A Review of Additive Manufacturing of Biodegradable Fe and Zn Alloys for Medical Implants Using Laser Powder Bed Fusion (LPBF)
Abstract
1. Introduction
1.1. Context and Background

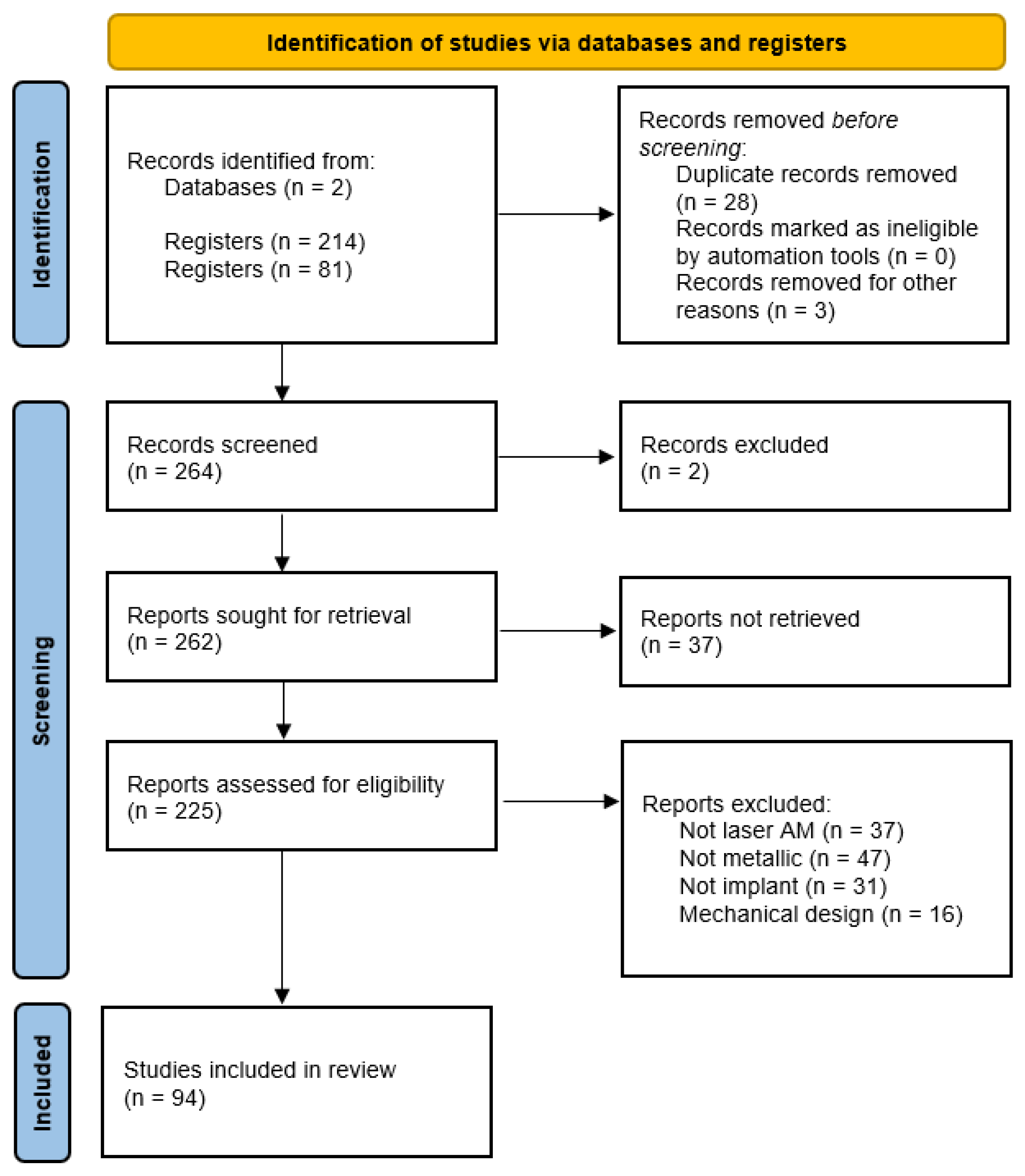
1.2. PRISMA
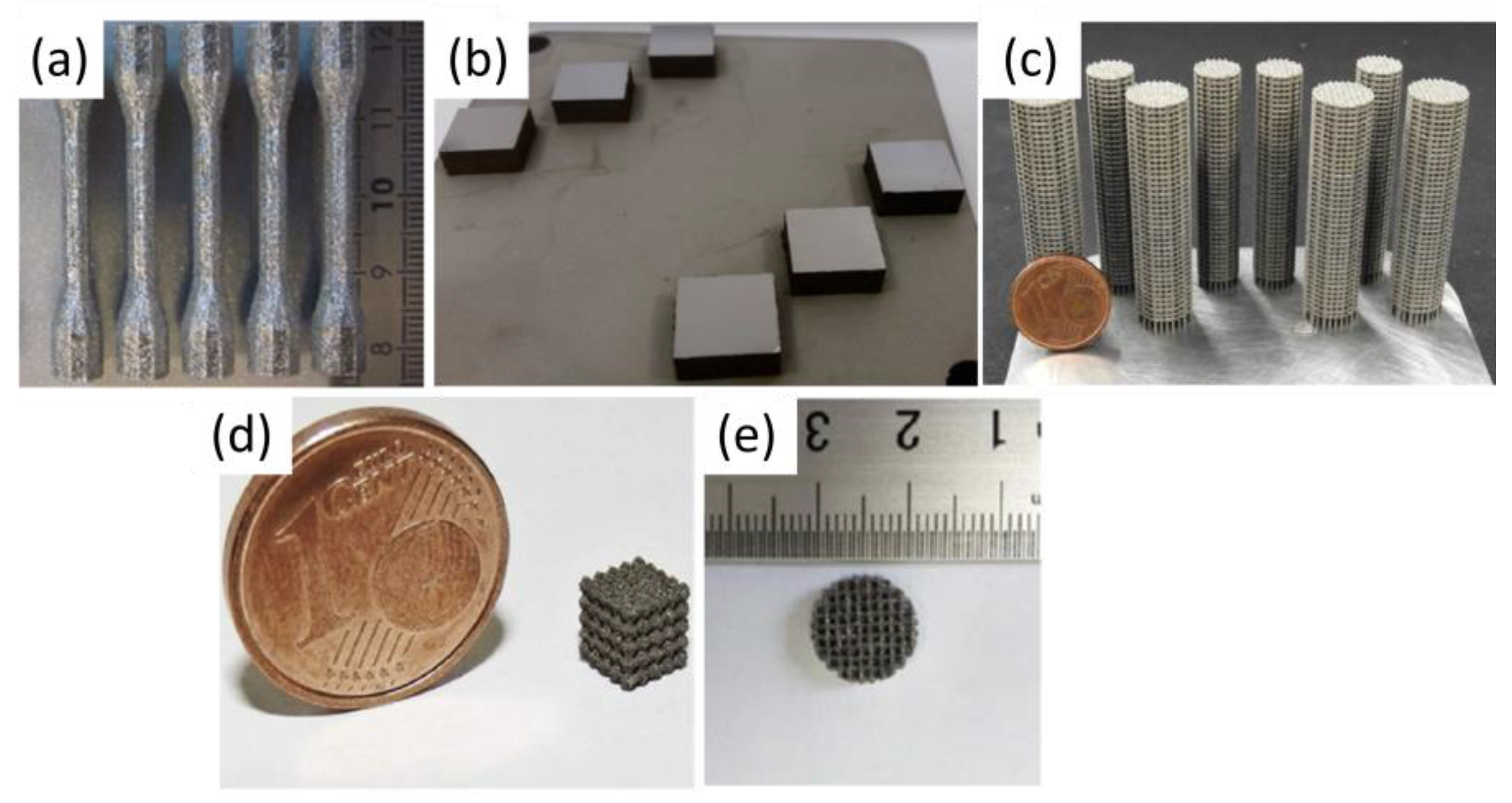
1.3. Additive Manufacturing—Laser Powder Bed Fusion
| Properties | Unit | Value | ||
|---|---|---|---|---|
| Zn | Fe | Mg | ||
| Density (20 °C) | g/cm3 | 7.14 | 7.874 | 1.74 |
| Melting point | °C | 419.5 | 1538 | 650 |
| Boiling point | °C | 907 | 2862 | 1091 |
| Heat conductivity (20 °C) | W/m·K | 113 | 80 | 158 |
| Heat conductivity (melting point) | W/m·K | 61 | 40 | 78 |
| Specific heat (20 °C) | J/kg K | 382 | 444 | 1360 |
| Surface tension (melting point) | mN/m | 782 | 1835 | 559 |
| Viscosity (melting point) | mPa·s | 3.85 | 6.93 | 1.25 |
| Laser absorptivity (powder, 20 °C) | % | 70 | 75 | / |

2. Additive Manufacturing of Iron-Based Alloys
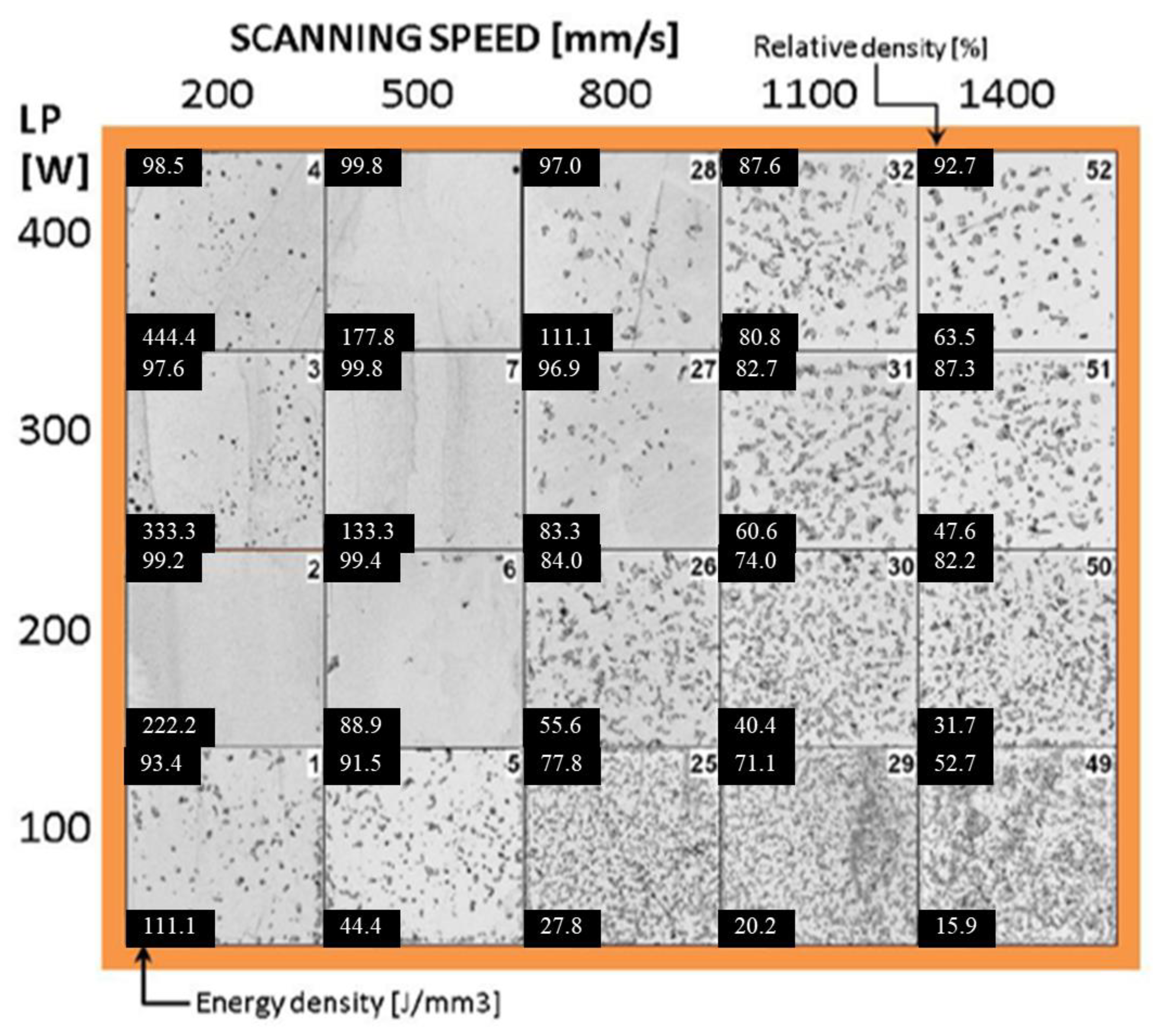
2.1. Linking Processing Parameters and Energy Density to Densification


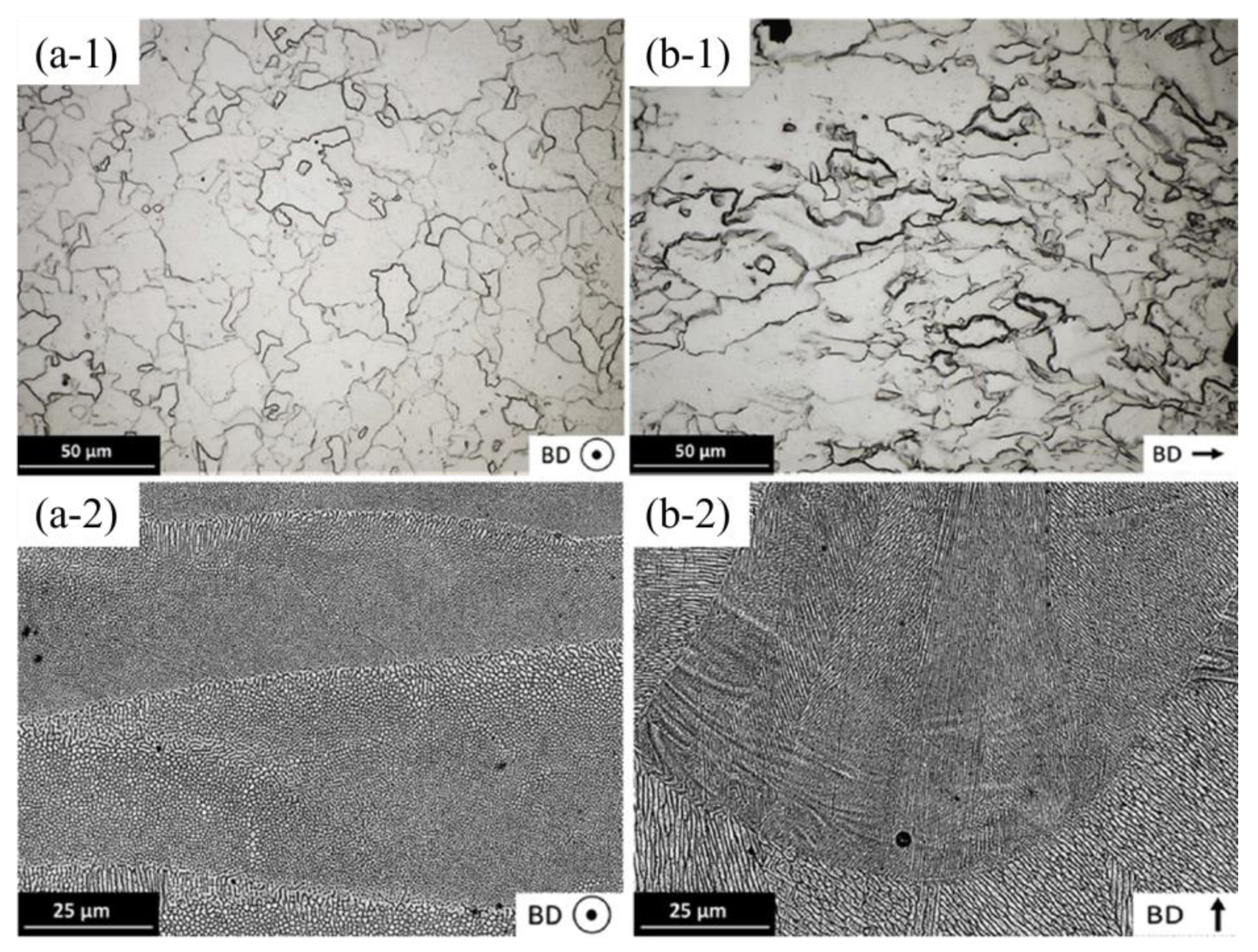
2.2. Influence of Microstructure on Hardness
| Human Bone | 40–79 | ||
|---|---|---|---|
| Material | SLM | Cast | Wrought |
| Pure Fe | 150 ± 6.5 | 130 (Mild steel) | 150 (Mild steel) |
| Fe35Mn | 163 ± 4.0 | n/a | n/a |
| AISI 316L | 245 ± 6.0 | 175 | 220 |
| Pure Zn | 45 ± 5.4 | n/a | 34 ± 2 |
| Pure Mg | 78 ± 8.2 | 30 ± 2 | 40 ± 2 |
2.3. Mechanical Properties of Dense Structures and Scaffolds
| Material | Energy Density (J/mm3) | E (GPa) | σ0.2 (MPa) | UTS (MPa) | Compressive Strength at 20% Strain (MPa) | Ref. |
|---|---|---|---|---|---|---|
| Human cortical bone | - | 1–35 | 1–20 | 103–140 | [53] | |
| Cast Fe | - | 202.5 ± 6.70 | 157.1 ± 7.7 | 497.8 ± 7.5 | [52] | |
| Cast Fe35Mn | - | 240 | 440 | [53] | ||
| SLM pure Fe | 185 | 205.67 ± 16 | 245.87 ± 17 | 354.27 ± 18 | - | [51] |
| SLM pure Fe | 152 | 208.77 ± 16 | 256.57 ± 17 | 356.67 ± 22 | - | |
| SLM pure Fe | 143 | 210.57 ± 18 | 285.47 ± 20 | 402.77 ± 24 | - | |
| SLM pure Fe | 125 | 215.87 ± 20 | 305.37 ± 22 | 411.57 ± 25 | - | |
| SLM pure Fe | 67 | 199.70 ± 6.70 | 421.1 ± 16 | - | 760.2 ± 6.5 | [52] |
| Material | Energy Density (J/mm3) | Struct Size (µm)/Pore Size (µm) | Geometry | E (GPa) | σ0.2 (MPa) | UTS (MPa) | Ref. | |
|---|---|---|---|---|---|---|---|---|
| LPBF pure Fe | 400/600 | Diamond | 2.82 ± 0.17 | 53.1 ± 0.9 |  | [60] | ||
| LPBF pure Fe | 200/800 | Diamond | 0.89 ± 0.09 | 10.70 ± 0.40 |  | [60] | ||
| LPBF pure Fe | 200–400/800–600 | Diamond | 1.77 ± 0.05 | 32.9 ± 1.6 |  | [60] | ||
| LPBF pure Fe | 400–200/600–800 | Diamond | 1.75 ± 0.03 | 30.5 ± 0.3 |  | [60] | ||
| LPBF pure Fe | 10 | 600–800/- | - | 70.3 ± 4.2 | 135 ± 5.2 |  | [55] | |
| LPBF Fe25Mn | 10 | 600–800/- | - | 137 ± 8.4 | 221.7 ± 10.9 |  | [55] | |
| LPBF Fe35Mn | 62 | 600/400 | Primitive Surface p-unit | 33.5 ± 1.70 | 89.2 ± 1.9 | - |  | [53] |
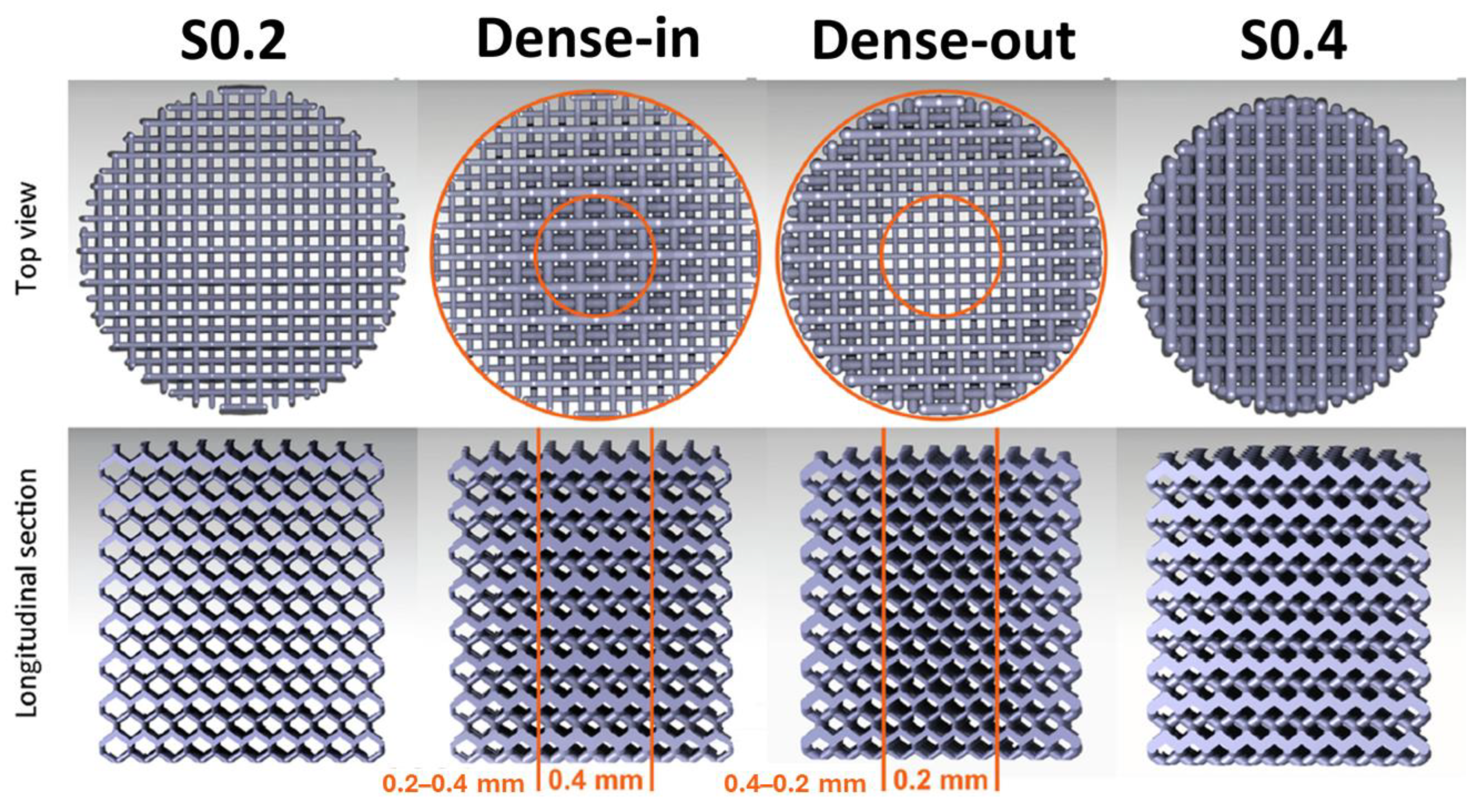
2.4. Corrosion Behavior
