mele, M.A. Degradation of Bioabsorbable Mg-Based Alloys: Assessment of the Effects of Insoluble Corrosion Products and Joint Effects of Alloying Components on Mammalian Cells. Mater. Sci. Eng. C 2016, 58, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Martinez Sanchez, A.H.; Luthringer, B.J.C.; Feyerabend, F.; Willumeit, R. Mg and Mg Alloys: How Comparable Are in Vitro and in Vivo Corrosion Rates? A Review. Acta Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Almajidi, Y.Q.; Ali, E.; Jameel, M.F.; Saleh, L.H.; Aggarwal, S.; Zearah, S.A.; Alamula, A.F.; Alsaalamy, A.; Sharifianjazi, F.; Bathaei, M.S. Unveiling the Effect of Particle Incorporation in PEO Coatings on the Corrosion and Wear Performance of Magnesium Implants. Lubricants 2023, 11, 519. [Google Scholar] [CrossRef]
- Esmaily, M.; Shahabi-Navid, M.; Svensson, J.E.; Halvarsson, M.; Nyborg, L.; Cao, Y.; Johansson, L.G. Influence of Temperature on the Atmospheric Corrosion of the Mg-Al Alloy AM50. Corros. Sci. 2015, 90, 420–433. [Google Scholar] [CrossRef]
- Sebastián, F.J. The Application of X-Ray Photoelectron Spectroscopy in Understanding Corrosion Mechanisms of Magnesium and Mg-Al Alloys. Open Surf. Sci. J. 2011, 3, 1–14. [Google Scholar] [CrossRef]
- Esmaily, M.; Blücher, D.B.; Svensson, J.E.; Halvarsson, M.; Johansson, L.G. New Insights into the Corrosion of Magnesium Alloys—The Role of Aluminum. Scr. Mater. 2016, 115, 91–95. [Google Scholar] [CrossRef]
- Taheri, M.; Danaie, M.; Kish, J.R. TEM Examination of the Film Formed on Corroding Mg Prior to Breakdown. J. Electrochem. Soc. 2014, 161, C89–C94. [Google Scholar] [CrossRef]
- Bowen, P.K.; McNamara, C.T.; Mills, O.P.; Drelich, J.; Goldman, J. FIB-TEM Study of Magnesium Corrosion Products after 14 Days in the Murine Artery. ACS Biomater. Sci. Eng. 2015, 1, 919–926. [Google Scholar] [CrossRef]
- Grieb, T.; Krause, F.F.; Mahr, C.; Zillmann, D.; Müller-Caspary, K.; Schowalter, M.; Rosenauer, A. Optimization of NBED Simulations for Disc-Detection Measurements. Ultramicroscopy 2017, 181, 50–60. [Google Scholar] [CrossRef]
- Usuda, K.; Numata, T.; Irisawa, T.; Hirashita, N.; Takagi, S. Strain Characterization in SOI and Strained-Si on SGOI MOSFET Channel Using Nano-Beam Electron Diffraction (NBD). Mater. Sci. Eng. B 2005, 124–125, 143–147. [Google Scholar] [CrossRef]
- Yu, S.; Gao, Y.; Liu, C.; Han, X. Effect of Aging Temperature on Precipitation Behavior and Mechanical Properties of Extruded AZ80-Ag Alloy. J. Alloys Compd. 2015, 646, 431–436. [Google Scholar] [CrossRef]
- Bordbar-Khiabani, A.; Yarmand, B.; Mozafari, M. Effect of ZnO Pore-Sealing Layer on Anti-Corrosion and in-Vitro Bioactivity Behavior of Plasma Electrolytic Oxidized AZ91 Magnesium Alloy. Mater. Lett. 2020, 258, 126779. [Google Scholar] [CrossRef]
- Brooks, E.K.; Der, S.; Ehrensberger, M.T. Corrosion and Mechanical Performance of AZ91 Exposed to Simulated Inflammatory Conditions. Mater. Sci. Eng. C 2016, 60, 427–436. [Google Scholar] [CrossRef]