| Material | Part | Energy Density (J/mm3) | Corrosion Test | Conditions | CR (mm/year) | Icorr (mA/cm2) | Rp (Ω cm2) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cast Fe | - | - | Electrochemical test | OCP: 150 min; EIS: 10 mV, 100 kHz–10 mHz; Lp: ±0.25 V (vs. SCE), 0.166 mVs−1. In HBSS. | 0.047 ± 0.003 | 4.05 ± 0.3 | 1410 | [52] |
| Cold-Rolled Iron | Electrochemical test | OCP: 60 min; EIS: 10 mV; 100 kHz–10 mHz; Lp: −0.3–+0.5 V (vs. SCE),0.5 mVs−1. In r-SBF. | 0.10 ± 0.01 | 0.0086 ± 0.0009 | - | [63] | ||
| Cold-Rolled Iron | Immersion test | Samples immersed for 28 days in r-SBF. | - | [63] | ||||
| LPBF Pure Fe | Dense | 67 | Electrochemical test | OCP: 150 min; EIS: 10 mV, 100 kHz–10 mHz; Lp: ±0.25 V (vs. SCE), 0.166 mVs−1. In HBSS. | 0.072 ± 0.001 | 6.2 ± 0.1 | 1035 | [52] |
| LPBF Pure Fe | Scaffold | 10 | Electrochemical test | EIS: 10 Mv, 100 kHz–10 mHz. In SBF. | - | 0.00738 ± 0.00321 | [55] | |
| LPBF Pure Fe | Scaffold | 10 | Immersion test | Samples immersed for 30 days in SBF with a pH of 7.4 | 0.09 ± 0.02 | [55] | ||
| LPBF Pure Fe | Scaffold | - | Electrochemical test | OCP: 60 min; EIS:10 mV; 100 kHz–10 mHz; Lp: −0.3–+0.5 V (vs. SCE), 0.5 mVs−1. In r-SBF. | 1.18 ± 0.22 | 0.1028 ± 0.0192 | - | [63] |
| LPBF Pure Fe | Scaffold | - | Immersion test | Samples immersed for 28 days in r-SBF | 0.03 | - | [63] | |
| LPBF Pure Fe | Scaffold S0.2 | - | Immersion test | Samples immersed for 28 days in r-SBF | 0.19446 | - | - | [60] |
| LPBF Pure Fe | Scaffold (Dense-in) | - | Immersion test | Samples immersed for 28 days in r-SBF | 0.1389 | - | - | [60] |
| LPBF Pure Fe | Scaffold (Dense-out) | - | Immersion test | Samples immersed for 28 days in r-SBF | 0.17131 | - | - | [60] |
| SLM Pure Fe | Scaffold S0.4 | - | Immersion test | Samples immersed for 28 days in r-SBF | 0.12501 | - | - | [60] |
| LPBF Pure Fe | Scaffold | Electrochemical test | OCP: 60 min; Lp: −0.2–+0.5 V (vs. SCE),0.1 mVs−1. In HBSS. | 0.049 | 0.0042 | [64] | ||
| LPBF Fe25Mn | Scaffold | 10 | Electrochemical test | EIS: 10 mV,100 kHz–10 mHz. In SBF. | - | 0.05125 ± 0.00752 | [55] | |
| LPBF Fe25Mn | Scaffold | 10 | Immersion test | Samples immersed for 30 days in SBF with a pH of 7.4 | 0.23 ± 0.05 | [55] | ||
| LPBF Fe30Mn | Scaffold | Electrochemical test | OCP: 60 min; Lp: −0.2–+0.5 V (vs. SCE),0.1 mVs−1. In HBSS. | 0.142 | 0.01191 | [64] | ||
| LPBF Fe35Mn | Scaffold | 62 | Electrochemical test | OCP: 150 min; EIS: 10 mV,100 kHz–10 mHz; Lp: ±0.25 V (vs. SCE),0.166 mVs−1. In HBSS. | 0.8 | [53] | ||
| LPBF Fe35Mn | Scaffold | 62 | Immersion test | Samples immersed for 28 days in HBSS with a pH of 7.4 | 0.42 ± 0.03 | [53] |

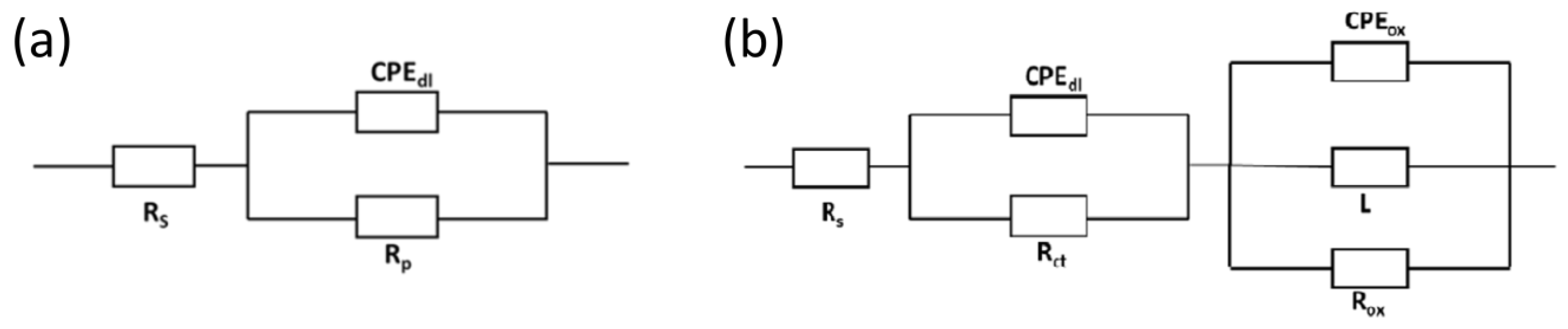
2.5. In Vitro Cytocompatibility
| Material | Cell Assay | Cell Line | Duration | Results | Ref. |
|---|---|---|---|---|---|
| Fe | Indirect | MG-63 | 3 days | good cytocompatibility, with cells growing normally on the scaffolds | [55] |
| Fe25Mn | Indirect | MG-63 | 3 days | good cytocompatibility, with cells growing normally on the scaffolds | [55] |
| Fe30Mn | Indirect; cell proliferation | MC3T3-E1 | 7 days | RGR grade 0. The extract is not cytotoxic. | [64] |
| Fe30Mn | Direct; live/dead test | MC3T3-E1 | 7 days | seven days co-culture results in many live cells and only a few dead cells | [64] |
| Fe35Mn | Indirect; cell proliferation and MTT tests | MC3T3-E1 | 3 days | the scaffold displayed biocompatibility, high viability towards mammalian cells, and filopodia on the scaffold indicated that the alloy is suitable for osteoblast adhesion | [53] |
| Fe | Indirect; cell proliferation | MG-63 | 3 days | MG-63 viability in extended, long-term extracts (72 h) of iron specimens dropped to below 50%. | [63] |
| Fe | Direct; live/dead test | MG-63 | 1 day | revealed substantial and almost instant cytotoxicity | [63] |
2.6. In Vivo Studies
| Material | Shape | Animal | Implantation Place | Duration | Results | Reference |
|---|---|---|---|---|---|---|
| Fe30Mn | Scaffold | Rabbit | lateral femoral condyle | 48 weeks | biocompatibility and osseointegration performances in the repair of load-bearing bone defects | [64] |
| Fe35Mn | Scaffold | Rat | cranium | 4 weeks | the implant integrated with the original bone, and even stimulated bone formation | [53] |
3. Additive Manufacturing of Zn-Based Alloys
3.1. Linking Processing Parameters and Energy Density to Densification

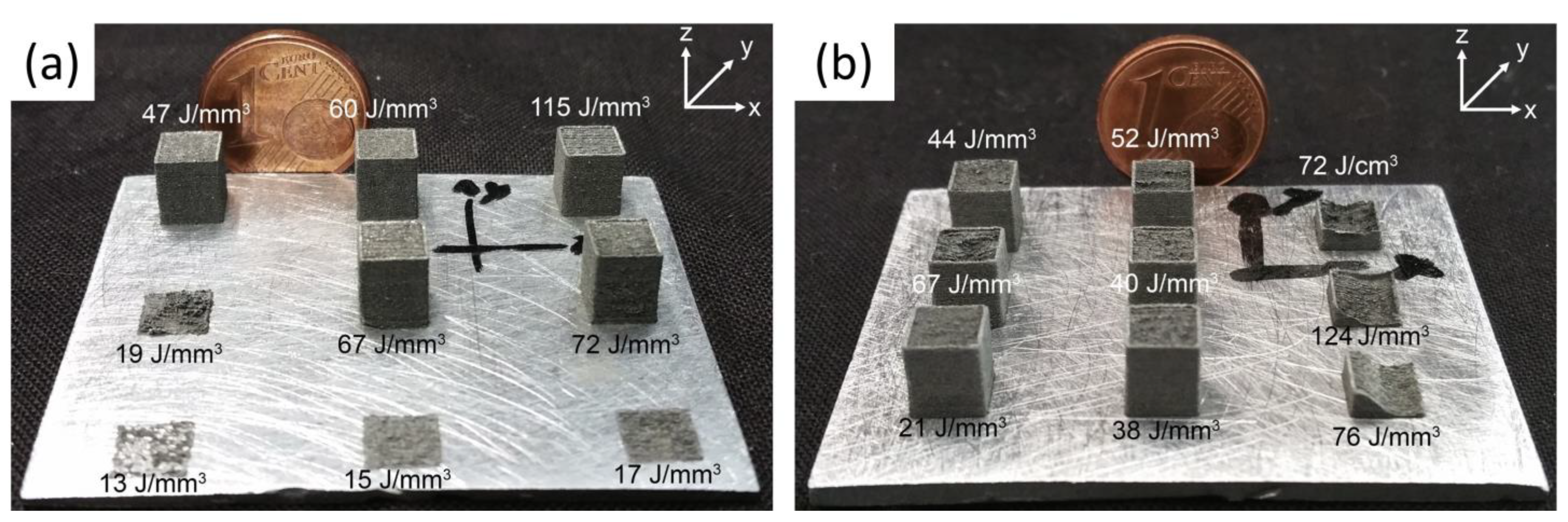
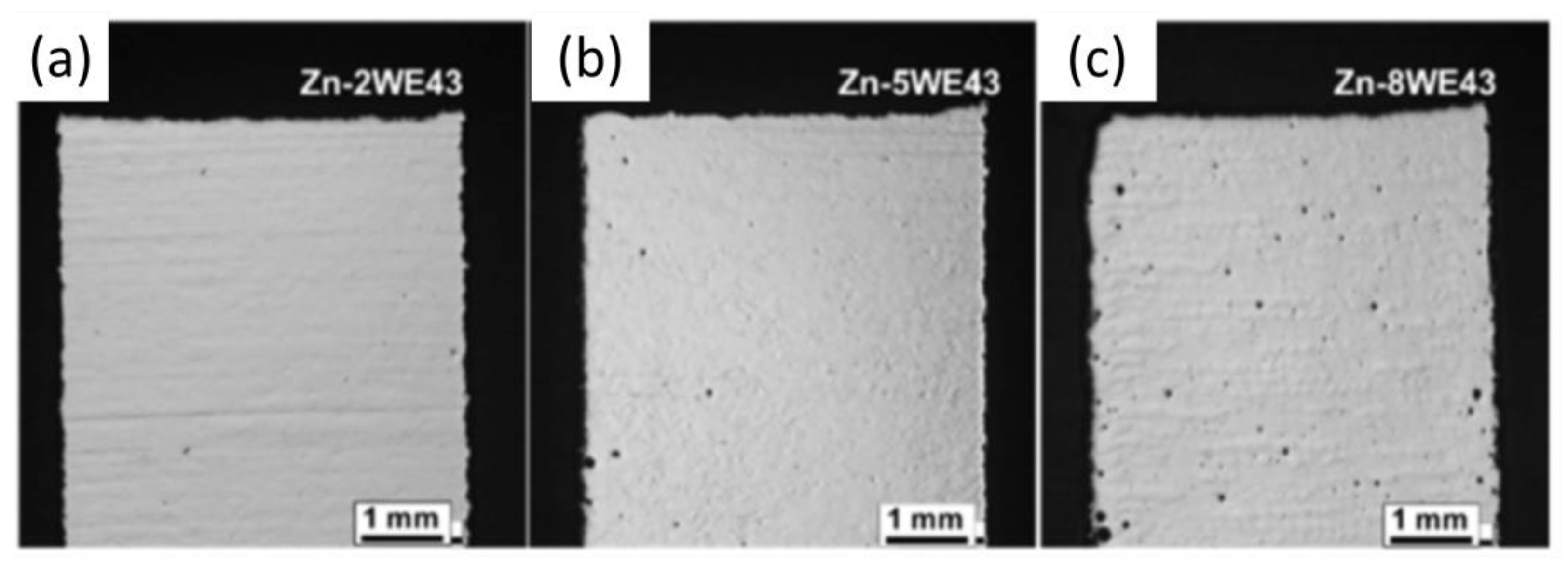
3.2. Influence of the Microstructure on Hardness
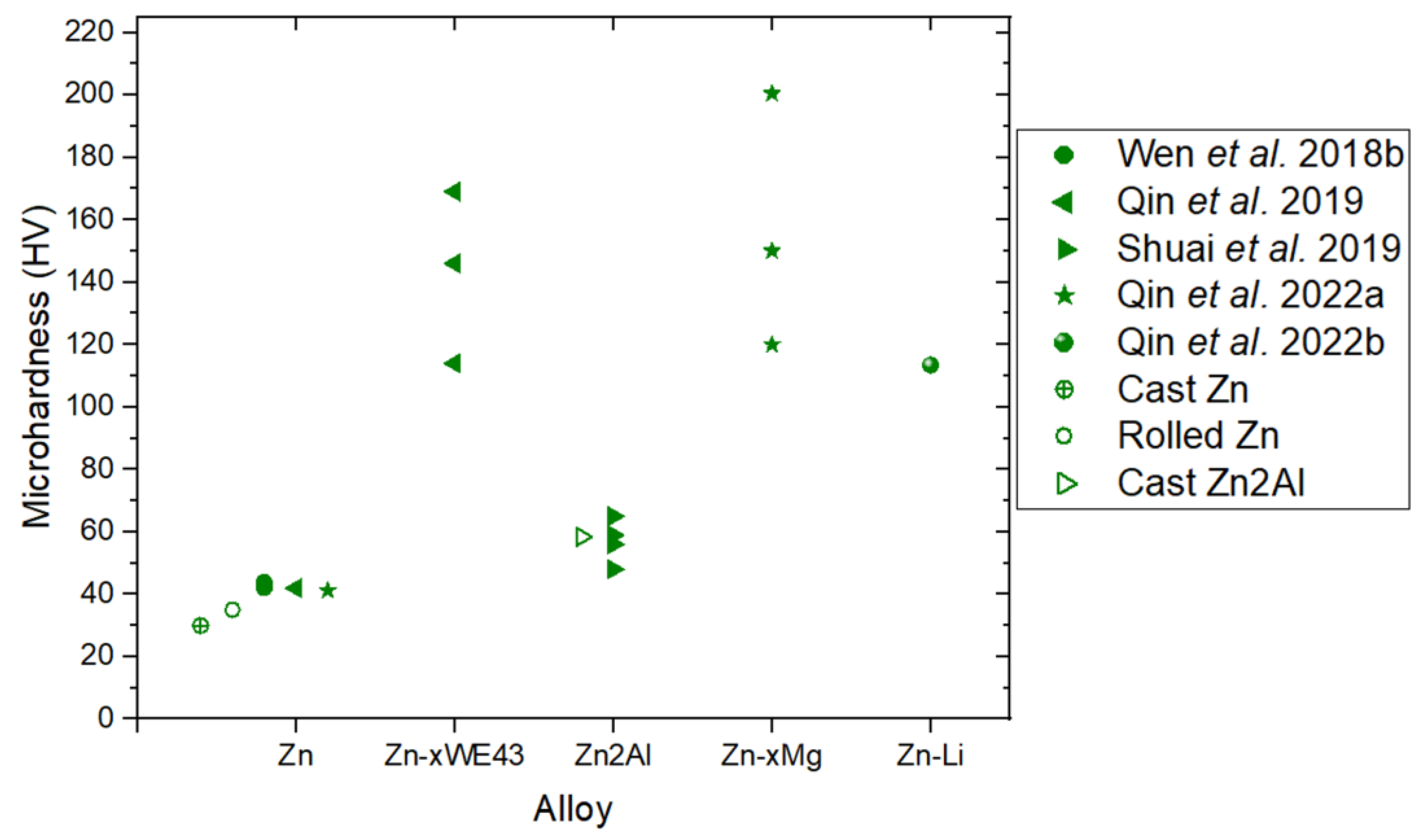
3.3. Mechanical Properties of Dense Structures and Scaffolds
| Material | Ev (J/mm3) | Yield Strength (MPa) | UTS (MPa) | Elongation (%) | Ref. |
|---|---|---|---|---|---|
| Zn | 66.7 | 134 | 10 | [80] | |
| Zn2WE43 | 298.5 | 1.8 | |||
| Zn5WE43 | 335.4 | 1 | |||
| Zn8WE43 | 154.1 | 0.9 | |||
| Zn | 125 | 43.2 | 61.3 | 1.7 | [83] |
| Zn1Mg | 74 | 126 | 3.6 | ||
| Zn2Mg | 117 | 162 | 4.1 | ||
| Zn3Mg | 152 | 222 | 7.2 | ||
| Zn4Mg | 132 | 166 | 3.1 | ||
| Zn2Al | 76.19 | 120 | 170 | 9 | [81] |
| 95.24 | 135 | 185 | 10 | ||
| 114.28 | 140 | 190 | 12 | ||
| 133.33 | 138 | 188 | 11 | ||
| Zn | 55.55 | 79.9 | 103.6 | 5.10 | [82] |
| Zn1Ce | 140 | 210 | 6 | ||
| Zn2Ce | 180.6 | 247.4 | 7.5 | ||
| Zn3Ce | 182 | 230 | 6.8 | ||
| Zn (Vertical) | 127(300 mm/s) | 94 | 119 | 2.6 | [87] |
| 76.19 (500 mm/s) | 108 | 130 | 8 | ||
| 54.42(700 mm/s) | 110.3 | 132 | 7 | ||
| Zn (Horizontal) | 127(300 mm/s) | 72 | 90 | 2.5 | |
| 54.42 (700 mm/s) | 75 | 92.3 | 5 | ||
| Zn (Vertical) | 39 | 78 | 100 | 10 | [86] |
| Zn (Horizontal) | 55 | 79 | 12 |
| Material | Ev (J/mm3) | Structural Porosity/Pore Size | Geometry | Ultimate Compressive Strength (MPa) | Yield Strength (MPa) | Elastic Modulus (GPa) | Ref. |
|---|---|---|---|---|---|---|---|
| Zn | 39 | 73%/700 μm | Diamond | 4 | 0.4 | [60] | |
| 69%/Graded pore size 600–800 μm | Diamond | 6 | 0.5 | ||||
| 62%/600 μm | Diamond | 11 | 0.8 | ||||