- Razavi, M. In Vitro Evaluations of Anodic Spark Deposited AZ91 Alloy as Biodegradable Metallic Orthopedic Implant. Annu. Res. Rev. Biol. 2014, 4, 3716–3733. [Google Scholar] [CrossRef]
- Zainal Abidin, N.I.; Martin, D.; Atrens, A. Corrosion of High Purity Mg, AZ91, ZE41 and Mg2Zn0.2Mn in Hank’s Solution at Room Temperature. Corros. Sci. 2011, 53, 862–872. [Google Scholar] [CrossRef]
- Zainal Abidin, N.I.; Rolfe, B.; Owen, H.; Malisano, J.; Martin, D.; Hofstetter, J.; Uggowitzer, P.J.; Atrens, A. The in Vivo and in Vitro Corrosion of High-Purity Magnesium and Magnesium Alloys WZ21 and AZ91. Corros. Sci. 2013, 75, 354–366. [Google Scholar] [CrossRef]
- Wang, L.; Shinohara, T.; Zhang, B. Corrosion Behavior of Mg, AZ31, and AZ91 Alloys in Dilute NaCl Solutions. J. Solid State Electrochem. 2010, 14, 1897–1907. [Google Scholar] [CrossRef]
- Mena-morcillo, E.; Veleva, L. Degradation of AZ31 and AZ91 Magnesium Alloys in Different Physiological Media: Effect of Surface Layer Stability on Electrochemical Behaviour. J. Magnes. Alloy. 2020, 8, 667–675. [Google Scholar] [CrossRef]
- Gonzalez, J.; Hou, R.Q.; Nidadavolu, E.P.S.; Willumeit-Römer, R.; Feyerabend, F. Magnesium Degradation under Physiological Conditions—Best Practice. Bioact. Mater. 2018, 3, 174–185. [Google Scholar] [CrossRef]
- Zeng, G.; Liu, C.; Gao, Y.; Jiang, S. Microstructure and Mechanical Properties of AZ80-Ag Alloy Processed by Hot Ring Rolling. Mater. Sci. Eng. A 2016, 674, 491–497. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, G.; Li, Y.; Yu, X.; Yuan, S.; Nie, Z.; Wang, J.; Han, J.; Tan, C.; Guo, C. Degradation Behavior, Transport Mechanism and Osteogenic Activity of Mg–Zn–RE Alloy Membranes in Critical-Sized Rat Calvarial Defects. Coatings 2020, 10, 496. [Google Scholar] [CrossRef]
- Zhu, Y.; Hou, D.; Li, Q. Quasi In-Situ EBSD Analysis of Twinning-Detwinning and Slip Behaviors in Textured AZ31 Magnesium Alloy Subjected to Compressive-Tensile Loading. J. Magnes. Alloy. 2022, 10, 956–964. [Google Scholar] [CrossRef]
- Drynda, A.; Hassel, T.; Hoehn, R.; Perz, A.; Bach, F.; Peuster, M. Development and Biocompatibility of a Novel Corrodible Fluoride-coated Magnesium-calcium Alloy with Improved Degradation Kinetics and Adequate Mechanical Properties for Cardiovascular Applications. J. Biomed. Mater. Res. Part A 2010, 93A, 763–775. [Google Scholar] [CrossRef]
- Liu, G.; Wang, K.; Pan, Y.; Yang, D.; Yu, X.; Nie, Z.; Zhu, J.; Han, J.; Tan, C. Effect of Ag Content and Extrusion on the Microstructure and Mechanical Properties of Mg–Ag Alloys. J. Mater. Res. Technol. 2024, 30, 5916–5926. [Google Scholar] [CrossRef]
- Cihova, M.; Schmutz, P.; Schäublin, R.; Löffler, J.F. Biocorrosion Zoomed In: Evidence for Dealloying of Nanometric Intermetallic Particles in Magnesium Alloys. Adv. Mater. 2019, 31, 1903080. [Google Scholar] [CrossRef]
- Liao, J.; Hotta, M. Corrosion Products of Field-Exposed Mg-Al Series Magnesium Alloys. Corros. Sci. 2016, 112, 276–288. [Google Scholar] [CrossRef]
- Bowen, P.K.; Drelich, J.; Goldman, J. Magnesium in the Murine Artery: Probing the Products of Corrosion. Acta Biomater. 2014, 10, 1475–1483. [Google Scholar] [CrossRef]