| Zn | 39 | 20–40% | Diamond | 7–15 * | [86] | ||
| 22–40% | Dodecahedron | 8–25 * | |||||
| 25–45% | FCC | 10–50 * | |||||
| 22–35% | Kagome | 15–50 * | |||||
| 30–50% | Octet Truss | 9–30 * | |||||
| Zn | 66.7 | 45%/600 μm | Diamond | 23 | 13 | 0.95 | [80] |
| Zn2WE43 | 60 | 51 | 1.91 | ||||
| Zn5WE43 | 73 | 66 | 2.48 | ||||
| Zn8WE43 | 51 | 51 | 2.54 | ||||
| Zn | 100 | 50% | Diamond | [84] | |||
| Zn1Mg | 40 | 1.2 | |||||
| Zn2Mg | 35 | 1.3 | |||||
| Zn5Mg | 24 | 1 | |||||
| Zn0.7Li | 35.7 | 80%/820 μm | Gyroid | 18.2 | 0.298 | [85] |
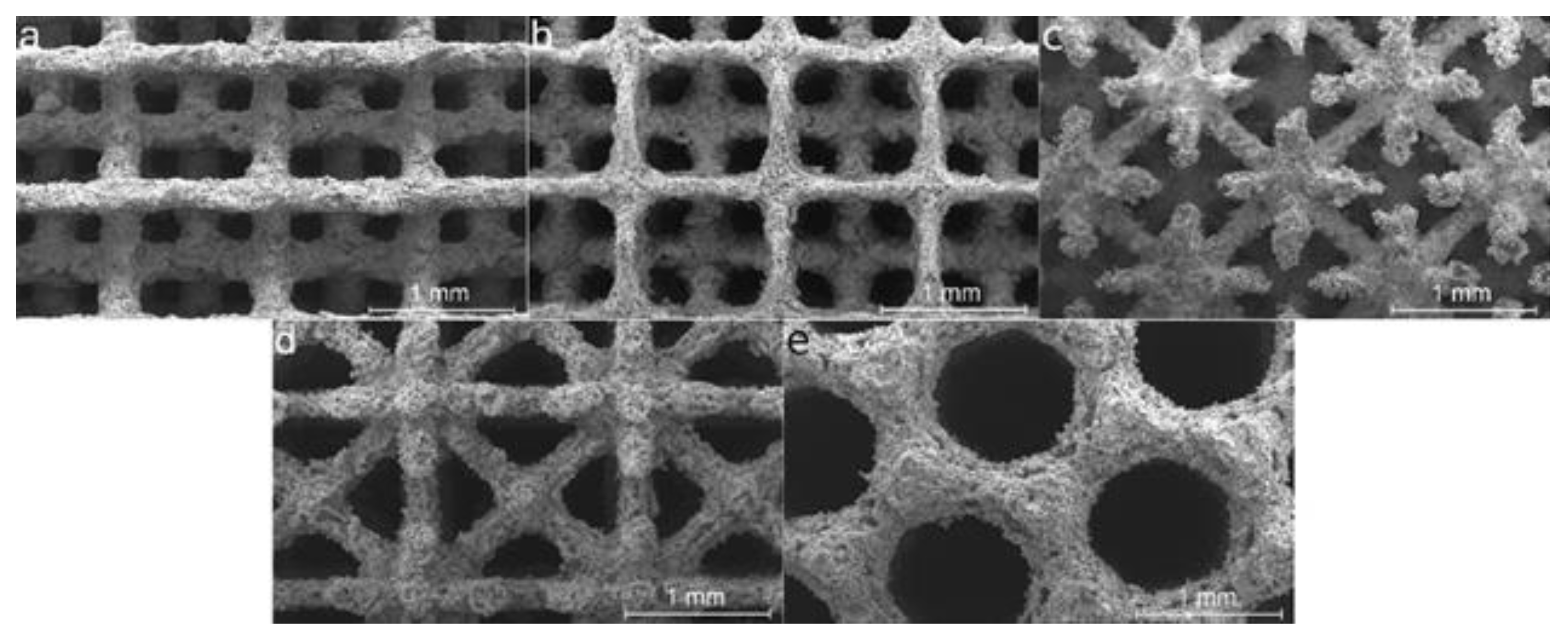
3.4. Corrosion Behavior
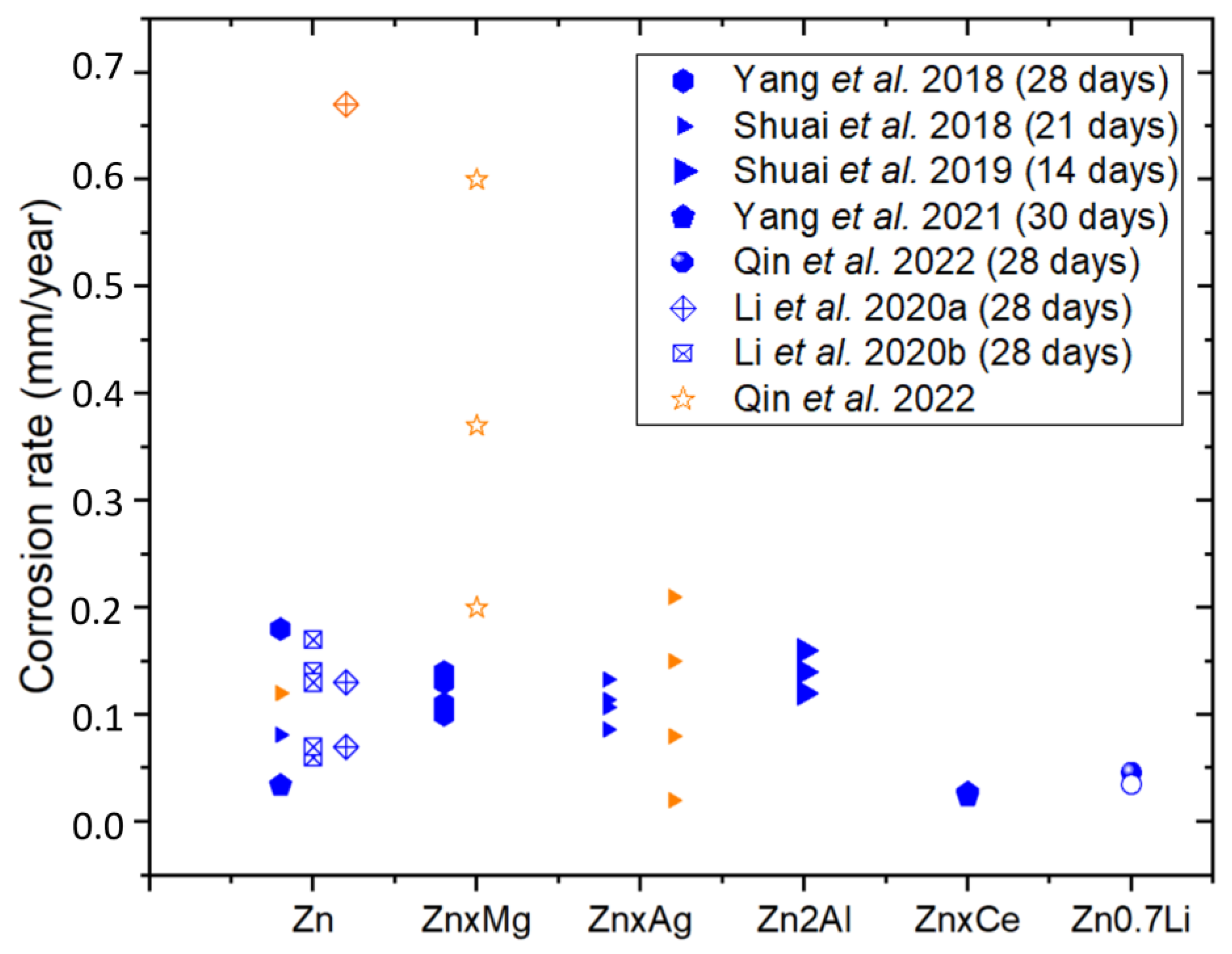
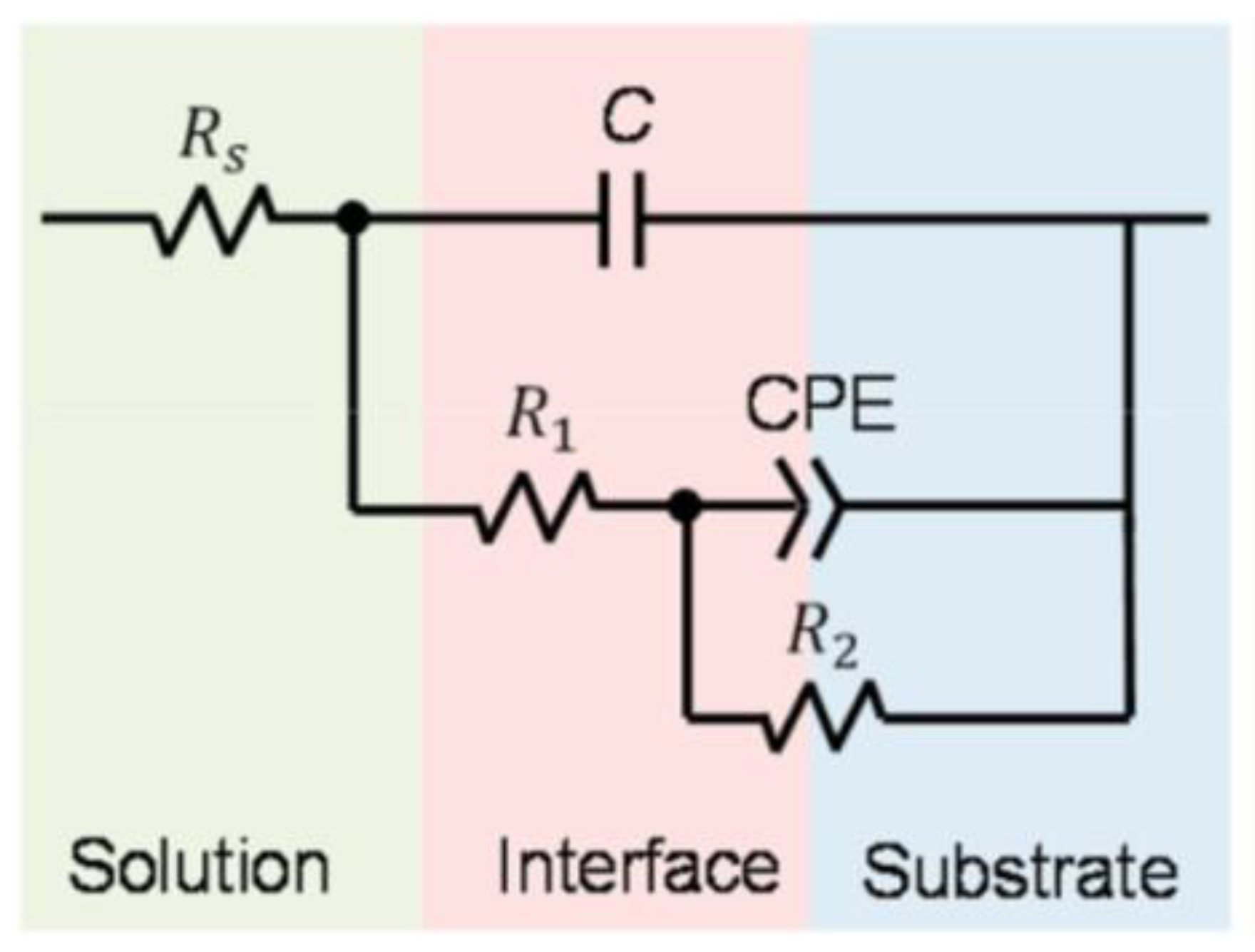
| Material | Part | Energy Density (J/mm3) | Electrochemical Test | Immersion Test | Ref | |||
|---|---|---|---|---|---|---|---|---|
| Conditions | CR (mm/year) | icorr (μA/cm2) | Conditions | CR (mm/year) | ||||
| Zn | Dense | 125 | Samples soaked in SBF at 37 °C to obtain an OCP and polarization curves were recorded. | 0.14 * | 9.24 ± 1.21 | Samples immersed in SBF at 37 °C for 4 weeks | 0.18 ± 0.03 | [83] |
| Zn1Mg | 0.09 * | 5.86 ± 1.42 | 0.14 ± 0.01 | |||||
| Zn2Mg | 0.07 * | 4.63 ± 0.95 | 0.13 ± 0.03 | |||||
| Zn3Mg | 0.05 * | 3.62 ± 0.76 | 0.10 ± 0.02 | |||||
| Zn4Mg | 0.06 * | 3.71 ± 0.87 | 0.11 ± 0.04 | |||||
| Zn | Dense | OCP: measured for 90 min (SBF). Scanning rate 1 mV/s | 0.12 | 7.76 | Samples immersed in SBF at 37 °C for 21 days | 0.081 | [88] | |
| Zn2Ag | 0.08 | 5.01 | 0.086 | |||||
| Zn4Ag | 0.02 | 1.47 | 0.107 | |||||
| Zn6Ag | 0.15 | 9.56 | 0.114 | |||||
| Zn8Ag | 0.21 | 13.94 | 0.133 | |||||
| Zn2Al | Dense | 95.24 | Samples immersed in SBF at 37 °C. OCP ± 300 mV | 0.18 * | 11.75 | Samples immersed in SBF at 37 °C for 14 days | 0.16 | [81] |
| 114.28 | 0.12 * | 8 | 0.14 | |||||
| 133.33 | 0.10 * | 7.07 | 0.12 | |||||
| Zn | Dense | OCP: 50 min, −200 mV to 200 mV at 0.05 mV/s. EIS: 10−2–106 Hz, 10 mV (SBF) | 0.13 * | 9 | Samples immersed in SBF for 30 days | 0.034 | [82] | |
| Zn1Ce | 0.12 * | 8 | 0.027 | |||||
| Zn2Ce | 0.11 * | 7.2 | 0.025 | |||||
| Zn3Ce | 0.10 * | 6.9 | 0.024 | |||||
| Zn0.7Li | Dense as built | EIS: frequency range 10−2–105 Hz 10 mV (Hank’s solution) | 1.5 * | 101 ± 4.1 | Samples immersed in Hank’s solution at 37 °C for 28 days | [85] | ||
| Dense polished | 0.43 * | 28.5 ± 1.6 | 0.046 | |||||
| Scaffold (Porosity 80%) | 1.6 * | 111.2 ± 12.2 | 0.035 | |||||
| Zn Scaffolds | Porosity 73% | Samples immersed in r-SBF for 28 days. Static and dynamic tests. Dynamic tests at a flow rate of 0.3 mL/min. | 0.17 (Dynamic) 0.07 (Static) | [90] | ||||
| Porosity 69% | 0.14 (Dynamic) 0.06 (Static) | |||||||
| Porosity 62% | 0.13 (Dynamic) 0.07 (Static) | |||||||
| Zn Scaffolds | Porosity 62% | r-SBF at 37 °C. Specimen was polarized from −0.2 V to +0.5 V potential versus OCP at 0.5 mV/s scan rate. For EIS: 1, 2, 7, 14, 21 and 28 days, 10 mV, 100 kHz | 0.67 ± 0.04 | 45 ± 2 | r-SBF at 37 °C for 28 days | 0.13 (Dynamic) 0.07 (Static) | [91] | |
| Zn | Scaffold (Porosity 50%) | Sample immersed in Hank’s solution for 1.5 h | [84] | |||||
| Zn1Mg | 0.20 * | 13.5 ± 5.7 | ||||||
| Zn2Mg | 0.37 * | 24.9 ± 10.6 | ||||||
| Zn5Mg | 0.60 * | 40 ± 11.3 | ||||||
3.5. In Vitro Cytocompatibility
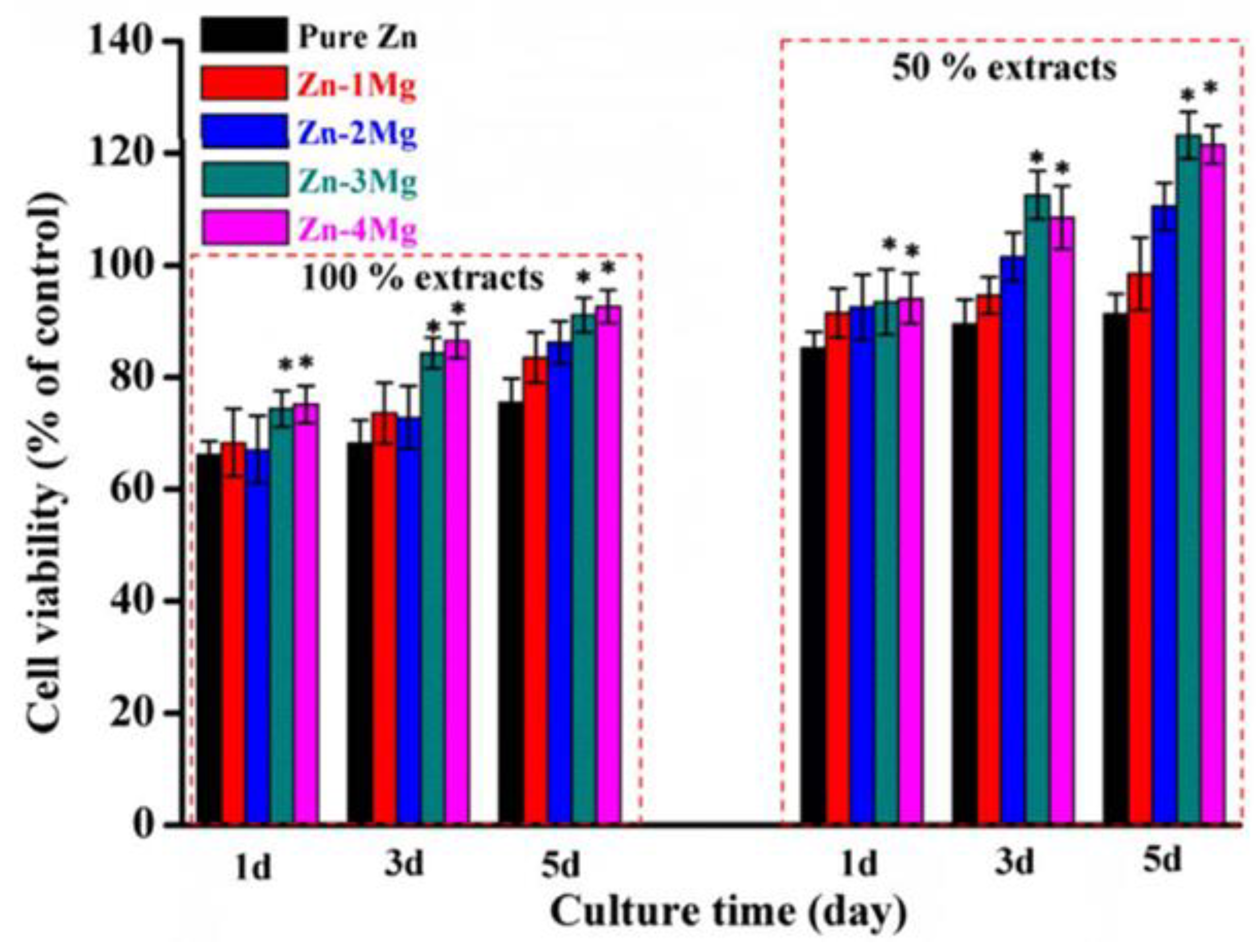
| Material | Cell Assay | Cell Line | Duration | Medium | Conditions | Results | Ref. |
|---|---|---|---|---|---|---|---|
| ZnxMg | Indirect CCK-8 | MG-63 cells | 6 h, 1, 3, and 5 days | DMEM + FBS | Dense samples 100% and 50% extracts. (1.25 cm2/mL). | Good viability; better in 50% extracts. Mg content increases cell viability. Zn-3Mg best viability. | [83] |
| ZnxCe | Indirect CCK-8 | MG-63 | 1, 3, and 7 days | DMEM + FBS, antibiotics | Dense samples. Extracts (1.25 cm2/mL). | Zn2Ce viability (80.6%), slightly lower than Zn (83.75%). | [82] |
| Zn0.7Li | Direct (cytoskeletal and nuclear staining) | MC3T3-E1 | 2 h | Cell suspension over the surface of samples. | Bulk and porous samples. | Better cell adhesion, viability and healthier morphology on porous samples. | [85] |
| Zn2Al | Indirect CCK-8 | MG-63 | 1, 4, and 7 days | DMEM + FBS, antibiotics | Dense samples. 100% and 50% extracts. (1.25 cm2/mL). | Viability 67.5% (100% extracts); >80% (50% extracts). Viability increased over time. | [81] |
| Zn scaffolds | Direct live/dead | MG-63 | 24 h | DMEM + 1 g/L glucose + FBS | Most cells were viable (>70%) | [90] | |
| indirect CellTiter 96® | 24, 48, and 72 h | Extract 0.2 g Zn/mL for 72 h | After 24 h, viability > 95% for all specimens | ||||
| Zn scaffolds | Direct Calcein staining and DNA measurement | hTERT-MSCs | 14 days | high glucose DMEM + FBS + 1% PenStrep | Static seeding and dynamic seeding in a bioreactor | No cell attachment and growth for the Zn scaffolds. | [86] |
| Zn scaffolds | Indirect CCK-8 and ALP activity | MC3T3-E1 | 1, 3, and 5 days | α-MEM + 10% FBS | 100%, 50%, and 10% extracts | Viability < 75% (100% extract afetr 1 day); viability > 75% after 3 and 5 days. | [92] |
| Zn scaffolds | Direct live/dead | MG-63 | 24 h | DMEM + 1 g/L glucose + 10% FBS | Most cells were viable, results similar to Ti6Al4V | [91] | |
| Indirect CellTiter 96® | 24, 48, and 72 h | Extract 0.2 g Zn/mL for 72 h | Viability > 95% at 24, decreased to 85% at 72 h |
3.6. In Vivo Studies
| Material | Shape | Animal | Implantation Site | Duration | Results | Ref. |
|---|---|---|---|---|---|---|
| Zn | Scaffold | Rabbit | Femur | 24 weeks | Successful osseointegration of the scaffold | [92] |
| Zn and Zn1Mg | Scaffold | Rabbit | Femur | 12 weeks | Osseointegration of Zn1Mg scaffolds. Fibrous connective tissue between bone tissue and Zn scaffold | [83] |
4. Discussion
4.1. Linking Processing Parameters and Energy Density to Densification
4.2. Influence of the Microstructure on Hardness
4.3. Mechanical Properties of Dense Structures and Scaffolds
4.4. Corrosion Behavior
4.5. In Vitro and In Vivo Studies