- Taheri, M.; Kish, J.R.; Birbilis, N.; Danaie, M.; McNally, E.A.; McDermid, J.R. Towards a Physical Description for the Origin of Enhanced Catalytic Activity of Corroding Magnesium Surfaces. Electrochim. Acta 2014, 116, 396–403. [Google Scholar] [CrossRef]
- Jung, J.; Kwon, S.; Han, H.; Lee, J.; Ahn, J.; Yang, S.; Cho, S.; Cha, P.; Kim, Y.; Seok, H. In Vivo Corrosion Mechanism by Elemental Interdiffusion of Biodegradable Mg–Ca Alloy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100B, 2251–2260. [Google Scholar] [CrossRef]
- Walter, R.; Kannan, M.B. A Mechanistic in Vitro Study of the Microgalvanic Degradation of Secondary Phase Particles in Magnesium Alloys. J. Biomed. Mater. Res. Part A 2015, 103, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zanna, S.; Ardelean, H.; Frateur, I.; Schmutz, P.; Song, G.; Atrens, A.; Marcus, P. A First Quantitative XPS Study of the Surface Films Formed, by Exposure to Water, on Mg and on the Mg–Al Intermetallics: Al3Mg2 and Mg17Al12. Corros. Sci. 2009, 51, 1115–1127. [Google Scholar] [CrossRef]
- Milazzo, G.; Caroli, S.; Braun, R.D. Tables of Standard Electrode Potentials. J. Electrochem. Soc. 1978, 125, 261C. [Google Scholar] [CrossRef]
- SenGupta, A.K. Ion Exchange in Environmental Processes; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Atrens, A.; Johnston, S.; Shi, Z.; Dargusch, M.S. Viewpoint—Understanding Mg Corrosion in the Body for Biodegradable Medical Implants. Scr. Mater. 2018, 154, 92–100. [Google Scholar] [CrossRef]
- Wang, J.; Giridharan, V.; Shanov, V.; Xu, Z.; Collins, B.; White, L.; Jang, Y.; Sankar, J.; Huang, N.; Yun, Y. Flow-Induced Corrosion Behavior of Absorbable Magnesium-Based Stents. Acta Biomater. 2014, 10, 5213–5223. [Google Scholar] [CrossRef]
| Element | Al | Zn | Mn | Ce | Si | Cu | Fe | Ni | Mg |
|---|---|---|---|---|---|---|---|---|---|
| Content | 9.03 ± 0.01 | 0.50 ± 0.01 | 0.27 ± 0.01 | 0.019 ± 0.002 | <0.01 | <0.01 | <0.01 | <0.01 | Bal. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Yue, H.; Zhu, J.; Han, J. In Vivo Degradation Behavior of AZ91 Magnesium Alloy: Comprehensive Microstructural and Crystallographic Characterization by TEM and NBED. Materials 2025, 18, 1500. https://doi.org/10.3390/ma18071500
Liu Z, Yue H, Zhu J, Han J. In Vivo Degradation Behavior of AZ91 Magnesium Alloy: Comprehensive Microstructural and Crystallographic Characterization by TEM and NBED. Materials. 2025; 18(7):1500. https://doi.org/10.3390/ma18071500
Chicago/Turabian StyleLiu, Zhichao, Honglei Yue, Jianhua Zhu, and Jianmin Han. 2025. "In Vivo Degradation Behavior of AZ91 Magnesium Alloy: Comprehensive Microstructural and Crystallographic Characterization by TEM and NBED" Materials 18, no. 7: 1500. https://doi.org/10.3390/ma18071500
APA StyleLiu, Z., Yue, H., Zhu, J., & Han, J. (2025). In Vivo Degradation Behavior of AZ91 Magnesium Alloy: Comprehensive Microstructural and Crystallographic Characterization by TEM and NBED. Materials, 18(7), 1500. https://doi.org/10.3390/ma18071500