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Ryu, H.; Seo, M.H.; Rogers, J.A. Bioresorbable Metals for Biomedical Applications: From Mechanical Components to Electronic Devices. Adv. Healthc. Mater. 2021, 10, 2002236. [Google Scholar] [CrossRef]
- Han, H.S.; Loffredo, S.; Jun, I.; Edwards, J.; Kim, Y.C.; Seok, H.K.; Witte, F.; Mantovani, D.; Glyn-Jones, S. Current status and outlook on the clinical translation of biodegradable metals. Mater. Today 2019, 23, 57–71. [Google Scholar] [CrossRef]
- Cifuentes, S.C.; San-Miguel, V.; Wang, Y.; García-Peñas, A. Bioresorbable metals for cardiovascular and fracture repair implants. In Nanohybrids Future Materials for Biomedical Applications; Materials Research Foundations: Millersville, PA, USA, 2020; pp. 134–155. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Zhou, J.; Zadpoor, A.A. Additively manufactured biodegradable porous metals. Acta Biomater. 2020, 115, 29–50. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Yokoi, H.; Nakagawa, Y.; Tamura, T.; Kaburagi, S.; Sawada, Y.; Sato, Y.; Yokoi, H.; Hamasaki, N.; Nosaka, H.; et al. Three-Year Follow-up after Implantation of Metallic Coronary-Artery Stents. N. Engl. J. Med. 1996, 334, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Moravej, M.; Mantovani, D. Biodegradable metals for cardiovascular stent application: Interests and new opportunities. Int. J. Mol. Sci. 2011, 12, 4250–4270. [Google Scholar] [CrossRef]
- Agrawal, C.M.; Haas, K.F.; Leopold, D.A.; Clark, H.G. Evaluation of poly(L-lactic acid) as a material for intravascular polymeric stents. Biomaterials 1992, 13, 176–182. [Google Scholar] [CrossRef]
- Zhou, C.; Li, H.; Yin, Y.; Shi, Z.; Li, T.; Feng, X.; Zhang, J.; Song, C.; Cui, X.; Xu, K.; et al. Long-term in vivo study of biodegradable Zn-Cu stent: A 2-year implantation evaluation in porcine coronary artery. Acta Biomater. 2019, 97, 657–670. [Google Scholar] [CrossRef]
- Nica, M.; Cretu, B.; Ene, D.; Antoniac, I.; Gheorghita, D.; Ene, R. Failure Analysis of Retrieved Osteosynthesis Implants. Materials 2020, 13, 1201. [Google Scholar] [CrossRef]
- Prediger, B.; Mathes, T.; Probst, C.; Pieper, D. Elective removal vs. retaining of hardware after osteosynthesis in asymptomatic patients—A scoping review. Syst. Rev. 2020, 9, 225. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shi, Y.; Lu, Y.; Li, X.; Zhou, J.; Zadpoor, A.A.; Wang, L. Additive manufacturing of vascular stents. Acta Biomater. 2023, 167, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qin, L.; Yang, K.; Ma, Z.; Wang, Y.; Cheng, L.; Zhao, D. Materials evolution of bone plates for internal fixation of bone fractures: A review. J. Mater. Sci. Technol. 2020, 36, 190–208. [Google Scholar] [CrossRef]
- Barati, D. Biodegradable Hybrid Tissue Engineering Scaffolds for Reconstruction of Large Bone Defects. Ph.D. Thesis, University of South Carolina, Columbia, SC, USA, 2016. Available online: https://scholarcommons.sc.edu/cgi/viewcontent.cgi?article=4831&context=etd (accessed on 29 November 2024).
- Li, H.; Zheng, Y.; Qin, L. Progress of biodegradable metals. Prog. Nat. Sci. Mater. Int. 2014, 24, 414–422. [Google Scholar] [CrossRef]
- Tilton, M.; Lewis, G.S.; Bok Wee, H.; Armstrong, A.; Hast, M.W.; Manogharan, G. Additive manufacturing of fracture fixation implants: Design, material characterization, biomechanical modeling and experimentation. Addit. Manuf. 2020, 33, 101137. [Google Scholar] [CrossRef]
- Milewski, J.O. Additive Manufacturing of Metals; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-58205-4. [Google Scholar]
- Bedmar, J.; Riquelme, A.; Rodrigo, P.; Torres, B.; Rams, J. Comparison of different additive manufacturing methods for 316l stainless steel. Materials 2021, 14, 6504. [Google Scholar] [CrossRef]
- Yang, L.; Hsu, K.; Baughman, B.; Godfrey, D.; Medina, F.; Menon, M.; Wiene, S. Additive Manufacturing of Metals: The Technology, Materials, Design and Production; Springer: Cham, Switzerland, 2017; ISBN 978-3-319-55127-2. [Google Scholar] [CrossRef]
- Pérez-Ruiz, J.D.; Marin, F.; Martínez, S.; Lamikiz, A.; Urbikain, G.; López de Lacalle, L.N. Stiffening near-net-shape functional parts of Inconel 718 LPBF considering material anisotropy and subsequent machining issues. Mech. Syst. Signal Process. 2022, 168, 1–18. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Fonseca, J.; Peixinho, N.; Alves, N.; Gasik, M.; Silva, F.S.; Miranda, G. Predicting the output dimensions, porosity and elastic modulus of additive manufactured biomaterial structures targeting orthopedic implants. J. Mech. Behav. Biomed. Mater. 2019, 99, 104–117. [Google Scholar] [CrossRef]
- Krakhmalev, P.; Yadroitsev, I.; Yadroitsava, I.; de Smidt, O. Functionalization of biomedical Ti6Al4V via in situ alloying by Cu during laser powder bed fusion manufacturing. Materials 2017, 10, 1154. [Google Scholar] [CrossRef]
- Gatto, M.L.; Cerqueni, G.; Groppo, R.; Santecchia, E.; Tognoli, E.; Defanti, S.; Mattioli-Belmonte, M.; Mengucci, P. Improved biomechanical behavior of 316L graded scaffolds for bone tissue regeneration produced by laser powder bed fusion. J. Mech. Behav. Biomed. Mater. 2023, 144, 105989. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Yap, C.Y.; Chua, C.K.; Dong, Z.L.; Liu, Z.H.; Zhang, D.Q.; Loh, L.E.; Sing, S.L. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015, 2, 41101. [Google Scholar] [CrossRef]
- Liu, J.H.; Shi, Y.S.; Lu, Z.L.; Xu, Y.; Chen, K.H.; Huang, S.H. Manufacturing metal parts via indirect SLS of composite elemental powders. Mater. Sci. Eng. A 2007, 444, 146–152. [Google Scholar] [CrossRef]
- Maeda, K.; Childs, T.H.C. Laser sintering (SLS) of hard metal powders for abrasion resistant coatings. J. Mater. Process. Technol. 2004, 149, 609–615. [Google Scholar] [CrossRef]
- Xu, W. Direct Additive Manufacturing Techniques for Metal Parts: SLM, EBM, Laser Metal Deposition. Encycl. Mater. Met. Alloys 2022, 3, 290–318. [Google Scholar] [CrossRef]
- Zhu, H.; Fu, X.; Fan, S.; Liang, L.; Lin, X.; Ning, Y. The conversion from a Gaussian-like beam to a flat-top beam in the laser hardening processing using a fiber coupled diode laser source. Opt. Laser Technol. 2020, 125, 106028. [Google Scholar] [CrossRef]
- Lee, H.; Lim, C.H.J.; Low, M.J.; Tham, N.; Murukeshan, V.M.; Kim, Y.J. Lasers in additive manufacturing: A review. Int. J. Precis. Eng. Manuf.-Green Technol. 2017, 4, 307–322. [Google Scholar] [CrossRef]
- Prabakaran, M.P.; Kannan, G.R. Optimization of CO2 Laser Beam Welding Process Parameters to Attain Maximum Weld Strength in Dissimilar Metals. Mater. Today Proc. 2018, 5, 6607–6616. [Google Scholar] [CrossRef]
- Wen, P.; Voshage, M.; Jauer, L.; Chen, Y.; Qin, Y.; Poprawe, R.; Schleifenbaum, J.H. Laser additive manufacturing of Zn metal parts for biodegradable applications: Processing, formation quality and mechanical properties. Mater. Des. 2018, 155, 36–45. [Google Scholar] [CrossRef]
- Thomas, M.; Baxter, G.J.; Todd, I. Normalised model-based processing diagrams for additive layer manufacture of engineering alloys. Acta Mater. 2016, 108, 26–35. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Zhou, Z. Influence of hatch distance on processing, microstructure and mechanical properties of AlMgScZr alloy fabricated by laser powder bed fusion. J. Manuf. Process. 2022, 81, 78–91. [Google Scholar] [CrossRef]
- Afrasiabi, M.; Lüthi, C.; Bambach, M.; Wegener, K. Multi-resolution SPH simulation of a laser powder bed fusion additive manufacturing process. Appl. Sci. 2021, 11, 2962. [Google Scholar] [CrossRef]
- Hermawan, H.; Alamdari, H.; Mantovani, D.; Dubé, D. Iron–manganese: New class of metallic degradable biomaterials prepared by powder metallurgy. Powder Metall. 2008, 51, 38–45. [Google Scholar] [CrossRef]
- Sikora-jasinska, M.; Chevallier, P.; Turgeon, S.; Paternoster, C.; Mostaed, E.; Vedani, M.; Mantovani, D. Understanding the e ff ect of the reinforcement addition on corrosion behavior of Fe/Mg 2 Si composites for biodegradable implant applications. Mater. Chem. Phys. 2019, 223, 771–778. [Google Scholar] [CrossRef]
- Moravej, M.; Purnama, A.; Fiset, M.; Couet, J.; Mantovani, D. Electroformed pure iron as a new biomaterial for degradable stents: In vitro degradation and preliminary cell viability studies q. Acta Biomater. 2010, 6, 1843–1851. [Google Scholar] [CrossRef]
- Chuan-hao, N.I.; Qiang, X.U.; Fu-chi, W. Grain refinement process of pure iron target under hypervelocity impact. Trans. Nonferrous Met. Soc. China 2010, 21, 1029–1034. [Google Scholar] [CrossRef]
- Hermawan, H.; Purnama, A.; Dube, D.; Couet, J.; Mantovani, D. Fe–Mn alloys for metallic biodegradable stents: Degradation and cell viability studies q. Acta Biomater. 2010, 6, 1852–1860. [Google Scholar] [CrossRef]
- Drynda, A.; Hassel, T.; Bach, F.W.; Peuster, M. In vitro and in vivo corrosion properties of new iron–manganese alloys designed for cardiovascular applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 649–660. [Google Scholar] [CrossRef]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Löf, J.F.; Uggowitzer, P.J. Degradation performance of biodegradable FeMnC (Pd) alloys. Mater. Sci. Eng. C 2013, 33, 1882–1893. [Google Scholar] [CrossRef]
- Schinhammer, M.; Gerber, I.; Hänzi, A.C.; Uggowitzer, P.J. On the cytocompatibility of biodegradable Fe-based alloys. Mater. Sci. Eng. C 2013, 33, 782–789. [Google Scholar] [CrossRef]
- Peuster, M.; Hesse, C.; Schloo, T.; Fink, C.; Beerbaum, P.; Von Schnakenburg, C. Long-term biocompatibility of a corrodible peripheral iron stent in the porcine descending aorta. Biomaterials 2006, 27, 4955–4962. [Google Scholar] [CrossRef] [PubMed]
- Oliver, A.A.; Sikora-jasinska, M.; Gökhan, A.; Guillory, R.J. Recent advances and directions in the development of bioresorbable metallic cardiovascular stents: Insights from recent human and in vivo studies. Acta Biomater. 2021, 127, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Qiu, H.; Tian, Y.; Hu, X.; Luo, T.; Wu, C.; Tian, Y.; Tang, Y.; Song, L.; Li, L.; et al. Preclinical Evaluation of a Novel Sirolimus-Eluting Iron Bioresorbable Coronary Scaffold in Porcine Coronary Artery at 6 Months. Cardiovasc. Interv. 2019, 12, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xi, Z.; Li, Y.; Li, J.; Qiu, H.; Hu, X.; Luo, T.; Wu, C.; Wang, X.; Song, L.; et al. Long-term safety and absorption assessment of a novel bioresorbable nitrided iron scaffold in porcine coronary artery. Bioact. Mater. 2022, 17, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Wegener, B.; Sievers, B.; Utzschneider, S.; Müller, P.; Jansson, V.; Rößler, S.; Nies, B.; Stephani, G.; Kieback, B.; Quadbeck, P. Microstructure, cytotoxicity and corrosion of powder-metallurgical iron alloys for biodegradable bone replacement materials. Mater. Sci. Eng. B 2011, 176, 1789–1796. [Google Scholar] [CrossRef]
- Yang, C.; Huan, Z.; Wang, X.; Wu, C.; Chang, J. 3D Printed Fe Scaffolds with HA Nanocoating for Bone Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 608–616. [Google Scholar] [CrossRef]
- Song, B.; Dong, S.; Deng, S.; Liao, H.; Coddet, C. Microstructure and tensile properties of iron parts fabricated by selective laser melting. Opt. Laser Technol. 2014, 56, 451–460. [Google Scholar] [CrossRef]
- Carluccio, D.; Bermingham, M.; Kent, D.; Demir, A.G.; Previtali, B.; Dargusch, M.S. Comparative Study of Pure Iron Manufactured by Selective Laser Melting, Laser Metal Deposition, and Casting Processes. Adv. Eng. Mater. 2019, 21, 1900049. [Google Scholar] [CrossRef]
- Carluccio, D.; Xu, C.; Venezuela, J.; Cao, Y.; Kent, D.; Bermingham, M.; Demir, A.G.; Previtali, B.; Ye, Q.; Dargusch, M. Additively manufactured iron-manganese for biodegradable porous load-bearing bone scaffold applications. Acta Biomater. 2020, 103, 346–360. [Google Scholar] [CrossRef]
- Carluccio, D.; Demir, A.G.; Caprio, L.; Previtali, B.; Bermingham, M.J.; Dargusch, M.S. The influence of laser processing parameters on the densification and surface morphology of pure Fe and Fe-35Mn scaffolds produced by selective laser melting. J. Manuf. Process. 2019, 40, 113–121. [Google Scholar] [CrossRef]
- Shuai, C.; Yang, W.; Yang, Y.; Pan, H.; He, C.; Qi, F.; Xie, D.; Liang, H. Selective laser melted Fe-Mn bone scaffold: Microstructure, corrosion behavior and cell response. Mater. Res. Express 2019, 7, 015404. [Google Scholar] [CrossRef]
- Palousek, D.; Pantelejev, L.; Zikmund, T.; Koutny, D. Processing of nearly pure iron using 400W selective laser melting—Initial study. MM Sci. J. 2017, 2017, 1738–1743. [Google Scholar] [CrossRef]
- Song, B.; Dong, S.; Liu, Q.; Liao, H.; Coddet, C. Vacuum heat treatment of iron parts produced by selective laser melting: Microstructure, residual stress and tensile behavior. Mater. Des. 2014, 54, 727–733. [Google Scholar] [CrossRef]
- Hansen, N. Hall–Petch relation and boundary strengthening. Scr. Mater. 2004, 51, 801–806. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwish, D.G. Materials Science and Engineering an Introduction; Wiley: Hoboken, NJ, USA, 2014; ISBN 978-1-118-32457-8. [Google Scholar]
- Li, Y.; Jahr, H.; Pavanram, P.; Bobbert, F.S.L.; Puggi, U.; Zhang, X.; Pouran, B.; Leeflang, M.A.; Weinans, H.; Zhou, J.; et al. Additively manufactured functionally graded biodegradable porous iron. Acta Biomater. 2019, 96, 646–661. [Google Scholar] [CrossRef]
- Misch, C.E.; Qu, Z.; Bidez, M.W. Mechanical Properties of Trabecular Bone in the Human Mandible: Implications for Dental Implant Treatment Planning and Surgical Placement. J. Oral Maxillofac. Surg. 1999, 57, 700–706. [Google Scholar] [CrossRef]
- Gabriela, G. Biodegradable Iron-Based Materials—What Was Done and What More Can Be Done? Materials 2021, 14, 3381. [Google Scholar] [CrossRef]
- Li, Y.; Jahr, H.; Lietaert, K.; Pavanram, P.; Yilmaz, A.; Fockaert, L.I.; Leeflang, M.A.; Pouran, B.; Gonzalez-garcia, Y.; Weinans, H.; et al. Additively manufactured biodegradable porous iron. Acta Biomater. 2018, 77, 380–393. [Google Scholar] [CrossRef]
- Nie, Y.; Chen, G.; Peng, H.; Tang, S.; Zhou, Z.; Pei, F.; Shen, B. In vitro and 48 weeks in vivo performances of 3D printed porous Fe-30Mn biodegradable scaffolds. Acta Biomater. 2021, 121, 724–740. [Google Scholar] [CrossRef]
- Dargusch, M.S.; Dehghan-Manshadi, A.; Shahbazi, M.; Venezuela, J.; Tran, X.; Song, J.; Liu, N.; Xu, C.; Ye, Q.; Wen, C. Exploring the Role of Manganese on the Microstructure, Mechanical Properties, Biodegradability, and Biocompatibility of Porous Iron-Based Scaffolds. ACS Biomater. Sci. Eng. 2019, 5, 1686–1702. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Pantopoulos, K. Iron metabolism and toxicity. Toxicol. Appl. Pharmacol. 2005, 202, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and Electron-Beam Powder-Bed Additive Manufacturing of Metallic Implants: A Review on Processes, Materials and Designs. J. Orthop. Res. 2016, 34, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Vallee, B.L.; Falchuk, H. The Biochemical Basis of Zinc Physiology. Physiol. Rev. 1993, 73, 79–118. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Zhang, W.; Tian, P.; Meng, F.; Zhu, H.; Jiang, X.; Liu, X.; Chu, P.K. Biomaterials Stimulation of bone growth following zinc incorporation into biomaterials. Biomaterials 2014, 35, 6882–6897. [Google Scholar] [CrossRef] [PubMed]
- Mcclain, C.J. Antiatherogenic Properties of Zinc: Implications in Endothelial Cell Metabolism. Nutrition 1996, 12, 711–717. [Google Scholar]
- Bowen, P.K.; Drelich, J.; Goldman, J. Zinc Exhibits Ideal Physiological Corrosion Behavior for Bioabsorbable Stents. Adv. Mater. 2013, 25, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, G.; Jia, Q.; Bian, D.; Guan, S.; Kulyasova, O.; Valiev, R.Z.; Rau, J.V.; Zheng, Y. Recent advances on the mechanical behavior of zinc based biodegradable metals focusing on the strain softening phenomenon. Acta Biomater. 2022, 152, 1–18. [Google Scholar] [CrossRef]
- Li, H.F.; Xie, X.H.; Zheng, Y.F.; Cong, Y.; Zhou, F.Y.; Qiu, K.J.; Wang, X.; Chen, S.H. Development of biodegradable Zn-1X binary alloys with nutrient alloying elements Mg, Ca and Sr. Sci. Rep. 2015, 5, 10719. [Google Scholar] [CrossRef]
- Bowen, P.K.; Guillory, R.J.; Shearier, E.R.; Seitz, J.; Drelich, J.; Bocks, M.; Zhao, F.; Goldman, J. Metallic zinc exhibits optimal biocompatibility for bioabsorbable endovascular stents. Mater. Sci. Eng. C 2015, 56, 467–472. [Google Scholar] [CrossRef]
- Lietaert, K.; Baekelant, W.; Thijs, L.; Vleugels, J. Direct Metal Printing of Zinc: From Single Laser Tracks to High Density Parts. In Proceedings of the European Congress and Exhibition on Powder Merallurgy, European PM Conference Proceedings, Lisbon, Portugal, 1–4 October 2016. [Google Scholar]
- Montani, M.; Demir, A.G.; Mostaed, E.; Vedani, M.; Previtali, B. Processability of pure Zn and pure Fe by SLM for biodegradable metallic implant manufacturing. Rapid Prototyp. J. 2017, 23, 514–523. [Google Scholar] [CrossRef]
- Demir, A.G.; Monguzzi, L.; Previtali, B. Selective laser melting of pure Zn with high density for biodegradable implant manufacturing. Addit. Manuf. 2017, 15, 20–28. [Google Scholar] [CrossRef]
- Wen, P.; Jauer, L.; Voshage, M.; Chen, Y.; Poprawe, R.; Henrich, J. Densi fi cation behavior of pure Zn metal parts produced by selective laser melting for manufacturing biodegradable implants. J. Mater. Process. Technol. 2018, 258, 128–137. [Google Scholar] [CrossRef]
- Wen, P.; Qin, Y.; Chen, Y.; Voshage, M.; Jauer, L.; Poprawe, R.; Henrich, J. Laser additive manufacturing of Zn porous scaffolds: Shielding gas flow, surface quality and densification. J. Mater. Sci. Technol. 2019, 35, 368–376. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, P.; Voshage, M.; Chen, Y.; Georg, P.; Jauer, L.; Xia, D.; Guo, H.; Zheng, Y.; Henrich, J. Additive manufacturing of biodegradable Zn-xWE43 porous scaffolds: Formation quality, microstructure and mechanical properties. Mater. Des. 2019, 181, 107937. [Google Scholar] [CrossRef]
- Shuai, C.; Cheng, Y.; Yang, Y.; Peng, S.; Yang, W. Laser additive manufacturing of Zn-2Al part for bone repair: Formability, microstructure and properties. J. Alloys Compd. 2019, 798, 606–615. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, M.; He, C.; Qi, F.; Wang, D.; Peng, S.; Shuai, C. Rare earth improves strength and creep resistance of additively manufactured Zn implants. Compos. Part B 2021, 216, 108882. [Google Scholar] [CrossRef]
- Yang, Y.; Yuan, F.; Gao, C.; Feng, P.; Xue, L.; He, S. A combined strategy to enhance the properties of Zn by laser rapid solidi fi cation and laser alloying. J. Mech. Behav. Biomed. Mater. 2018, 82, 51–60. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, A.; Guo, H.; Shen, Y.; Wen, P.; Lin, H. Additive manufacturing of Zn-Mg alloy porous scaffolds with enhanced osseointegration: In vitro and in vivo studies. Acta Biomater. 2022, 145, 403–415. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, H.; Liu, A.; Dai, J.; Wen, P.; Zheng, Y. Processing optimization, mechanical properties, corrosion behavior and cytocompatibility of additively manufactured Zn-0.7Li biodegradable metals. Acta Biomater. 2022, 142, 388–401. [Google Scholar] [CrossRef]
- Lietaert, K.; Zadpoor, A.A.; Sonnaert, M.; Schrooten, J.; Weber, L.; Mortensen, A.; Vleugels, J. Mechanical properties and cytocompatibility of dense and porous Zn produced by laser powder bed fusion for biodegradable implant applications. Acta Biomater. 2020, 110, 289–302. [Google Scholar] [CrossRef]
- Qin, Y.; Wen, P.; Xia, D.; Guo, H.; Voshage, M.; Jauer, L.; Zheng, Y.; Schleifenbaum, J.H.; Tian, Y. Effect of grain structure on the mechanical properties and in vitro corrosion behavior of additively manufactured pure Zn. Addit. Manuf. 2020, 33, 101134. [Google Scholar] [CrossRef]
- Shuai, C.; Xue, L.; Gao, C.; Yang, Y.; Peng, S.; Zhang, Y. Selective laser melting of Zn–Ag alloys for bone repair: Microstructure, mechanical properties and degradation behaviour. Virtual Phys. Prototyp. 2018, 13, 146–154. [Google Scholar] [CrossRef]
- Li, Z.; Shi, Z.Z.; Yan, Y.; Zhang, D.; Yang, K.; Li, H.F.; Zhang, H.; Wang, L.N. Suppression mechanism of initial pitting corrosion of pure Zn by Li alloying. Corros. Sci. 2021, 189, 109564. [Google Scholar] [CrossRef]
- Li, Y.; Pavanram, P.; Zhou, J.; Lietaert, K.; Bobbert, F.S.L.; Kubo, Y.; Leeflang, M.A.; Jahr, H.; Zadpoor, A.A. Additively manufactured functionally graded biodegradable porous zinc. Biomater. Sci. 2020, 8, 2404–2419. [Google Scholar] [CrossRef]
- Li, Y.; Pavanram, P.; Zhou, J.; Lietaert, K.; Taheri, P.; Li, W.; San, H.; Leeflang, M.A.; Mol, J.M.C.; Jahr, H.; et al. Additively manufactured biodegradable porous zinc. Acta Biomater. 2020, 101, 609–623. [Google Scholar] [CrossRef]
- Xia, D.; Qin, Y.; Guo, H.; Wen, P.; Lin, H.; Voshage, M.; Schleifenbaum, J.H.; Cheng, Y.; Zheng, Y. Additively manufactured pure zinc porous scaffolds for critical-sized bone defects of rabbit femur. Bioact. Mater. 2023, 19, 12–23. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Arlington, VA, USA, 2009.
- ISO 10993-4:2017; Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood. International Organization for Standardization: Arlington, VA, USA, 2017.
| Amount in Human Organism (g) | Blood Serum Level | Daily Allowance | Young’s Modulus (GPa) | In Vitro Corrosion Rate (mm/Year) | |
|---|---|---|---|---|---|
| Fe | 5 | 5–17.6 g/L | 10–20 mg | 200 | 0.012 |
| Mg | 25 | 1.6–2.5 mg/dL | 0.7 g | 41–45 | 0.10 ± 0.07 |
| Zn | 2 | 60–120 µg/dL | 12–15 mg | 96 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limón, I.; Bedmar, J.; Fernández-Hernán, J.P.; Multigner, M.; Torres, B.; Rams, J.; Cifuentes, S.C. A Review of Additive Manufacturing of Biodegradable Fe and Zn Alloys for Medical Implants Using Laser Powder Bed Fusion (LPBF). Materials 2024, 17, 6220. https://doi.org/10.3390/ma17246220
Limón I, Bedmar J, Fernández-Hernán JP, Multigner M, Torres B, Rams J, Cifuentes SC. A Review of Additive Manufacturing of Biodegradable Fe and Zn Alloys for Medical Implants Using Laser Powder Bed Fusion (LPBF). Materials. 2024; 17(24):6220. https://doi.org/10.3390/ma17246220
Chicago/Turabian StyleLimón, Irene, Javier Bedmar, Juan Pablo Fernández-Hernán, Marta Multigner, Belén Torres, Joaquín Rams, and Sandra C. Cifuentes. 2024. "A Review of Additive Manufacturing of Biodegradable Fe and Zn Alloys for Medical Implants Using Laser Powder Bed Fusion (LPBF)" Materials 17, no. 24: 6220. https://doi.org/10.3390/ma17246220
APA StyleLimón, I., Bedmar, J., Fernández-Hernán, J. P., Multigner, M., Torres, B., Rams, J., & Cifuentes, S. C. (2024). A Review of Additive Manufacturing of Biodegradable Fe and Zn Alloys for Medical Implants Using Laser Powder Bed Fusion (LPBF). Materials, 17(24), 6220. https://doi.org/10.3390/ma17246220









